Synthetic chemists, myself included, are eager to tell anyone who will listen about the virtues of building complex molecules. Truth be told, most molecules themselves are not particularly interesting, save for a precious few that are predisposed to either glow or explode. Rather, the most fascinating aspects of molecules are the myriad ways in which they interact with each other. In a dramatic example of “basic science meets application,” Clackson and colleagues (1) report in this issue of PNAS a point mutant of a known protein that causes this protein to self-associate. This interaction provides the molecular basis for a platform technology to control the secretion of therapeutic proteins from cells by using orally available drugs (2). Along more fundamental lines, the self-associating protein ultimately may teach us more about the forces that govern molecular interactions.
Molecular interactions are the basis of life. A single polypeptide chain has the ability to fold into a discrete, well-defined enzyme structure from an almost infinite number of possibilities. Such an enzyme may catalyze a particular chemical reaction 5, 10, even 15 orders of magnitude faster than the reaction proceeds in the absence of enzyme. Intramolecular interactions control how the enzyme folds into its three-dimensional structure, and intermolecular interactions control how the enzyme associates with its substrate or cofactors. Molecular interactions (both intermolecular and intramolecular) ultimately provide the fundamental structural and mechanistic framework for the formation of complex systems such as cells and organisms. Cells communicate both intercellularly and intracellularly by using signal transduction pathways, and protein–protein interactions are the lingua franca of these pathways (3).
A complete understanding of the forces that govern molecular interactions should allow us to reliably predict molecular associations, and there is little doubt that such predictive power would have far-reaching implications. Whether one is trying to understand the intricacies of a nerve cell, design and synthesize drugs, or invent new materials with desirable properties, a clear understanding of why certain molecules prefer to associate and why others would rather leave each other alone would take much of the trial and error out of these endeavors. Unfortunately, we have a long way to go before we can quantitatively predict molecular interactions a priori, especially intermolecular associations.
We do know a few things about the forces that govern molecular interactions. We know that opposite charges attract each other and that hydrogen bonds and van der Waals forces provide similar attractive potentials. We also realize that nonpolar molecules (e.g., hydrocarbons) would rather cluster together when placed in a polar environment such as water. Individually, these forces are relatively weak (1–5 kcal/mol) compared with covalent bonds (50–120 kcal/mol). However, molecular associations typically are influenced by many of these forces, which means that a large number of relatively weak forces are responsible for an interaction.
To complicate matters further, the majority of these interactions occur in the liquid phase, so one must always consider the interactions of solvent (often water) with the molecules in question. We are getting better all of the time, but the complex nature of these molecular interactions prevents us from being able to reliably predict the precise three-dimensional structure that a single polypeptide chain will form when allowed to fold. This “protein folding problem” is of special interest because the ability to design a polypeptide with a particular structure and function would be useful for both understanding and controlling cellular processes.
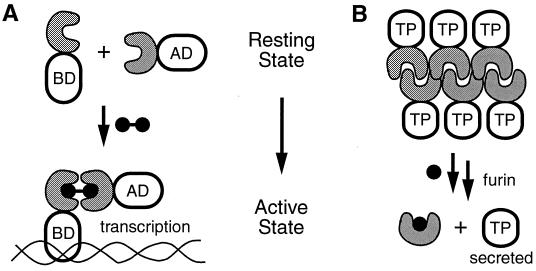
The ability to design and predict molecular interactions is steadily improving, but scientists are impatient. Although this field continues to reach new milestones, other methods have been devised to control molecular interactions in cells. Several naturally occurring proteins bind to small cell-permeable ligands. One can use molecular biology to append a ligand-binding domain to a protein of interest and subsequently add exogenous synthetic ligand to control a cellular process. Spencer et al. (4) used such a strategy to control the transcription of a specific gene through the addition of a dimeric synthetic molecule that binds to a target protein in cells. Additional “dimerizer systems” have been developed to control gene transcription (5–8), and Clackson and his colleagues (1) at ARIAD have been at the forefront of this field since the beginning. Monomeric proteins provide the resting state for their dimerizer system (Fig. 1A). Dimerization (or oligomerization) of the target protein is responsible for the desired cellular response. It might be desirable to have a complementary system in which oligomeric proteins represent the resting state and addition of a synthetic ligand could be used to dissociate the protein aggregates to form monomeric proteins (Fig. 1B). Such a strategy would be very useful for turning on cellular events that are inactivated by protein oligomerization.
