Abstract
OBJECTIVE
Non-obese diabetic (NOD) mice develop an autoimmune exocrinopathy that shows similarities with Sjögren’s syndrome. They provide an experimental model to study the pathoetiogenesis of this disease.
MATERIALS AND METHODS
Salivary gland (SG) function and salivary sodium content were measured in 8-, 12-, 16- and 20-week-old NOD and age-matched CB6 mice. In NOD mice, SG expression of phenotypic cell markers, B cell-stimulating and costimulatory molecules were evaluated. Cytokine levels were measured in serum and SG homogenates.
RESULTS
Microscopically evident SG inflammation in NOD mice was preceded by expression of intercellular adhesion molecule 1 on epithelial cells in the presence of macrophages and relatively high levels of cytokines. Next, an influx consisting of mainly T, B, natural killer, plasma and dendritic cells was seen. Most cytokines, except for interleukin (IL)12 /IL23p40 and B cell-activating factor, decreased or remained stable over time, while glandular function deteriorated from 16 weeks of age onward compared with CB6 mice.
CONCLUSION
Sjögren’s syndrome-like disease in NOD mice occurs in multiple stages; immunological and physiological abnormalities can be detected before focal inflammation appears and salivary output declines. Extrapolating this knowledge to human subjects could help in understanding the pathogenesis and aid the identification of potential therapeutic targets.
Keywords: Sjögren’s syndrome, salivary gland, autoimmune sialadenitis, non-obese diabetic mouse, immunology
Introduction
Sjögren’s syndrome (SS) is a chronic, systemic autoimmune disorder of unknown aetiology with a poorly understood pathogenesis. The disease is characterised by inflammation and dysfunction of secretory glands leading to ocular and oral dryness. There is currently no cure for SS, and treatment options are mostly limited to palliation of symptoms (Fox, 2005). Immune cells, such as macrophages, dendritic cells and lymphocytes and cytokines, immunoglobulins and autoantibodies are all thought to play a role in the disease development and progression. One of the hallmarks of SS is the presence of typical focal, periductal and perivascular lymphocytic infiltrates in the secretory glands. In human salivary glands (SGs), T cells initially outnumber B cells in small inflammatory aggregates, but in more severe lesions, the B to T cell ratio is increased (Adamson et al, 1983; Christodoulou et al, 2010). Eventually, focal infiltrates may organise into germinal centre-like formations, which is regarded as an intermediate state that could lead to the potentially lethal development of B cell lymphomas (reviewed in Voulgarelis and Moutsopoulos, 2008). In addition, the infiltration of certain types of inflammatory cells is associated with several clinical features, including systemic disease manifestations, such as C4 hypocomplementaemia, cryoglobulinaemia and purpura (Molina et al, 1985; Bombardieri et al, 2004; Manoussakis et al, 2007; Christodoulou et al, 2010).
Most patients are not diagnosed until a relatively late disease stage, when many glandular changes have already occurred, and disease parameters are often not followed longitudinally. Therefore, only limited data regarding the early stages and subsequent progression are available, making it difficult to discern the mechanisms of the disease and to develop appropriate treatment. The non-obese diabetic (NOD) mouse could provide more insight; NOD mice spontaneously develop an inflammatory syndrome affecting the exocrine glands, which shows many histological parallels with its human counterpart (reviewed in Humphreys-Beher and Peck, 1999), coinciding with a progressive impairment of secretory function (reviewed in Chiorini et al, 2009). Female (more so than male) NOD mice develop focal infiltrates in the SGs typically between 8 and 12 weeks of age, and SG output declines between 12 and 24 weeks (Yamano et al, 1999; Rosignoli et al, 2005; Jonsson et al, 2006). The loss of salivary flow was associated with changes in cytokine levels in serum and saliva and to a lesser degree with the glandular influx of lymphocytes in NOD mice followed up to 24 weeks (Jonsson et al, 2006). In studies on SS-like disease in NOD mice, salivary sodium content or SG cytokines, B cell-stimulating factors and costimulatory molecules were not assessed.
In this descriptive study, we examined the NOD mouse and followed several pathological and physiological processes during disease development, starting at a preclinical phase, and continuing up to late-stage inflammation and secretory dysfunction. The salivary flow rate (SFR) and salivary levels of sodium, expression of cytokines in SGs and serum and additional local inflammatory biomarkers were assessed. Translating this knowledge to SS will help to unravel its pathogenesis and could potentially lead to identification of therapeutic targets.
Material and methods
Mice
Female NOD (001976 NOD/ShiLtJ; Jackson Laboratories, ME, USA) and female CB6 mice (CB6F1 /Cr, BALB /CAnNCr_x C57BL /6NCr_; National Cancer Institute Mouse Repository, Frederick, MD, USA) were obtained at 6 weeks of age and housed under specific pathogen-free conditions in our animal facility. Animal studies were approved by the National Institute of Dental and Craniofacial Research (NIDCR) Animal Care and Use Committee and the National Institutes of Health (NIH) Biosafety Committee. All procedures were conducted in compliance with the NIH guidelines on the use of animals in research. Strains were housed separately with a maximum of five mice per cage on a 12-h light /12-h dark cycle and had access to food and water ad libitum. Blood glucose levels of NOD mice were measured weekly, starting at 12 weeks of age, using a OneTouch monitor (LifeScan, Milpitas, CA, USA). A subcutaneous injection of long-acting Humalin N (1 U per mouse, every 24 h; Eli Lilly, Indianapolis, IN, USA) was administered to mice with blood glucose levels ≥250 mg dl−1 to treat hyperglycaemia and related dehydration. In our facility, the incidence of diabetes is normally 50–70% by 20 weeks of age using this cut-off value.
Saliva collection and assessment of sodium concentration
Five days prior to sacrificing, whole stimulated saliva of NOD mice was collected, as previously described (Yamano et al, 1999; Kok et al, 2003). For CB6 mice, saliva was collected at the same age as NOD mice. Briefly, mild anaesthesia was induced with ketamine (100 mg ml−1, 1 ml kg−1 body weight; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (20 mg ml−1, 0.7 ml kg−1 body weight; Phoenix Scientific, St. Joseph, MO, USA) solution given intramuscularly, and saliva secretion was stimulated by a subcutaneous injection of pilocarpine (0.5 mg kg−1; Sigma-Aldrich, St Louis, MO, USA). Next, whole saliva was collected from the oral cavity for 20 min with a haematocrit tube (Drummond Scientific Company, Broomall, PA, USA) placed in a preweighed 0.5-ml microcentrifuge tube and volume was determined gravimetrically. Saliva was stored at −80°C until analysis. Saliva samples were diluted 1000 times in a solution containing 5 g l−1 CsCl, and sodium concentrations were measured by atomic absorption spectrometry using a K-Na lamp (AAnalyst 200 Atomic Absorption spectrophotometer; PerkinElmer, Waltham, MA, USA). Calibrations for sodium were performed by linear regression analysis using 1, 2 and 3 ppm (mg l−1) as standards.
