Abstract
Context:
Information regarding the structure, composition, and function of the knee menisci has been scattered across multiple sources and fields. This review contains a concise, detailed description of the knee menisci—including anatomy, etymology, phylogeny, ultrastructure and biochemistry, vascular anatomy and neuroanatomy, biomechanical function, maturation and aging, and imaging modalities.
Evidence Acquisition:
A literature search was performed by a review of PubMed and OVID articles published from 1858 to 2011.
Results:
This study highlights the structural, compositional, and functional characteristics of the menisci, which may be relevant to clinical presentations, diagnosis, and surgical repairs.
Conclusions:
An understanding of the normal anatomy and biomechanics of the menisci is a necessary prerequisite to understanding the pathogenesis of disorders involving the knee.
Keywords: knee, meniscus, anatomy, function
Once described as a functionless embryonic remnant,162 the menisci are now known to be vital for the normal function and long-term health of the knee joint.§ The menisci increase stability for femorotibial articulation, distribute axial load, absorb shock, and provide lubrication and nutrition to the knee joint.4,91,152,153
Injuries to the menisci are recognized as a cause of significant musculoskeletal morbidity. The unique and complex structure of menisci makes treatment and repair challenging for the patient, surgeon, and physical therapist. Furthermore, long-term damage may lead to degenerative joint changes such as osteophyte formation, articular cartilage degeneration, joint space narrowing, and symptomatic osteoarthritis.36,45,92 Preservation of the menisci depends on maintaining their distinctive composition and organization.
Anatomy of Menisci
Meniscal Etymology
The word meniscus comes from the Greek word mēniskos, meaning “crescent,” diminutive of mēnē, meaning “moon.”
Meniscal Phylogeny and Comparative Anatomy
Hominids exhibit similar anatomic and functional characteristics, including a bicondylar distal femur, intra-articular cruciate ligaments, menisci, and asymmetrical collateral.40,66 These similar morphologic characteristics reflect a shared genetic lineage that can be traced back more than 300 million years.40,66,119
In the primate lineage leading to humans, hominids evolved to bipedal stance approximately 3 to 4 million years ago, and by 1.3 million years ago, the modern patellofemoral joint was established (with a longer lateral patellar facet and matching lateral femoral trochlea).164 Tardieu investigated the transition from occasional bipedalism to permanent bipedalism and observed that primates contain a medial and lateral fibrocartilaginous meniscus, with the medial meniscus being morphologically similar in all primates (crescent shaped with 2 tibial insertions).163 By contrast, the lateral meniscus was observed to be more variable in shape. Unique in Homo sapiens is the presence of 2 tibial insertions—1 anterior and 1 posterior—indicating a habitual practice of full extension movements of the knee joint during the stance and swing phases of bipedal walking.20,134,142,163,168
Embryology and Development
The characteristic shape of the lateral and medial menisci is attained between the 8th and 10th week of gestation.53,60 They arise from a condensation of the intermediate layer of mesenchymal tissue to form attachments to the surrounding joint capsule.31,87,110 The developing menisci are highly cellular and vascular, with the blood supply entering from the periphery and extending through the entire width of the menisci.31 As the fetus continues to develop, there is a gradual decrease in the cellularity of the menisci with a concomitant increase in the collagen content in a circumferential arrangement.30,31 Joint motion and the postnatal stress of weightbearing are important factors in determining the orientation of collagen fibers. By adulthood, only the peripheral 10% to 30% have a blood supply.12,31
Despite these histologic changes, the proportion of tibial plateau covered by the corresponding meniscus is relatively constant throughout fetal development, with the medial and lateral menisci covering approximately 60% and 80% of the surface areas, respectively.31
Gross Anatomy
Gross examination of the knee menisci reveals a smooth, lubricated tissue (Figure 1). They are crescent-shaped wedges of fibrocartilage located on the medial and lateral aspects of the knee joint (Figure 2A). The peripheral, vascular border (also known as the red zone) of each meniscus is thick, convex, and attached to the joint capsule. The innermost border (also known as the white zone) tapers to a thin free edge. The superior surfaces of menisci are concave, enabling effective articulation with their respective convex femoral condyles. The inferior surfaces are flat to accommodate the tibial plateau (Figure 1).28,175
Figure 1.
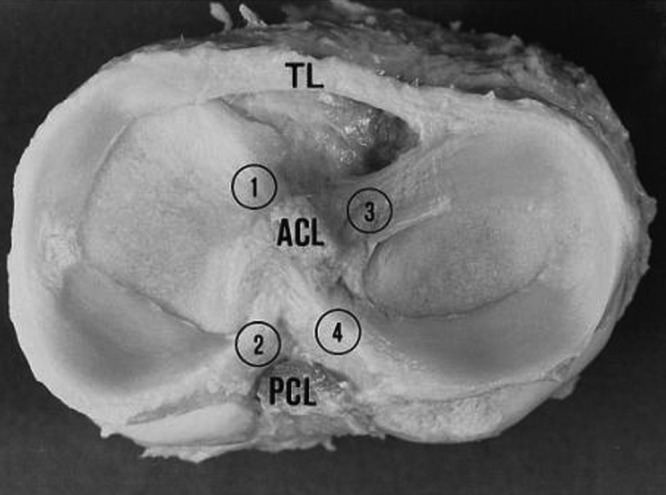
Gross photograph of human tibial plateau demonstrating the relative size and attachments of the medial and lateral menisci. The medial and lateral menisci (left side and right side of image, respectively) are connected by a transverse ligament (TL). 1, anterior insertional ligament of the medial meniscus; 2, posterior insertional ligament of the medial meniscus; 3, anterior insertional ligament of the lateral meniscus; 4, posterior insertional ligament of the lateral meniscus. ACL, anterior cruciate ligament; PCL, posterior cruciate ligament. Reprinted with permission from Messner and Gao.115
Figure 2.
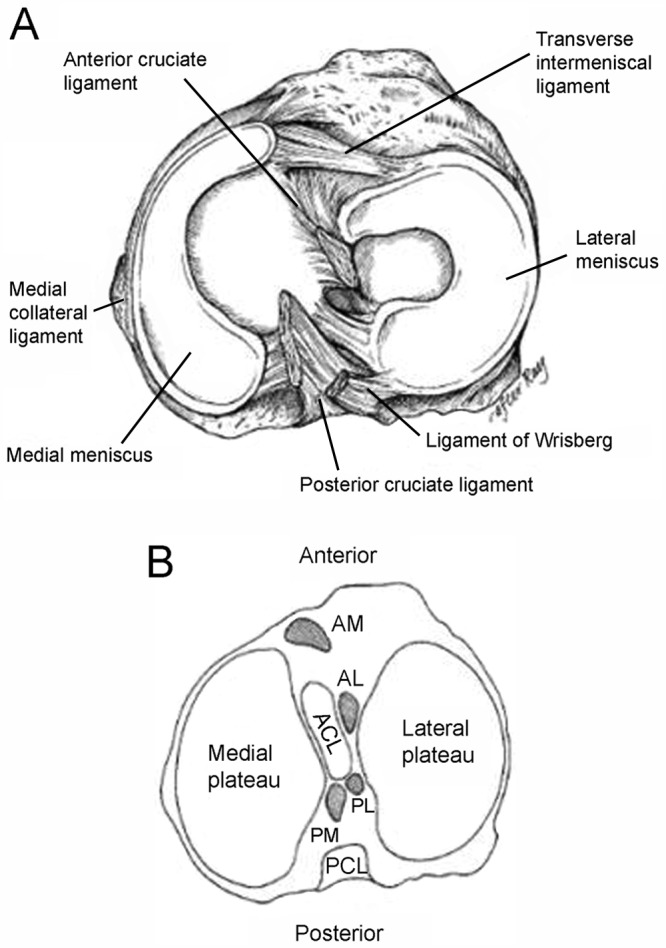
(A) Anatomy of the meniscus viewed from above (adapted image reprinted with permission from Greis et al63; original from Pagnani et al129). (B) Axial view of a right tibial plateau showing sections of the meniscus and their relationship to the cruciate ligaments. AL, anterior horn lateral meniscus; AM, anterior horn medial meniscus; PCL, posterior cruciate ligament; PL, posterior horn lateral meniscus; PM, posterior horn medial meniscus. Reprinted with permission from Johnson et al.83
Medial meniscus
The semicircular medial meniscus measures approximately 35 mm in diameter (anterior to posterior) and is significantly broader posteriorly than it is anteriorly.175 The anterior horn is attached to the tibia plateau near the intercondylar fossa anterior to the anterior cruciate ligament (ACL). There is significant variability in the attachment location of the anterior horn of the medial meniscus. The posterior horn is attached to the posterior intercondylar fossa of the tibia between the lateral meniscus and the posterior cruciate ligament (PCL; Figures 1 and 2B). Johnson et al reexamined the tibial insertion sites of the menisci and their topographic relationships to surrounding anatomic landmarks of the knee.82 They found that the anterior and posterior horn insertion sites of the medial meniscus were larger than those of the lateral meniscus. The area of the anterior horn insertion site of the medial meniscus was the largest overall, measuring 61.4 mm2, whereas the posterior horn of the lateral meniscus was the smallest, at 28.5 mm2.82
The tibial portion of the capsular attachment is the coronary ligament. At its midpoint, the medial meniscus is more firmly attached to the femur through a condensation in the joint capsule known as the deep medial collateral ligament.175 The transverse, or “intermeniscal,” ligament is a fibrous band of tissue that connects the anterior horn of the medial meniscus to the anterior horn of the lateral meniscus (Figures 1 and 2A).
