Abstract
This study assessed the impact of serotonin transporter genotype (5-HTTLPR) on regional responses to emotional faces in the amygdala and subgenual cingulate cortex (sgACC), while subjects performed a gender discrimination task. Although we found no evidence for greater amygdala reactivity or reduced amygdala–sgACC coupling in short variant 5-HTTLPR homozygotes (s/s), we observed an interaction between genotype and emotion in sgACC. Only long variant homozygotes (la/la) exhibited subgenual deactivation to fearful versus neutral faces, whereas the effect in s/s subjects was in the other direction. This absence of subgenual deactivation in s/s subjects parallels a recent finding in depressed subjects [Grimm, S., Boesiger, P., Beck, J., Schuepbach, D., Bermpohl, F., Walter, M., et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology, 34, 932–943, 2009]. Taken together, the findings suggest that subgenual cingulate activity may play an important role in regulating the impact of aversive stimuli, potentially conferring greater resilience to the effects of aversive stimuli in la/la subjects. Using dynamic causal modeling of functional magnetic resonance imaging data, we explored the effects of genotype on effective connectivity and emotion-specific changes in coupling across a network of regions implicated in social processing. Viewing fearful faces enhanced bidirectional excitatory coupling between the amygdala and the fusiform gyrus, and increased the inhibitory influence of the amygdala over the sgACC, although this modulation of coupling did not differ between the genotype groups. The findings are discussed in relation to the role of sgACC and serotonin in moderating responses to aversive stimuli [Dayan, P., & Huys, Q. J., Serotonin, inhibition, and negative mood. PLoS Comput Biol, 4, e4, 2008; Mayberg, H. S., Liotti, M., Brannan, S. K., McGinnis, S., Mahurin, R. K., Jerabek, P. A., et al. Reciprocal limbic–cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry, 156, 675–682, 1999].
INTRODUCTION
Processing facial emotions depends on a distributed network of cortical and subcortical structures (Haxby, Hoffman, & Gobbini, 2000). This network, comprising the lateral occipital cortex (LOC), fusiform gyrus (FG), amygdala, inferior frontal gyrus (IFG), posterior superior temporal sulcus (pSTS), and anterior cingulate cortex, plays a pivotal role in the detection and decoding of socially relevant visual stimuli (Castelli, Happe, Frith, & Frith, 2000; Baron-Cohen et al., 1999; Brothers, Ring, & Kling, 1990). Functional specialization across this network has been explored extensively with respect to face processing. Dorsal components (pSTS and IFG) appear to process dynamic aspects of facial stimuli, including gaze direction and expression (Pelphrey, Morris, & McCarthy, 2004; George, Driver, & Dolan, 2001). Ventral components (LOC, FG, and amygdala) are thought to process invariant features (Haxby et al., 2000; Kanwisher, McDermott, & Chun, 1997) such as large eye whites, which signal fear (Whalen et al., 2004).
In a seminal study, Fairhall and Ishai (2007) used dynamic causal modeling (DCM) to explore connectivity across this network during face processing. They identified hierachial organization in the “core” network, with LOC exerting direct influence over both the FG and pSTS, with no evidence that including feedback or lateral connections increased model fit. Responses in the “extended” components of the network (IFG, amygdala, and orbito-frontal cortex) were predominantly driven by inputs from the FG, with a reduction in model fit observed when connections were modeled from the STS.
In addition to examining connectivity related to viewing face stimuli, Fairhall and Ishai also examined the modulatory effects of emotional valence on coupling. Viewing emotional faces increased effective connectivity in the ventral component of this network, from the LOC to the FG, and from the FG to the amygdala. Their findings are consistent with the wealth of evidence indicating the centrality of the amygdala in processing emotions, particularly fear; consistent with its more general role as a threat-detection system (Dolan, 2002; Adolphs, Tranel, Damasio, & Damasio, 1994).
A plethora of studies have demonstrated that genetic factors contribute to individual differences in responsivity to threat-relevant stimuli at the behavioral and neural levels (Gotlib, Joormann, Minor, & Hallmayer, 2008; Strange, Kroes, Roiser, Tan, & Dolan, 2008; Roiser, Cook, Cooper, Rubinsztein, & Sahakian, 2005; Hariri et al., 2002; Garpenstrand, Annas, Ekblom, Oreland, & Fredrikson, 2001). In particular, exaggerated amygdala response to emotional faces, particularly those displaying fear or anger, has been associated with the short allele of a polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) (Dannlowski et al., 2010; Bertolino et al., 2005; Pezawas et al., 2005; Hariri et al., 2002), although others have failed to report this effect (Thomason et al., 2010; Shah et al., 2009). Differential responses to aversive stimuli related to 5-HTTLPR genotype have also been observed in subgenual cingulate cortex (Fortier et al., 2010; Fukudo et al., 2009; Shah et al., 2009), a region strongly implicated in mood disorders and pivotal in the regulation of amygdala response (Schardt et al., 2010; Grimm et al., 2009; Drevets et al., 1997). Volumetric reductions in both the amygdala and sgACC, as well as altered functional and structural connectivity between the two, have also been linked with the short allele (Schardt et al., 2010; Pacheco et al., 2009; Pezawas et al., 2005).
Although convergent evidence suggests modulation of limbic and medial prefrontal response related to 5-HTTLPR genotype, evidence for transient expression of serotonin transporters across diverse glutamatergic cortical networks during pre- and postnatal development raises the possibility that the effects of 5-HTTLPR may extend to diverse neural pathways (Gaspar, Cases, & Maroteaux, 2003). However, to date, studies assessing the influence of 5-HTTLPR polymorphism on functional coupling in the brain have utilized methods that only permit the assessment of connectivity between two regions at a time (i.e., “functional connectivity”; e.g., Schardt et al., 2010; Canli et al., 2006; Pezawas et al., 2005). These studies have also tended to focus on coupling with the amygdala. Moreover, such approaches do not provide insights into the direction of connectivity.