Histological assessment of salivary gland inflammation
NOD mice were sacrificed at 8, 12, 16 and 20 weeks of age, and the submandibular SGs were removed, cleaned of any adjacent (possibly lymphoid) tissue and cut in three cross-sectional parts. The first part was collected in formalin, embedded in paraffin and cut in 5-μm sections. Three sections (each 50 μm apart from the previous) were mounted on glass slides and stained with haematoxylin and eosin. The number of foci [where one focus is defined as an aggregate of ≥50 infiltrated cells per 4 mm2 (Greenspan et al, 1974)] on each section was counted blindly by two different examiners, and the mean focus score (FS) was determined.
Immunohistochemical analysis of salivary gland tissue
The second submandibular SG part was emerged in Tissue-Tek optimal-cutting temperature (OCT) compound (Miles, Elkhart, IN, USA) and quickly frozen on dry ice. Sections (7 μm) were cut with a cryostat, mounted on glass slides and stored at −80°C until further use. For staining, frozen sections were thawed and fixated in acetone for 10 min, except for anti-BAFF [B cell-activating factor belonging to the tumour necrosis factor (TNF) family] that was used on formalin-fixed, paraffin-embedded tissue, which had undergone heat-induced antigen retrieval in a citrate buffer (pH 6.0) after deparaffinisation with xylene and alcohol. Endogenous peroxidase was blocked, slides were washed in phosphate-buffered saline (PBS) and further blocked with 1% bovine serum albumin (BSA) and 10% normal goat serum (NGS; Dako, Glostrup, Denmark) in PBS overnight. The next day, slides were incubated with primary antibodies in 1% PBS and 5% NGS at room temperature for 1 h. Monoclonal primary antibodies used were anti-CD4 (clone L3T4; eBioscience, San Diego, CA, USA) to detect T helper (Th) 1 and 2 cells; anti-CD8 (clone 53-6.7; eBioscience) for cytotoxic T cells; anti-CD11c (clone N418; Abcam, Cambridge, MA, USA) for plasmacytoid dendritic cells; anti-CD19 (clone 1D3; BD Biosciences, Franklin Lakes, NJ, USA) for B cells; anti-CD138 (clone 281-2; BD Biosciences) for plasma cells; anti-IgD [clone 11-26c (11–26); eBioscience] for mature, non-switched, peripheral B cells; anti-CD68 (clone FA11; Abcam) for monocytes /macrophages; anti-CD49b (BD Biosciences) for natural killer cells; and anti-CD40 (clone 3 /23; BD Biosciences) and anti-ICAM1 (intercellular adhesion molecule 1; clone YN 1 /1.7.4; Abcam) for cell adhesion and costimulatory molecules. Anti-BAFF (clone buffy-2; Enzo Life Sciences, Farmingdale, NY, USA) antibody was used to detect the cellular B cell-stimulating factor BAFF. For anti-Foxp3 antibody (clone FJK-16s; eBioscience), to detect T regulatory cells (Tregs) in the SG tissue, a 1:50 dilution and an additional amplification step with biotin-labelled tyramide (PerkinElmer) followed by streptavidin-labelled horse-radish peroxidase (HRP; PerkinElmer) was performed. All primary antibodies used were of the rat IgG class of immunoglobulins, except for anti-CD11c and anti-CD49b (hamster IgG), and anti-BAFF (rat IgM) antibodies, and used at a 1:100 dilution, except for anti-CD3 (1:200), anti-CD4 (1:250) and anti-CD138 (1:50). Staining was visualised with HRP-labelled goat anti-rat secondary antibody (Southern Biotechnology, Birmingham, AL, USA) or, for anti-CD11c antibody only, goat anti-hamster secondary antibody (Jackson ImmunoResearch, Suffolk, UK) in a 1:100 dilution, and developed with aminoethylcarbazole (AEC) substrate (Dako). To be able to analyse SGs before focal inflammation had appeared, five 8-week-old mice without an FS were analysed in more detail. From week 12, when all mice showed inflammatory aggregation, five randomly selected mice were assessed. For control sections, immunoglobulin class- and species-matched control antibodies instead of the primary antibody were used.
For all markers, images of foci and adjacent tissue of in total 18 high-power fields were assessed using the Qwin digital image analysis system (Leica, Cambridge, UK), as previously described (Haringman et al, 2005). For Foxp3, only staining in foci was examined, because amplification with tyramide resulted in non-specific staining of surrounding epithelial tissue. For BAFF, CD40 and ICAM1, the staining for foci and tissue was digitally separated after which they were analysed. Staining was expressed as internal optical density (IOD) mm−2, an arbitrary unit representing the intensity of staining per mm2 (van der Hall et al, 2007).
Preparation of salivary gland tissue homogenates
The third submandibular SG part was snap-frozen on dry ice and stored at −80°C until further use. Prior to homogenisation, samples were thawed and kept on ice. Samples were crushed, placed in 2-ml tubes containing 1 ml HEPES lysis buffer (20 mM HEPES, 0.5 M NaCl, 0.25% Triton X-100 and 1 mM EDTA) and complete protease inhibitor (Roche, Mannheim, Germany) and lysed by shaking at 4°C overnight. The next day, samples were centrifuged at 1500 g at 4°C for 10 min. Protein content of supernatants was measured using a bicinchoninic acid (BCA) protein detection kit (Pierce, Rockford, IL, USA) and stored at −80°C until further use.
Cytokine detection in salivary gland homogenates and serum
Blood was collected by heart puncture immediately postmortem, left to clot on ice for 3 h and centrifuged at 2500 g for 25 min at 4°C to obtain serum. Levels of interleukin (IL)1β, IL2, IL4, IL6, IL10, IL12 /IL23p40, IL17, IL18, IL23p19, interferon (IFN)γ, TNFα and transforming growth factor (TGF)β were measured in serum and SG homogenates using a multiplex sandwich ELISA (Aushon Biosystems, Billerica, MA, USA). This assay does not distinguish between the p40 subunit of IL12 and IL23, or free p40. Results for SG homogenates were corrected for protein concentration. Duplicates for each sample were tested in three dilutions, and the mean values of the duplicates from every optimal dilution were reported. Detection levels (pg ml−1) were for IL1β and TNFα > 0.9, IL2 and IL4 > 1.2, IL6 > 8.2, IL10 and IL17 > 1.3, IL12 /IL23p40 > 0.2, IL18 > 2.4, IL23p19 > 33.7, IFNγ > 4.3, TGFβ > 8.0.
