Abstract
Objective
To demonstrate the usefulness of 3-tesla (3T) magnetic resonance imaging (MRI) including T2-weighted imaging (T2WI), diffusion weighted imaging (DWI), time-of-flight (TOF) magnetic resonance angiography (MRA), T2*-weighted gradient recalled echo (GRE), and susceptibility weighted imaging (SWI) in diagnosing brain death.
Materials and Methods
Magnetic resonance imaging findings for 10 patients with clinically verified brain death (group I) and seven patients with comatose or stuporous mentality who did not meet the clinical criteria of brain death (group II) were retrospectively reviewed.
Results
Tonsilar herniation and loss of intraarterial flow signal voids (LIFSV) on T2WI were highly sensitive and specific findings for the diagnosis of brain death (p < 0.001 and < 0.001, respectively). DWI, TOF-MRA, and GRE findings were statistically different between the two groups (p = 0.015, 0.029, and 0.003, respectively). However, cortical high signal intensities in T2WI and SWI findings were not statistically different between the two group (p = 0.412 and 1.0, respectively).
Conclusion
T2-weighted imaging, DWI, and MRA using 3T MRI may be useful for diagnosing brain death. However, SWI findings are not specific due to high false positive findings.
Keywords: CNS, MR imaging, Brain, Adult, Brain death
INTRODUCTION
The definition of brain death is "complete irreversible cessation of all brain functions, including the brainstem" but the diagnostic criteria of brain death vary widely among countries (1-6). On the other hand, the diagnosis of brain death becomes more and more important because the demand for organs for transplantation is increasing worldwide (2). In most countries (United States, Canada, United Kingdom, Germany, Russia, China, etc), the diagnosis of brain death is based on clinical criteria (1, 4). Technical examinations serve exclusively as ancillary tools in the diagnosis of brain death (4). In other countries (France, Italy, The Netherland, Japan, Korea, etc), various technical examinations are obligatory (1). In Korea, electroencephalography (EEG) is the obligatory test for brain death in addition to clinical criteria (5). An EEG, however, has several limitations (2, 4, 6). First, there are artifacts within that hinder the interpretation of brain death. Second, isoelectric EEG findings can occur in patients with hypothermia and drug or toxic ingestion. Third, the use of an EEG makes it difficult to judge brain death in patients with traumatic injuries or who have skull defects. Fourth, there is residual EEG activity in clinically verified brain death in approximately 19.6% of patients (6). Therefore, if only residual activity seen on EEG, ancillary tests may replace EEG in exceptional circumstances. It is known that verifying the loss of brain blood flow is a more accurate ancillary diagnostic tool for assessing brain death (4). Magnetic resonance imaging (MRI) is not yet accepted as an accurate ancillary test for brain death (7).
Clinical applications of 3-tesla (3T) MRI increased after Food and Drug Administration provided approval of 3T MRI, especially in neuroradiology (8). Furthermore, T2*-weighted gradient recalled echo (GRE) findings for brain death are unknown, although this sequence is included in routine imaging protocols. One description of brain death using susceptibility weighted imaging (SWI) was recently published (9), but SWI findings for brain death are not fully analyzed since it is a relatively new imaging protocol.
The purpose of this study was to verify 3T MRI including T2 weighted imaging (T2WI), diffusion weighted image (DWI), time-of-flight (TOF) magnetic resonance angiography (MRA), GRE, and SWI in diagnosing brain death.
Therefore, we hypothesize that conventional MRI findings are valid on a 3T machine and GRE and SWI findings are specific for brain death. The current study is the first to address the assessment of brain death using 3T MRI.
MATERIALS AND METHODS
This retrospective study was approved by our hospital's institutional review board.
