Abstract
Objective
To retrospectively evaluate the intermediate results of radiofrequency ablation (RFA) of small renal masses (SRMs).
Materials and Methods
Percutaneous or laparoscopic RFA was performed on 48 renal tumors in 47 patients. The follow-up studies included a physical examination, chest radiography, creatinine level, and contrast-enhanced CT or MRI. To confirm the pathologic criteria of complete ablation, 35 patients underwent a follow-up biopsy. Recurrence was defined as contrast enhancement on imaging studies after 3 months, lesion growth at subsequent imaging, or viable cancer cells on follow-up biopsy.
Results
Technical success was achieved in 43 (89.6%) of 48 renal tumors. The mean tumor size was 2.3 cm and the mean follow-up period was 49.6 months. Repeated RFA was necessary in 5 tumors due to incomplete ablation. The overall complication rate was 35.8%, of which 96.2% were mild complications. Serum creatinine levels at 12 months after RFA did not differ from those before RFA (1.28 vs. 1.36 mg/dL). Four patients were found to have recurrence at various follow-up intervals, and distant metastasis was not found in any cases.
Conclusion
RFA appears to be a useful treatment for selected patients with SRMs. Our 4-year follow-up results disclose an excellent therapeutic outcome with RFA, while achieving effective local tumor control.
Keywords: Radiofrequency ablation, Kidney tumors, Minimally invasive surgical procedures
INTRODUCTION
The incidences of small renal masses (SRMs) are increasing due to screening with sectional imaging for the evaluation of other abdominal conditions. Incidentally, discovered SRMs are typically at a low stage, slow growing, and are almost uniformly confined to the kidney at the initial diagnosis (1). For decades, radical nephrectomy (RN) was considered the "gold standard" of treatment for localized renal cell carcinoma. However, it has been reported that a significant number of patients who are rendered with a single kidney after RN, are under increased risk of developing chronic kidney disease (2, 3). Also, RN may certainly be over treating many of these SRMs. Recent advances in surgical techniques have brought the use of nephron-sparing (NS) surgery such as an open, laparoscopic, and robot-assisted laparoscopic partial nephrectomy. The American Urological Association's guidelines for the management of SRMs have advocated the partial nephrectomy as the "New Gold Standard" for the treatment of SRMs (4). In recent years, many reports have demonstrated that these NS procedures have been shown to confer equivalent oncologic and functional outcomes compared to RN for patients with renal tumors smaller than 4 cm (5-8). However, NS surgery is a technically challenging procedure that has been correlated with increased perioperative complications and patient morbidity (7). Therefore, investigations into in situ ablative methods have expanded considerably with the use of cryoablation (CA), radiofrequency ablation (RFA), high-intensity focused ultrasound, and microwave thermotherapy. These ablation technologies, including RFA, offer several benefits over the extirpative approach: 1) lower complication rate; 2) shorter convalescence; and 3) absence of an ischemic insult on the kidney (5, 7-13). The most attractive merit of the ablative technique would be to offer NS treatment to patients who are otherwise poor surgical candidates.
Radiofrequency ablation is a minimally invasive treatment that has been used for liver tumors. The first report of RFA for renal tumors was published in 1997 by Zlotta et al. (10). Since then, many reports have been published on RFA for renal tumors, and RFA has shown favorable outcomes in terms of local tumor control in addition to preserving renal function (11, 12, 14-16). We have been performing both percutaneous and laparoscopic RFA on select patients since August 2004 and have been following these patients with serial laboratory, imaging studies, and repeated biopsies of the ablated lesions. Herein, we report our experience with RFAs of SRMs in the management of 47 patients over a mean follow-up time of 49.6 months.