Statistical analysis
Data were described as mean ± standard deviation (s.d.) and displayed in dot plots with individual values for each mouse and a mean (line) or in bar graphs with mean ± s.d. A one-way ANOVA was used to detect differences within the groups for the various ages followed by a post hoc Tukey’s test. Depending on the data distribution, an unpaired Student’s t test or a Mann–Whitney test was used to compare the mouse strains at each time point. The Pearson’s correlation coefficient was used to investigate the relationship between FS and SFR, between cytokines and SFR, between cytokines and FS or between Th1 and Th2 cytokines. All analyses were performed with GraphPad Prism v5.01 statistical software (GraphPad Software, La Jolla, CA, USA). A P-value ≤0.05 was considered statistically significant. In figures, an * indicates a P-value ≤0.05 and >0.01, ** for 0.01 ≤ P > 0.001, *** for P ≤ 0.001 and P = ns was used for non-significant values.
Results
Salivary flow rate of NOD mice is decreased after 16 weeks of age
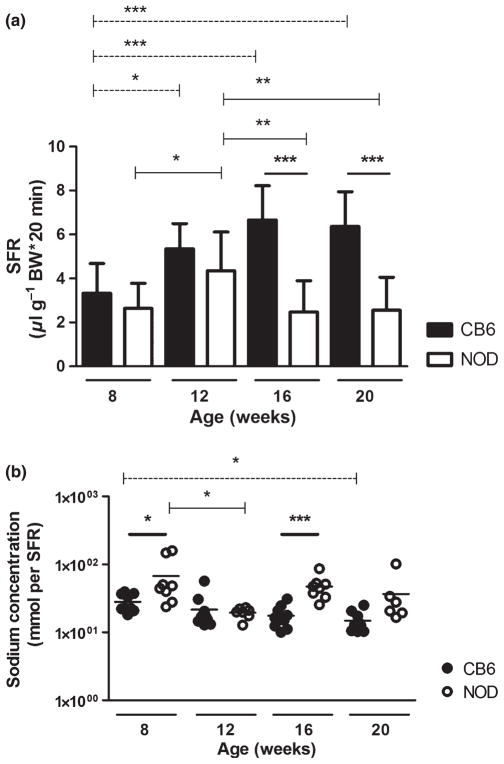
The progressive loss of salivary function in NOD mice starts between 12 and 24 weeks (Yamano et al, 1999; Rosignoli et al, 2005; Jonsson et al, 2006). To determine when salivary function declines at our facility, stimulated SFR was measured at age 8, 12, 16 and 20 weeks. As shown in Figure 1a, at 8 weeks, SFR (mean ± s.d.)—corrected for body weight (μl per gram body weight*20 min)—for NOD and CB6 mice was similar at and had significantly increased more than 50% by 12 weeks in both mouse strains (from 3.3 ± 1.4 to 5.3 ± 1.2 and from 2.6 ± 1.1 to 4.3 ± 1.8 for CB6 and NOD mice, respectively). At 16 and 20 weeks, NOD mice showed a significant decrease in SFR to a level that was comparable to 8-week-old mice (2.5 ± 1.4 and 2.6 ± 1.5, respectively). In contrast, SFR was unaltered in CB6 mice after 12 weeks. As was previously described (Hu et al, 1992), the decline in SFR was independent of diabetic state (data not shown).
Figure 1.
Changes in salivary gland function and salivary sodium concentration of NOD and age-matched CB6 mice. Pilocarpine-stimulated saliva was collected for 20 min at age 8, 12, 16 and 20 weeks. Mean ± standard deviation (s.d.) salivary flow rate (SFR) of non-obese diabetic (NOD; n = 10–16 mice per time point) and CB6 (n = 10) mice is shown; volume was corrected for body weight (BW, a). Salivary sodium concentration was measured in NOD (n = 6–10) and CB6 (n = 10) mice and corrected for SFR. Dot plot shows the value for each individual mouse (line is mean, b, shown on 10-log scale). Significant differences within the groups were indicated with dashed lines for CB6 and solid lines for NOD mice. Bold lines indicate differences between CB6 and NOD mice at the indicated time points
Altered salivary sodium levels in saliva of NOD mice
Saliva is initially produced as an isotonic solution by acinar cells. During transportation through the ducts, sodium is actively reabsorbed by the ductal epithelium, resulting in a hypotonic solution by the time the saliva reaches the oral cavity (Young and van Lennep, 1979). In the healthy gland, a lower SFR results in a lower salivary sodium concentration (Dawes, 1974), caused by prolonged exposure of the saliva to the epithelial sodium channel ENaC in the apical membrane of ductal epithelium (Catalan et al, 2010). In SGs of patients with SS, sodium reabsorption is disturbed and saliva, despite the lower SFR, has a relatively high sodium content, indicating ductal cell dysfunction. This parameter can be used as an indirect indicator for inflammation of the ductal epithelium and can aid the diagnosis of SS (Kalk et al, 2002). We tested saliva from NOD and CB6 mice for sodium content corrected for SFR. Overall, sodium levels (mean ± s.d., mmol /SFR) of CB6 mice slightly decreased over time, reaching significance at 20 weeks (27.4 ± 8.4, 21.6 ± 13.4, 17.5 ± 6.5 and 14.8 ± 5.1 for 8, 12, 16 and 20 weeks, respectively). The sodium levels of NOD mice showed a different pattern: the sodium concentration at 8 weeks was significantly higher in NOD than in CB6 mice, but at 12 weeks, had dropped to the same level as that of CB6 mice (27.4 ± 8.4 vs 67.6 ± 53.8 and 21.6 ± 13.4 vs 19.5 ± 3.4, respectively). Thereafter, it significantly increased again (17.5 ±6.5 vs 47.3 ± 18.4 at 16 weeks). At 20 weeks, NOD mice still showed a trend towards higher sodium levels (14.8 ± 5.1 vs 36.6 ± 32.3, P = 0.06; Figure 1b).