Patients
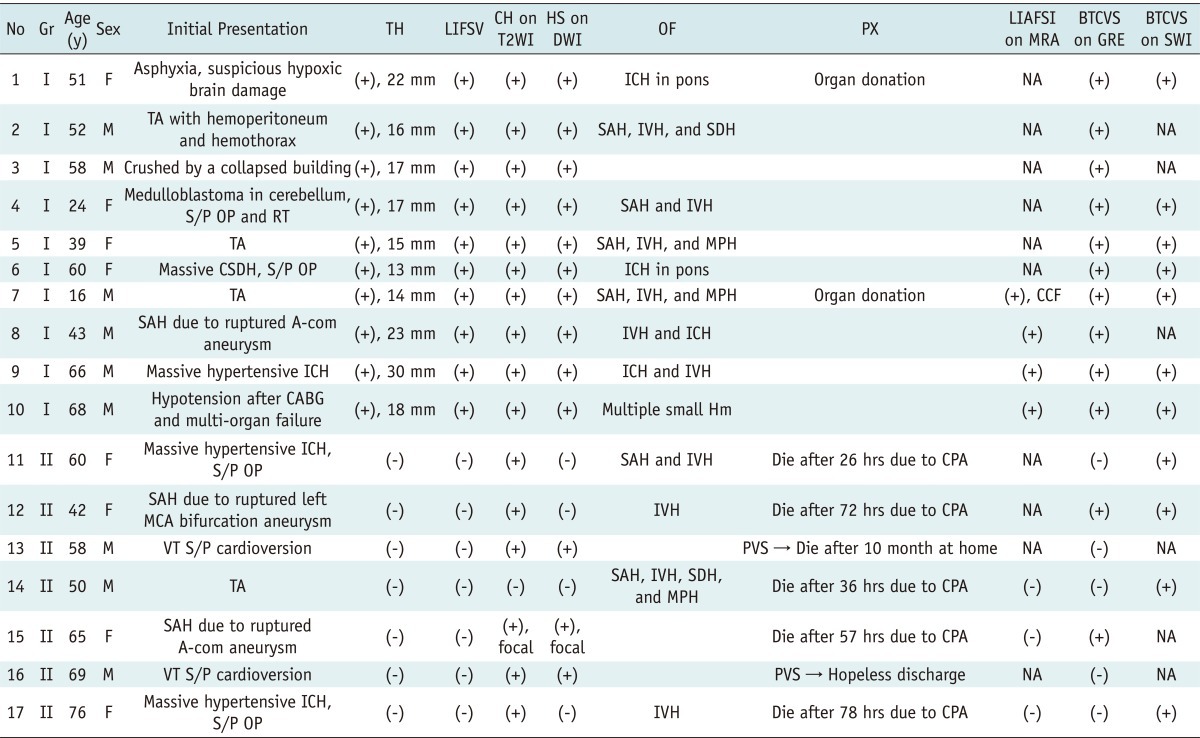
Between June 2007 and October 2009, 17 patients (eight females and nine males; mean age, 52.9 years; range, 16-76 years) were evaluated for the diagnosis of brain death. The underlying conditions and clinical data are shown in Table 1.
Table 1.
Patient Characteristics, Clinical Data, and Magnetic Resonance Imaging (MRI) Findings of 17 Patients
Note.- A-com = anterior communicating artery, BTCVS = bilateral transcerebral and cortical vein sign, CABG = coronary artery bypass graft, CCF = carotid-cavernous fistula, CH = cortical high signal intensity, CPA = cardiopulmonary arrest, CSDH = chronic subdural hemorrhage, DWI = diffusion weighted image, F = female, Gr = group, GRE = T2*-weighted gradient recalled echo, Hm = hemorrhage, hrs = hours, HS = high signal intensity, ICH = intracerebral hemorrhage, IVH = intraventricular hemorrhage, LIAFSI = loss of intracranial arterial flow signal intensity, LIFSV = loss of intraarterial flow signal void, M = male, MCA = middle cerebral artery, MPH = multiple petechial hemorrhage, MRA = magnetic resonance angiography, NA = not available, No = number, OF = other findings, OP = operation, PVS = persistent vegetative state, PX = prognosis, RT = radiotherapy, SAH = subarachnoid hemorrhage, SDH = subdural hemorrhage, S/P = status post, SWI = susceptibility weighted image, T2WI = T2 weighted image, TA = traffic accident, TH = tonsillar herniation, y = years, VT = ventricular tachycardia
In group 1 (n = 10), all patients met the Korean Medical Association criteria for brain death but did not meet EEG criteria due to the presence of residual waves (5). Comatose and stuporous patients in group 2 (n = 7) were enrolled, and none of them met the clinical criteria of brain death.
Criteria of Brain Death in Korea
The criteria of brain death in Korea are as follows. First, metabolic disorders, hypothermia, drug intoxication, and hypotension must be ruled out. Second, coma, unresponsiveness, complete loss of brainstem-mediated reflexes (light, corneal, oculocephalic, vestibular-cephalic, ciliocephalic, gag, and cough) and the apnea test were fulfilled. Third, neurologic exam and reflexes were repeated after 6 hours. Fourth, after these processes, a flat wave on EEG must persist for 30 minutes.