MATERIALS AND METHODS
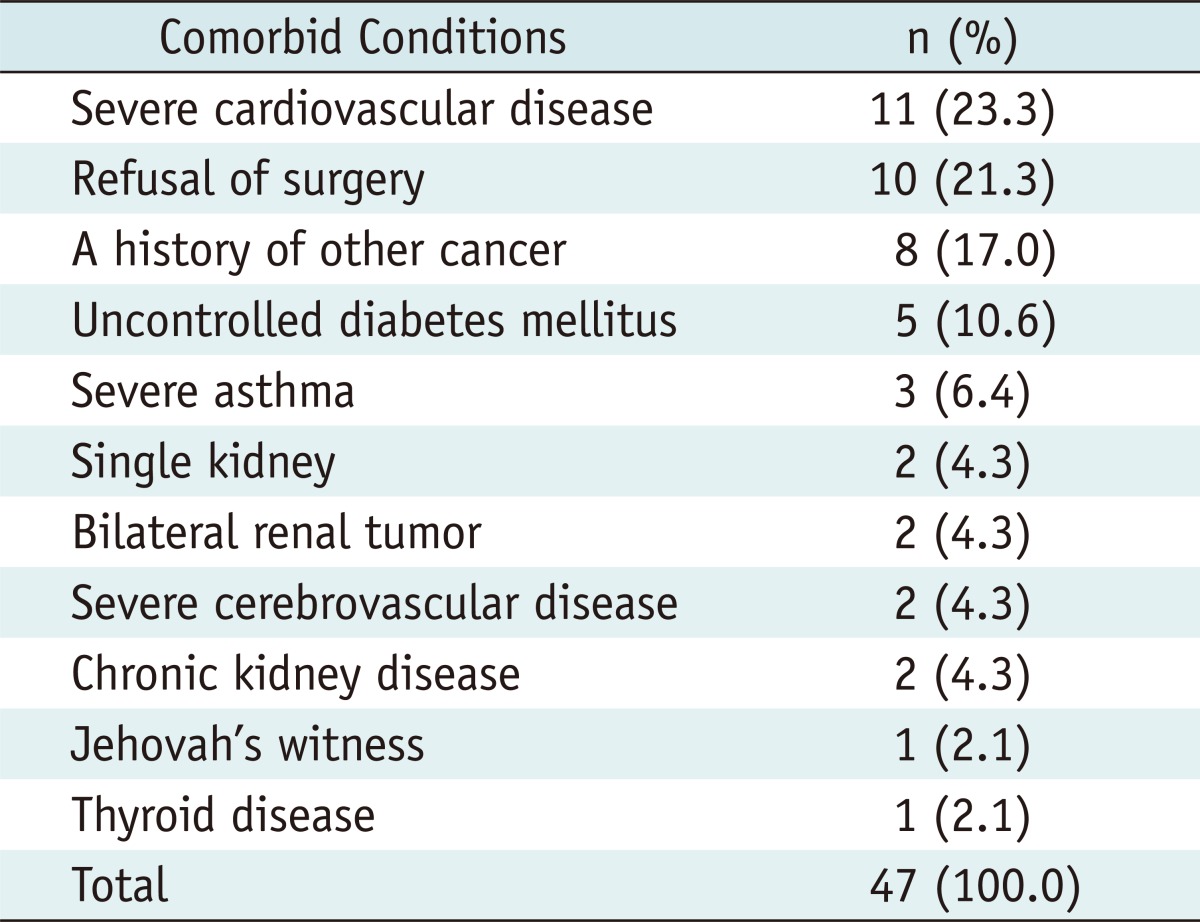
Forty seven patients with 48 renal tumors were treated by either percutaneous or laparoscopic-assisted RFA from August 2004 to July 2010. All of the patients were followed for at least one year after the RFA procedures. All patients underwent preoperative imaging with contrast-enhanced CT or MRI and were suspicious of renal cell carcinoma. Patient demographics and tumor characteristics are shown in Table 1. Reasons for undergoing RFA included coexistent morbidities, bilateral renal tumors, high surgical or anesthetic risks, religious observances and patient preference (Table 2). Informed consent to perform RFA was obtained from each patient after the surgeon reviewed the other treatment options as well as perioperative complications and morbidities associated with RFA.
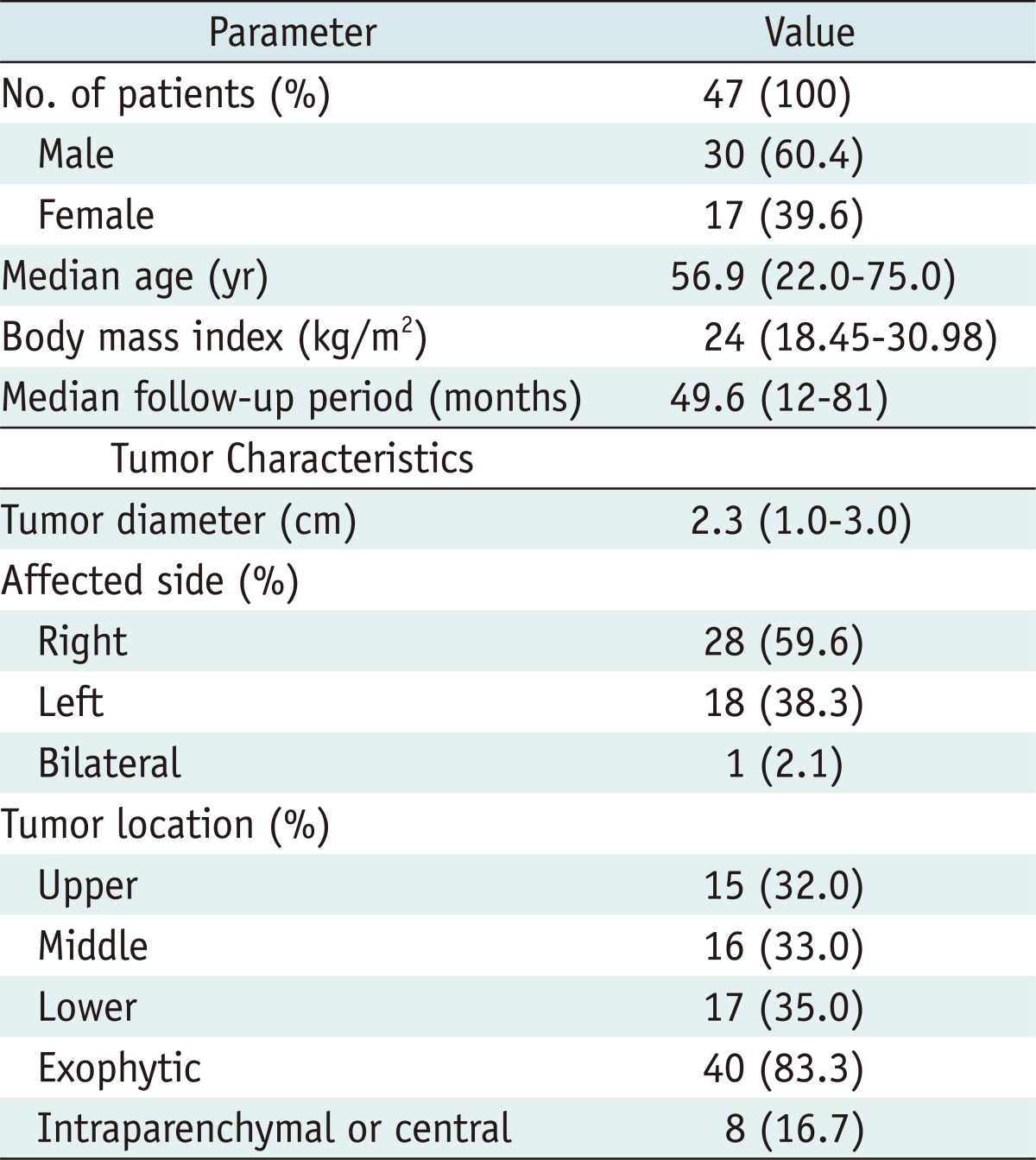
Table 1.