Figure 1.
Schematic representations of two cellular processes that are regulated by cell-permeable synthetic molecules. (A) Two chimeric proteins, each containing an FKBP domain (gray C-shaped structures) as well as either a DNA binding domain (BD) or a transcriptional activation domain (AD) are monomeric in the resting state. Addition of a dimeric synthetic ligand for FKBP causes association of the two chimeras and subsequent activation of transcription. (B) The FKBP F36M mutant domains are fused to a therapeutic protein (TP). In the absence of ligand, the FKBP F36M domains aggregate in the ER. Addition of a monomeric synthetic ligand causes the aggregates to dissociate. Subsequent vesicle transport and furin-mediated proteolysis ultimately results in rapid secretion of the therapeutic protein from cells.
The ARIAD dimerizer systems use the FK506-binding protein (FKBP) as their ligand binding domain. FKBP is an abundant cellular protein that binds tightly to peptidomimetic drugs (FK506 and rapamycin) as well as structurally related synthetic ligands (9). The presence of endogenous cellular FKBP potentially can dilute the potency of their dimerizers, so Clackson and colleagues (1) used protein engineering to mutate single amino acids to create “holes” in the FKBP domains of their signaling proteins. They then used synthetic chemistry to build complementary “bumps” onto their small molecule ligands, so that their activating dimerizer ligands bind tightly to the engineered FKBP mutants and weakly to the endogenous pool of cellular FKBP. Guided by the three-dimensional structure of FKBP, they made several point mutants of FKBP and assayed them for activity by using a cell-based assay similar to a two-hybrid screen. In the absence of dimeric ligands the FKBP chimeras are monomeric and no signal is produced (Fig. 1A). Addition of dimeric ligands causes association of the FKBP chimeras and production of a reporter gene. One of their mutants (Phe-36 to Met = F36M) gave a strong positive signal in the absence of dimeric ligand. This contrary behavior was not the goal of the screen, but chance favors the prepared mind, and the authors were on the lookout for a system with an oligomeric ground state.
They expressed and purified both wild-type FKBP and the F36M FKBP mutant. Gel filtration experiments showed that FKBP is monomeric but that the F36M mutant exists in a monomer-dimer equilibrium. They pursued this finding and characterized both proteins by using analytical ultracentrifugation. Equilibrium data sets for the F36M mutant support a monomer-dimer equilibrium with a dissociation constant of 30 μM. To understand the molecular basis for this association, they solved the x-ray structure of the F36M mutant, which revealed two FKBP proteins binding to each other. A loop belonging to one protein molecule is packed into the ligand binding site of the other protein molecule and vice versa.
When proteins interact with each other, surface loops and individual residues often undergo subtle rearrangements to maximize shared surface area. This behavior, referred to as the plasticity of protein surfaces, is believed to contribute to the thermodynamic stability of these complexes (10). Overall, the F36M FKBP mutant closely resembles wild-type FKBP (1.24-Å rms deviation for all atoms). However, the majority of the differences between the monomeric and dimeric FKBPs involve side chains at the protein–protein interface, which may provide further support for protein plasticity at protein–protein interfaces.
FKBP is an enzyme that catalyzes the isomerization of peptidyl-prolyl amide bonds, and FKBP's substrate specificity is rather broad (11). The plasticity of its protein surface may be critical for interacting with other proteins. This property of FKBP recently was recruited to enhance the affinity of a small molecule ligand for its cognate protein target, presumably by contributing additional stabilizing protein–protein interactions (12). Upon inspection, there does not appear to be anything special about the FKBP surface, but it has evolved as a protein that is able to bind to a variety of other protein surfaces. Perhaps there really is something unique about the surface of FKBP, but we do not yet know exactly what to look for.
Building on their fundamental discovery, Rivera and Clackson and colleagues (2) used the self-associating F36M FKBP mutant to control the exocytosis of therapeutic proteins from cells by using cell-permeable small molecules. Individual F36M FKBP domains bind weakly to each other, but by fusing four FKBPs together, the authors created tetrameric constructs that self-associate with high avidity into oligomeric aggregates. By adding a signal sequence to the N terminus of the tetrameric FKBP construct, the protein aggregates are targeted to the endoplasmic reticulum (ER). The FKBP aggregates are presumably too large to be packaged into vesicles for transport through the secretory pathway, so they accumulate in large bunches in the ER.