MRI Examinations
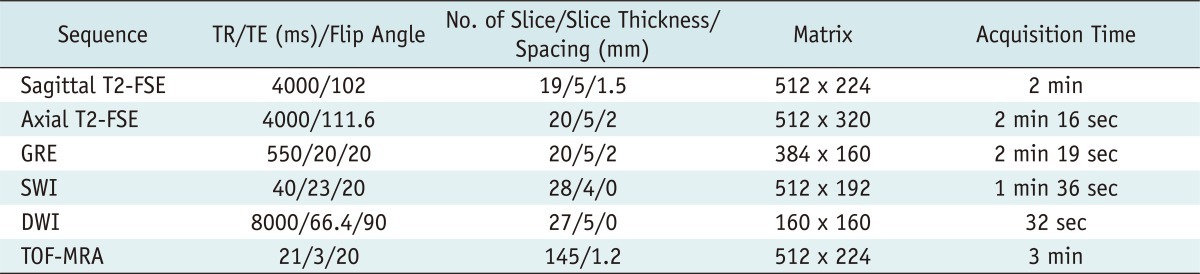
All MRI studies were performed using a 3T MR system (Signa VHi; GE Medical Systems, Milwaukee, WI, USA) with an 8-channel high-resolution brain coil. No intravenous contrast media was administered. The MRI examination consisted of a T2WI fast-spin echo sequence in the sagittal plane, a T2WI fast-spin echo sequence in the axial plane, a GRE, and a DWI (a single-shot, spin echo, echo-planar pulse sequence with b values of 0 and 1000 s/mm2) in the axial plane. An additional 3D TOF-MRA was done in each of the lasts seven patients. SWI was performed for additional evaluation in 11 patients. The imaging parameters of the pulse sequences are shown in Table 2.
Table 2.
Imaging Parameters of Pulse Sequences
Note.- T2 indicates T2-weighted image. FSE = fast-spin echo, GRE = T2*-weighted gradient echo image, SWI = susceptibility weighted image, DWI = diffusion-weighted image, TOF = time-of-flight, TR = repetition time, TE = echo time, ms = millisecond, No. = number
Image Processing and Analysis
The 3D raw data from MRA were post-processed with a maximum intensity projection algorithm at a work station included in the MRI system (FuncTool PF; GE Medical Systems Milwaukee, WI, USA). On SWI, both magnitude and phase information were used. The post-processing of SWI images was also done in the work station. All MRI studies and the included source images were evaluated by two neuroradiologists (C-HS and H-WC, with 15 and 5 years of experience, respectively) who did not have access to the patients' clinical information.
Imaging Criteria of Brain Death and Vascular Signs
We evaluated each conventional MRI finding and bilateral transcerebral and cortical vein signs (BTCVS) on both GRE and SWI in brain dead patients (group I) and comatose or stuporous patients without a diagnosis of brain death (group II). The conventional diagnostic criteria for brain death on MRI are well known and as follows (10): tonsillar herniation; absent intracranial vascular flow void in both conventional MRI and MRA; diffuse cortical high signal intensity and swelling of the cerebral sulci on T2WI; diffuse hemispheric hyperintensities on DWI; and a drop in the apparent diffusion coefficient (ADC) due to cytotoxic edema.
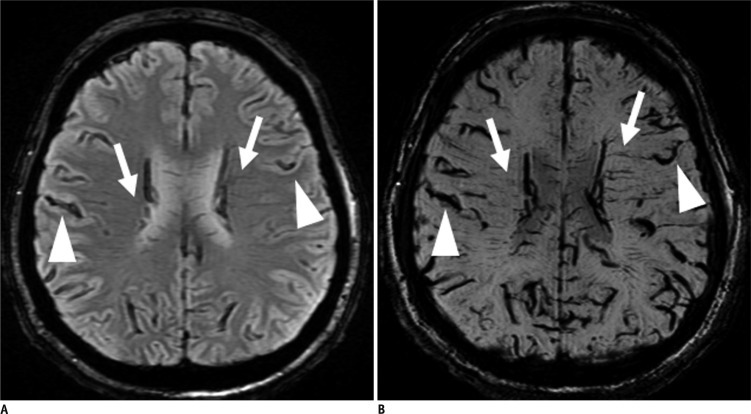
The bilateral transcerebral vein sign (Fig. 1) is defined as multiple and/or branching structures extending through the cerebral hemisphere parallel or perpendicular to the outer wall of both lateral ventricles (11). The bilateral cortical vein sign (Fig. 1) is defined as visualization of both cerebral hemisphere cortical veins (12-15).