The primary aim of this study was to investigate whether 5-HTTLPR genotype influences responses to emotional faces in the amygdala and sgACC, using an incidental task. We also used DCM to explore whether effective connectivity was influenced by 5-HTTLPR genotype across a distributed network that participates in emotional face processing. Using DCM allowed us to ascertain not only the strength and direction of connectivity but also task-specific effects on coupling, providing insight into the mechanisms behind changes in regional responses (Friston, Harrison, & Penny, 2003). Based on previous findings, we predicted greater amygdala responses to fearful versus neutral faces and reduced amygdala–sgACC coupling in s/s homozygotes while viewing fearful faces (Pezawas et al., 2005; Hariri et al., 2002). As both enhanced and attenuated sgACC response to emotional stimuli have been associated with the short variant, we explored changes in response in this region without a directional hypothesis (Fukudo et al., 2009; Shah et al., 2009). Given that transient serotonin transporter expression influences the development of diverse cortical networks (Gaspar et al., 2003), we predicted differences in connectivity related to serotonin transporter genotype across the network.
METHODS
Subjects
Thirty right-handed volunteers of European ancestry selected for 5-HTTLPR genotype took part in the study. None of these subjects carried a low-frequency A-to-G substitution within the long allele (Hu et al., 2006; Nakamura, Ueno, Sano, & Tanabe, 2000). Fifteen subjects were homozygous for the short allele (s/s) and 15 for the long allele (la/la). Subjects were screened for previous psychiatric disorders using the Mini International Neuropsychiatric Inventory (Sheehan et al., 1998). IQ was measured using the Weschler Test of Adult Reading (Wechsler, 2001). All subjects provided written informed consent before participation, as approved by the National Hospital for Neurology and Neurosurgery and the Institute of Neurology joint ethics committee.
Experimental Paradigm
Subjects were told they would see a series of faces and were instructed to indicate the gender of the face via an MRI-compatible keypad. The task comprised four cycles of three blocks. Each block consisted of faces representing one single emotion (happy, fearful, or neutral faces). Each facial emotion block consisted of eight trials, each lasting 2 sec. Between blocks, there was a 16-sec period during which a central fixation cross was displayed. The scanning session lasted 6 min and 24 sec in total.
Image Acquisition and Analysis
A 3-T head scanner (Magnetom Allegra, Siemens Medical) was used to acquire gradient-echo T2*-weighted images. There were 32 slices per volume, positioned to ensure maximum coverage of prefrontal cortex and subcortical regions. The 30° tilted sequence was designed to minimize dropout in ventral prefrontal cortex and amygdala (Weiskopf, Hutton, Josephs, & Deichmann, 2006). Echo time was 30 msec, repetition time per slice was 65 msec, slice thickness was 2 mm, interslice gap was 1 mm, and in-plane resolution was 2 × 2 × 2 mm. fMRI data were analyzed using Statistical Parametric Mapping (SPM5; Wellcome Trust Centre for Neuroimaging, London; www. fil.ion.uck.ac.uk/spm) in Matlab 7.1. The first six image volumes were removed from the analysis to allow for T1 equilibration. The volumes were realigned to the seventh image, normalized into standardized space [Montreal Neurological Institute (MNI) template], and smoothed using an 8-mm FWHM, to allow for anatomical variability across subjects.
Regressors for each facial emotion were constructed (happy, fearful, and neutral) and convolved with a synthetic hemodynamic response function, with onsets modeled as box-car functions at the presentation of the first face in each 16-sec block. There was one box-car per block. For subjects who made errors or omissions in the gender judgment task, an extra regressor was included to model instances where they may not have been attending to the task. For the five subjects who made no errors, the non-attending regressor was not included. The 16-sec fixation period between blocks constituted an implicit baseline. A high-pass filter with a cutoff of 128 sec was used to remove low-frequency fluctuations. Regression coefficients (parameter estimates, or betas) were then estimated using the general linear model, creating a beta image for each regressor. Parameter estimate images for the facial emotion regressors were linearly combined to calculate the contrasts of interest (happy vs. neutral, fear vs. neutral, and happy vs. fear). These were then used to compare s/s and la/la subjects in random-effects group-level analyses using the t statistic.
The aim of the group-level comparison was to measure differences in response to fearful versus neutral faces associated with 5-HTTLPR genotype in our primary ROIs: the amygdala and sgACC. To define our amygdala ROI, we used a bilateral mask from the Wake Forest University PickAtlas toolbox with the Automated Anatomical Labeling (AAL) atlas (Maldjian, Laurienti, Kraft, & Burdette, 2003). For the sgACC ROI, we constructed an 8-mm sphere surrounding the peak coordinate identified by Drevets et al. (1997) (Talairach and Tournoux coordinates: x = −2, y = 32, z = −2; maximum volumetric reduction across unmedicated unipolar and bipolar depressed subjects, relative to controls). The rationale behind the selection of this ROI was that s/s subjects may be more vulnerable to depression in the context of stress (Uher & McGuffin, 2010), thus by choosing the group maxima of changes associated with depression reported by Drevets et al., we hoped to maximize our chances of detecting group differences in functional response at a potential biomarker for susceptibility. We used the small-volume family-wise error corrected (SV-FWE) p value at the voxel level reported by SPM to determine statistical significance (threshold p = .05, SV-FWE).
For these ROIs, we extracted parameter estimates for each of the regressors (happy, fearful, and neutral) relative to the implicit baseline at the peak voxel for the fear versus neutral contrast (either the main effect of emotion or the Genotype group × Emotion interaction, as appropriate). Where a significant interaction was detected, these parameter estimates were used to identify simple main effects in post hoc analyses. In addition, for completeness and descriptive purposes, we also include the results of whole-brain comparisons at p < .001 (uncorrected) with a spatial extent threshold of 5 voxels (Supplementary Table S3). Although the above analyses were conducted in the context of the mass-univariate framework, we have also included descriptive statistics relating to the mean response across anatomically defined amygdala ROIs (left, right, and combined) for each of the relevant contrasts in the supplementary materials for future meta-analytic studies (Supplementary Table S4). In Table 1, we restrict our reporting of the main effects of visual stimulation (all faces vs. baseline) to the regions or nodes considered by the DCM (see below), along with those in the amygdala and sgACC. The p values were SV-FWE corrected and a threshold of p < .05 was adopted, although without correction for multiple comparisons across regions. The ROIs in this instance comprised spheres of between 4 and 8 mm centered on locations determined a priori from Fairhall and Ishai (2007).