Patient Demographics and Tumor Characteristics of 47 Patients
Note.- No. = number
Table 2.
Patient Comorbidities in 47 Patients
In general, posterior and posterolateral tumors were treated percutaneously and anterior and medially placed tumors were treated by the laparoscopic approach. Percutaneous RFA was performed in a prone or modified lateral position based on tumor location. The patients were sedated intravenously with 3-5 mg of midazolam hydrochloride (Roche, Fontenaysous-Bois, France) and 100-300 µg of fentanyl citrate (Hana Pharm.Co., Hwaseong, Korea). Local analgesia with 2% lidocaine (Huons, Hwaseong, Korea) was administered in combination with intravenous analgesia. The guidance methods used were sonography and CT for the percutaneous approach. Based on the size and location of the tumor, some patients were required to perform overlapping ablations by repositioning the electrode to completely ablate the tumor. As our experiences increase, some of the anterior or medially placed tumors were selectively approached percutaneously. In these select tumors; position change and hydrodissection with dextrose 5% in water were used to displace bowel and adjacent structures to target the tumors.
For the laparoscopic-assisted approach, general anesthesia was conducted and the three- to four-port transperitoneal approach was used to perform laparoscopic-assisted RFA. A kidney surrounding tumor was exposed and the perirenal fat covering the tumor was removed and sent for pathology testing. A steerable laparoscopic ultrasound (US) probe was introduced to visualize the tumor size and location. The electrode probe was placed in the deepest part of the renal tumor under real-time laparoscopic US guidance. RFA was performed using a 200 W generator (Radionics, Burlington, MA, USA) and single (with one 2.0-3.0 cm tip) internally cooled electrodes (Radionics, Burlington, MA, USA) with an impedance-controlled pulsed current. The selection of the tip size was based on the tumor size and location. Ablation time was a maximum of 12 minutes for one cycle, and the ablation cycle was repeated if the target temperature achieved was suboptimal. A follow-up for each patient included a physical examination, chest radiography, creatinine measurement, and a contrast-enhanced CT or MRI. In the early period of the study, contrast-enhanced CT or MRI was obtained at 1 day, 1 week, 1 month, 3 months, 6 months and at least bi-annually thereafter. After January 2008, contrast-enhanced CT or MRI was obtained at 1 month, 3 months, 6 months and bi-annually. To confirm the pathologic criteria of complete ablation, a follow-up biopsy was recommended at 6 months or 1-year follow-up. In addition, a follow-up biopsy was performed on the lesion that had been suspected of growth at the previously ablated tumor on subsequent contrast-enhanced CT or MRI. If the tumor was enhanced within one month, this lesion was considered as an incomplete ablation and RFA was repeated. The ablation zone was defined by measuring the difference in the minimal contrast enhancement (i.e. < 20 Housfield units for CT) as observed on a contrast-enhanced CT after the ablation. The benign periablation enhancement (halo sign) was defined as a thin, concentric, and uniform rim peripheral enhancement with smooth inner margins on contrast-enhanced CT. Technical success was defined as the complete ablation of tumor following the initial session or additional sessions within a month following RFA. Recurrence was deemed probable in cases where nodular or crescentic enhancement was seen on 3 months CT or MRI after confirmed non-enhancement of the initial RFA lesion. Complications were categorized into minor (Clavien Grade I & II) and major (Clavien Grade III, IV and V) complications.
Statistical analyses were performed using Fisher's exact test on the outcome of RFA, and paired t test on the effects of RFA on renal function. For each analysis, a p value of less than 0.05 was considered statistically significant.