Lateral meniscus
The lateral meniscus is almost circular, with an approximately uniform width from anterior to posterior (Figures 1 and 2A). It occupies a larger portion (~80%) of the articular surface than the medial meniscus (~60%) and is more mobile.10,31,165 Both horns of the lateral meniscus are attached to the tibia. The insertion of the anterior horn of the lateral meniscus lies anterior to the intercondylar eminence and adjacent to the broad attachment site of the ACL (Figure 2B).9,83 The posterior horn of the lateral meniscus inserts posterior to the lateral tibial spine and just anterior to the insertion of the posterior horn of the medial meniscus (Figure 2B).83 The lateral meniscus is loosely attached to the capsular ligament; however, these fibers do not attach to the lateral collateral ligament. The posterior horn of the lateral meniscus attaches to the inner aspect of the medial femoral condyle via the anterior and posterior meniscofemoral ligaments of Humphrey and Wrisberg, respectively, which originate near the origin of the PCL (Figures 1 and 2).75
Meniscofemoral ligaments
The literature reports significant inconsistencies in the presence and size of meniscofemoral ligaments of the lateral meniscus. There may be none, 1, 2, or 4.‖ When present, these accessory ligaments transverse from the posterior horn of the lateral meniscus to the lateral aspect of the medial femoral condyle. They insert immediately adjacent to the femoral attachment of the PCL (Figures 1 and 2).
In a series of studies, Harner et al measured the cross-sectional area of the ligaments and found that the meniscofemoral ligament averaged 20% of the size of the PCL (range, 7%-35%).69,70 However, the size of the insertional area alone without knowledge of the insertional angle or collagen density does not indicate their relative strength.115 The function of these ligaments remains unknown; they may pull the posterior horn of the lateral meniscus in an anterior direction to increase the congruity of the meniscotibial fossa and the lateral femoral condyle.75
Ultrastructure and Biochemistry
Extracellular Matrix
The meniscus is a dense extracellular matrix (ECM) composed primarily of water (72%) and collagen (22%), interposed with cells.9,55,56,77 Proteoglycans, noncollagenous proteins, and glycoproteins account for the remaining dry weight.¶ Meniscal cells synthesize and maintain the ECM, which determines the material properties of the tissue.
The cells of the menisci are referred to as fibrochondrocytes because they appear to be a mixture of fibroblasts and chondrocytes.111,177 The cells in the more superficial layer of the menisci are fusiform or spindle shaped (more fibroblastic), whereas the cells located deeper in the meniscus are ovoid or polygonal (more chondrocytic).55,56,178 Cell morphology does not differ between the peripheral and central locations in the menisci.56
Both cell types contain abundant endoplasmic reticulum and Golgi complex. Mitochondria are only occasionally visualized, suggesting that the major pathway for energy production of fibrochondrocytes in their avascular milieu is probably anaerobic glycolysis.112
Water
In normal, healthy menisci, tissue fluid represents 65% to 70% of the total weight. Most of the water is retained within the tissue in the solvent domains of proteoglycans. The water content of meniscal tissue is higher in the posterior areas than in the central or anterior areas; tissue samples from surface and deeper layers had similar contents.135
Large hydraulic pressures are required to overcome the drag of frictional resistance of forcing fluid flow through meniscal tissue. Thus, interactions between water and the matrix macromolecular framework significantly influence the viscoelastic properties of the tissue.
Collagens
Collagens are primarily responsible for the tensile strength of menisci; they contribute up to 75% of the dry weight of the ECM.77 The ECM is composed primarily of type I collagen (90% dry weight) with variable amounts of types II, III, V, and VI.43,44,80,112,181 The predominance of type I collagen distinguishes the fibrocartilage of menisci from articular (hyaline) cartilage. The collagens are heavily cross-linked by hydroxylpyridinium aldehydes.44
The collagen fiber arrangement is ideal for transferring a vertical compressive load into circumferential “hoop” stresses (Figure 3).57 Type I collagen fibers are oriented circumferentially in the deeper layers of the meniscus, parallel to the peripheral border. These fibers blend the ligamentous connections of the meniscal horns to the tibial articular surface (Figure 3).10,27,49,156 In the most superficial region of the menisci, the type I fibers are oriented in a more radial direction. Radially oriented “tie” fibers are also present in the deep zone and are interspersed or woven between the circumferential fibers to provide structural integrity (Figure 3).# There is lipid debris and calcified bodies in the ECM of human menisci.54 The calcified bodies contain long, slender crystals of phosphorous, calcium, and magnesium on electron-probe roentgenographic analysis.54 The function of these crystals in not completely understood, but it is believed that they may play a role in acute joint inflammation and destructive arthropathies.
Figure 3.
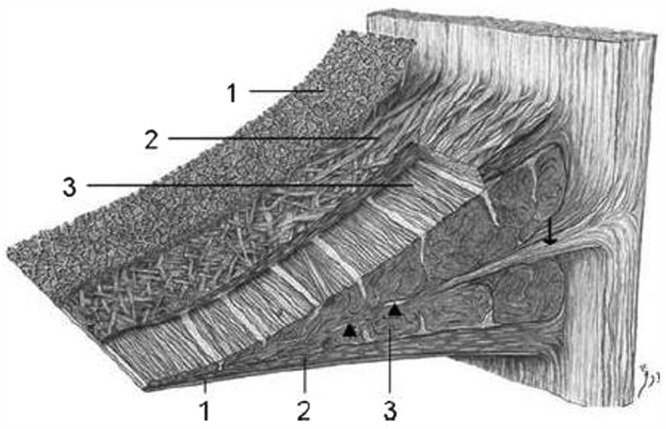
Schematic diagram demonstrating the collagen fiber ultrastructure and orientation within the meniscus: 1, superficial network; 2, lamellar layer; 3, central main layer. Arrowheads, radial interwoven fibers; arrow, loose connective tissue. Reprinted with permission from Petersen and Tillmann.132
Noncollagenous matrix proteins, such as fibronectin, contribute 8% to 13% of the organic dry weight. Fibronectin is involved in many cellular processes, including tissue repair, embryogenesis, blood clotting, and cell migration/adhesion. Elastin forms less than 0.6% of the meniscus dry weight; its ultrastructural localization is not clear. It likely interacts directly with collagen to provide resiliency to the tissue.**
Proteoglycans
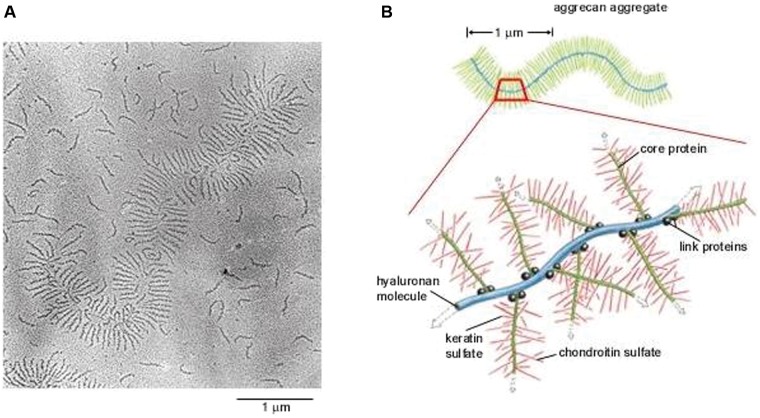
Located within a fine meshwork of collagen fibrils, proteoglycans are large, negatively charged hydrophilic molecules, contributing 1% to 2% of dry weight.58 They are formed by a core protein with 1 or more covalently attached glycosaminoglycan chains (Figure 4).122 The size of these molecules is further increased by specific interaction with hyaluronic acid.67,72 The amount of proteoglycans in the meniscus is one-eighth that of articular cartilage,2,3 and there may be considerable variation depending on the site of the sample and the age of the patient.49
Figure 4.
Extracellular matrix. (A) Electron micrograph of an aggrecan aggregate shadowed by platinum. Many free aggrecan molecules are also seen. (B) Schematic drawing of an aggrecan aggregate shown in part A. Reprinted with permission from Alberts et al.6
By virtue of their specialized structure, high fixed-charge density, and charge-charge repulsion forces, proteoglycans in the ECM are responsible for hydration and provide the tissue with a high capacity to resist compressive loads.‡ The glycosaminoglycan profile of the normal adult human meniscus consists of chondroitin-6-sulfate (40%), chondroitin-4-sulfate (10% to 20%), dermatan sulfate (20% to 30%), and keratin sulfate (15%; Figure 4).65,77,99,159 The highest glycosaminoglycan concentrations are found in the meniscal horns and the inner half of the menisci in the primary weightbearing areas.58,77
Aggrecan is the major proteoglycan found in the human menisci and is largely responsible for their viscoelastic compressive properties (Figure 5). Smaller proteoglycans, such as decorin, biglycan, and fibromodulin, are found in smaller amounts.124,151 Hexosamine contributes 1% to the dry weight of ECM.57,74 The precise functions of each of these small proteoglycans on the meniscus have yet to be fully elucidated.
Figure 5.