Fig. 1.
Bilateral transcerebral and cortical vein signs on T2*-weighted gradient recalled echo (GRE) and susceptibility weighted image (SWI) in patient 1.
A. GRE image shows multiple and branching low signal intensities extending through cerebral hemisphere parallel or perpendicular to outer wall of both lateral ventricles (arrows, bilateral transcerebral vein sign) and abnormal low signal intensities in both cerebral hemisphere cortical areas (arrow heads, bilateral cortical vein sign). B. Similar, but more prominent low signal intensities are visualized on SWI.
Statistical Analysis
For statistical analysis, we used the SPSS software package for Windows (SPSS, Inc., Chicago, IL, USA). Results were statistically analyzed using Fisher's exact test and considered significant at p < 0.05.
RESULTS
All group I patients (n = 10) showed tonsillar herniation, loss of intraarterial flow signal voids (LIFSV), diffuse cortical high signal intensity and swelling of cerebral sulci on T2WI, high signal intensity in cerebral hemisphere on DWI due to cytotoxic edema and BTCVS on GRE. All MRA in group I (n = 4) showed loss of intracranial arterial flow signal intensities (LIAFSI). All SWI in group I (n = 7) showed BTCVS (Fig. 2).
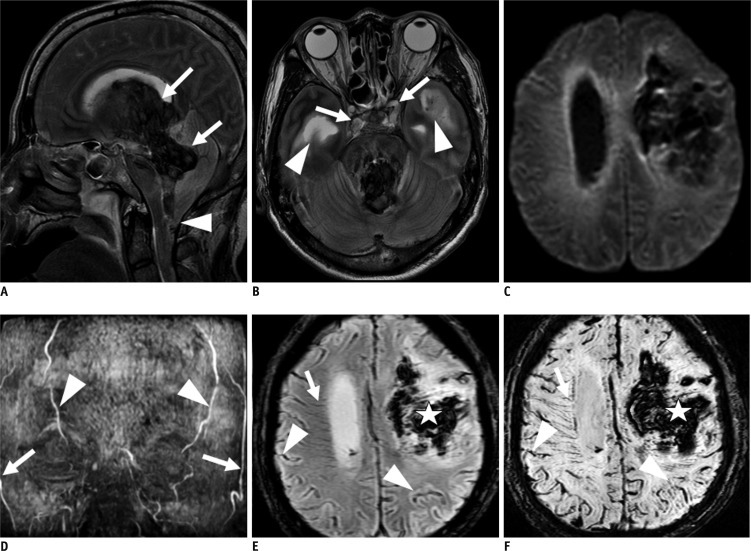
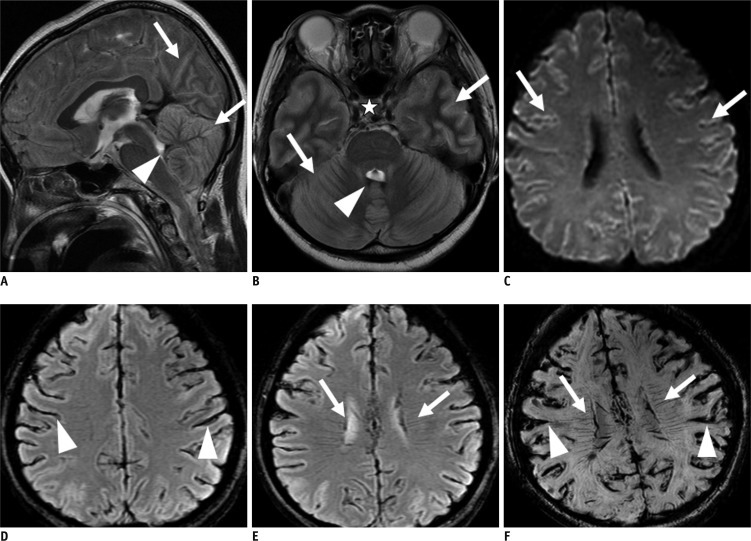
Fig. 2.
Group I (patient 9). 66-year-old male with massive intracerebral hemorrhage (ICH).