RESULTS
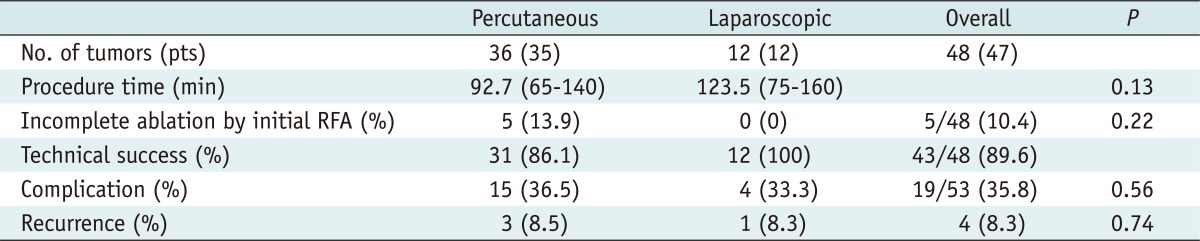
We summarized the results of RFA in Table 3. RFA was performed a total of 53 sessions in 48 tumors and technical success was achieved in 43 of 48 cases (89.6%). Mean age at the time of ablation was 56.9 years (range, 22-75 years). Mean tumor size was 2.3 cm (range, 1.0-3.0 cm). Tumors were evenly distributed at the upper, middle and lower pole (upper, middle, lower: 32, 33, 35 %) of the kidney. The majority (n = 40) of the tumors were exophytic and 8 tumors (16.7%) were intraparenchymal or centrally located. Thirty six (75.0%) tumors underwent percutaneous RFA and 12 tumors (25%) underwent laparoscopic-assisted RFA. The mean procedure time was 92.7 min (range, 65-140 min) for the percutaneous approach and 123.5 min (range, 75-160 min) for the laparoscopic-assisted approach (p = 0.13). Incomplete tumor ablation was observed in 5 of 36 tumors (13.9%) for the percutaneous approach, but all of the 12 tumors for the laparoscopic-assisted approach were successfully ablated following the first RFA session (p = 0.22). Technical success was achieved in 31 of 36 tumors (86.1%) for the percutaneous approach and in all of 12 patients (100%) for the laparoscopic-assisted approach (Fig. 1). The mean follow-up period was 49.6 months (range, 12-81 months).
Table 3.
Results of RFA Procedures in 48 Tumors from 47 Patients
Note.- No. = number, RFA = radiofrequency ablation
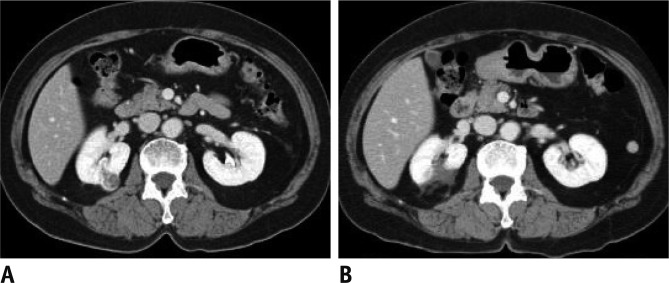
Fig. 1.
Well ablated, small intraparenchymal renal cell carcinoma in 62-year-old male who underwent radiofrequency ablation (RFA).
A. Contrast-enhanced CT scan before RFA demonstrates 2.1 cm solid enhancing renal tumor located at upper pole posterior region of right kidney. B. One month follow-up contrast-enhanced CT scan demonstrates absence of periablation enhancement or residual contrast enhancement within tumor bed indicating technical success.
A total of 19 complications were noted in 17 sessions, for an overall complication rate of 35.8%. Complications occurred in 15 of 41 tumors (36.5%) for the percutaneous approach and in 4 of 12 tumors (33.3%) for the laparoscopic-assisted approach (p = 0.56). Minor complications (Clavien I & II) occurred in 32.1% of the procedures and accounted for 94.8% of the overall complications. Major complications (Clavien ≥ III) occurred in 1.9% of the procedures and accounted for 5.2% of the overall complications. Complications included a perinephric hematoma in 11, gross hematuria in 1, perinephric fluid collection in 3, a mild thermal injury of the psoas muscle in 1, a bowel injury with mild hydronephrosis in 1, and a liver injury and a thermal injury of the pelvocalyceal system in 1. In one patient, a major complication (Clavien Grade III) occurred during laparoscopic-assisted RFA. Inadvertent bowel injury with mild hydronephrosis occurred in a patient who had a tumor in the anterior mid pole of the kidney. Since this patient underwent a subtotal radical gastrectomy three years prior to RFA and had severe adhesion, we decided to perform laparoscopic-assisted RFA. During the laparoscopic dissection of bowel adhesion, inadvertent thermal injury has occurred in a small bowel by monopolar hook cautery. After completion of RFA, the patient underwent emergent ileostomy diversion, and 4 months later, the ileostomy was reversed. Mild hydronephrosis was resolved within a month (Table 4).
Table 4.