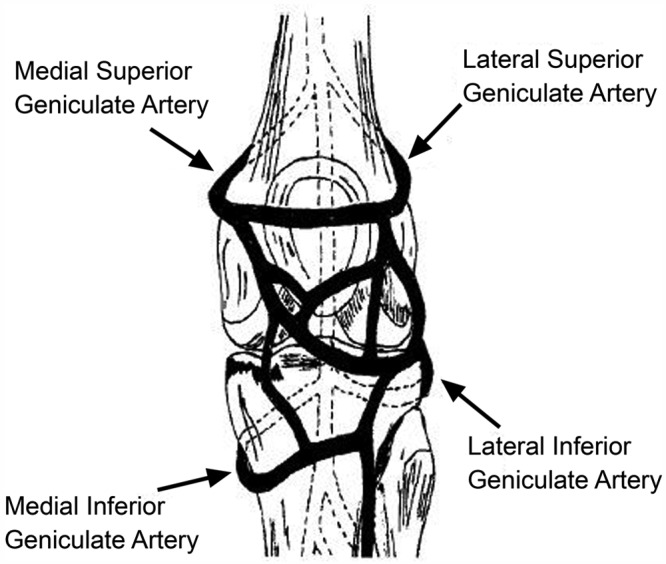
Confluence of geniculate arteries (anterior view). Reprinted with permission from Brindle et al.26
Matrix Glycoproteins
Meniscal cartilage contains a range of matrix glycoproteins, the identities and functions of which have yet to be determined. Electrophoresis and subsequent staining of the polyacrylamide gels reveals bands with molecular weights varying from a few kilodaltons to more than 200 kDa.112 These matrix molecules include the link proteins that stabilize proteoglycan–hyaluronic acid aggregates and a 116-kDa protein of unknown function.46 This protein resides in the matrix in the form of disulfide-bonded complex of high molecular weight.46 Immunolocalization studies suggest that it is predominantly located around the collagen bundles in the interterritorial matrix.47
The adhesive glycoproteins constitute a subgroup of the matrix glycoproteins. These macromolecules are partly responsible for binding with other matrix molecules and/or cells. Such intermolecular adhesion molecules are therefore important components in the supramolecular organization of the extracellular molecules of the meniscus.150 Three molecules have been identified within the meniscus: type VI collagen, fibronectin, and thrombospondin.112,118,181
Vascular Anatomy
The meniscus is a relatively avascular structure with a limited peripheral blood supply. The medial, lateral, and middle geniculate arteries (which branch off the popliteal artery) provide the major vascularization to the inferior and superior aspects of each meniscus (Figure 5).9,12,33-35,148 The middle geniculate artery is a small posterior branch that perforates the oblique popliteal ligament at the posteromedial corner of the tibiofemoral joint. A premeniscal capillary network arising from the branches of these arteries originates within the synovial and capsular tissues of the knee along the periphery of the menisci. The peripheral 10% to 30% of the medial meniscus border and 10% to 25% of the lateral meniscus are relatively well vascularized, which has important implications for meniscus healing (Figure 6).12,33,68 Endoligamentous vessels from the anterior and posterior horns travel a short distance into the substance of the menisci and form terminal loops, providing a direct route for nourishment.33 The remaining portion of each meniscus (65% to 75%) receives nourishment from synovial fluid via diffusion or mechanical pumping (ie, joint motion).116,120
Figure 6.
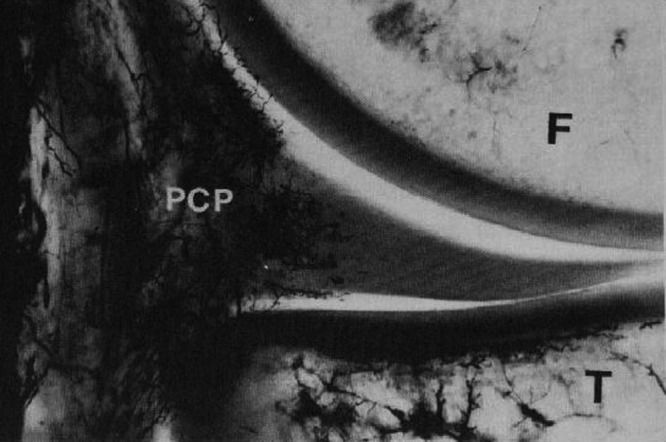
Microvasculature of the medial meniscus (superior aspect), following vascular perfusion with India ink and tissue clearing using a modified Spälteholz technique. Perimeniscal capillary plexus (PCP) can be seen penetrating the peripheral border of the medial meniscus. F, femur; T, tibia. Reprinted with permission from Arnoczky and Warren.12
Bird and Sweet examined the menisci of animals and humans using scanning electron and light microscopy.23,24 They observed canal-like structures opening deep into the surface of the menisci. These canals may play a role in the transport of fluid within the meniscus and may carry nutrients from the synovial fluid and blood vessels to the avascular sections of the meniscus.23,24 However, further study is needed to elucidate the exact mechanism by which mechanical motion supplies nutrition to the avascular portion of the menisci.
Neuroanatomy
The knee joint is innervated by the posterior articular branch of the posterior tibial nerve and the terminal branches of the obturator and femoral nerves. The lateral portion of the capsule is innervated by the recurrent peroneal branch of the common peroneal nerve. These nerve fibers penetrate the capsule and follow the vascular supply to the peripheral portion of the menisci and the anterior and posterior horns, where most of the nerve fibers are concentrated.52,90 The outer third of the body of the meniscus is more densely innervated than the middle third.183,184 During extremes of flexion and extension of the knee, the meniscal horns are stressed, and the afferent input is likely greatest at these extreme positions.183,184
The mechanoreceptors within the menisci function as transducers, converting the physical stimulus of tension and compression into a specific electrical nerve impulse. Studies of human menisci have identified 3 morphologically distinct mechanoreceptors: Ruffini endings, Pacinian corpuscles, and Golgi tendon organs.‡‡ Type I (Ruffini) mechanoreceptors are low threshold and slowly adapting to the changes in joint deformation and pressure. Type II (Pacinian) mechanoreceptors are low threshold and fast adapting to tension changes.§§ Type III (Golgi) are high-threshold mechanoreceptors, which signal when the knee joint approaches the terminal range of motion and are associated with neuromuscular inhibition. These neural elements were found in greater concentration in the meniscal horns, particularly the posterior horn.
The asymmetrical components of the knee act in concert as a type of biological transmission that accepts, transfers, and dissipates loads along the femur, tibia, patella, and femur.41 Ligaments act as an adaptive linkage, with the menisci representing mobile bearings. Several studies have reported that various intra-articular components of the knee are sensate, capable of generating neurosensory signals that reach spinal, cerebellar, and higher central nervous system levels.‖‖ It is believed that these neurosensory signals result in conscious perception and are important for normal knee joint function and maintenance of tissue homeostasis.42
Biomechanical Function
The biomechanical function of the meniscus is a reflection of the gross and ultrastructural anatomy and of its relationship to the surrounding intra-articular and extra-articular structures. The menisci serve many important biomechanical functions. They contribute to load transmission,¶¶ shock absorption,10,49,94,96,170 stability,51,100,101,109,155 nutrition,23,24,84,141 joint lubrication,102-104,141 and proprioception.5,15,81,88,115,147 They also serve to decrease contact stresses and increase contact area and congruity of the knee.91,172
Meniscal Kinematics
In a study on ligamentous function, Brantigan and Voshell reported the medial meniscus to move an average 2 mm, while the lateral meniscus was markedly more mobile with approximately 10 mm of anterior-posterior displacement during flexion.25 Similarly, DePalma reported that the medial meniscus undergoes 3 mm of anterior-posterior displacement, while the lateral meniscus moves 9 mm during flexion.37 In a study using 5 cadaveric knees, Thompson et al reported the mean medial excursion to be 5.1 mm (average of anterior and posterior horns) and the mean lateral excursion, 11.2 mm, along the tibial articular surface (Figure 7).165 The findings from these studies confirm a significant difference in segmental motion between the medial and lateral menisci. The anterior and posterior horn lateral meniscus ratio is smaller and indicates that the meniscus moves more as a single unit.165 Alternatively, the medial meniscus (as a whole) moves less than the lateral meniscus, displaying a greater anterior to posterior horn differential excursion. Thompson et al found that the area of least meniscal motion is the posterior medial corner, where the meniscus is constrained by its attachment to the tibial plateau by the meniscotibial portion of the posterior oblique ligament, which has been reported to be more prone to injury.143,165 A reduction in the motion of the posterior horn of the medial meniscus is a potential mechanism for meniscal tears, with a resultant “trapping” of the fibrocartilage between the femoral condyle and the tibial plateau during full flexion. The greater differential between anterior and posterior horn excursion may place the medial meniscus at a greater risk of injury.165
Figure 7.