To take their system to the next level of sophistication, the authors appended a therapeutic protein (either insulin or growth hormone) to the C terminus of the FKBP F36M tetramer. They also engineered a recognition site for the furin protease between the FKBPs and the therapeutic protein. Furin resides in the trans-Golgi and cleaves proteins that are destined for exocytosis. As a result, cells that express these chimeric proteins store them in the ER as large oligomeric aggregates. Upon treatment with monomeric FKBP ligands, the aggregates dissociate and the monomeric proteins are packaged into vesicles and transported through the secretory pathway. Before exocytosis, furin cleaves the construct to generate the free therapeutic protein, which is rapidly secreted from the cells. In the most ambitious experiment, cells harboring this secretory machinery for insulin were implanted into diabetic mice. Serum levels of both glucose and insulin were monitored, and treatment with a synthetic FKBP ligand resulted in insulin production that allowed glucose to be maintained at normal levels.
Sequestration of the FKBP aggregates in the ER does not appear to harm the cells. The rate of secretion in the absence of FKBP ligand is very low (250-fold difference between the on and off states), and the secretory machinery responds to FKBP ligands in a dose-dependent and reversible manner. The time scale for protein secretion is 5–20 min with this system, whereas the time required to produce protein using a transcriptional switch is closer to 24 h. To borrow a phrase from the marketing world, the rapidity and reversibility of this secretory strategy make it an attractive model for the “just in time” production of therapeutic proteins.
At least one prepared mind noticed an FKBP mutant with interesting properties. Rather than dismissing it as a negative result in a cell-based screen, subsequent biophysical studies revealed that a single conservative point mutation leads to self-association of FKBP. There is apparently a fine line between monomeric and dimeric forms of FKBP. This simple intermolecular interaction, coupled with an appreciation of vesicle transport and secretion, allowed Rivera and colleagues to invent a new method to control the production of therapeutic proteins by using orally available drugs. Their system appears to tolerate a variety of C-terminal proteins as cargo and, coupled with improved methods to introduce genes into human cells, the ARIAD secretory strategy ultimately may prove to be a powerful platform for regulated protein production that is applicable to different disease states. But perhaps the discovery of this self-associating FKBP mutant also provides us with a new chance to probe fundamental aspects of molecular interactions.
Footnotes
See companion article on page 7096.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110149697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110149697
References
- 1.Rollins C T, Rivera V M, Woolfson D N, Keenan T, Hatada M, Adams S E, Andrade L J, Yaeger D, van Schravendijk M R, Holt D A, et al. Proc Natl Acad Sci USA. 2000;97:7096–7101. doi: 10.1073/pnas.100101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera V M, Wang X, Wardwell S, Courage N L, Volchuk A, Keenan T, Holt D A, Gilman M, Orci L, Cerasoli F, et al. Science. 2000;287:826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- 3.Mayer B J. Mol Biotechnol. 1999;13:201–213. doi: 10.1385/MB:13:3:201. [DOI] [PubMed] [Google Scholar]
- 4.Spencer D M, Wandless T J, Schreiber S L, Crabtree G R. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 5.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y L, O'Malley B W, Jr, Tsai S Y, O'Malley B W. Proc Natl Acad Sci USA. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.No D, Yao T P, Evans R M. Proc Natl Acad Sci USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera V M, Clackson T, Natesan S, Pollock R, Amara J F, Keenan T, Magari S R, Phillips T, Courage N L, Cerasoli R, Holt D A. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber S L. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 10.Atwell S, Ultsch M, DeVos A M, Wells J A. Science. 1997;278:1125–1128. doi: 10.1126/science.278.5340.1125. [DOI] [PubMed] [Google Scholar]
- 11.Albers M W, Walsh C T, Schreiber S L. J Org Chem. 1990;55:4984–4986. [Google Scholar]
- 12.Briesewitz R, Ray G T, Wandless T J, Crabtree G R. Proc Natl Acad Sci USA. 1999;96:1953–1958. doi: 10.1073/pnas.96.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]