Complications That Occurred during and after Radiofrequency Ablation in 48 Renal Tumors
In 48 tumors, secondary RFA session was necessary in 5 tumors (10.4%) after the initial session due to irregular peripheral enhancement, indicating incomplete ablation in a one month follow-up contrast-enhanced CT. These tumors were initially treated by percutaneous RFA. Among the 5 tumors for which RFA was necessary, 3 were located in the central area of the kidney. In another five tumors, benign periablation enhancement were observed, but subsequently resolved within 3 months of ablation. A pre-RFA biopsy was performed in eight tumors, which have shown other possible features of renal tumors on preoperative kidney CT. Five tumors were diagnosed as renal cell carcinoma (RCC) and three tumors had either fibrotic tissue or normal renal parenchyma on pathology (Table 5). These 3 tumors were included in the study because of probable radiological features of RCC and the possibility of false-negative results. In the forty remaining tumors, pre-RFA biopsy was not performed due to clear radiographic findings of renal cell carcinoma. To confirm the pathologic criteria of complete ablation, 35 tumors underwent a 6-month or 1-year follow-up biopsy (Table 5). In the remaining 13 tumors, a post RFA biopsy was not performed due to patient refusal and the risk of bleeding.
Table 5.
Results of Renal Tumor Biopsy
Note.- RFA = radiofrequency ablation, No. = number
Local tumor recurrence was observed in 4 patients (8.3%) at various follow-up intervals (6, 26, 30, 60 months). Tumor recurrence was observed in 3 of 35 patients (8.5%) for the percurtaneous approach and in 1 of 12 patients (8.3%) for the laparoscopic-assisted approach (p = 0.74). One patient had a viable renal tumor on a 6 months follow-up biopsy despite non-enhancement of the ablated lesion on a 6-months follow-up CT. This patient successfully underwent repeated RFA and no signs of local recurrence were observed at the 27 months follow-up period. Another patient who had been treated by RFA for bilateral renal tumors was found to have a hyperechoic lesion of the left upper pole of the kidney on a 26 months follow-up US. A subsequent renal biopsy confirmed the recurrent tumor and the patient underwent radical nephrectomy. Another patient who had received successful RFA for a left low pole renal tumor developed a new cystic renal tumor with enhancement on the mid pole of the left kidney on a 30 months follow-up CT. Repeated RFA was performed successfully and no recurrence has been observed for 11 months since the last RFA. Another patient with bilateral RCCs (left: 6 cm, right: 3 cm) initially underwent right percutaneous RFA, followed by a left radical nephrectomy without any complications. On a 60 months follow-up CT, a recurrent renal tumor on the right upper pole was detected, and repeated RFA was performed successfully. No recurrence has been observed for 6 months since the last RFA performed on this patient.
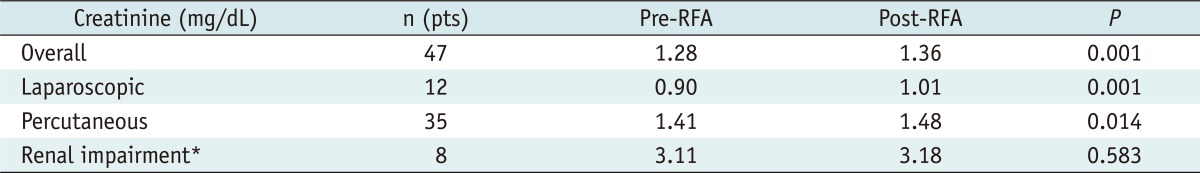
The median creatinine levels before and after 12 months of RFA were 1.28 and 1.36 mg/dL, respectively (p = 0.001). Additionally, in 8 patients with renal impairment preoperatively, serum creatinine levels 12 months after RFA did not differ from those before RFA (3.11 vs. 3.18 mg/dL; p = 0.583) (Table 6).
Table 6.
Creatinine Change before and after 12 Months of RFA
Note.- *Single kidney; bilateral renal tumor; chronic kidney disease. RFA = radiofrequency ablation
In our study, no distant metastasis was observed during the mean follow-up time of 49.6 months and all of the patients were still alive at the time of this writing.
DISCUSSION
For decades, radical nephrectomy was considered the gold standard treatment for localized RCC and was the only curative option. Recently, advanced radiologic imaging techniques have led to the increased incidence of small and localized renal tumors. Refinements in surgical techniques with better imaging modalities have resulted in an evolution of NS surgeries such as an open and laparoscopic partial nephrectomy (5-8). Despite the excellent results for the surgical excision, advancements in imaging technology have resulted in the increased use of more conservative, thermal ablative techniques as an alternative to surgical extirpation (17). Since more tumors are diagnosed in elderly patients with medical comorbidities, some patients may have renal insufficiency or other comorbidities that require a maximal nephron conserving approach. Since Zlotta et al. (10) initially described the clinical application of RFA in human RCC in 1997, many authors have been reporting favorable experiences on RFA for SRMs.