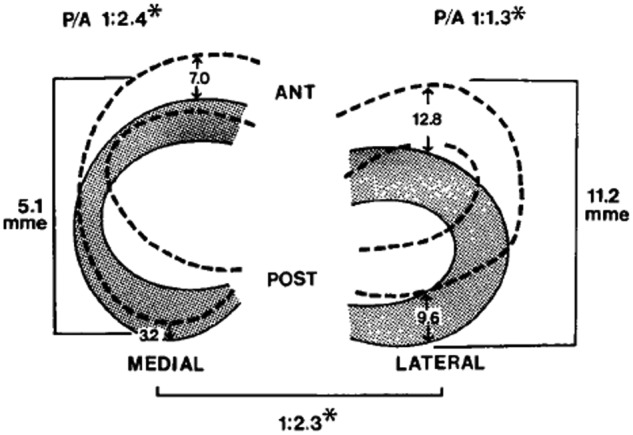
Diagrams showing the mean movement (mm) in each meniscus during flexion (shaded) and extension (hashed). ANT, anterior; POST, posterior; mme, mean meniscal excursion; P/A, ratio of posterior to anterior meniscal translation during flexion. Reproduced with permission from Thomspon et al.165
*P < 0.05 (Student t test analysis).
The differential of anterior horn to posterior horn motion allows the menisci to assume a decreasing radius with flexion, which correlates to the decreased radius of curvature of the posterior femoral condyles.165 This change of radius allows the meniscus to maintain contact with the articulating surface of both the femur and the tibia throughout flexion.
Load Transmission
The function of the menisci has been clinically inferred by the degenerative changes that accompany its removal. Fairbank described the increased incidence and predictable degenerative changes of the articular surfaces in completely meniscectomized knees.45 Since this early work, numerous studies have confirmed these findings and have further established the important role of the meniscus as a protective, load-bearing structure.
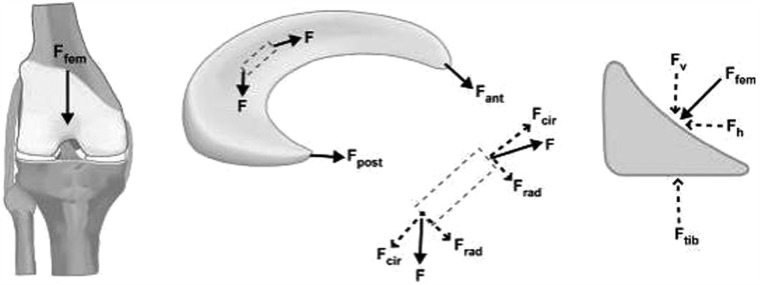
Weightbearing produces axial forces across the knee, which compress the menisci, resulting in “hoop” (circumferential) stresses.170 Hoop stresses are generated as axial forces and converted to tensile stresses along the circumferential collagen fibers of the meniscus (Figure 8). Firm attachments by the anterior and posterior insertional ligaments prevent the meniscus from extruding peripherally during load bearing.94 Studies by Seedhom and Hargreaves reported that 70% of the load in the lateral compartment and 50% of the load in the medial compartment is transmitted through the menisci.153 The menisci transmit 50% of compressive load through the posterior horns in extension, with 85% transmission at 90° flexion.172 Radin et al demonstrated that these loads are well distributed when the menisci are intact.137 However, removal of the medial meniscus results in a 50% to 70% reduction in femoral condyle contact area and a 100% increase in contact stress.4,50,91 Total lateral meniscectomy results in a 40% to 50% decrease in contact area and increases contact stress in the lateral component to 200% to 300% of normal.18,50,76,91 This significantly increases the load per unit area and may contribute to accelerated articular cartilage damage and degeneration.45,85
Figure 8.
Free body diagram of forces acting on the meniscus during loading. As the femur presses down on the meniscus during normal loading, the meniscus deforms radially but is anchored by its anterior and posterior horns (Fant and Fpost). During loading, tensile, compressive, and shear forces are generated. A tensile hoop stress (Fcir) results from the radial deformation, while vertical and horizontal forces (Fv and Fh) result from the femur pressing on the curved superior surface of the tissue. A radial reaction force (Frad) balances the femoral horizontal force (Fh). Reprinted with permission from Athanasiou and Sanchez-Adams.17
Shock Absorption
The menisci play a vital role in attenuating the intermittent shock waves generated by impulse loading of the knee with normal gait.94,96,153 Voloshin and Wosk showed that the normal knee has a shock-absorbing capacity about 20% higher than knees that have undergone meniscectomy.170 As the inability of a joint system to absorb shock has been implicated in the development of osteoarthritis, the meniscus would appear to play an important role in maintaining the health of the knee joint.138
Joint Stability
The geometric structure of the menisci provides an important role in maintaining joint congruity and stability.## The superior surface of each meniscus is concave, enabling effective articulation between the convex femoral condyles and flat tibial plateau. When the meniscus is intact, axial loading of the knee has a multidirectional stabilizing function, limiting excess motion in all directions.9
Markolf and colleagues have addressed the effect of meniscectomy on anterior-posterior and rotational knee laxity. Medial meniscectomy in the ACL-intact knee has little effect on anterior-posterior motion, but in the ACL-deficient knee, it results in an increase in anterior-posterior tibial translation of up to 58% at 90o of flexion.109 Shoemaker and Markolf demonstrated that the posterior horn of the medial meniscus is the most important structure resisting an anterior tibial force in the ACL-deficient knee.155 Allen et al showed that the resultant force in the medial meniscus of the ACL-deficient knee increased by 52% in full extension and by 197% at 60° of flexion under a 134-N anterior tibial load.7 The large changes in kinematics due to medial meniscectomy in the ACL-deficient knee confirm the important role of the medial meniscus in knee stability. Recently, Musahl et al reported that the lateral meniscus plays a role in anterior tibial translation during the pivot-shift maneuver.123
Joint Nutrition and Lubrication
The menisci may also play a role in the nutrition and lubrication of the knee joint. The mechanics of this lubrication remains unknown; the menisci may compress synovial fluid into the articular cartilage, which reduces frictional forces during weightbearing.13
There is a system of microcanals within the meniscus located close to the blood vessels, which communicates with the synovial cavity; these may provide fluid transport for nutrition and joint lubrication.23,24
Proprioception
The perception of joint motion and position (proprioception) is mediated by mechanoreceptors that transduce mechanical deformation into electric neural signals. Mechanoreceptors have been identified in the anterior and posterior horns of the menisci.*** Quick-adapting mechanoreceptors, such as Pacinian corpuscles, are thought to mediate the sensation of joint motion, and slow-adapting receptors, such as Ruffini endings and Golgi tendon organs, are believed to mediate the sensation of joint position.140 The identification of these neural elements (located mostly in the middle and outer third of the meniscus) indicates that the menisci are capable of detecting proprioceptive information in the knee joint, thus playing an important afferent role in the sensory feedback mechanism of the knee.61,88,90,158,169
Maturation and Aging of The Meniscus
The microanatomy of the meniscus is complex and certainly demonstrates senescent changes. With advancing age, the meniscus becomes stiffer, loses elasticity, and becomes yellow.78,95 Microscopically, there is a gradual loss of cellular elements with empty spaces and an increase in fibrous tissue in comparison with elastic tissue.74 These cystic areas can initiate a tear, and with a torsional force by the femoral condyle, the superficial layers of the meniscus may shear off from the deep layer at the interface of the cystic degenerative change, producing a horizontal cleavage tear. Shear between these layers may cause pain. The torn meniscus may directly injure the overlying articular cartilage.74,95
Ghosh and Taylor found that collagen concentration increased from birth to 30 years and remained constant until 80 years of age, after which a decline occurred.58 The noncollagenous matrix proteins showed the most profound changes, decreasing from 21.9% ± 1.0% (dry weight) in neonates to 8.1% ± 0.8% between the ages of 30 to 70 years.80 After 70 years of age, the noncollagenous matrix protein levels increased to 11.6% ± 1.3%. Peters and Smillie observed an increase in hexosamine and uronic acid with age.131
McNicol and Roughley studied the variation of meniscal proteoglycans in aging113; small differences in extractability and hydrodynamic size were observed. The proportions of keratin sulfate relative to chondroitin-6-sulfate increased with aging.146
Petersen and Tillmann immunohistochemically investigated human menisci (ranging from 22 weeks of gestation to 80 years), observing the differentiation of blood vessels and lymphatics in 20 human cadavers. At the time of birth, nearly the entire meniscus was vascularized. In the second year of life, an avascular area developed in the inner circumference. In the second decade, blood vessels were present in the peripheral third. After 50 years of age, only the peripheral quarter of the meniscal base was vascularized. The dense connective tissue of the insertion was vascularized but not the fibrocartilage of the insertion. Blood vessels were accompanied by lymphatics in all areas.†††
Arnoczky suggested that body weight and knee joint motion may eliminate blood vessels in the inner and middle aspects of the menisci.9 Nutrition of meniscal tissue occurs via perfusion from blood vessels and via diffusion from synovial fluid. A requirement for nutrition via diffusion is the intermittent loading and release on the articular surfaces, stressed by body weight and muscle forces.130 The mechanism is comparable with the nutrition of articular cartilage.22
Magnetic Resonance Imaging of The Meniscus
Magnetic resonance imaging (MRI) is a noninvasive diagnostic tool used in the evaluation, diagnosis, and monitoring of the menisci. MRI is widely accepted as the optimal imaging modality because of superior soft tissue contrast.
On cross-sectional MRI, the normal meniscus appears as a uniform low-signal (dark) triangular structure (Figure 9). A meniscal tear is identified by the presence of an increased intrameniscal signal that extends to the surface of this structure.
Figure 9.