Table 1.
Coordinates of the Nodes for the DCM Analysis, and Corresponding Effects of Visual Stimulation, Emotional Valence, or Emotion × Genotype Interaction: s/s (n = 15), la/la (n = 15)
| Coordinate |
||||||
|---|---|---|---|---|---|---|
| Region | x | y | z | Contrast | Z score | SV-FWE p Value |
| LOC | −42 | −78 | −12 | all faces–baseline | 6.25 | <.001 |
| FG | −42 | −57 | −21 | all faces–baseline | 6.60 | <.001 |
| pSTS | −51 | −54 | 12 | all faces–baseline | 3.37 | .023 |
| IFG | −42 | 15 | 12 | all faces–baseline | 3.26 | .032 |
| AMG | −21 | −6 | −15 | fear–neutral | 3.42 | .020 |
| sgACC | −6 | 30 | 0 | fear–neutral (Genotype × Emotion interaction) | 3.22 | .032 |
LOC = lateral occipital cortex; FG = fusiform gyrus; pSTS = superior temporal sulcus; IFG = inferior frontal gyrus; AMG = amygdala; sgACC = subgenual anterior cingulate cortex.
Power Analysis
Given that we were attempting to replicate the findings of Hariri et al. (2002), the power of the present study to detect an effect of the size they reported warrants particular consideration. In their sample, mean response across the amygdala ROI was 0.28 (SD 0.299) for the s-carriers (n = 14), and 0.03 (SD 0.187) for the l/l group (n = 14); this corresponds to an effect size of d = 1.0016 (Munafò, Brown, & Hariri, 2009). Power calculations indicate that in the present study, at α = .05, an effect of this size was detected at 75% power with a two-tailed test and at 85% power with a one-tailed test, warranted on the grounds of a replication study.
Dynamic Causal Modeling
We selected the nodes for our DCM analysis from the network used by Fairhall and Ishai (2007) in their analysis of effective connectivity during face processing. The decision to use the anatomical regions described by Fairhall and Ishai as nodes for the DCM was based on evidence that viewing faces versus nonface objects or scrambled faces elicits a preferential response across these regions that is independent of valence and task (Ishai, 2008). First, a model was specified consisting of fixed connections across the network and modulators (changes in coupling) related to viewing particular emotional expressions. It should be noted that the modulators on the connections in the models examined changes in coupling related to viewing a particular emotion per se, rather than for a contrast (e.g., fear vs. neutral) as in the univariate analysis.
Having established the position of the nodes for each subject (described in detail in the Results section), we constructed models to describe the dynamic connectivity across the network. We referred to anatomical evidence when specifying the connections for our models, supporting the existence of the fixed connectivity model we assumed (Schmahmann et al., 2007; Croxson et al., 2005; Molnar-Szakacs, Iacoboni, Koski, & Mazziotta, 2005; Catani, Jones, Donato, & Ffytche, 2003; Ongur & Price, 2000).
We elaborated a model space that contained several plausible models. Parameter estimates for the fixed connections and modulators were estimated by fitting the model to each subject’s time series. Following an initially exploratory model identification strategy (Models 1 and 2), models were identified using a heuristic search by successively removing connections/modulators from a model with a fairly complete connectivity structure (Model 3 onward; see Supplementary Figure 1). We continued this process until the model evidence pooled over subjects (see below) stopped increasing, and we were unable to supersede the pooled model evidence for the best model. One-sample t tests on the mean parameter estimates across subjects determined whether the connections were significantly different from zero. Connections were removed from subsequent models if they failed to survive uncorrected thresholds of p < .005 or p < .01 for fixed connections and modulatory effects, respectively.
A formal comparison of the alternative models was based on the model evidence. The model evidence indicates which model provides the best fit to the measured time-series data for each subject. It penalizes model complexity to prevent “overfitting,” whereby the model does not generalize well to other datasets (Friston et al., 2003). This analysis was performed using the group Bayesian Model Comparison tool in SPM8 (Stephan, Penny, Daunizeau, Moran, & Friston, 2009).
Once we had established the best model across all subjects (Model 11), we conducted analyses to compare parameter estimates for connections and modulators between the genotype groups, using independent-samples t tests. In these analyses, we corrected for multiple comparisons according to the number of fixed connections (n = 14) and modulators (n = 5) using the Bonferroni method. However, we report results reaching a nominal threshold of p < .05 using two-tailed p values in Table 2.
Table 2.
Connection Parameters Included in Model 11 for Each of the Genotype Groups
| Parameter Estimates for s/s (n = 15) and la/la (n = 15) |
Evidence for Connection across Subjects |
|||
|---|---|---|---|---|
| Connection | s/s (SD) | la/la (SD) | t Statistic | p |
| Fixed Connections | ||||
| LOC to FG | 0.384 (0.063) | 0.440 (0.076) | 30.48 | 1.406 × 10−23 |
| LOC to pSTS | 0.093 (0.073) | 0.140 (0.062) | 9.08 | 5.571 × 10−10 |
| FG to LOC | 0.064 (0.088) | 0.053 (0.080) | 3.87 | 5.732 × 10−4 |
| FG to pSTS | 0.052 (0.083) | 0.056 (0.066) | 4.04 | 3.618 × 10−4 |
| FG to AMG | 0.131 (0.117) | 0.081 (0.088) | 5.53 | 5.781 × 10−6 |
| FG to IFG | 0.165 (0.093) | 0.198 (0.107) | 9.95 | 7.375 × 10−11 |
| pSTS to LOC | 0.021 (0.034) | 0.026 (0.043) | 3.35 | .002 |
| pSTS to FG | 0.045 (0.030) | 0.070 (0.036) | 9.00 | 6.853 × 10−10 |
| pSTS to AMG | 0.043 (0.046) | 0.032 (0.030) | 5.31 | 1.082 × 10−5 |
| pSTS to IFG | 0.051 (0.063) | 0.071 (0.043) | 6.15 | 1.066 × 10−6 |
| AMG to FG | 0.024 (0.024) | 0.021 (0.028) | 4.85 | 3.832 × 10−5 |
| AMG to IFG | 0.019 (0.027) | 0.026 (0.030) | 4.33 | 1.617 × 10−4 |
| AMG to sgACC | −0.003 (0.034) | −0.005 (0.021) | −0.85 | .404 |
| IFG to AMG | 0.029 (0.031) | 0.010 (0.019) | 4.04 | 3.600 × 10−4 |
| Modulatory Connections | ||||
| FG to AMG | 0.041 (0.083) | 0.044 (0.065) | 3.19 | .003 |
| FG to pSTS | 0.039 (0.110) | 0.055 (0.062) | 2.94 | .006 |
| FG to AMG | 0.035 (0.056) | 0.050 (0.052) | 4.29 | .000 |
| AMG to FG | 0.007 (0.012) | 0.010 (0.018) | 3.04 | .005 |
| AMG to sgACC | −0.009 (0.014) | −0.004 (0.014) | −2.75 | .010 |
Modulatory effects: modulation of the connection strength related to viewing happy faces denoted by an asterisk (*), all other modulatory effects were related to viewing fearful faces. LOC = lateral occipital cortex; FG = fusiform gyrus; pSTS = superior temporal sulcus; IFG = inferior frontal gyrus; AMG = amygdala; sgACC = subgenual anterior cingulate cortex. Given that multiple tests were conducted, Bonferroni correction for multiple comparisons indicates the threshold for α = .05 should be p < .0036 for basic connections and p < .01 for modulators.