The focus score of NOD mice increases with age, but does not correlate with salivary flow rate
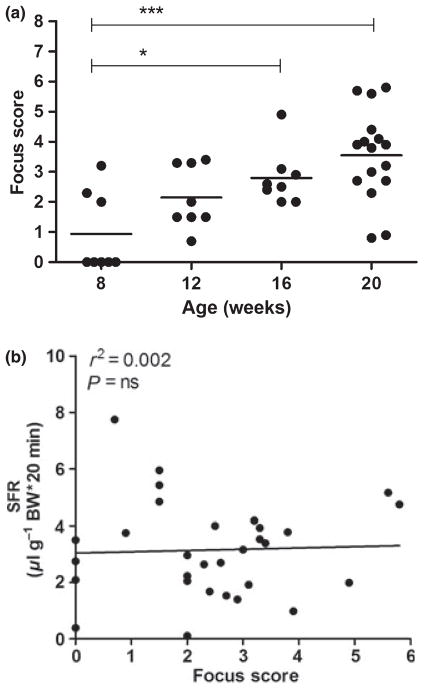
The major histological hallmark of SS is the presence of clustered lymphocytic infiltrates in the SG, which are thought to progressively increase in number, size and organisation (Jonsson et al, 1993). Occasionally, focal infiltrates can be found in the major and minor SGs of healthy individuals (Kurashima and Hirokawa, 1986; Radfar et al, 2002). Non-autoimmune prone mice only sporadically develop small non-focal infiltrates regardless of age (Hu et al, 1992; Rosignoli et al, 2005; Jonsson et al, 2006), and the CB6 mice in our study did not show any other sign of disturbed SG physiology. Therefore, we continued with the analysis of SG inflammation and infiltrating cells of NOD mice only. The majority (5 /8) of 8-week-old NOD mice did not have focal infiltrates, whereas at the age of 12 weeks, all mice did. The FS (mean ± s.d.) was 0.9 ± 1.3, 2.1 ± 1.0, 2.8 ± 0.9 and finally increased to 3.5 ± 1.5 at 8, 12, 16 and 20 weeks, respectively. Compared with 8-week-old mice, mice at 16 and 20 weeks displayed significantly more infiltrates (Figure 2a).
Figure 2.
Inflammation in salivary glands (SGs) from NOD mice. SGs were obtained from non-obese diabetic (NOD) mice at 8, 12, 16 (each n = 8) and 20 (n = 16) weeks of age, and focus score (FS) was determined in paraffin-embedded tissue sections by two independent examiners. Dot plots with distribution and mean values for individual animals, the group mean for each time point and significant P-values are shown (a). Correlation between individual mean FS and pilocarpine- stimulated salivary flow rate (SFR) of mice from all ages (n = 8; b)
The relationship between SG inflammation and dysfunction in SS is complex. Although an FS ≥ 1 and objective SG dysfunction in humans make the diagnosis of SS very likely (Vitali et al, 2002), severely reduced salivary output can be seen in patients with only minor infiltration of the gland and vice versa (Nikolov et al, 2009). Moreover, in NOD mice, secretory function can abruptly deteriorate without obvious inflammatory progression (Jonsson et al, 2006). As in human subjects, the FS of the NOD mice also did not correlate with SFR, confirming no direct correlation with the extent of focal glandular inflammation and the volume of stimulated saliva (Figure 2b).
Focal inflammation of the salivary gland is preceded by the presence of macrophages and is quickly followed by a massive influx of T, B and other immune cells
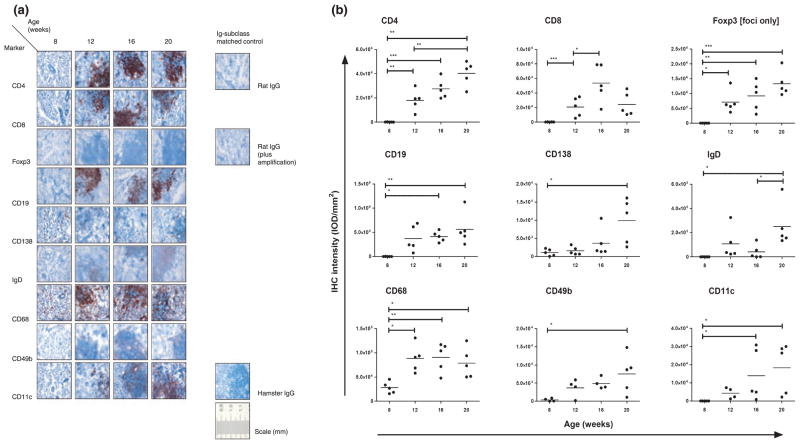
Assessing the sequence of inflammatory processes in humans requires longitudinal follow-up with frequent, repetitive SG biopsies, which can lead to practical and /or ethical objections. In NOD mice, the order of cell influx and the composition of infiltrates at different disease stages can be studied. Immunohistochemical staining for different immune cell types showed that SGs of 8-week-old NOD mice without focal infiltrates contained a considerable amount of CD68+ monocytes /macrophages (Figure 3a,b). Staining for this cell marker was significantly elevated when infiltrates were present in all mice (12 weeks), but did not increase thereafter (Figure 3b). Other immune cells detectable at the age of 8 weeks were CD138+ plasma cells, found distributed throughout the tissue with a preference for periductual tissue, and CD49b+ NK cells that were found sparsely distributed throughout the tissue (Figure 3a). At week 12, CD4+ and CD8+ T cells, Foxp3+ Tregs, CD11c+ plasmacytoid dendritic cells, CD19+ B cells, and IgD+ mature, non-switched, peripheral B cells had also infiltrated into the SG and were found concentrated in distinct foci. The IOD mm−2 for some cell subsets, including CD4+ T cells, NK cells, IgD+ B cells and Foxp3+ Tregs (foci only), steadily increased with age. Expression for plasma and plasmacytoid dendritic cells was high in some mice at 16 and 20 weeks, whereas other mice showed levels comparable with 12 weeks. The staining intensity for CD8+ cells increased significantly up to 16 weeks, but at 20 weeks, showed a reduction trend to the 12-week level (P = 0.06; Figure 3b).
Figure 3.
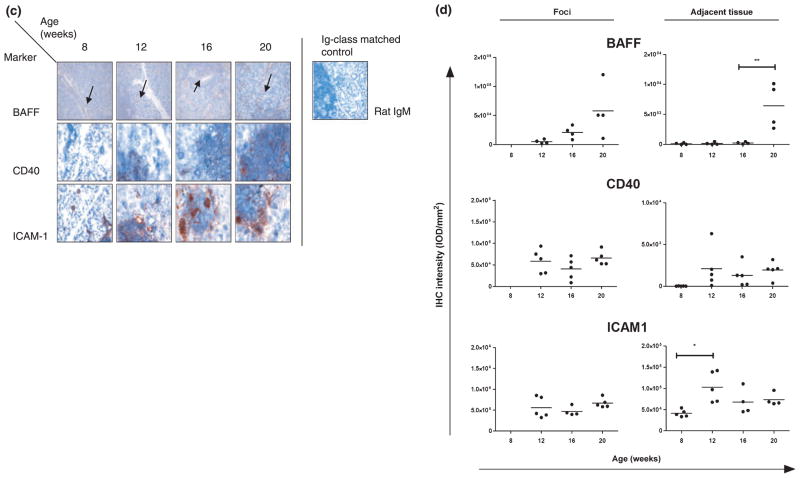
Immunohistochemical staining of salivary glands (SGs) from NOD mice. Frozen and paraffin-embedded sections of SGs obtained from non-obese diabetic (NOD) mice at age 8, 12, 16 and 20 weeks (n = 5) were used. Immunohistochemical staining with antibodies against cellular markers (a) or immunomodulatory molecules (c), including immunoglobulin class- and species-matched control antibodies (negative control), are shown (scale bar in mm). Images of in total 18 high-power fields were assessed for foci and adjacent tissue, except for Foxp3, using digital image analysis. For cellular markers, dot plots with distribution for individual mice, mean staining intensity per area [expressed as internal optical density (IOD) mm−2] of foci and adjacent tissue together (foci only for Foxp3) and significant P-values are shown (b); for BAFF, CD40 and ICAM1, foci and adjacent tissue were digitally separated, analysed and displayed in separate graphs. Significant P-values are shown. (d). For reference, arrows indicate ductal epithelium in tissue stained for BAFF. IHC – immunohistochemical; BAFF – B cell-activating factor belonging to the tumour necrosis factor (TNF) family; ICAM1 – intercellular adhesion molecule 1
Increased BAFF, CD40 and ICAM1 levels in salivary glands of NOD mice
The cytokine BAFF is aberrantly expressed in SGs of patients with SS (Daridon et al, 2007). It is thought that BAFF is important in ectopic germinal centre formation and may contribute to lymphomagenesis, but data are still inconclusive (Mariette et al, 2003; Gottenberg et al, 2005; Jonsson et al, 2005). In 8-week-old NOD mice, low levels of BAFF were detected, confined to the ductal epithelium. When foci had formed, BAFF was seen in the infiltrating cells, and this expression was higher for three out of four mice at 20 weeks compared with 16 weeks. In the ductal epithelium, expression levels (mean ± s.d., IOD mm−2) remained stable up to 16 weeks until an increase was seen at 20 weeks (251 ± 172 vs 6423 ± 3762 for 16 and 20 weeks, respectively; Figure 3c,d).