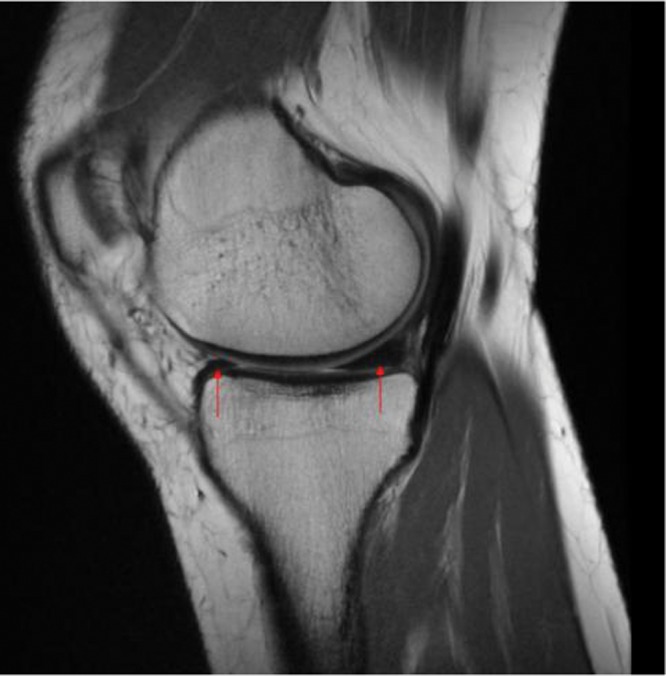
A sagittal magnetic resonance (proton-density) image of a healthy knee depicting the medial menisci (arrows). The concave superior meniscal surface improves contact with the femoral epicondyles, and a flat undersurface improves contact with the tibial plateau. The periphery is thicker than the central portion, allowing for firm attachment to the joint capsule.
Several studies have evaluated the clinical utility of MRI for meniscal tears. In general, MRI is highly sensitive and specific for tears of the meniscus. The sensitivity of MRI in detecting meniscal tears ranges from 70% to 98%, and the specificity, from 74% to 98%.48,62,105,107,117 The MRI of 1014 patients before an arthroscopic examination had an accuracy of 89% for pathology of the medial meniscus and 88% for the lateral meniscus.48 A meta-analysis of 2000 patients with an MRI and arthroscopic examination found 88% sensitivity and 94% accuracy for meniscal tears.105,107
There have been discrepancies between MRI diagnoses and the pathology identified during arthroscopic examination.‡‡‡ Justice and Quinn reported discrepancies in the diagnosis of 66 of the 561 patients (12%).86 In a study of 92 patients, discrepancies between the MRI and arthroscopic diagnoses were noted in 22 of the 349 (6%) cases.106 Miller conducted a single-blind prospective study comparing clinical examinations and MRI in 57 knee examinations.117 He found no significant difference in sensitivity between the clinical examination and MRI (80.7% and 73.7%, respectively). Shepard et al assessed the accuracy of MRI in detecting clinically significant lesions of the anterior horn of the meniscus in 947 consecutive knee MRI154 and found a 74% false-positive rate. Increased signal intensity in the anterior horn does not necessarily indicate a clinically significant lesion.154
Conclusions
The menisci of the knee joint are crescent-shaped wedges of fibrocartilage that provide increased stability to the femorotibial articulation, distribute axial load, absorb shock, and provide lubrication to the knee joint. Injuries to the menisci are recognized as a cause of significant musculoskeletal morbidity. Preservation of the menisci is highly dependent on maintaining its distinctive composition and organization.
Acknowledgments
We wish to thank Tom Cichonski for his assistance in the formatting of this manuscript
Footnotes
References
- 1. Adams ME, Hukins DWL. The extracellular matrix of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW. eds. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992:15-282016 [Google Scholar]
- 2. Adams ME, McDevitt CA, Ho A, Muir H. Isolation and characterization of high-buoyant-density proteoglycans from semilunar menisci. J Bone Joint Surg Am. 1986;68:55-64 [PubMed] [Google Scholar]
- 3. Adams ME, Muir H. The glycosaminoglycans of canine menisci. Biochem J. 1981;197:385-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints: part I. Tibial surface of the knee. J Biomech Eng. 1983;185:290-294 [DOI] [PubMed] [Google Scholar]
- 5. Akgun U, Kogaoglu B, Orhan EK, Baslo MB, Karahan M. Possible reflex pathway between medial meniscus and semi-membranous muscle: an experimental study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):809-814 [DOI] [PubMed] [Google Scholar]
- 6. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. Bethesda, MD: National Center for Biotechnology Information; 2002 [Google Scholar]
- 7. Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000;18(1):109-115 [DOI] [PubMed] [Google Scholar]
- 8. Arnoczky SP. Building a meniscus: biologic considerations. Clin Orthop Relat Res. 1999;367S:244-253 [PubMed] [Google Scholar]
- 9. Arnoczky SP. Gross and vascular anatomy of the meniscus and its role in meniscal healing, regeneration and remodeling. In: Mow VC, Arnoczky SP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992:1-14 [Google Scholar]
- 10. Arnoczky SP, Adams ME, DeHaven KE, Eyre DR, Mow VC. The meniscus. In: Woo SL-Y, Buckwalter J, eds. Injury and Repair of Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1987:487-537 [Google Scholar]
- 11. Arnoczky SP, Warren RF. Anatomy of the cruciate ligaments. In: Feagin JA, ed. The Crucial Ligaments. New York, NY: Churchill Livingstone; 1988:179-195 [Google Scholar]
- 12. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90-95 [DOI] [PubMed] [Google Scholar]
- 13. Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using exogenous fibrin clot: an experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209-1217 [PubMed] [Google Scholar]
- 14. Aspden RM, Yarker YE, Hukins DWL. Collagen orientations in the meniscus of the knee joint. J Anat. 1985;140:371. [PMC free article] [PubMed] [Google Scholar]
- 15. Assimakopoulos AP, Katonis PG, Agapitos MV, Exarchou EI. The innervations of the human meniscus. Clin Orthop Relat Res. 1992;275:232-236 [PubMed] [Google Scholar]
- 16. Atencia LJ, McDevitt CA, Nile WB, Sokoloff L. Cartilage content of an immature dog. Connect Tissue Res. 1989;18:235-242 [DOI] [PubMed] [Google Scholar]
- 17. Athanasiou KA, Sanchez-Adams J. Engineering the Knee Meniscus. San Rafael, CA: Morgan & Claypool Publishers; 2009 [Google Scholar]
- 18. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on the intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270-275 [DOI] [PubMed] [Google Scholar]
- 19. Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17:1-6 [DOI] [PubMed] [Google Scholar]
- 20. Beaufils P, Verdonk R, eds. The Meniscus. Heidelberg, Germany: Springer-Verlag; 2010 [Google Scholar]
- 21. Beaupre A, Choukroun R, Guidouin R, Carneau R, Gerardin H. Knee menisci: correlation between microstructure and biomechanics. Clin Orthop Relat Res. 1986;208:72-75 [PubMed] [Google Scholar]
- 22. Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Erste Mitteilung: Die modellierenden und formerhaltenden Faktoren des Knorpelreliefs. Z Anat Entwickl Gesch. 1925;76:4263 [Google Scholar]
- 23. Bird MDT, Sweet MBE. Canals of the semilunar meniscus: brief report. J Bone Joint Surg Br. 1988;70:839. [DOI] [PubMed] [Google Scholar]
- 24. Bird MDT, Sweet MBE. A system of canals in semilunar menisci. Ann Rheum Dis. 1987;46:670-673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brantigan OC, Voshell AF. The mechanics of the ligaments and menisci of the knee joint. J Bone Joint Surg Am. 1941;23:44-66 [PubMed] [Google Scholar]
- 26. Brindle T, Nyland J, Johnson DL. The meniscus: review of basic principles with application to surgery and rehabilitation. J Athl Train. 2001;32(2):160-169 [PMC free article] [PubMed] [Google Scholar]
- 27. Bullough PG, Munuera L, Murphy J, et al. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1979;52:564-570 [PubMed] [Google Scholar]
- 28. Bullough PG, Vosburgh F, Arnoczky SP, et al. The menisci of the knee. In: Insall JN, ed. Surgery of the Knee. New York, NY: Churchill Livingstone; 1984:135-149 [Google Scholar]
- 29. Burr DB, Radin EL. Meniscal function and the importance of meniscal regeneration in preventing late medial compartment osteoarthrosis. Clin Orthop Relat Res. 1982;171:121-126 [PubMed] [Google Scholar]
- 30. Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiol Rev. 1988;68:858-910 [DOI] [PubMed] [Google Scholar]
- 31. Clark CR, Ogden JA. Development of the menisci of the human knee joint. J Bone Joint Surg Am. 1983;65:530. [PubMed] [Google Scholar]
- 32. Clark FJ, Horsh KW, Bach SM, Larson GF. Contributions of cutaneous and joint receptors to static knee-position sense in man. J Neurophysiol. 1979;42:877-888 [DOI] [PubMed] [Google Scholar]
- 33. Danzig L, Resnik D, Gonsalves M, Akeson WH. Blood supply to the normal and abnormal meniscus of the human knee. Clin Orthop Relat Res. 1983;172:271-276 [PubMed] [Google Scholar]
- 34. Davies D, Edwards D. The vascular and nerve supply of the human meniscus. Am R Coll Surg Engl. 1948;2:142-156 [PMC free article] [PubMed] [Google Scholar]
- 35. Day B, Mackenzie WG, Shim SS, Leung G. The vascular and nerve supply of the human meniscus. Arthroscopy. 1985;1:58-62 [DOI] [PubMed] [Google Scholar]
- 36. DeHaven KE. Meniscectomy versus repair: clinical experience. In: Mow VC, Arnoczky SP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992:131-139 [Google Scholar]