Analysis of Behavioral and Demographic Data
Behavioral and demographic data were analyzed with the Statistical Package for the Social Sciences (SPSS) 16, using repeated measures analyses of variance or t tests as appropriate (Supplementary Tables S1 and S2).
RESULTS
Demographic Data
There were no differences in age, gender, or IQ between the genotype groups (Supplementary Table S1).
Behavioral Data
There were no differences between the genotype groups for either response times or gender judgment accuracy, and no evidence for an Emotion × Genotype interaction for either response times [F(2, 56) < 1] or accuracy [F(2, 56) = 2.14, p = .13] (Supplementary Table S2).
Univariate fMRI Analysis
Amygdala
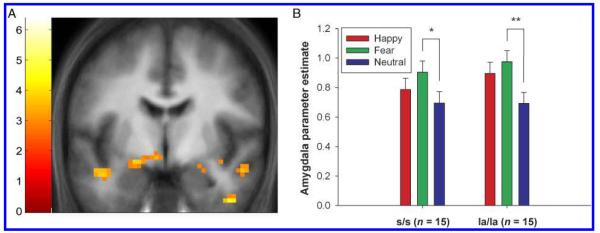
Left amygdala responses were greater for fearful relative to neutral faces, irrespective of genotype (main effect of emotion depicted in Figure 1A; MNI coordinates x = 21, y = −6, z = −15; Z = 3.42, p < .05, SV-FWE). t tests on the parameter estimates at the peak voxel for this contrast confirmed that the effect was present in both genotype groups [Figure 1B; s/s: t(14) = 2.28, p = .04 (uncorrected); la/la: t(14) = 3.07, p = .008 (uncorrected)]. There was no evidence that the amygdala response to fearful relative to neutral faces was greater in s/s subjects within the bilateral amygdala ROI, with no voxels surviving even the liberal threshold of p < .005, uncorrected.
Figure 1.
(A) Left amygdala response to fearful versus neutral faces across all subjects, image thresholded at p < .005 (uncorrected). MNI coordinates for peak voxel: x = −21, y = −6, z = −15. The color bar to the left of the image represents t values. (B) Parameter estimates for each face type relative to baseline at the peak voxel in the left amygdala for fearful versus neutral faces. Both s/s and la/la showed significantly greater amygdala response to fearful versus neutral faces, with no effect of genotype on amygdala response. *p < .05; **p < .01. Error bars represent standard error of the mean.
Because Hariri et al. (2002) did not use a neutral face condition as a baseline, we repeated these analyses comparing the fearful faces versus fixation contrast between groups for comparability with their study. Again, when comparing the genotype groups, no amygdala voxels survived SV-FWE correction, or even the liberal threshold of p < .005 (uncorrected), for this contrast.
Subgenual Cingulate
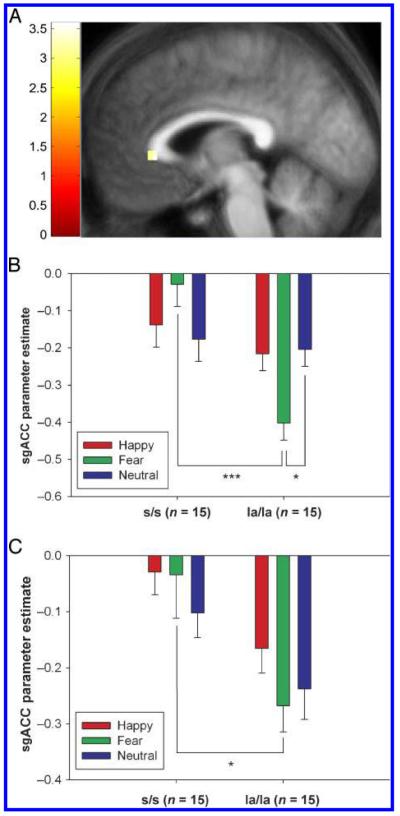
sgACC responses to fearful versus neutral faces differed significantly between the genotype groups (Genotype × Emotion interaction depicted in Figure 2A; MNI coordinates x = −6, y = 30, z = 0; Z = 3.22, p = .032 SV-FWE). Post hoc analysis revealed that in la/la subjects the sgACC response was decreased for fearful relative to neutral faces [Figure 2B; t(14) = 2.89, p = .01, uncorrected], and was increased in the s/s group [Figure 2B; t(14) = 2.21, p = .04, uncorrected]. la/la subjects showed significantly reduced responses to fearful faces (vs. baseline) compared to the s/s subjects at this voxel [t(28) = 5.51, p < .001, uncorrected], while the groups did not differ in terms of responses to neutral faces (t < 1) (Figure 2B).
Figure 2.