Several factors affect the outcome of RFA: tumor size and location; tissue impedance; ablation time; as well as the amount of energy delivered and surface area of the electrodes. First, tumor size is an important consideration for patient selection. Gervais et al. (11) reported in a multivariate analysis of 85 patients, who underwent percutaneous RFA, that small tumor size and the non-central location of the tumor were independent significant predictors of complete necrosis after a single RFA session. Furthermore, Mylona et al. (18) reported a complete response of 85.7% for tumors less than 3 cm after the first RFA session, but reported a noticeably lower response rate with tumors greater than 5 cm in size. Tumors greater than 3 cm were technically challenging to completely ablate on the initial attempt and required multiple overlapping ablation techniques. Therefore, smaller renal tumors are ideal candidates to obtain complete responses for the first RFA session. In our study, all of the tumors were less than 3 cm in size and 20 tumors were greater than 2.5 cm. In these patients, two sessions of RFA with overlapping techniques were performed for the complete ablation of the tumor.
Tumor location is another factor that may influence ablative outcome. It is reported that tumors adjacent to large vessels will suffer a 'heat sink', in which a regional vascular flow reduces the extent of the thermally induced coagulation (11, 19). By contrast, the ablative effect of exophytic tumors are higher, as they are easy to target with an RFA probe and because the insulating effect of surrounding perirenal fat allows for higher temperatures during RFA (11, 19). In our series, the tumors were evenly distributed in the upper, middle and lower pole of the kidney (upper, middle, lower: 32, 33, 35%). Tumors adjacent to the renal hilum were relatively difficult to completely ablate using the primary procedure compared to other tumors. Also, tumors located in the upper pole of the kidney tend to be more difficult to compare to those located in the middle or lower pole. This outcome may be due to the difficulty of needle insertion as the upper pole of the kidney is always covered by ribs, spleen, lung and liver. The location of the tumors such as exophytic or endophytic tumors, was not related to the outcome except when the tumor was endophytic in the medial central location. However, complete tumor ablation was achieved in all tumors after the second RFA session regardless of tumor location. Repeatability is clearly an advantage of RFA. A laparoscopy-assisted US guidance or open intraoperative RFA should be considered as a treatment option for patients with difficult access, such as multiple lesions in a solitary kidney, an intervening lung, parenchyma, bowel or thin patients with an anterior and medial lesion.
In five patients, we have utilized a position change and hydrodissection with dextrose 5% in water to protect adjacent structures before targeting the tumors. Twelve patients whose tumors were not in an ideal location for percutaneous approach were treated with a laparoscopy-assisted US guided RFA to isolate the targeted tumors away from adjacent normal structures, such as liver, colon, spleen or psoas muscle. In our series, the percutaneous approach for RFA ablation showed a higher incomplete ablation rate (13.9%) compared with the laparoscopic approach after the initial RFA session. A secondary RFA session was performed successfully in 5 tumors with incomplete ablation within one month of percutaneous RFA.
The technical success rate of the initial RFA procedures has been reported to be from 90 to 100% (20). We defined technical success as the absence of enhancement inside the tumor as observed by contrast-enhanced CT or MRI, the criterion also used in several other reports (18-22). In our series, technical success was achieved in 43 of 48 renal tumors (89.6%), which is comparable with previous studies (18-22). The local recurrence rate varies from 0.0% to 11.1% in cases where technical success has been possible at the initial RFA (11, 15, 23). Local tumor recurrence has occurred in 4 patients (8.5%) in our study. Three of 35 patients (8.5%) recurred for the percutaneous approach and one of 12 patients (8.3%) recurred for the laparoscopic-assisted approach. Each patient developed local recurrences after 6, 26, 30, and 60 months, respectively. This data illustrates the importance of long term follow-up of patients treated by RFA. Of the 4 patients who developed local recurrence, 3 had recurrences detected after 2 years of the initial RFA procedure. With a mean follow-up of 49.6 months, no distant metastasis has been observed. Despite our favorable intermediate follow-up results, it may require a longer follow-up period to detect recurrence radiologically following RFA. Also, T1a renal masses are generally known to have low malignant potential. Therefore, it may take quite a while to have a metastatic extension from local recurrence following RFA.
Another important issue is the use of the post-ablation of tumor specimens to question the validity of a radiographic definition of ablative success.
The importance of the post-RFA biopsy was recently demonstrated by Weight et al. (24). In 6 of 13 patients who underwent post-RFA follow-up biopsy at 6 months, viable tumor cells were observed, where they would otherwise have no evidence of enhancement on follow-up CT or MRI. However, the same authors have not seen any viable tumor at the 6 months follow-up biopsy following cryoablation, and any contrast enhancement was obtained on a follow-up CT. The authors concluded that after RFA, radiologic findings were not reliable and follow-up biopsies had an impact on further decision making. Thus, the authors recommended post RFA at a follow-up biopsy due to the significant risk of residual cancer cells without radiographic evidence. Also, Arima et al. examined the ablated tumor specimens removed 6 weeks after RFA and discovered that tumor specimens showed well-preserved cancer cells under Hematoxylin and Eosin staining. However, almost all the cells were stained with terminal deoxynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate nick end labeling (TUNEL), which is used to detect apoptosis (22). Since these studies have been reported, we have been performing follow-up biopsies after an initial RFA. Of the 35 patients who underwent a 6-month or 1-year follow-up biopsy, one patient was discovered to have recurrent tumor. This patient showed viable cancer cells on biopsy who otherwise demonstrated no evidence of enhancement on a 6 months follow-up CT. However, in a recent study, Tracy et al. (25) raised concerns by emphasizing that the majority of these studies have used traditional hematoxylin and eosin staining in evaluating post-treatment biopsies, which is inadequate for assessing cellular viability, because the heat fixation during RFA results in the preservation of the cellular architecture. To further address this issue, Raman and colleagues recently reported the biopsy data of radiologically negative lesions in 19 patients obtained more than 1 year after RFA. In this study, multiple core biopsies had consistently shown non-viable cells within RF treated lesions in all specimens (26). The authors concluded that the early-ablative biopsy may be unreliable. Thus, particularly for RFA, an early-ablative biopsy and staining may not accurately reflect cell death or viability. Because of these studies and the difficulties associated with obtaining routine post-ablative biopsies in patients who have no radiological evidence of recurrence, currently we do not recommend postablative biopsy for patients who have normal imaging studies (absence of contrast enhancement or growth) after RFA.