- 37. DePalma AF. Diseases of the Knee. Philadelphia, PA: JB Lippincott Co; 1954 [Google Scholar]
- 38. De Smet AA, Graf BK. Meniscal tears missed on MR imaging: relationship to meniscal tear patterns and anterior cruciate ligament tears. AJR Am J Roentgenol. 1994;162:905-911 [DOI] [PubMed] [Google Scholar]
- 39. De Smet AA, Norris MA, Yandow DR, et al. MR diagnosis of meniscal tears of the knee: importance of high signal in the meniscus that extends to the surface. AJR Am J Roentgenol. 1993;161:101-107 [DOI] [PubMed] [Google Scholar]
- 40. Dye SF. Functional morphologic features of the human knee: an evolutionary perspective. Clin Orthop Relat Res. 2003;410:19-24 [DOI] [PubMed] [Google Scholar]
- 41. Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res. 1996;325:10-18 [DOI] [PubMed] [Google Scholar]
- 42. Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773-777 [DOI] [PubMed] [Google Scholar]
- 43. Eyre DR, Koob TJ, Chun LE. Biochemistry of the meniscus: unique profile of collagen types and site dependent variations in composition. Orthop Trans. 1983;8:56 [Google Scholar]
- 44. Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983;158:265. [DOI] [PubMed] [Google Scholar]
- 45. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:664-670 [PubMed] [Google Scholar]
- 46. Fife RS. Identification of the link proteins and a 116,000-dalton matrix protein in canine meniscus. Arch Biochem Biophys. 1985;240:682. [DOI] [PubMed] [Google Scholar]
- 47. Fife RS, Hook GL, Brandt KD. Topographic localization of a 116,000 dalton protein in cartilage. J Histochem Cytochem. 1985;33:127. [DOI] [PubMed] [Google Scholar]
- 48. Fischer SP, Fox JM, Del Pizzo W, et al. Accuracy of diagnoses from magnetic resonance imaging of the knee: a multi-center analysis of one thousand and fourteen patients. J Bone Joint Surg Am. 1991;73:2-10 [PubMed] [Google Scholar]
- 49. Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19-31 [PubMed] [Google Scholar]
- 50. Fukubayashi T, Kurosawa H. The contact area and pressure distribution pattern of the knee: a study of normal and osteoarthritic knee joints. Acta Orthop Scand. 1980;51:871-879 [DOI] [PubMed] [Google Scholar]
- 51. Fukubayashi T, Torzilli PA, Sherman MF, Warren RF. An in vivo biomechanical analysis of anterior-posterior motion of the knee, tibial displacement rotation and torque. J Bone Joint Surg Am. 1982;64:258-264 [PubMed] [Google Scholar]
- 52. Gardner E. The innervations of the knee joint. Anat Rec. 1948;101:109-130 [DOI] [PubMed] [Google Scholar]
- 53. Gardner E, O’Rahilly R. The early development of the knee joint in staged human embryos. J Anat. 1968;102:289-299 [PMC free article] [PubMed] [Google Scholar]
- 54. Ghadially FN, LaLonde JMA. Intramatrical lipidic debris and calcified bodes in human semilunar cartilages. J Anat. 1981;132:481. [PMC free article] [PubMed] [Google Scholar]
- 55. Ghadially FN, LaLonde JMA, Wedge JH. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983;136:773-791 [PMC free article] [PubMed] [Google Scholar]
- 56. Ghadially FN, Thomas I, Yong N, LaLonde JMA. Ultrastructure of rabbit semilunar cartilage. J Anat. 1978;125:499. [PMC free article] [PubMed] [Google Scholar]
- 57. Ghosh P, Ingman AM, Taylor TK. Variations in collagen, non-collagenous proteins, and hexosamine in menisci derived from osteoarthritic and rheumatoid arthritic knee joints. J Rheumatol. 1975;2:100-107 [PubMed] [Google Scholar]
- 58. Ghosh P, Taylor TKF. The knee joint meniscus: a fibrocartilage of some distinction. Clin Orthop Relat Res. 1987;224:52-63 [PubMed] [Google Scholar]
- 59. Ghosh P, Taylor TKF, Pettit GD, Horsburgh BA, Bellenger CR. Effect of postoperative immobilization on the regrowth of knee joint semilunar cartilage: an experimental study. J Orthop Res. 1983;1:153. [DOI] [PubMed] [Google Scholar]
- 60. Gray DJ, Gardner E. Pre-natal development of the human knee and superior tibial fibula joints. Am J Anat. 1950;86:235-288 [DOI] [PubMed] [Google Scholar]
- 61. Gray JC. Neural and vascular anatomy of the menisci of the human knee. J Orthop Sports Phys Ther. 1999;29(1):23-30 [DOI] [PubMed] [Google Scholar]
- 62. Gray SD, Kaplan PA, Dussault RG. Imaging of the knee: current status. Orthop Clin North Am. 1997;28:643-658 [DOI] [PubMed] [Google Scholar]
- 63. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10:168-176 [DOI] [PubMed] [Google Scholar]
- 64. Gronblad M, Korkala O, Liesi P, Karaharju E. Innervation of synovial membrane and meniscus. Acta Orthop Scand. 1985;56:484-486 [DOI] [PubMed] [Google Scholar]
- 65. Habuchi H, Yamagata T, Iwata H, Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J Biol Chem. 1973;248:6019-6028 [PubMed] [Google Scholar]
- 66. Haines RW. The tetrapod knee joint. J Anat. 1942;76:270-301 [PMC free article] [PubMed] [Google Scholar]
- 67. Hardingham TE, Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973;135 (4):905-908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Harner CD, Janaushek MA, Kanamori A, Yagi AKM, Vogrin TM, Woo SL. Biomechanical analysis of a double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2000;28:144-151 [DOI] [PubMed] [Google Scholar]
- 69. Harner CD, Kusayama T, Carlin G, et al. Structural and mechanical properties of the human posterior cruciate ligament and meniscofemoral ligaments. In: Transactions of the 40th Annual Meeting of the Orthopaedic Research Society; 1992 [Google Scholar]
- 70. Harner CD, Livesgay GA, Choi NY, et al. Evaluation of the sizes and shapes of the human anterior and posterior cruciate ligaments: a comparative study. Trans Orthop Res Soc. 1992;17:123. [DOI] [PubMed] [Google Scholar]
- 71. Hascall VC. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7:101-120 [DOI] [PubMed] [Google Scholar]
- 72. Hascall VC, Heinegård D. Aggregation of cartilage proteoglycans: I. The role of hyaluronic acid. J Biol Chem. 1974;249(13):4205-4256 [PubMed] [Google Scholar]
- 73. Heinegard D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042-2051 [DOI] [PubMed] [Google Scholar]
- 74. Helfet AJ. Osteoarthritis of the knee and its early arrest. Instr Course Lect. 1971;20:219-230 [Google Scholar]
- 75. Heller L, Langman J. The meniscofemoral ligaments of the human knee. J Bone Joing Surg Br. 1964;46:307-313 [PubMed] [Google Scholar]
- 76. Henning CE, Lynch MA, Clark JR. Vascularity for healing of meniscal repairs. Arthroscopy. 1987;3:13-18 [DOI] [PubMed] [Google Scholar]
- 77. Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Höpker WW, Angres G, Klingel K, Komitowksi D, Schuchardt E. Changes of the elastin compartment in the human meniscus. Virchows Arch A Pathol Anat Histopathol. 1986;408:575-592 [DOI] [PubMed] [Google Scholar]
- 79. Humphry GM. A Treatise on the Human Skeleton Including the Joints. Cambridge, UK: Macmillan; 1858:545-546 [Google Scholar]
- 80. Ingman AM, Ghosh P, Taylor TKF. Variation of collagenous and non-collagenous proteins of human knee joint menisci with age and degeneration. Gerontologia. 1974;20:212-233 [DOI] [PubMed] [Google Scholar]
- 81. Jerosch J, Prymka M, Castro WH. Proprioception of the knee joints with a lesion of the medial meniscus. Acta Orthop Belg. 1996;62(1):41-45 [PubMed] [Google Scholar]
- 82. Johnson DL, Swenson TD, Harner CD. Arthroscopic meniscal transplantation: anatomic and technical considerations. Presented at: Nineteenth Annual Meeting of the American Orthopaedic Society for Sports Medicine; July 12-14, 1993; Sun Valley, ID [Google Scholar]
- 83. Johnson DL, Swenson TM, Livesay GA, Aizawa H, Fu FH, Harner CD. Insertion-site anatomy of the human menisci: gross, arthroscopic, and topographical anatomy as a basis for meniscal transplantation. Arthroscopy. 1995;11:386-394 [DOI] [PubMed] [Google Scholar]
- 84. Johnson RJ, Pope MH. Functional anatomy of the meniscus. In: Symposium on Reconstruction of the Knee of the American Academy of Orthopaedic Surgeons. St Louis, MO: Mosby; 1978:3 [Google Scholar]
- 85. Jones RE, Smith EC, Reisch JS. Effects of medial meniscectomy in patients older than forty years. J Bone Joint Surg Am. 1978;60:783-786 [PubMed] [Google Scholar]
- 86. Justice WW, Quinn SF. Error patterns in the MR imaging evaluation of the menisci of the knee. Radiology. 1995;196:617-621 [DOI] [PubMed] [Google Scholar]