A. T2-weighted image (T2WI) sagittal scan shows intraventricular hemorrhage (IVH) in 3rd and 4th ventricles (arrows) and tonsillar herniation (arrowhead). Diffuse swelling with effacement of cortical gyri is noted. B. T2WI axial image reveals loss of intraarterial flow signal voids in both cavernous and paraclinoid internal carotid arteries (arrows). There is hydrocephalus in both lateral ventricles due to IVH (arrowheads). C. Diffusion weighted image (b value = 1000) shows diffuse increased signal intensities in both periventricular white matters. D. Maximum intensity projection reconstruction of time-of-flight magnetic resonance angiography shows loss of intracranial arterial flow signal intensities. There is visualization of both superficial temporal arteries (arrows) and occipital arteries (arrowhead). E, F. T2*-weighted gradient recalled echo and susceptibility weighted imaging show visualization of transcerebral vein sign in right cerebral hemisphere (arrow) and bilateral cortical vein sign (arrowhead). Transcerebral vein sign in left cerebral hemisphere is not visualized due to massive ICH (asterisk).
In contrast, all group II patients (n = 7) showed neither tonsillar herniation nor LIFSV (Fig. 3). None of the MRA in group II (n = 3) showed LIAFSI (Fig. 4). However, T2WI, DWI, GRE, and SWI findings of the patients in group II were variable (Table 1).
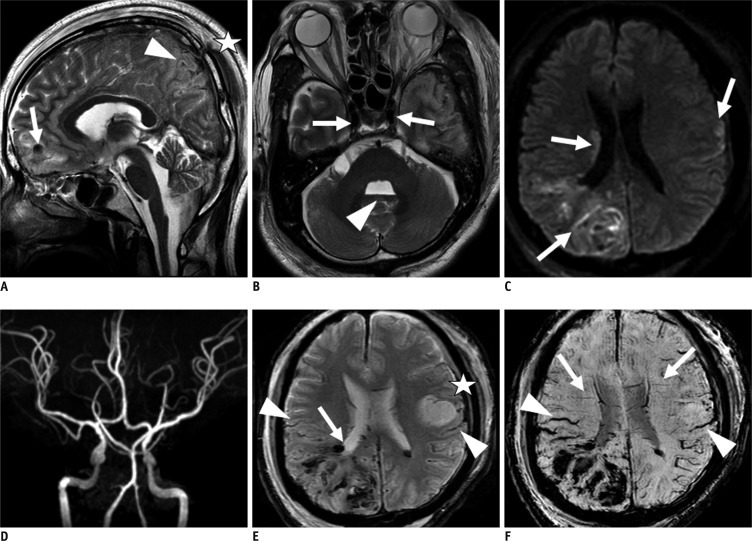
Fig. 3.
Group II (patient 12). 42-year-old female with ruptured left middle coronary artery bifurcation aneurysm and subarachnoid hemorrhage (SAH).
A, B. T2 weighted image sagittal and axial scans reveal diffuse swelling of both cerebral hemisphere and cerebellum (arrows) and intraventricular hemorrhage in 4th ventricle (arrowhead), but there is no evidence of definite tonsillar herniation or loss of intraarterial flow signal voids (asterisk). C. Diffusion weighted imaging (b value = 1000) shows increased signal intensity in cerebral sulci, possibly due to SAH (arrows), but there was no evidence of definite increased signal intensity in brain parenchyma. D, E. T2*-weighted gradient recalled echo (GRE) reveals transcerebral (arrows) and cortical vein (arrowheads) signs in both cerebral hemispheres. F. Susceptibility weighted image reveals bilateral bilateral transcerebral and cortical vein sign (BTCVS) (arrows and arrowheads). In this case, we could not discriminate SAH from BTCVS on GRE and SWI due to an increased oxygen extraction fraction and increase in deoxyhemoglobin in capillaries and veins in setting of SAH and subsequent vascular spasm or increased intracranial pressure.
Fig. 4.
Group II (patient 14). 50-year-old male admitted due to injuries sustained in traffic accident.
A. T2 weighted imaging sagittal scan reveals focal intracerebral hemorrhage (ICH) in frontal lobe (arrow) and subarachnoid hemorrhage (SAH) in frontal and parietal lobe sulci (arrowhead), fracture in parietal bone and massive hematoma in scalp (asterisk). However, there is no evidence of definite tonsillar herniation. B. T2WI axial image reveals normal intravascular flow void signal in both cavernous ICAs (arrow). Minimal IVH in 4th ventricle (arrowhead). C. Diffusion weighted image (b value = 1000) shows multifocal high signal intensities in right corona radiata, right parieto-occipital lobe, and left frontoparietal lobe sulci, possibly due to shearing injury (arrows). D. Minimum-intensity projection reconstruction of TOF-MRA visualizes intracranial vasculature. E. T2*-weighted gradient recalled echo image reveals petechial hemorrhage in right periventricular white matter (arrow), minimal SAH in right parietal lobe sulci, and subdural hemorrhage in left frontoparietal convexity. There is no evidence of bilateral transcerebral and cortical vein signs (BTCVS). F. Susceptibility weighted imaging (SWI) shows bilateral transcerebral (arrows) and cortical vein (arrowheads) signs. In this case, we could not discriminate traumatic SAH and petechial hemorrhage from BTCVS on SWI.