Complications of RFA can be divided as minor (Clavien Grade I or II) and major (Clavien Grade III, IV, V). The reported complications include a perinephric hematoma, gross hematuria, pyonephrosis, ureteral stricture, damage to adjacent organs, pain, and paresthesis (27, 28). In the present study, 19 complications occurred in 17 sessions for an overall complication rate of 35.8%. Most complications were managed conservatively, and just one patient required a repeat surgery.
According to several studies regarding preservation of renal function after RFA, there is minimal change in renal function before and after RFA. Reported mean serum creatinine change ranged from +0.06 to +0.24 mg/dL at 24 months when compared with pre-RFA creatinine levels (29-32). In our series, the median creatinine levels of 47 patients before and 12 months after RFA was 1.28 and 1.36 mg/dL, respectively. Additionally, in 8 patients with renal impairment preoperatively, serum creatinine levels did not differ from those before RFA at 12 months after RFA (3.11 vs. 3.18 mg/dL) (Table 6).
Our study results are in accordance with those reported in the medical literature and supports the RFs of SRMs as a safe, effective, reproducible and well tolerated procedure for the treatment of stage T1 renal tumors.
However, this study has some limitations. First, our series is considered a retrospective review despite a 4-year mean follow-up. Second, pre-RFA biopsy was not routinely performed in most cases, thus lacking histological proofs. As such, despite the intermediate follow-up report, the definitive success of oncological control remains unclear. In our early experience, pre-RFA renal biopsy was considered as a controversial procedure because of the risk of hemorrhage and tumor seeding (33). Also, we relied on the imaging characteristics of RCC on contrast-enhanced CT or MRI to make a clinical diagnosis. Therefore, a pre-RFA renal biopsy was performed in the patients, who have shown other possible features of the renal tumors on a preoperative kidney CT.
Recently, with the increased use of percutaneous ablative technologies to treat renal tumors, many investigators have addressed the important role of confirmatory preablative biopsy, as ablation lacks the histological analysis as afforded by surgical resection. Pathologic confirmation will provide an accurate treatment and follow-up plan, reduce over-estimation of the treatment effectiveness and minimize unnecessary follow-up. Therefore, currently we have included pre-RFA renal biopsy in our routine protocol.
Despite these limitations, 4-year mean follow-up results suggests an excellent therapeutic outcome with RFA in select patients with SRMs, while achieving effective local tumor control and preserving normal renal parenchyma. Nevertheless, long term follow-up should be performed to define durable efficacy after successful RFA.
Acknowledgments
The authors thank Won Yeol Cho and Tae Hyo Kim for their assistance in the writing of this manuscript.
Footnotes
This study was supported by research funds from Dong-A University.