(A) Difference in response to fearful versus neutral faces between the genotype groups in sgACC. MNI coordinates for peak voxel: x = −6, y = 30, z = 0. la/la subjects showed a significant reduction in sgACC response to fearful faces relative to s/s subjects. The image is thresholded at p < .005, uncorrected, and the color bar to the left of the image represents t values. (B) Parameter estimates for each face type relative to baseline at the peak voxel for the Genotype × Emotion interaction in left sgACC. la/la subjects showed a reduced response to fearful relative to neutral faces. *p < .05; ***p < .001. Error bars represent standard error of the mean. (C) Parameter estimates for each face type relative to baseline at the subject specific peaks for the nodes extracted in the DCM for left sgACC. The interaction for fearful versus neutral faces was nonsignificant at these voxels; however, there was a significant difference between the genotypes in response to fearful faces versus implicit baseline. *p < .05; ***p < .001. Error bars represent standard error of the mean.
Dynamic Causal Modeling
Having established that contrasting fearful and neutral faces produced robust responses in the amygdala and an interaction with group in sgACC, we next used DCM to explore the mechanisms underpinning these effects by examining functional coupling across regions involved in social processing. The coordinates of the nodes of interest are listed in Table 1. These were the main effects of our task (all faces minus baseline) identified within the anatomical regions described by Fairhall and Ishai (2007). Note that because we had identified a Genotype × Emotion interaction in sgACC, and because sgACC–amygdala coupling was of particular interest in our analysis, we substituted the OFC node used by Fairhall and Ishai (2007) with sgACC. We restricted our connectivity modeling to the left hemisphere, as the emotion-specific effects we aimed to understand in the amygdala and sgACC were both in the left hemisphere.
Identifying the Position of the Nodes for Each Subject
We identified the location of each node for each subject on the basis of their own responses and anatomy using a two-stage process. For the LOC, FG, pSTS, and IFG, an automated procedure (using a custom-written Matlab script) identified each subject’s peak response for all faces versus baseline within a specified distance of the group maximum (code available from the corresponding author on request). The size of the spheres around the group maxima used to constrain the search varied according to the size of the region (LOC: 12 mm; FG: 10 mm; pSTS: 10 mm; IFG: 16 mm). Given our interest in detecting changes in connectivity in emotion processing networks when viewing fearful faces, the automated procedure identified each subject’s peak responses for the fear versus neutral contrast for the amygdala and sgACC nodes. For the amygdala, the AAL atlas was used to generate a mask (PickAtlas toolbox; Maldjian et al., 2003), which constrained the search for the subject-specific peaks. For sgACC, an 8-mm sphere around the peak for the Genotype × Emotion interaction was used (MNI coordinates x = −6, y = 30, z = 0). Given that only la/la subjects exhibited sgACC deactivation to fearful versus neutral faces, while the effect in the s/s subjects was, if anything, in the opposite direction (Figure 2B), we used an F-contrast to identify responses in sgACC (because t contrasts in SPM are unidirectional). We used the peak response within each region without a statistical threshold. This allowed us to include all nodes in each subject’s DCM, and hence to fit DCMs for all subjects, alleviating the potential difficulty that a differential response related to emotion may be stronger in one genotype, as for very few cases there was no response significant at a threshold of p < .05, uncorrected within the appropriate anatomical region.
Once the location of the nodes had been identified for each subject, their responses were overlaid onto their own anatomical scan to manually check that responses were located in the correct anatomical location. Where necessary, a more anatomically appropriate peak was selected. This procedure was carried out blind to genotype. Once each subject’s nodes had been selected, we used the SPM eigenvariate tool to extract the principal component across the time series, adjusting for effects of interest.
Our strategy of selecting subject-specific peaks for the nodes meant that the voxel where the Emotion × Genotype interaction was observed (x = −6, y = 30, z = 0) was not the center for the DCM eigenvariate extraction. As a result, at the extracted subject-specific voxels, the Genotype × Emotion interaction was nonsignificant for the fear versus neutral contrast [F(1, 28) = 1.072, p > .1]. However, for the fear versus baseline contrast, the difference between the genotype groups was significant [F(1, 29) = 6.689, p < .02; Figure 3C].
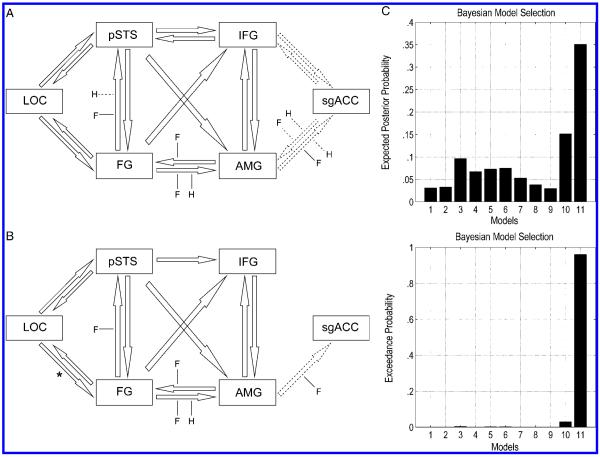
Figure 3.
(A) Specification of the second best model (Model 10) and (B) best model (Model 11). AMG = amygdala; LOC = lateral occipital cortex; FG = fusiform gyrus; pSTS = posterior superior temporal sulcus; IFG = inferior frontal gyrus; sgACC = subgenual cingulate cortex. Arrows with solid edge represent fixed connections modeled across the whole time series significant at p < .005. Dashed edge arrows represent nonsignificant fixed connection included in the model ( p > .005). Solid lines leading to arrows represent modulators significant at p < .01, labeled F (fear) and H (happy). Dashed lines leading to arrows represent nonsignificant modulators included in the model. * connection parameter stronger in la/la versus s/s subjects, nominally significant ( p < .05, 2-tailed, uncorrected). (C) Expected posterior probability and exceedance probability for the 11 models specified. The expected posterior probability is the probability that each model provides the best fit to the measured data across subjects. The expected posterior probability was .3507 for the winning model, and the summed posterior probability was .6469 for the other models. The exceedance probability is the probability that each model provides the best fit to the data relative to other models. The exceedance probability was .9609 for the winning model, and the summed exceedance probability was .0391 for the other models. Both statistics provide overwhelming evidence that Model 11 was the best model.