On T2WI, 6 patients showed cortical high signal intensity and swelling of the cerebral sulci while one did not. On DWI, 3 patients showed high signal intensity, whereas 4 did not. On GRE, 2 patients showed BTCVS, whereas 5 did not. All SWI in group II (n = 4) showed BTCVS.
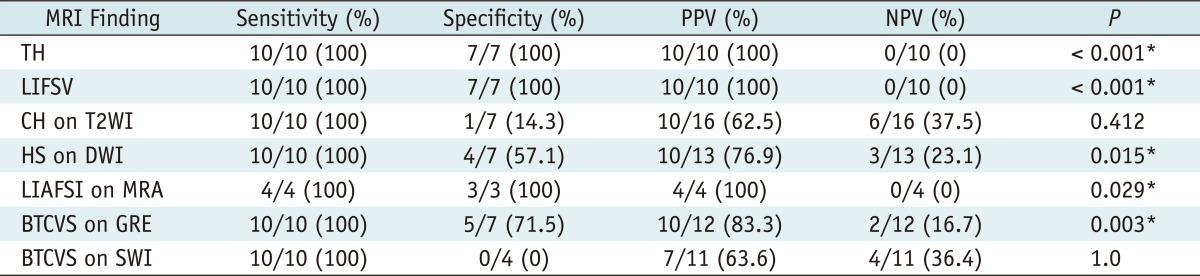
Table 3 summarizes the statistical analysis of each MRI finding of brain death.
Table 3.
Statistical Analysis in Each Magnetic Resonance Imaging (MRI) Finding of Brain Death
Note.- *p < 0.05 for Fisher's exact test. TH = tonsillar herniation, LIFSV = loss of intraarterial flow signal void, CH = cortical high signal intensity, T2WI = T2 weighted image, HS = high signal intensity, DWI = diffusion-weighted image, LIAFSI = loss of intracranial arterial flow signal intensity, MRA = magnetic resonance angiography, BTCVS = bilateral transcerebral and cortical vein sign, GRE = T2*-weighted gradient recalled echo, SWI = susceptibility weighted image, PPV = positive predictive value, NPV = negative predictive value
DISCUSSION
The guidelines for brain death were proposed by an ad hoc committee from the Harvard Medical School faculty in 1968 (16). Unfortunately, there are no internationally accepted "guidelines for brain death" (1). In fact, the recommendations of the Harvard committee have never become an internationally accepted guideline (1-3). The definition and handling of brain death is more a political, ethical, and even religious issue than a medical issue (17). Therefore, brain death is defined by legal authorities following recommendations of medical institutions in almost all countries, and the diagnostic criteria for brain death vary among countries (1-3). However, the diagnostic criteria for brain death are all based on the following three neurologic characteristics (3): comatose mentality; loss of brainstem-mediated reflexes; and apnea. As previously described, in Korea, EEG is the obligatory test for brain death in addition to the three clinical criteria, but it has several limitations (2, 4-6). Therefore, it is known that verifying the loss of brain blood flow is a more accurate ancillary diagnostic tool for assessing brain death (4). Single photon emission computed tomography (SPECT), transcranial Doppler (TCD), conventional angiography, computed tomographic angiography (CTA), and MRI with MRA have all been studied for their ability to provide a more accurate and objective diagnosis of brain death (2, 4, 7).
Magnetic resonance imaging has no advantage over CTA or SPECT in that the patient has to be transported to the MRI suite and the accessibility of critically sick patients remains a serious problem, but there is no need for contrast media injection and it is always available (4).
The recently reported guidelines of the American Academy of Neurology show insufficient evidence for determining if newer ancillary tests (such as MRI and MRA, CTA, somatosensory evoked potentials, and bispectral index) accurately confirm the cessation of the entire brain function (7), but controversy remains in other countries worldwide.
In diagnosing brain death, a short scan time is very important due to probable serious problems in critically sick patients. As such, a high field strength scanner is more important. In this study, we evaluated each of the MRI findings separately to diagnose brain death.