CD40 and ICAM1 are also overexpressed in SGs of patients with SS (Kapsogeorgou et al, 2001; Dimitriou et al, 2002; Turkcapar et al, 2005). These integral membrane proteins function as costimulatory molecules, and ICAM1 has in addition an important role in adhesion and migration of lymphocytes in(to) the inflammatory milieu. Both immunomodulatory molecules are involved in the autoimmune pathogenesis of many diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and SS (reviewed in Grewal and Flavell, 1996; Anderson and Siahaan, 2003). In NOD mice, ICAM1 was present in ductal epithelial and endothelial cells at 8 weeks, before infiltrates were detected, and expression was significantly increased both in epithelium and in scattered infiltrating cells appearing throughout the tissue at 12 weeks (Figure 3c). Within foci, ICAM1 was found highly and stably expressed on the infiltrating cells through 20 weeks. In contrast, specific CD40 staining could not be detected in adjacent tissue at the age of 8 weeks, but this marker was clearly expressed from 12 weeks and onward, mostly in infiltrates. Individual mice showed higher CD40 staining at 12 weeks compared with 8 weeks, but this increase was not significant (P = 0.10; Figure 3c,d).
Salivary gland cytokine levels indicate an early, local proinflammatory environment
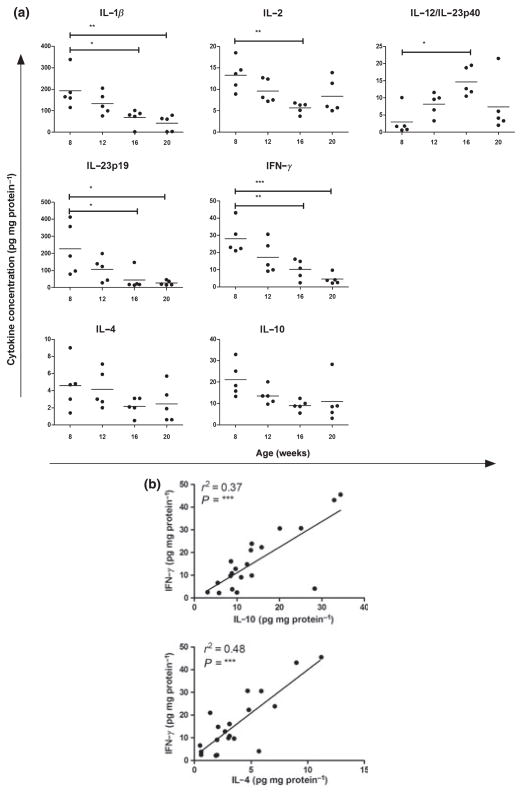
Many pro-inflammatory cytokines are found to be upregulated in SGs of patients with SS compared with healthy controls, and anti-inflammatory cytokines are mostly absent or expressed at low levels (reviewed in Roescher et al, 2009). We measured SG levels of different classical and novel pro- and anti-inflammatory cytokines in NOD mice at age 8 (only mice without SG infiltrates), 12, 16 and 20 weeks. At 8 weeks, pro- and anti-inflammatory cytokines could be detected in the SG homogenates, but they revealed different expression patterns during follow-up. The pro-inflammatory cytokines IFNγ, IL1β, IL2 and IL23p19 showed an overall decrease over time. IL12 /IL23p40 levels increased up to 16 weeks, but at 20 weeks, four of five mice had levels comparable to 12 weeks. Mean levels of the other proinflammatory cytokines IL6, IL17, IL18 and TNFα, and anti-inflammatory TGFβ remained stable (data not shown). Typical Th2 cytokines IL4 and IL10 also exhibited a constant mean expression level (Figure 4a).
Figure 4.
Expression of cytokines in salivary gland (SG) tissue from NOD mice. Cytokine levels were analysed in homogenates of SG tissue obtained from non-obese diabetic (NOD) mice at 8, 12, 16 and 20 weeks of age (n = 5) by multiplex sandwich ELISA. Values were normalised for total protein, and final concentrations are displayed. Per cytokine, dot plots for individual mice and mean concentrations for individual animals are shown (a). Correlation between Th1 cytokine interferon (IFN)γ and Th2 cytokines interleukin (IL)10 or IL4 levels of mice from all ages (b). Significant P-values are shown
In contrast to local SG expression, most cytokines could not be detected above threshold in serum of the same NOD mice. At 8 weeks, only serum levels (mean ± s.d., pg ml−1) of IL12 /IL23p40 (46.4 ± 17.0), IL18 (630.3 ± 133.0) and TGFβ (623.9 ± 266.6) were measurable. The mean levels of these cytokines did not change significantly over time, although four of five mice showed higher systemic IL18 levels by week 20 (mean ± s.d. of all five mice, 2414.0 ± 2128.6). None of the cytokines, measured in SG or serum, was correlated with SFR or FS (data not shown).
Th1 and Th2 responses are not mutually exclusive in salivary glands of NOD mice
A Th1 type immune response, or cellular response, is characterised by high levels of IFNγ, whereas high levels of IL4 and IL10 mark the Th2 type, or humoral, response. In SS, activation of B cells and elevated immunoglobulin production are seen concomitantly with high IFNγ levels, indicating both Th1 and Th2 type responses (reviewed in Roescher et al, 2009). We analysed the correlation of IFNγ with IL10 or IL4 in the SGs of NOD mice of all ages and found simultaneous activation of Th1 and Th2 type responses (Figure 4b).