Model Estimation and Comparison
Our DCM specification procedure (see Methods) furnished 11 models, which were then compared using group Bayesian Model Comparison (Stephan et al., 2009), with random effects on models over subjects. Both the exceedance and posterior probability indicated overwhelmingly that Model 11 was the best model (Figure 3B and C), even in comparison to extremely similar models (Model 10; Figure 3A). Images of the other nine models are displayed in Supplementary Figure 1. Conducting the Bayesian Model Comparison procedure separately for each genotype group demonstrated that Model 11 was the best model for both s/s and la/la subjects (Supplementary Table 5). All the fixed connections in the model were highly significant (save for one; see below), with significant modulatory effects of emotion identified on connections from the FG to amygdala, FG to pSTS, amygdala to FG, and amygdala to sgACC (Table 2). Note that the fixed connection from the amygdala to sgACC was nonsignificant, but this was retained because the modulator on this connection was significant.
Having established that Model 11 was the best model (independent of genotype), we compared the parameter estimates for the connections and modulators between the genotype groups (Table 2 and Figure 3B). The la/la subjects exhibited stronger fixed connections from LOC to the FG [t(28) = 2.21, p = .035, 2-tailed) at a nominally significant threshold, with trends toward nominal significance for LOC to the pSTS [t(28) = 1.90, p = .068, 2-tailed] and from the pSTS to the FG [t(28) = 2.03, p = .052, 2-tailed], whereas s/s subjects showed a nominal trend toward stronger connectivity from the IFG to the amygdala [t(28) = 2.035, p = .051, 2-tailed]. However, none of these results survived correction for multiple comparisons. No differences in modulators between the genotype groups approached even the nominally significant level.
Model estimation was repeated with the sgACC node extracted using an 8-mm sphere around the peak voxel for the Genotype × Emotion interaction for the fear versus neutral contrast from the categorical univariate analysis (i.e., instead of subject-specific peaks, which did not themselves show a significant Genotype × Emotion interaction for the fear versus neutral contrast; Figure 2C). This made very little difference to the results: Model 11 remained the best model with an exceedance probability > 90%. In the majority of cases, the fixed and modulatory coupling parameters were almost identical. However, the modulation by fear of the amygdala–sgACC connection was no longer even nominally significant across all subjects, and the modulation by fear of the amygdala–FG connection no longer survived correction for multiple comparisons. As in the previous analysis, there were no statistically significant differences in the modulatory parameters between the genotype groups.
DISCUSSION
This study assessed the impact of 5-HTTLPR genotype on neural responses to emotional faces using an incidentaltask. We identified an interaction between genotype and emotion in sgACC, a region strongly implicated in depression and negative mood states (Harrison et al., 2009; Drevets, Savitz, & Trimble, 2008; Ongur, Drevets, & Price, 1998), which shares reciprocal connections with the amygdala (Ongur & Price, 2000), although we were unable to replicate previous reports of amygdala hyperreactivity to emotional stimuli in 5-HTTLPR s/s carriers. Using DCM, we examined coupling across a network of regions implicated in social processing, finding strong evidence for functional integration across this network and its modulation by emotion. However, in contrast with previous reports (e.g., Pezawas et al., 2005), there was only weak evidence in our dataset that connectivity in this network differed between the 5-HTTLPR genotype groups, with no results surviving correction for multiple comparisons. Below, we consider separately the effects of genotype on regional responses and functional coupling, and the findings of the DCM with regard to interplay during face processing independent of genotype.
Effects of 5-HTTLPR on Functional Response in the Amygdala and sgACC
Consistent with previous reports of enhanced sgACC response in s-carriers in the context of aversive stimulation (Fortier et al., 2010; Fukudo et al., 2009), we observed an interaction between genotype and emotion in sgACC, with s/s subjects demonstrating increased response to fearful versus neutral faces relative to la/la (Figure 2). Post hoc analyses revealed sgACC deactivation to fearful versus neutral faces in la/la subjects, and increased activation for the same contrast in s/s subjects. Our findings differ from those recently reported by Shah et al. (2009), where no significant Genotype × Emotion interaction was identified in sgACC, using a similar incidental emotional processing task. However, s/s and s/l subjects were pooled into a single s-carrying group, and the rare A-to-G substitution in 5-HTTLPR was not taken into account, which may have decreased sensitivity in this analysis (Shah et al., 2009).
The absence of subgenual deactivation to fearful versus neutral faces in s/s subjects parallels a recent finding in depressed subjects. Although subgenual deactivation was proportional to the subjective aversiveness of the stimuli in controls, there was no relationship between aversiveness and signal change in depressed subjects (Grimm et al., 2009). Several studies have reported evidence for an association between the s-allele and susceptibility to depression, particularly in the context of stressful life events (Uher & McGuffin, 2010; Caspi et al., 2003; Lesch et al., 1996), consistent with greater sensitivity to aversive emotional stimuli assessed cognitively (Strange et al., 2008). Taken together, these findings suggest that subgenual reactivity may be important in emotion regulation, and could explain exaggerated sensitivity to aversive stimuli in short allele carriers (Thomason et al., 2010; Roiser et al., 2009; Strange et al., 2008).
Convergent evidence suggests that the subgenual cingulate may serve to dampen affective responses to support cognitive functioning. Subgenual deactivation is typically observed during cognitive tasks (e.g., inhibitory processing). However, whereas healthy controls display more subgenual deactivation for harder task levels, depressed patients show less (Matthews et al., 2009). This suggests a failure of inhibitory control, which could explain worse performance on cognitive tasks in depressed patients (Eshel & Roiser, 2010). In short, subgenual deactivation may curtail excessive emotional response for the benefit of higher-level cognitive functioning.