- 87. Kaplan EB. The embryology of the menisci of the knee joint. Bull Hosp Joint Dis. 1955;6:111-124 [PubMed] [Google Scholar]
- 88. Karahan M, Kocaoglu B, Cabukoglu C, Akgun U, Nuran R. Effect of partial medial meniscectomy on the proprioceptive function of the knee. Arch Orthop Trauma Surg. 2010;130:427-431 [DOI] [PubMed] [Google Scholar]
- 89. Kempson GE, Tuke MA, Dingle JT, Barrett AJ, Horsfield PH. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976;428(3):741-760 [DOI] [PubMed] [Google Scholar]
- 90. Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. Am J Sports Med. 1982;10:329-335 [DOI] [PubMed] [Google Scholar]
- 91. Kettelkamp DB, Jacobs AW. Tibiofemoral contact area: determination and implications. J Bone Joint Surg Am. 1972;54:349-356 [PubMed] [Google Scholar]
- 92. King D. The function of the semilunar cartilages. J Bone Joint Surg Br. 1936;18:1069-1076 [Google Scholar]
- 93. Kohn D, Moreno B. Meniscus insertion anatomy as a basis for meniscus replacement: a morphological cadaveric study. Arthroscopy. 1995;11:96-103 [DOI] [PubMed] [Google Scholar]
- 94. Krause WR, Pope MH, Johnson RJ, Wilder DG. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58:599-604 [PubMed] [Google Scholar]
- 95. Kulkarni VV, Chand K. Pathological anatomy of the aging meniscus. Acta Orthop Scand. 1975;46:135-140 [DOI] [PubMed] [Google Scholar]
- 96. Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;149:283-290 [PubMed] [Google Scholar]
- 97. LaPrade RF, Burnett QM, II, Veenstra MA, et al. The prevalence of abnormal magnetic resonance imaging findings in asymptomatic knees: with correlation of magnetic resonance imaging to arthroscopic finding in symptomatic knees. Am J Sports Med. 1994;22:739-745 [DOI] [PubMed] [Google Scholar]
- 98. Last RJ. Some anatomical details of the knee joint. J Bone Joint Surg Br. 1948;30:368-688 [PubMed] [Google Scholar]
- 99. Lehtonen A, Viljanto J, Kärkkäinen J. The mucopolysaccharides of herniated human intervertebral discs and semilunar cartilages. Acta Chir Scand. 1967;133(4):303-306 [PubMed] [Google Scholar]
- 100. Levy IM, Torzilli PA, Warren RF. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg Am. 1989;71:401-406 [PubMed] [Google Scholar]
- 101. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883-888 [PubMed] [Google Scholar]
- 102. MacConaill MA. The function of intra-articular fibrocartilages with special reference to the knee and inferior radio-ulnar joints. J Anat. 1932;6:210-227 [PMC free article] [PubMed] [Google Scholar]
- 103. MacConaill MA. The movements of bones and joints: III. The synovial fluid and its assistants. J Bone Joint Surg Br. 1950;32:244. [DOI] [PubMed] [Google Scholar]
- 104. MacConaill MA. Studies in the mechanics of synovial joints: II. Displacements on articular surfaces and the significance of saddle joints. Ir J Med Sci. 1946;6:223-235 [PubMed] [Google Scholar]
- 105. Mackenzie R, Dixon AK, Keene GS, et al. Magnetic resonance imaging of the knee: assessment of effectiveness. Clin Radiol. 1996;41:245-250 [DOI] [PubMed] [Google Scholar]
- 106. Mackenzie R, Keene GS, Lomas DJ, Dixon AK. Errors at knee magnetic resonance imaging: true or false? Br J Radiol. 1995;68:1045-1051 [DOI] [PubMed] [Google Scholar]
- 107. Mackenzie R, Palmer CR, Lomas DJ, et al. Magnetic resonance imaging of the knee: diagnostic performance studies. Clin Radiol. 1996;51:251-257 [DOI] [PubMed] [Google Scholar]
- 108. Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee instability. J Bone Joint Surg Am. 1981;63:570-585 [PubMed] [Google Scholar]
- 109. Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee: the contributions of the supporting structures. J Bone Joint Surg Am. 1976;58:583-597 [PubMed] [Google Scholar]
- 110. McDermott LJ. Development of the human knee joint. Arch Surg. 1943;46:705-719 [Google Scholar]
- 111. McDevitt CA, Miller RR, Sprindler KP. The cells and cell matrix interaction of the meniscus. In: Mow VC, Arnoczky SP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992:29-36 [Google Scholar]
- 112. McDevitt CA, Webber RJ. Ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990;252:8-18 [PubMed] [Google Scholar]
- 113. McNicol D, Roughley PJ. Extraction and characterization of proteoglycan from human meniscus. Biochem J. 1980;185:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Merkel KHH. The surface of human menisci and its aging alterations during age: a combined scanning and transmission electron microscopic examination (SEM, TEM). Arch Orthop Trauma Surg. 1980;97:185-191 [DOI] [PubMed] [Google Scholar]
- 115. Messner K, Gao J. The menisci of the knee joint: anatomical and functional characteristics, and a rationale for clinical treatment. J Anat. 1998;193:161-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Meyers E, Zhu W, Mow V. Viscoelastic properties of articular cartilage and meniscus. In: Nimni M, ed. Collagen: Chemistry, Biology and Biotechnology. Boca Raton, FL: CRC; 1988 [Google Scholar]
- 117. Miller GK. A prospective study comparing the accuracy of the clinical diagnosis of meniscal tear with magnetic resonance imaging and its effect on clinical outcome. Arthroscopy. 1996;12:406-413 [DOI] [PubMed] [Google Scholar]
- 118. Miller GK, McDevitt CA. The presence of thrombospondin in ligament, meniscus and intervertebral disc. Glycoconjugate J. 1988;5:312. [DOI] [PubMed] [Google Scholar]
- 119. Mossman DJ, Sargeant WAS. The footprints of extinct animals. Sci Am. 1983;250:78-79 [Google Scholar]
- 120. Mow V, Fithian D, Kelly M. Fundamentals of articular cartilage and meniscus biomechanics. In: Ewing JW, ed. Articular Cartilage and Knee Joint Function: Basic Science and Arthroscopy. New York, NY: Raven Press; 1989:1-18 [Google Scholar]
- 121. Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties or articular cartilage: a review. J Biomech. 1984;17:377. [DOI] [PubMed] [Google Scholar]
- 122. Muir H. The structure and metabolism of mucopolysaccharides (glycosaminoglycans) and the problem of the mucopolysaccharidoses. Am J Med. 1969;47 (5):673-690 [DOI] [PubMed] [Google Scholar]
- 123. Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38(8):1591-1597 [DOI] [PubMed] [Google Scholar]
- 124. Nakano T, Dodd CM, Scott PG. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res. 1997;15:213-222 [DOI] [PubMed] [Google Scholar]
- 125. Newton RA. Joint receptor contributions to reflective and kinaesthetic responses. Phys Ther. 1982;62:22-29 [DOI] [PubMed] [Google Scholar]
- 126. O’Connor BL. The histological structure of the dog knee menisci with comments on its possible significance. Am J Anat. 1976;147:407-417 [DOI] [PubMed] [Google Scholar]
- 127. O’Connor BL, McConnaughey JS. The structure and innervation of cat knee menisci, and their relation to a “sensory hypothesis” of meniscal function. Am J Anat. 1978;153:431-442 [DOI] [PubMed] [Google Scholar]
- 128. Oretorp N, Gillquist J, Liljedahl S-O. Long term results of surgery for non-acute anteromedial rotatory instability of the knee. Acta Orthop Scand. 1979;50:329-336 [DOI] [PubMed] [Google Scholar]
- 129. Pagnani MJ, Warren RF, Arnoczky SP, Wickiewicz TL. Anatomy of the knee. In: Nicholas JA, Hershman EB, eds. The Lower Extremity and Spine in Sports Medicine. 2nd ed. St Louis, MO: Mosby; 1995:581-614 [Google Scholar]
- 130. Pauwels F. [Developmental effects of the functional adaptation of bone]. Anat Anz. 1976;139:213-220 [PubMed] [Google Scholar]
- 131. Peters TJ, Smillie IS. Studies on the chemical composition of the menisci of the knee joint with special reference to the horizontal cleavage lesion. Clin Orthop Relat Res. 1972;86:245-252 [DOI] [PubMed] [Google Scholar]
- 132. Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl). 1998;197:317-324 [DOI] [PubMed] [Google Scholar]
- 133. Poynton AR, Javadpour SM, Finegan PJ, O’Brien M. The meniscofemoral ligaments of the knee. J Bone Joint Surg Br. 1997;79:327-330 [DOI] [PubMed] [Google Scholar]
- 134. Preuschoft H, Tardieu C. Biomechanical reasons for divergent morphology of the knee joint and the distal epiphyseal suture in hominoids. Folia Primatol (Basel). 1996;66:82-92 [DOI] [PubMed] [Google Scholar]