In this way, TH, LIFSV, and LIAFSI on MRA are accurate findings of brain death with 100% accuracy, but LIFSV and LIAFSI on MRA are same phenomena in nature. As a result, we recommend T1 or T2WI sagittal scan and T2WI axial scan or TOF-MRA in diagnosing brain death, but further studies involving a larger number of patients are needed.
Other brain MRI and MRA findings of brain death not described here have been well documented as well (10, 18-22), including absent intracranial contrast enhancement, carotid artery enhancement, prominent nasal and scalp enhancement (MR "hot nose" sign), but we did not evaluate them since we did not use intravenous contrast media.
In brain death, regardless of the cause, increased intracranial pressure decreases cerebral blood flow. This phenomenon leads to cytotoxic edema and the progression of brain swelling. Finally, compression of the entire network of intracranial arteries is observed. As such, paradoxical and irreversible brain death occurs (10). Diffuse hyperintensities on DWI and ADC drop may be non-specific and can occur in other situations, such as bilateral carotid artery occlusion (21). With other findings, diffuse hyperintensities on DWI and ADC drop can be a finding of brain death.
This is the first series on brain death with 3T MRI to be performed, while GRE findings for brain death are not known, although the sequence is included in routine imaging protocols. There has already been one description of brain death for SWI (9). Tong et al. (9) described an 8-year-old boy who had prominent deep medullary veins throughout the bilateral hemispheres after a traffic accident. The findings were possibly due to a combination of increased oxygen extraction, venous stasis, and/or possible venous dilatation secondary to release of substances (such as adenosine) after cell death. However, this was only one case, and the study did not show other image protocol findings (9, 23).
The transcerebral vein sign on GRE in acute stroke is also well known (11, 12). The transcerebral vein sign on GRE in acute stroke is caused by an increased oxygen extraction fraction and an increase in deoxyhemoglobin in the capillaries and veins.
The cortical vein sign on GRE is defined as visualization of both cerebral hemisphere cortical veins. Similar imaging findings are encountered in acute stroke, subarachnoid hemorrhage, cortical vein thrombosis, vascular malformations such as developmental venous anomalies and arteriovenous malformations (12-15), and patients under general anesthesia.
The signal intensity on SWI is quite variable among patients and patients with different physiological conditions (24, 25). Imaging findings can be easily changed with different post-processing parameters (26). Our SWI parameters were optimized to reduce scan time because of the patients' conditions.
Our study had two limitations. First, we had only a small number of cases. Second, there was no confirmation of brain death by an independent method, such as conventional angiography.
conclusions
3-tesla MRI and MRA may be useful for diagnosing brain death. However, SWI findings are not specific due to false positive findings.
Acknowledgment
We want to thank all our MRI technologists: Bae Sung-Jin, Choi Chul Hwan, Kim Soon Hwan, Kwon Sang Hyuk, Seo Young Seok, Son Nam Gon.
Footnotes
This research was supported by the Bisa Research Grant from Keimyung University in 2009.
References
- 1.Wijdicks EF. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology. 2002;58:20–25. doi: 10.1212/wnl.58.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Baron L, Shemie SD, Teitelbaum J, Doig CJ. Brief review: history, concept and controversies in the neurological determination of death. Can J Anaesth. 2006;53:602–608. doi: 10.1007/BF03021852. [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks EF. Determining brain death in adults. Neurology. 1995;45:1003–1011. doi: 10.1212/wnl.45.5.1003. [DOI] [PubMed] [Google Scholar]
- 4.Young GB, Shemie SD, Doig CJ, Teitelbaum J. Brief review: the role of ancillary tests in the neurological determination of death. Can J Anaesth. 2006;53:620–627. doi: 10.1007/BF03021855. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Lee SB. Diagnostic criteria of brain death. J Korean Med Assoc. 1999;42:349–357. [Google Scholar]