Consistent with this hypothesis, increased sgACC metabolism related to disturbed emotion regulation has been implicated in mood disorders (Drevets et al., 2008; Greicius et al., 2007; Botteron, Raichle, Drevets, Heath, & Todd, 2002; Drevets, 1999). Previous studies in healthy subjects reported elevated sgACC responses associated with induced sadness and recall of aversive memories (Kross, Davidson, Weber, & Ochsner, 2009; Drevets et al., 2008; Mayberg et al., 1999). In addition, a recent study observed increased sgACC responses to emotional versus neutral faces as the result of a typhoid injection precipitating high levels of inflammatory cytokines, which correlated with the extent of induced depressive symptoms (Harrison et al., 2009). The decrease in sgACC response to fearful versus neutral faces observed in la/la subjects may therefore reflect a compensatory mechanism to aversive stimuli that may confer resilience, and warrants further investigation.
The foregoing discussion argues that the 5-HTTLPR short variant renders individuals susceptible to cognitive and emotion processing biases associated with depression. This is despite consistent lack of evidence for differential serotonin transporter expression related to 5-HTTLPR genotype anywhere in the adult brain (Murthy et al., 2010), potentially indicating that structural and functional differences associated with this polymorphism reflect a developmental effect (e.g., Pacheco et al., 2009; Pezawas et al., 2005). Indeed, Dayan and Huys (2008) have proposed that the level of serotonin-mediated inhibition during early learning impacts long-term sensitivity to serotonergic stimulation, resulting in differential sensitivity to serotonergic manipulations in adulthood. This hypothesis is consistent with findings that s/s individuals are more sensitive to serotonin depletion (Roiser, Muller, Clark, & Sahakian, 2007; Roiser et al., 2005, 2006). Although the neural basis of this sensitivity has yet to be investigated using fMRI, our data are consistent with suggestions that sgACC may play a key role in mediating this vulnerability (Neumeister et al., 2006).
We were unable to replicate previous reports of increased amygdala reactivity to fearful faces in s/s subjects (Dannlowski et al., 2008; Hariri et al., 2002, 2005). A potential explanation for our nonreplication is that we used an incidental facial emotion processing task, which directed attention toward gender-related cues. In support of this hypothesis, a recent study reported greater amygdala response to neutral faces judged as “to be avoided” compared to those deemed “approachable,” but only found this effect in the context of a social judgment task: There was no differential amygdala response to the same faces when judging gender (Blasi et al., 2009). Other recent studies that employed incidental tasks also failed to report an effect of 5-HTTLPR genotype on amygdala response to fear (e.g., Thomason et al., 2010; Shah et al., 2009), and a meta-analysis has suggested that making responses of any kind can affect amygdala reactivity (Costafreda, Brammer, David, & Fu, 2008). Nonetheless, it is possible that our failure to identify this difference represents a false negative, as even with 85% power, there remains a 15% chance of a false negative.
Effects of 5-HTTLPR on Amygdala–sgACC Connectivity
We also examined changes in amygdala–sgACC connectivity during fearful face processing, and the effects of 5-HTTLPR genotype on coupling changes. In contrast with a previous report, we found no evidence for differential amygdala–sgACC coupling between the genotypes (Pezawas et al., 2005), perhaps reflecting differences in the task demands or analytic methods employed. Notably, Pezawas et al. (2005) reported differential functional coupling across task duration (not specifically during fearful/angry face processing), and did not specify the direction of coupling. Further studies will be required to disambiguate drivers of the differential response during fearful face processing observed in sgACC related to genotype.
Nominally significant differences were observed between the genotype groups in the fixed connections between other regions that participate in face processing, although none survived correction for multiple comparisons. This is interesting given the evidence that serotonin transporter expression facilitates plasticity across glutamatergic networks (Gaspar et al., 2003; Bruning & Liangos, 1997). However, although these findings are of potential importance, they should be treated with caution until independently replicated.
Effective Connectivity during Face Processing: Fixed Connections
Our connectivity analysis sheds new light on interactions across regions implicated in social processing associated with viewing faces and facial emotions. The Bayesian Model Selection procedure identified a model with bidirectional fixed connectivity along the dorsal and ventral pathway (Figure 3B), in preference to models with only feedforward or feedforward and lateral connections (e.g., FG–pSTS) (Models 1 and 7). This contrasts with Fairhall and Ishai’s findings, where model identification indicated that including lateral and backwards connections in the “core” network did not increase model fit. The connections in common with the model identified by Fairhall and Ishai were stronger than the other connections in our model, suggesting this might be due to increased sensitivity of new improvements to DCM to detect weak connections, as well as the subject-specific placement of nodes based on anatomical location. This is likely to have reduced noise related to anatomical heterogeneity, increasing the sensitivity of the analysis (Smith, Pillai, Chen, & Horwitz, 2010).
In support of Fairhall and Ishai’s findings, connectivity parameters indicated that responses in the “extended” network, including the amygdala and the IFG, were driven predominantly by the FG; although the selected model included connections from the pSTS to the extended network, surpassing model evidence of a model with connections to the extended network routed solely via the FG (Model 8) (Fairhall & Ishai, 2007). The importance of the FG in driving responses may be attributable to the use of static facial stimuli, thought to exert lesser processing demands on regions in the dorsal stream compared to dynamic facial stimuli (James, Culham, Humphrey, Milner, & Goodale, 2003).
One of the strengths of quantifying interactions across neural systems using DCM is the sensitivity of this method (Stephan et al., 2008). This is evident in the large exceedance probability for Model 11 relative to Model 10, despite the similarity of the models (Figure 3). It should be noted that the exceedance probability of Model 11 surpassed that of less as well as more complex models. As such, we can conclude that the result justifies the sampled model space in terms of the numbers of parameters included. Modeling connectivity from regions receiving visual input across the network reduced the likelihood that the parameter estimates for direct connections were contaminated by indirect effects. In addition, selecting the position of the DCM nodes for each subject according to their own peak response greatly increased the sensitivity of the analysis. These factors improved our power to detect weak connections and provided an extremely detailed picture of interactions across the face processing network.