Discussion
Sjögren’s syndrome is a common, but poorly understood, heterogeneous autoimmune disease for which there is currently no adequate treatment. Several therapies that were effective in other autoimmune diseases have failed for SS, e.g., TNF inhibitors for RA. Identification of new therapeutic targets is essential, and finding a successful treatment will benefit from a better understanding of the pathological process(es). Because patients are often diagnosed when loss of salivary function and focal infiltration is already present, little is known about the early stages of the disease, impeding the development of new treatments. Mouse strains that either spontaneously or after experimental induction develop an SS-like phenotype provide models to study the disease parameters longitudinally. The NOD mouse is an especially useful model to study SG inflammation, because its infiltrates resemble those in humans, and it also develops SG dysfunction (reviewed in Chiorini et al, 2009). We performed an extensive, descriptive study of the disease progression from before symptom onset to secretory dysfunction and focal SG inflammation in NOD mice and focused on salivary output and content, SG focal infiltrates and their cellular composition and expression of local cytokines and costimulatory molecules.
Between 8 and 12 weeks, both NOD and the healthy control mice showed a significant increase in stimulated salivary output. The reason for this rise in SFR was not studied herein, but is possibly due to a not fully developed responsiveness to secretagogues, such as pilocarpine, at an early age. Interestingly, despite a similar SFR at 8 weeks, saliva of NOD mice contained significantly higher sodium levels, which normalised to age-matched CB6 mice levels at 12 weeks, but thereafter increased again. Elevated sodium levels were also found in submandibular and parotid saliva of patients with SS compared with healthy controls (Kalk et al, 2001); treatment with rituximab normalised sodium levels concomitant with improved histology of the SGs (Pijpe et al, 2009). Because saliva can be acquired non-invasively, the measurement of sodium levels in humans and mice may provide a useful tool in determining disease status and the effect of treatment. The elevated sodium levels in 8-week-old NOD mice indicate epithelial dysfunction despite a normal SFR. At this age, focal infiltrates were not present in most mice, but we found markedly raised levels of the pro-inflammatory cytokines IFNγ, IL1β, IL2 and IL23p19 in the gland. Additionally, we observed upregulation of ICAM1 on SG ducts and endothelium at 8 weeks, suggesting an active role for epithelium in the early stages of inflammation. These events may have caused or contributed to the impaired Na+ reabsorption by the ducts. Support for this hypothesis can be found, for instance, in the fact that IL2 treatment in cancer patients resulted in mucositis, reduced SFR and high salivary sodium levels (Marmary et al, 1992), and in vitro stimulation of human SG cells with IFNγ and TNFα led to altered calcium signalling (Wu et al, 1996), which illustrates the glandular epithelium’s sensitivity to certain cytokines. The early presence of these inflammatory markers and high sodium levels could be the result of a (sub)clinical infection of the ductal epithelium that may be cleared by 12 weeks (based on the normalised sodium levels and reduction of many pro-inflammatory cytokines), followed by the triggering of an (auto)immune response. It could also be due to the emergence of autoantigens based on early developmental disorders of the SGs (Cha et al, 2001) leading to the production of (a variety of) autoantibodies. In this study, we did not assess the autoantibodies anti-Ro and -La commonly found in SS, as the penetrance of this feature is highly variable in NOD mice. Previously, we (unpublished observations) and others (Skarstein et al, 1995) found no or only low serum levels, making it unlikely that these autoantibodies contribute significantly to disease progression in this mouse model. However, it cannot be ruled out that other less commonly studied autoantibodies, such as anti-muscarinic receptor and anti-carbonic anhydrase antibodies, play a role in the late(r) stage disease. They may provide an explanation for disease progression despite overall reduction of many pro-inflammatory cytokines. Studying these phenomena could yield clues about the development of SS in humans.
The aetiology and pathogenesis of the salivary hypofunction in SS are still elusive. It has become apparent that the observed immune cell infiltration cannot be the only cause of dysfunction; the reduction in SFR does not always correlate with the degree of SG inflammation, as measured by FS, in patients with SS (Jonsson et al, 1993; Nikolov et al, 2009). The same holds true for the NOD mouse model. NOD–SCID mice, for example, developed SG dysfunction despite the absence of focal infiltrates (Robinson et al, 1996). Moreover, abnormalities in salivary control and signal transduction can be seen prior to focal inflammation and salivary volume decline (Robinson et al, 1996; Rosignoli et al, 2005), illustrating an uncoupling of focal infiltrates and decreased SFR. Comparable with a previous report on NOD mice (Jonsson et al, 2006), we observed an increase in inflammation at 12 weeks—based on FS and numbers of infiltrating immune cells—before an SFR decrease and salivary sodium concentration increase at 16 weeks took place without any further dramatic inflammatory changes; no relationship was found between FS and SFR. These data confirm the complexity of salivary flow and inflammation. Interestingly, although no correlation between SFR and SG cytokine levels was observed, expression of subunit p40, but not p19, peaked at 16 weeks, indicating that IL12 may contribute to the decline in SFR. This is also illustrated by IL12 transgenic mice, which showed reduced SFRs compared with wild-type mice (Vosters et al, 2009). Taken together, the possible role of IL12 and /or the p40 subunit in salivary dysfunction needs to be further explored.
Relatively high levels of pro- and anti-inflammatory cytokines were seen at 8 weeks, which, except for IL12 /IL23p40 and BAFF, diminished with age and progression of microscopic inflammation. Because lymphocytes had not yet infiltrated the gland of these mice, two other possible cytokine producers emerge: the numerous CD68+ monocytes /macrophages, which we and others (van Blokland et al, 2000) found, and /or the activated epithelial cells. Macrophages are potent cytokine secretors under inflammatory circumstances (Gordon, 1995), and SG epithelial cells in SS are known as important contributors to and orchestrators of the local autoimmune process (Moutsopoulos, 1994). Additional double staining for cytokines and cellular markers, or laser capture microscopy followed by microarray analysis could identify the responsible cell(s), and further studies regarding this research will be set up in our department.
The onset of the SS-like disease in the inbred NOD mouse strain varies, possibly because of environmental factors (Lodde et al, 2006). We and other authors found some focal infiltrates from the age of 8 weeks onward (Jonsson et al, 2006), whereas others only detected sporadic infiltrates at 16 weeks (Rosignoli et al, 2005). In addition, we did not detect CD11c+ dendritic cells at 8 weeks, which is in contrast to van Blokland et al (2000) who observed these cells already at 5 weeks. This variation may be explained by the fact that the mice of van Blokland et al were bred in their own facility, while our mice were purchased directly from a supplier. Differences in food, water content and /or the presence of pathogens in the housing facility may be involved as well. Exploring these diversities in disease presentation and the relation with the environment may give us interesting insights in SS, which also varies in its presentation.