- 135. Proctor CS, Schmidt MB, Whipple RR, Kelly MA, Mow VC. Material properties of the normal medial bovine meniscus. J Orthop Res. 1989;7:771-782 [DOI] [PubMed] [Google Scholar]
- 136. Proske U, Schaible H, Schmidt RF. Joint receptors and kinanesthesia. Exp Brain Res. 1988;72:219-224 [DOI] [PubMed] [Google Scholar]
- 137. Radin EL, de Lamotte F, Maquet P. Role of the menisci in the distribution of stress in the knee. Clin Orthop Relat Res. 1984;185:290-294 [PubMed] [Google Scholar]
- 138. Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;213:34-40 [PubMed] [Google Scholar]
- 139. Raszeja F. Untersuchungen Bber Entstehung und feinen Bau des Kniegelenkmeniskus. Bruns Beitr klin Chir. 1938;167:371-387 [Google Scholar]
- 140. Reider B, Arcand MA, Diehl LH, et al. Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy. 2003;19(1):2-12 [DOI] [PubMed] [Google Scholar]
- 141. Renstrom P, Johnson RJ. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9:523-538 [PubMed] [Google Scholar]
- 142. Retterer E. De la forme et des connexions que presentment les fibro-cartilages du genou chez quelques singes d’Afrique. Cr Soc Biol. 1907;63:20-25 [Google Scholar]
- 143. Ricklin P, Ruttimann A, Del Bouno MS. Diagnosis, Differential Diagnosis and Therapy. 2nd ed. Stuttgart, Germany: Verlag Georg Thieme; 1983 [Google Scholar]
- 144. Rodkey WG. Basic biology of the meniscus and response to injury. In: Price CT, ed. Instructional Course Lectures 2000. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000:189-193 [PubMed] [Google Scholar]
- 145. Rosenberg LC, Buckwalter JA, Coutts R, Hunziker E, Mow VC. Articular cartilage. In: Woo SLY, Buckwalter JA, eds. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeon; 1988:401 [Google Scholar]
- 146. Roughley PJ. Changes in cartilage proteoglycan structure during aging: origin and effects: a review. Agents Actions. 1986;518:19. [DOI] [PubMed] [Google Scholar]
- 147. Saygi B, Yildirim Y, Berker N, Ofluoglu D, Karadag-Saygi E, Karahan M. Evaluation of neurosensory function of the medial meniscus in humans. Arthroscopy. 2005;21(12):1468-1472 [DOI] [PubMed] [Google Scholar]
- 148. Scapinelli R. Studies on the vasculature of the human knee joint. Acta Anat. 1968;70:305-331 [DOI] [PubMed] [Google Scholar]
- 149. Schutte MJ, Dabezius EJ, Zimny ML, Happe LT. Neural anatomy of the human anterior cruciate ligament. J Bone Joint Surg Am. 1987;69:243-247 [PubMed] [Google Scholar]
- 150. Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6:2639-2645 [PubMed] [Google Scholar]
- 151. Scott PG, Nakano T, Dodd CM. Isolation and characterization of small proteoglycans from different zones of the porcine knee meniscus. Biochim Biophys Acta. 1997;1336:254-262 [DOI] [PubMed] [Google Scholar]
- 152. Seedhom BB. Loadbearing function of the menisci. Physiotherapy. 1976;62(7):223. [PubMed] [Google Scholar]
- 153. Seedhom BB, Hargreaves DJ. Transmission of the load in the knee joint with special reference to the role in the menisci: part II. Experimental results, discussion and conclusion. Eng Med. 1979;8:220-228 [Google Scholar]
- 154. Shepard MF, Hunter DM, Davies MR, Shapiro MS, Seeger LL. The clinical significance of anterior horn meniscal tears diagnosed on magnetic resonance images. Am J Sports Med. 2002;30(2):189-192 [DOI] [PubMed] [Google Scholar]
- 155. Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee: effects of partial versus total excision. J Bone Joint Surg Am. 1986;68(1):71-79 [PubMed] [Google Scholar]
- 156. Skaags DL, Mow VC. Function of the radial tie fibers in the meniscus. Trans Orthop Res Soc. 1990;15:248 [Google Scholar]
- 157. Skinner HB, Barrack RL. Joint position sense in the normal and pathologic knee joint. J Electromyogr Kinesiol. 1991;1(3):180-190 [DOI] [PubMed] [Google Scholar]
- 158. Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop Relat Res. 1984;184:208-211 [PubMed] [Google Scholar]
- 159. Solheim K. Glycosaminoglycans, hydroxyproline, calcium, and phosphorus in healing fractures. Acta Univ Lund. 1965;28:1-22 [PubMed] [Google Scholar]
- 160. Spilker RL, Donzelli PS. A biphasic finite element model of the meniscus for stress-strain analysis. In: Mow VC, Arnoczky SP, Jackson DW, eds. Knee Meniscus: Basic and Clinical Foundations. New York, NY: Raven Press; 1992:91-106 [Google Scholar]
- 161. Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. J Biomechanics. 1992;25:1027-1045 [DOI] [PubMed] [Google Scholar]
- 162. Sutton JB. Ligaments: Their Nature and Morphology. 2nd ed. London: HK Lewis; 1897 [Google Scholar]
- 163. Tardieu C. Ontogeny and phylogeny of femoral-tibial characters in humans and hominid fossils: functional influence and genetic determinism. Am J Phys Anthropol. 1999;110:365-377 [DOI] [PubMed] [Google Scholar]
- 164. Tardieu C, Dupont JY. The origin of femoral trochlear dysplasia: comparative anatomy, evolution, and growth of the patellofemoral joint. Rev Chir Orthop Reparatrice Appar Mot. 2001;87:373-383 [PubMed] [Google Scholar]
- 165. Thompson WO, Thaete FL, Fu FH, Dye SF. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance imaging. Am J Sports Med. 1991;19:210-216 [DOI] [PubMed] [Google Scholar]
- 166. Tissakht M, Ahmed AM. Tensile stress-strain characteristics of the human meniscal material. J Biomech. 1995;28:411-422 [DOI] [PubMed] [Google Scholar]
- 167. Tobler T. Zur normalen und pathologischen Histologie des Kniegelenkmeniscus. Arch Klin Chir. 1933;177:483-495 [Google Scholar]
- 168. Vallois H. Etude anatomique de l’articulation du genou chez les primates. Montpelier, France: L’Abeille; 1914 [Google Scholar]
- 169. Verdonk R, Aagaard H. Function of the normal meniscus and consequences of the meniscal resection. Scand J Med Sci Sports. 1999;9(3):134-140 [DOI] [PubMed] [Google Scholar]
- 170. Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157-161 [DOI] [PubMed] [Google Scholar]
- 171. Wagner H-J. Die kollagenfaserarchitecktur der menisken des menschlichen kniegelenkes. Z Mikrosk Anat Forsch. 1976;90:302. [PubMed] [Google Scholar]
- 172. Walker PS, Erkman MJ. The role of the meniscus in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184-192 [DOI] [PubMed] [Google Scholar]
- 173. Wan ACT, Felle P. The menisco-femoral ligaments. Clin Anat. 1995;8:323-326 [DOI] [PubMed] [Google Scholar]
- 174. Warren PJ, Olanlokun TK, Cobb AG, Bentley G. Proprioception after knee arthroplasty: the influence of prosthetic design. Clin Orthop Relat Res. 1993;297:182-187 [PubMed] [Google Scholar]
- 175. Warren RF, Arnoczky SP, Wickiewiez TL. Anatomy of the knee. In: Nicholas JA, Hershman EB, eds. The Lower Extremity and Spine in Sports Medicine. St Louis: Mosby; 1986:657-694 [Google Scholar]
- 176. Watanabe AT, Carter BC, Teitelbaum GP, et al. Common pitfalls in magnetic resonance imaging of the knee. J Bone Joint Surg Am. 1989;71:857-862 [PubMed] [Google Scholar]
- 177. Webber RJ, Norby DP, Malemud CJ, Goldberg VM, Moskowitz RW. Characterization of newly synthesized proteoglycans from rabbit menisci in organ culture. Biochem J. 1984;221(3):875-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Webber RJ, York JL, Vanderschildren JL, Hough AJ. An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393-400 [DOI] [PubMed] [Google Scholar]
- 179. Wilson AS, Legg PG, McNeu JC. Studies on the innervations of the medial meniscus in the human knee joint. Anat Rec. 1969;165:485-492 [DOI] [PubMed] [Google Scholar]
- 180. Wirth CJ. The meniscus: structure, morphology and function. Knee. 1996;3:57-58 [Google Scholar]
- 181. Wu JJ, Eyre DR, Slayter HS. Type VI collagen of the intervertebral disc: biochemical and electron microscopic characterization of the native protein. Biochem J. 1987;248:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yasui K. Three dimensional architecture of normal human menisci. J Jpn Ortho Assoc. 1978;52:391 [Google Scholar]
- 183. Zimny ML. Mechanoreceptors in articular tissues. Am J Anat. 1988;64:883-888 [DOI] [PubMed] [Google Scholar]
- 184. Zimny ML, Albright DJ, Dabezies E. Mechanoreceptors in the human medial meniscus. Acta Anat. 1988;133:35-40 [DOI] [PubMed] [Google Scholar]
- 185. Zivanovic S. Menisco-meniscal ligaments of the human knee joint. Anat Anz. 1974;145:35-42 [PubMed] [Google Scholar]