Effective Connectivity during Face Processing: Modulation by Emotion
Across all subjects, we observed increased bidirectional excitatory coupling between the amygdala and the FG during fearful face processing, providing a mechanistic explanation for the enhanced amygdala response elicited by fearful relative to neutral faces. This finding is consistent with previous reports using neuroimaging (Surguladze et al., 2003; Morris et al., 1998) or a combination of neuroimaging and lesion models (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). Viewing fearful faces also increased excitatory coupling between the FG and the pSTS to a degree similar to the enhancement of FG–amygdala coupling. Using DCM, it is not possible to determine whether facilitation of functional integration between dorsal and ventral pathways was mediated by direct or indirect connections (e.g., via attentional networks) (Vuilleumier & Driver, 2007). Viewing fearful faces also increased the inhibitory influence of the amygdala over sgACC, implying that interactions between these regions are important in the processing of threat-relevant stimuli. This finding is consistent with a previous report of negative connectivity between the right amygdala and sgACC during passive viewing of fearful faces (Williams et al., 2006).
Conclusion
We used an incidental facial emotion processing task to explore the effects of 5-HTTLPR on functional response in the amygdala and sgACC, as well as differences in network connectivity and task-specific changes in coupling related to genotype. Although we were unable to replicate differential amygdala response or amygdala–sgACC coupling related to genotype, we observed sgACC deactivation to fearful versus neutral faces in la/la subjects, which we speculate may represent an adaptive mechanism conferring resilience in this group. Across all subjects, we found evidence for bidirectional connectivity across dorsal and ventral visual pathways, as well as increased functional integration between them in the context of fearful faces. We also identified increased excitatory bidirectional FG–amygdala coupling and increased inhibitory amygdala–sgACC coupling related to the processing of threat-relevant stimuli. The absence of sgACC deactivation to fearful faces in s/s subjects is consistent with evidence for their increased sensitivity to aversive stimuli and warrants further investigation.
Acknowledgments
This work was supported by a Wellcome Trust Programme Grant (R. J. D). J. P. R. was supported by the Raymond Way Fund. We thank Ulrich Muller, Barbara Sahakian, and Trevor Robbins for assistance in recruiting participants. We also thank Geoff Tan, Nick Wood, David Rubinsztein, and Oana Sadiq for genotyping. We thank the Ben Seymour, Dharshan Kumaran, and Benedetto de Martino, as well as the Wellcome Trust Centre for Neuroimaging support staff, for help with data collection. Finally, we thank Karl Friston for discussion of the manuscript.
REFERENCES
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: An fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, et al. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biological Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, et al. Preferential amygdala reactivity to the negative assessment of neutral faces. Biological Psychiatry. 2009;66:847–853. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behavioural Brain Research. 1990;41:199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Bruning G, Liangos O. Transient expression of the serotonin transporter in the developing mouse thalamocortical system. Acta Histochemica. 1997;99:117–121. doi: 10.1016/S0065-1281(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. Journal of Neuroscience. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Konrad C, Kugel H, Zwitserlood P, Domschke K, Schoning S, et al. Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. Neuroimage. 2010;53:893–898. doi: 10.1016/j.neuroimage.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, et al. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology. 2008;33:418–424. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS Computational Biology. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical–amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr., Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fortier E, Noreau A, Lepore F, Boivin M, Perusse D, Rouleau GA, et al. Early impact of 5-HTTLPR polymorphism on the neural correlates of sadness. Neuroscience Letters. 2010;485:261–265. doi: 10.1016/j.neulet.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Kanazawa M, Mizuno T, Hamaguchi T, Kano M, Watanabe S, et al. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage. 2009;47:946–951. doi: 10.1016/j.neuroimage.2009.04.083. [DOI] [PubMed] [Google Scholar]
- Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behavioral Neuroscience. 2001;115:358–364. [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nature Reviews Neuroscience. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive–compulsive disorder. American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A. Let’s face it: It’s a cortical network. Neuroimage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- James TW, Culham J, Humphrey GK, Milner AD, Goodale MA. Ventral occipital lesions impair object recognition but not object-directed grasping: An fMRI study. Brain. 2003;126:2463–2475. doi: 10.1093/brain/awg248. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry. 2009;65:361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matthews S, Simmons A, Strigo I, Gianaros P, Yang T, Paulus M. Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Research. 2009;172:1–6. doi: 10.1016/j.pscychresns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic–cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Iacoboni M, Koski L, Mazziotta JC. Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cerebral Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri A. Erratum: Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2009;66:302. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52:50–54. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Archives of General Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal–limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. Journal of Neuroscience. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, et al. Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology. 2006;31:2264–2272. doi: 10.1038/sj.npp.1301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Cook LJ, Cooper JD, Rubinsztein DC, Sahakian BJ. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. American Journal of Psychiatry. 2005;162:609–612. doi: 10.1176/appi.ajp.162.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW, et al. A genetically mediated bias in decision making driven by failure of amygdala control. Journal of Neuroscience. 2009;29:5985–5991. doi: 10.1523/JNEUROSCI.0407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. International Journal of Neuropsychopharmacology. 2007;10:449–461. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardt DM, Erk S, Nusser C, Nothen MM, Cichon S, Rietschel M, et al. Volition diminishes genetically mediated amygdala hyperreactivity. Neuroimage. 2010;53:943–951. doi: 10.1016/j.neuroimage.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–1310. doi: 10.1038/npp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smith JF, Pillai A, Chen K, Horwitz B. Identification and validation of effective connectivity networks in functionalmagnetic resonance imaging using switching linear dynamic systems. Neuroimage. 2010;52:1027–1040. doi: 10.1016/j.neuroimage.2009.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Kasper L, Harrison LM, Daunizeau J, den Ouden HE, Breakspear M, et al. Nonlinear dynamic causal models for fMRI. Neuroimage. 2008;42:649–662. doi: 10.1016/j.neuroimage.2008.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Kroes MC, Roiser JP, Tan GC, Dolan RJ. Emotion-induced retrograde amnesia is determined by a 5-HTT genetic polymorphism. Journal of Neuroscience. 2008;28:7036–7039. doi: 10.1523/JNEUROSCI.0834-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SC, et al. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Henry ML, Paul Hamilton J, Joormann J, Pine DS, Ernst M, et al. Neural and behavioral responses to threatening emotion faces in children as a function of the short allele of the serotonin transporter gene. Biological Psychology. 2010;85:38–44. doi: 10.1016/j.biopsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 Update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading manual. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. Neuroimage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. Journal of Neuroscience. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]