B cell-targeting therapy has shown promising results in clinical trials with patients with SS with some residual SG function (Meijer et al, 2010), indicating a significant role for B cells in this disease and the importance of timing of therapy. At 12 weeks, B cells were already prominent in SG lesions of NOD mice. At the same age, we also detected the expression of BAFF, a cytokine important in B cell survival and stimulation, in the foci. BAFF transgenic mice exhibit B cell hyperplasia and hyperglobulinaemia resembling the autoimmune phenotype of SLE (Gross et al, 2000). At a later age, BAFF transgenic mice develop an SS-like disease with infiltrates in the SG (Mackay et al, 1999) and a loss of SFR (Groom et al, 2002). Local BAFF expression in our NOD mice showed a dichotomous progression: a steady expression of BAFF once the infiltrates were present vs a seemingly abrupt increase in ductal tissue at 20 weeks of age. This finding was rather unexpected, as BAFF is produced by epithelial cells in the initial (triggering) phase of innate immunity and forms a link with the adaptive immunity (Ittah et al, 2008), and this late change in epithelial BAFF has not been reported in patients with SS; in fact, epithelial BAFF was found to be similar for healthy controls and patients with SS (Daridon et al, 2007). Interestingly, epithelial BAFF augmentation at 20 weeks coincided with increases in IgD and CD138 staining, but not with CD19, indicating epithelial BAFF might be involved in specific recruitment and /or local differentiation of mature B and plasma cells in NOD mice. We did not assess BAFF expression in CB6 mice, and it can therefore not be ruled out that this increase is related to something else than an SS-like phenomenon, e.g., ageing. Whether epithelial BAFF expression develops similarly in human subjects will also have to be further examined.
With immunohistochemistry and digital image analysis, it is technically difficult to identify and count individual cells in foci, where many cells are packed closely together. We therefore chose to assess and compare the staining intensity instead of the cell count, which would have led to an underestimation of the numbers of cells. Although staining intensity can differ depending on the used primary antibody and additional amplification, we believe that a temporal comparison for each of the cell type markers is valid. One of the other limitations of our study is the relatively small number of mice per group, which may have restricted the statistical power. In addition, we did not study the expression of SG inflammatory parameters in CB6 mice. However, based on the data presented herein, we suggest that the next step should be exploring the (timely) modulation of local expression of ICAM1, BAFF, IL12 and /or recruitment of macrophages in NOD mice. We are currently examining the use of local gene therapy to address some of these factors and have recently shown that interfering with the ICAM1-ligand interaction early in the disease resulted in a lower FS, whereas later intervention led to increased CD4+ and CD8+ T cell numbers in the gland (Roescher et al, 2011). Translating results of animal models to the clinic requires careful considerations, but several therapeutic options are already available. Belimumab (an anti-BAFF fully human monoclonal antibody) is on the market for SLE and phase II clinical trials for SS are ongoing; atacicept (a soluble receptor that binds and neutralises BAFF) and a proliferation-inducing ligand (APRIL) are being investigated in phase II /III studies for SLE (http://clinicaltrials.gov, NCT00732940); and ustekinumab (an anti-IL12 /IL23p40 human monoclonal antibody) has been approved for psoriasis, while phase III trials for psoriatic arthritis are currently being undertaken (http://clinicaltrials.gov, NCT01009086).
In conclusion, our observations confirm the notion that SS-like disease in NOD mice develops in multiple stages: initially, macrophages infiltrate the SG when epithelium is activated as indicated by ICAM1 expression and high levels of salivary sodium and pro- and anti-inflammatory cytokines; second, there is an influx of other immune cells that seemingly quickly assemble into focal infiltrates; and in the last phase, salivary secretion declines while local IL12 /IL23p40 levels peak and epithelial BAFF increases.
Acknowledgments
This research was funded by an Intramural NIH grant to JAC and a grant from the Dutch Arthritis Association to JLV. We thank the NIH Fellows Editorial Board for editorial assistance, Corine van der Horst for technical assistance with the digital image analysis and Dr J. E. Melvin for assistance with the sodium measurements.
Footnotes
The authors declare no conflict of interest.
Author contribution
NR designed the study, collected the data, interpreted the results and wrote the manuscript. BML helped with interpretation of the data and the writing of the manuscript. JLV assisted in collecting the data. PPT revised the manuscript. MAC helped with the acquisition of the data and revised the manuscript. GGI and JAC participated in the design of the study and the interpretation of the data and JAC also revised the manuscript.
References
- Adamson TC, III, Fox RI, Frisman DM, Howell FV. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren’s syndrome using monoclonal antibodies. J Immunol. 1983;130:203–208. [PubMed] [Google Scholar]
- Anderson ME, Siahaan TJ. Targeting ICAM-1 /LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides. 2003;24:487– 501. doi: 10.1016/s0196-9781(03)00083-4. [DOI] [PubMed] [Google Scholar]
- van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, et al. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjogren’s syndrome: a differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80:575–585. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
- Bombardieri M, Barone F, Pittoni V, et al. Increased circulating levels and salivary gland expression of interleukin- 18 in patients with Sjogren’s syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 2004;6:R447–R456. doi: 10.1186/ar1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan MA, Nakamoto T, Gonzalez-Begne M, et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol. 2010;588:713– 724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S, van Blockland SC, Versnel MA, et al. Abnormal organogenesis in salivary gland development may initiate adult onset of autoimmune exocrinopathy. Exp Clin Immunogenet. 2001;18:143–160. doi: 10.1159/000049194. [DOI] [PubMed] [Google Scholar]
- Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009;33:190–196. doi: 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J Autoimmun. 2010;34:400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Daridon C, Devauchelle V, Hutin P, et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjogren’s syndrome. Arthritis Rheum. 2007;56:1134–1144. doi: 10.1002/art.22458. [DOI] [PubMed] [Google Scholar]
- Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human submandibular saliva. Arch Oral Biol. 1974;10:887–895. doi: 10.1016/0003-9969(74)90051-x. [DOI] [PubMed] [Google Scholar]
- Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. CD40 on salivary gland epithelial cells: high constitutive expression by cultured cells from Sjogren’s syndrome patients indicating their intrinsic activation. Clin Exp Immunol. 2002;127:386–392. doi: 10.1046/j.1365-2249.2002.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RI. Sjogren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- Gordon S. The macrophage. Bioessays. 1995;17:977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Busson M, Cohen-Solal J, et al. Correlation of serum B lymphocyte stimulator and beta2 microglobulin with autoantibody secretion and systemic involvement in primary Sjogren’s syndrome. Ann Rheum Dis. 2005;64:1050–1055. doi: 10.1136/ard.2004.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85– 106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- van der Hall PO, Kraan MC, Tak PP. Quantitative image analysis of synovial tissue. Methods Mol Med. 2007;135:121–143. doi: 10.1007/978-1-59745-401-8_8. [DOI] [PubMed] [Google Scholar]
- Haringman JJ, Vinkenoog M, Gerlag DM, Smeets TJ, Zwinderman AH, Tak PP. Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R862–R867. doi: 10.1186/ar1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263:E607–E614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- Humphreys-Beher MG, Peck AB. New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model. Arch Oral Biol. 1999;44(Suppl 1):S21–S25. doi: 10.1016/s0003-9969(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Ittah M, Miceli-Richard C, Gottenberg JE, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008;38:1058–1064. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- Jonsson R, Kroneld U, Backman K, Magnusson B, Tarkowski A. Progression of sialadenitis in Sjogren’s syndrome. Br J Rheumatol. 1993;32:578–581. doi: 10.1093/rheumatology/32.7.578. [DOI] [PubMed] [Google Scholar]
- Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren’s syndrome. J Clin Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- Jonsson MV, Delaleu N, Brokstad KA, Berggreen E, Skarstein K. Impaired salivary gland function in NOD mice: association with changes in cytokine profile but not with histopathologic changes in the salivary gland. Arthritis Rheum. 2006;54:2300–2305. doi: 10.1002/art.21945. [DOI] [PubMed] [Google Scholar]
- Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV. Sialometry and sialochemistry: diagnostic tools for Sjogren’s syndrome. Ann Rheum Dis. 2001;60:1110–1116. doi: 10.1136/ard.60.12.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk WW, Vissink A, Stegenga B, Bootsma H, Nieuw Amerongen AV, Kallenberg CG. Sialometry and sialochemistry: a non-invasive approach for diagnosing Sjogren’s syndrome. Ann Rheum Dis. 2002;61:137–144. doi: 10.1136/ard.61.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsogeorgou EK, Dimitriou ID, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Activation of epithelial and myoepithelial cells in the salivary glands of patients with Sjogren’s syndrome: high expression of intercellular adhesion molecule-1 (ICAM.1) in biopsy specimens and cultured cells. Clin Exp Immunol. 2001;124:126–133. doi: 10.1046/j.1365-2249.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok MR, Yamano S, Lodde BM, et al. Local adenoassociated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjogren’s syndrome. Hum Gene Ther. 2003;14:1605–1618. doi: 10.1089/104303403322542257. [DOI] [PubMed] [Google Scholar]
- Kurashima C, Hirokawa K. Age-related increase of focal lymphocytic infiltration in the human submandibular glands. J Oral Pathol. 1986;15:172–178. doi: 10.1111/j.1600-0714.1986.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Lodde BM, Mineshiba F, Kok MR, et al. NOD mouse model for Sjogren’s syndrome: lack of longitudinal stability. Oral Dis. 2006;12:566–572. doi: 10.1111/j.1601-0825.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697– 1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoussakis MN, Boiu S, Korkolopoulou P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmary Y, Shiloni E, Katz J. Oral changes in interleukin-2 treated patients: a preliminary report. J Oral Pathol Med. 1992;21:230–231. doi: 10.1111/j.1600-0714.1992.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:960–968. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- Molina R, Provost TT, Alexander EL. Two types of inflammatory vascular disease in Sjogren’s syndrome. Differential association with seroreactivity to rheumatoid factor and antibodies to Ro (SS-A) and with hypocomplementemia. Arthritis Rheum. 1985;28:1251–1258. doi: 10.1002/art.1780281109. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos HM. Sjogren’s syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–165. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- Nikolov NP, Alevizos I, Grisus M, et al. Uncoupling salivary gland dysfunction from inflammation in a large cohort of primary Sjögren’s syndrome patients. Arthritis Rheum (Abstract) 2009;60(Suppl 10):480. [Google Scholar]
- Pijpe J, Meijer JM, Bootsma H, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren’s syndrome. Arthritis Rheum. 2009;60:3251–3256. doi: 10.1002/art.24903. [DOI] [PubMed] [Google Scholar]
- Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47:520–524. doi: 10.1002/art.10668. [DOI] [PubMed] [Google Scholar]
- Robinson CP, Yamamoto H, Peck AB, Humphreys-Beher MG. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)-scid mouse in the absence of detectable lymphocytic infiltration: a potential trigger for sialoadenitis of NOD mice. Clin Immunol Immunopathol. 1996;79:50– 59. doi: 10.1006/clin.1996.0050. [DOI] [PubMed] [Google Scholar]
- Roescher N, Tak PP, Illei GG. Cytokines in Sjogren’s syndrome. Oral Dis. 2009;15:519–526. doi: 10.1111/j.1601-0825.2009.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roescher N, Vosters JL, Yin H, Illei GG, Tak PP, Chiorini JA. Effect of Soluble ICAM-1 on a Sjogren’s Syndromelike Phenotype in NOD Mice Is Disease Stage Dependent. PLoS ONE. 2011;6:e19962. doi: 10.1371/journal.pone.0019962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Perez Leiros C. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142:411–418. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstein K, Wahren M, Zaura E, Hattori M, Jonsson R. Characterization of T cell receptor repertoire and anti-Ro /SSA autoantibodies in relation to sialadenitis of NOD mice. Autoimmunity. 1995;22:9–16. doi: 10.3109/08916939508995294. [DOI] [PubMed] [Google Scholar]
- Turkcapar N, Sak SD, Saatci M, Duman M, Olmez U. Vasculitis and expression of vascular cell adhesion molecule- 1, intercellular adhesion molecule-1, and E-selectin in salivary glands of patients with Sjogren’s syndrome. J Rheumatol. 2005;32:1063–1070. [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosters JL, Landek-Salgado MA, Yin H, et al. Interleukin- 12 induces salivary gland dysfunction in transgenic mice, providing a new model of Sjogren’s syndrome. Arthritis Rheum. 2009;60:3633–3641. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulgarelis M, Moutsopoulos HM. Mucosa-associated lymphoid tissue lymphoma in Sjogren’s syndrome: risks, management, and prognosis. Rheum Dis Clin North Am. 2008;34:921–933. vii. doi: 10.1016/j.rdc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Wu AJ, Chen ZJ, Baum BJ, Ambudkar IS. Interferon gamma induces persistent depletion of internal Ca2+ stores in ahumansalivary gland cell line. Am J Physiol. 1996;270:C514–C521. doi: 10.1152/ajpcell.1996.270.2.C514. [DOI] [PubMed] [Google Scholar]
- Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–275. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- Young JA, van Lennep EW. Salivary and salt glands. In: Giebisch G, Tosteton DC, Ussing HH, editors. Membrane transport in biology. Springer-Verlag; Berlin: 1979. pp. 563–674. [Google Scholar]