References
- 1.Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995;197:589–597. doi: 10.1148/radiology.197.3.7480724. [DOI] [PubMed] [Google Scholar]
- 2.Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75–79. doi: 10.1016/j.juro.2007.08.156. discussion 79-80. [DOI] [PubMed] [Google Scholar]
- 3.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161:33–34. doi: 10.1016/s0022-5347(01)62052-4. discussion 34-35. [DOI] [PubMed] [Google Scholar]
- 6.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 7.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Park H, Byun SS, Kim HH, Lee SB, Kwon TG, Jeon SH, et al. Comparison of laparoscopic and open partial nephrectomies in t1a renal cell carcinoma: a Korean multicenter experience. Korean J Urol. 2010;51:467–471. doi: 10.4111/kju.2010.51.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasticier G, Timsit MO, Badet L, De La Torre Abril L, Halila M, Fassi Fehri H, et al. Nephron-sparing surgery for renal cell carcinoma: detailed analysis of complications over a 15-year period. Eur Urol. 2006;49:485–490. doi: 10.1016/j.eururo.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Zlotta AR, Wildschutz T, Raviv G, Peny MO, van Gansbeke D, Noel JC, et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11:251–258. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 11.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto ED, Johnson DB, Ogan K, Trimmer C, Sagalowsky A, Margulis V, et al. Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology. 2005;65:877–881. doi: 10.1016/j.urology.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Yoon SK, Cho JH, Oh JY, Nam KJ, Kwon HJ, et al. Radiofrequency ablation treatment for renal cell carcinoma: early clinical experience. Korean J Radiol. 2008;9:340–347. doi: 10.3348/kjr.2008.9.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell MA, Charboneau WJ, DiMarco DS, Chow GK, Zincke H, Callstrom MR, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003;180:1509–1513. doi: 10.2214/ajr.180.6.1801509. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol. 2006;20:569–573. doi: 10.1089/end.2006.20.569. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Kim TH, Kim SD, Lee KS, Sung GT. Radiofrequency ablation of renal tumors: our experience. Korean J Urol. 2011;52:531–537. doi: 10.4111/kju.2011.52.8.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingo MS, Leveillee RJ. Central and deep renal tumors can be effectively ablated: radiofrequency ablation outcomes with fiberoptic peripheral temperature monitoring. J Endourol. 2008;22:1261–1267. doi: 10.1089/end.2008.0135. [DOI] [PubMed] [Google Scholar]
- 18.Mylona S, Kokkinaki A, Pomoni M, Galani P, Ntai S, Thanos L. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with solitary kidney: 6 years experience. Eur J Radiol. 2009;69:351–356. doi: 10.1016/j.ejrad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Watkins TW, Parkinson R. Percutaneous radiofrequency ablation of renal tumours: case series of 11 tumours and review of published work. Australas Radiol. 2007;51:412–419. doi: 10.1111/j.1440-1673.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 20.Salas N, Ramanathan R, Dummett S, Leveillee RJ. Results of radiofrequency kidney tumor ablation: renal function preservation and oncologic efficacy. World J Urol. 2010;28:583–591. doi: 10.1007/s00345-010-0562-2. [DOI] [PubMed] [Google Scholar]
- 21.Breen DJ, Rutherford EE, Stedman B, Roy-Choudhury SH, Cast JE, Hayes MC, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30:936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arima K, Yamakado K, Kinbara H, Nakatsuka A, Takeda K, Sugimura Y. Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol. 2007;14:585–590. doi: 10.1111/j.1442-2042.2007.01740.x. discussion 590. [DOI] [PubMed] [Google Scholar]
- 23.Zagoria RJ, Traver MA, Werle DM, Perini M, Hayasaka S, Clark PE. Oncologic efficacy of CT-guided percutaneous radiofrequency ablation of renal cell carcinomas. AJR Am J Roentgenol. 2007;189:429–436. doi: 10.2214/AJR.07.2258. [DOI] [PubMed] [Google Scholar]
- 24.Weight CJ, Kaouk JH, Hegarty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179:1277–1281. doi: 10.1016/j.juro.2007.11.075. discussion 1281-1283. [DOI] [PubMed] [Google Scholar]
- 25.Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: experience from treating 243 small renal masses over 7.5 years. Cancer. 2010;116:3135–3142. doi: 10.1002/cncr.25002. [DOI] [PubMed] [Google Scholar]
- 26.Raman JD, Stern JM, Zeltser I, Kabbani W, Cadeddu JA. Absence of viable renal carcinoma in biopsies performed more than 1 year following radio frequency ablation confirms reliability of axial imaging. J Urol. 2008;179:2142–2145. doi: 10.1016/j.juro.2008.01.119. [DOI] [PubMed] [Google Scholar]
- 27.Aron M, Gill IS. Renal tumor ablation. Curr Opin Urol. 2005;15:298–305. doi: 10.1097/01.mou.0000177684.93531.85. [DOI] [PubMed] [Google Scholar]
- 28.Kwan KG, Matsumoto ED. Radiofrequency ablation and cryoablation of renal tumours. Curr Oncol. 2007;14:34–38. doi: 10.3747/co.2007.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arzola J, Baughman SM, Hernandez J, Bishoff JT. Computed tomography-guided, resistance-based, percutaneous radiofrequency ablation of renal malignancies under conscious sedation at two years of follow-up. Urology. 2006;68:983–987. doi: 10.1016/j.urology.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Levinson AW, Su LM, Agarwal D, Sroka M, Jarrett TW, Kavoussi LR, et al. Long-term oncological and overall outcomes of percutaneous radio frequency ablation in high risk surgical patients with a solitary small renal mass. J Urol. 2008;180:499–504. doi: 10.1016/j.juro.2008.04.031. discussion 504. [DOI] [PubMed] [Google Scholar]
- 31.Ahrar K, Matin S, Wood CG, Wallace MJ, Gupta S, Madoff DC, et al. Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol. 2005;16:679–688. doi: 10.1097/01.RVI.0000153589.10908.5F. [DOI] [PubMed] [Google Scholar]
- 32.Mahnken AH, Rohde D, Brkovic D, Günther RW, Tacke JA. Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol. 2005;46:208–214. doi: 10.1080/02841850510015938. [DOI] [PubMed] [Google Scholar]
- 33.Kümmerlin IP, Borrego J, Wink MH, Van Dijk MM, Wijkstra H, de la Rosette JJ, et al. Nephron-sparing surgery and percutaneous biopsies in renal-cell carcinoma: a global impression among endourologists. J Endourol. 2007;21:709–713. doi: 10.1089/end.2006.0409. [DOI] [PubMed] [Google Scholar]