- 6.Grigg MM, Kelly MA, Celesia GG, Ghobrial MW, Ross ER. Electroencephalographic activity after brain death. Arch Neurol. 1987;44:948–954. doi: 10.1001/archneur.1987.00520210048018. [DOI] [PubMed] [Google Scholar]
- 7.Wijdicks EF, Varelas PN, Gronseth GS, Greer DM American Academy of Neurology. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:1911–1918. doi: 10.1212/WNL.0b013e3181e242a8. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Linera J. 3T MRI: advances in brain imaging. Eur J Radiol. 2008;67:415–426. doi: 10.1016/j.ejrad.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29:9–17. doi: 10.3174/ajnr.A0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton BE. Brain death. In: Osborn AG, Salzman KL, Katzman G, Provenzale J, Castillo M, Hedlund G, et al., editors. Dignostic imaging. 1st ed. Salt Lake City, Utah: Amirsys; 2004. pp. 54–55. [Google Scholar]
- 11.Hermier M, Nighoghossian N, Derex L, Adeleine P, Wiart M, Berthezène Y, et al. Hypointense transcerebral veins at T2*-weighted MRI: a marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab. 2003;23:1362–1370. doi: 10.1097/01.WCB.0000091764.61714.79. [DOI] [PubMed] [Google Scholar]
- 12.Morita N, Harada M, Uno M, Matsubara S, Matsuda T, Nagahiro S, et al. Ischemic findings of T2*-weighted 3-tesla MRI in acute stroke patients. Cerebrovasc Dis. 2008;26:367–375. doi: 10.1159/000151640. [DOI] [PubMed] [Google Scholar]
- 13.Sohn CH, Baik SK, Lee HJ, Lee SM, Kim IM, Yim MB, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol. 2005;26:662–665. [PMC free article] [PubMed] [Google Scholar]
- 14.Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004;35:1989–1994. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 15.Boukobza M, Crassard I, Bousser MG, Chabriat H. MR imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR Am J Neuroradiol. 2009;30:344–348. doi: 10.3174/ajnr.A1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337–340. [PubMed] [Google Scholar]
- 17.DuBois JM, Anderson EE. Attitudes toward death criteria and organ donation among healthcare personnel and the general public. Prog Transplant. 2006;16:65–73. doi: 10.1177/152692480601600113. [DOI] [PubMed] [Google Scholar]
- 18.Orrison WW, Jr, Champlin AM, Kesterson OL, Hartshorne MF, King JN. MR 'hot nose sign' and 'intravascular enhancement sign' in brain death. AJNR Am J Neuroradiol. 1994;15:913–916. [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii K, Onuma T, Kinoshita T, Shiina G, Kameyama M, Shimosegawa Y. Brain death: MR and MR angiography. AJNR Am J Neuroradiol. 1996;17:731–735. [PMC free article] [PubMed] [Google Scholar]
- 20.Lövblad KO, Bassetti C. Diffusion-weighted magnetic resonance imaging in brain death. Stroke. 2000;31:539–542. doi: 10.1161/01.str.31.2.539. [DOI] [PubMed] [Google Scholar]
- 21.Phan TG, Wijdicks EF. Diffusion-weighted magnetic resonance imaging in brain death. Stroke. 2000;31:1458–1459. author reply 1459-1460. [PubMed] [Google Scholar]
- 22.Karantanas AH, Hadjigeorgiou GM, Paterakis K, Sfiras D, Komnos A. Contribution of MRI and MR angiography in early diagnosis of brain death. Eur Radiol. 2002;12:2710–2716. doi: 10.1007/s00330-002-1336-z. [DOI] [PubMed] [Google Scholar]
- 23.Bell MJ, Robertson CS, Kochanek PM, Goodman JC, Gopinath SP, Carcillo JA, et al. Interstitial brain adenosine and xanthine increase during jugular venous oxygen desaturations in humans after traumatic brain injury. Crit Care Med. 2001;29:399–404. doi: 10.1097/00003246-200102000-00033. [DOI] [PubMed] [Google Scholar]
- 24.Kesavadas C, Thomas B, Misra S, Saini J. Attenuation of cerebral veins in susceptibility-weighted MR imaging performed with the patient under general anesthesia. AJNR Am J Neuroradiol. 2008;29:e71. doi: 10.3174/ajnr.A1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedlacik J, Löbel U, Kocak M, Loeffler RB, Reichenbach JR, Broniscer A, et al. Attenuation of cerebral venous contrast in susceptibility-weighted imaging of spontaneously breathing pediatric patients sedated with propofol. AJNR Am J Neuroradiol. 2010;31:901–906. doi: 10.3174/ajnr.A1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori M, Mori H, Aoki S, Abe O, Masumoto T, Kunimatsu S, et al. Three-dimensional susceptibility-weighted imaging at 3 T using various image analysis methods in the estimation of grading intracranial gliomas. Magn Reson Imaging. 2010;28:594–598. doi: 10.1016/j.mri.2010.01.002. [DOI] [PubMed] [Google Scholar]