Abstract
Background:
Pain control is a factor in the sideline treatment of competitive athletes. Ketorolac injections by team physicians as a pain control measure are seemingly becoming more mainstream, although there have been very little data published on its use.
Hypothesis:
Intramuscular ketorolac injections are being used regularly by orthopaedic surgeons and primary care sports medicine physicians in their care of athletes.
Study Design:
Descriptive epidemiology study.
Methods:
A 19-question survey was generated online for physician members of the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, and the American Medical Society for Sports Medicine. The survey link was e-mailed, with reminders sent every 1 to 2 weeks, and results were collected from April to June 2011.
Results:
The survey was e-mailed to 6950 physicians, with 1100 respondents completing it (60% orthopaedic surgeons, 40% nonsurgical sports medicine physicians). Approximately 49% use intramuscular ketorolac in the treatment of athletes, primarily at the collegiate and professional levels; 95.8% reported effective pain control after administration; 2.9% reported bleeding complications; and 1.9% reported kidney complications from its use.
Conclusion:
Intramuscular ketorolac injections are used by approximately half of all team physicians in their sideline treatment of competitive athletes.
Keywords: Toradol, Ketorolac, NSAIDs, Pain Control
The majority of injuries in the athlete affect muscle, tendon, ligaments, periarticular bursa, and bone.17 The consistent component of this array of injury patterns is inflammation. In acute musculoskeletal injuries, the involved tissue is stretched, torn, or fractured, and the body’s immediate response is inflammation. The local vasculature of the tissue dilates and becomes more permeable, allowing an influx of inflammatory cells and immunologic mediators to the affected area.17 The inflammatory cells produce free radicals, which break down damaged cell membranes and walls, resulting in the release of arachidonic acid metabolites.14 The arachidonic acid substrate is subsequently broken down via lipoxygenase or cyclo-oxygenase (COX). Breakdown via the lipoxygenase pathway results in leukotriene production, whereas the COX pathway breakdown results in the production of prostacyclins, prostaglandins, and thromboxanes.14 Prostaglandins and prostacyclins can sensitize local pain receptors as well as increase vascular permeability resulting in edema; thromboxane is involved in platelet aggregation and stimulates the coagulation cascade.17,19
The COX pathway involves 2 isoenzymes: COX-1 and COX-2.15 The COX-1 enzyme is a noninducible constitutional enzyme found in most tissues in the body, and it is involved in normal cellular processes, including gastric cytoprotection, vascular hemostasis, platelet aggregation, and renal blood flow.15 The COX-2 enzyme is induced by an inflammatory response and is involved in sensitizing pain receptors, increasing body temperature, and recruiting inflammatory cells to injured tissue.15,24 Nonsteroidal anti-inflammatory drugs (NSAIDs) function by inhibiting the COX enzymes and blocking the breakdown of arachidonic acid into its by-products.15,17,24 Nonselective NSAIDS block both COX-1 and COX-2. In athletes, the intended goal of NSAIDs is to limit the COX-2 pathway.15
Ketorolac (Toradol, Roche Pharmaceuticals, Nutley, New Jersey) is a well-known NSAID used for short-term pain relief via an intravenous, intramuscular (IM), oral, or ophthalmologic route.12 Since its approval by the Food and Drug Administration in 1990, it was the only NSAID available in either the IM or intravenous form until the 2009 approval of intravenous ibuprofen (Caldolor, Cumberland Pharmaceuticals, Nashville, Tennessee).10,17 When administered IM, ketorolac has a rapid onset of action, with initial pain relief within 10 minutes, a peak plasma level at 50 minutes, and a half-life of 6.5 hours in young adults.31 It undergoes hepatic metabolism prior to renal excretion, and 90% of the drug is excreted within 24 hours.31 The recommended initial dose is 30 to 60 mg when administered parenterally.12 If administered on a regular schedule, subsequent dosing of 15 or 30 milligrams can be given every 6 hours for no longer than 5 consecutive days because of its risk of renal impairment.4,12,17,21,31 Dosing adjustments must be made for patients older than 65 years, for patients weighing less than 110 lb (50 kg), and with elevated creatinine.21,31 Absolute contraindications to its use include NSAID hypersensitivity, history of gastrointestinal bleeding, hepatic or renal impairment, and severe asthma.21,31 The major documented side effects include increased risk of bleeding, gastrointestinal side effects, and kidney dysfunction.12,17,20,29 Singer et al showed IM ketorolac administration increases bleeding time in young healthy adults by 50%.29 This increased bleeding time is secondary to the inhibition of platelet aggregation and thromboxane production.17,29 This effect is reversible, with platelet function and bleeding times returning to normal within 24 hours of administration.3,17 Gastrointestinal side effects occur in up to 20% of patients with prolonged use (> 5 days) and include dyspepsia, bleeding, and ulceration.17,36 Renal dysfunction can be seen with NSAIDs secondary to decreased prostaglandin production.7 This can result in a lower glomerular filtration rate, particularly in a state of dehydration.7
Ketorolac is indicated for the treatment of moderate to severe pain.12 Standard dosing (30 mg, IM) is comparable to 12 mg of IM morphine with a longer duration.12 Veenema et al found a single 60-mg IM ketorolac dose comparable in analgesic efficacy to a single IM dose of meperidine (1 mg/kg) in patients with severe musculoskeletal low back pain.33 However, Turturro et al found that a single 60-mg IM ketorolac dose had no greater analgesic efficacy than 800 mg of oral ibuprofen in patients with acute, traumatic musculoskeletal pain.32
With its rapid onset of action and ease of administration in providing nonsedating pain relief, IM ketorolac use has become prevalent in the care of athletes. A 2000 survey of National Football League team physicians and trainers by Tokish et al revealed that 28 teams (93%) administered Toradol to an average of 15 players per team, over the course of a 16-game season.31 The Charleston Post and Courier reported that the South Carolina football medical staff administered 169 Toradol injections to players on game days during the 13 games of the 2008 season.25
With IM ketorolac injections becoming a mainstream treatment option for athletes, our goal was to determine the prevalence, indications, administration patterns, and perceived efficacy of ketorolac at all competitive levels.
Materials and Methods
A 19-question survey was designed to explore the use of IM ketorolac as a treatment modality for athletes with musculoskeletal sports-related injuries (Table 1). This epidemiologic survey was generated online through SurveyMonkey.com and distributed to physician members of the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, and the American Medical Society for Sports Medicine. E-mail lists were obtained from the membership directories of each society and included all current international and national members as of January, 2011. Each e-mail address for the Arthroscopy Association of North America and American Orthopaedic Society for Sports Medicine was logged individually onto a spreadsheet prior to being transferred into the SurveyMonkey.com database established for this project. The American Medical Society for Sports Medicine e-mailed its members directly with the survey link. The survey was emailed to approximately 4750 orthopaedic surgeons and 2200 primary care sports medicine physicians at the end of April 2011, with reminders sent out every 1 to 2 weeks.
Table 1.
Intramuscular ketorolac injections in the athlete questionnaire.
| 1. Which specialty do you practice? |
| a) Orthopaedic Surgery b) Primary Care-Sports Medicine c) Other___________________ |
| 2. Are you involved in the direct care of competitive athletes, in either a team or individual competition environment? |
| a) Yes b) No |
| If answered Yes to Question #2, please continue to Question #3. If No to Question #2, survey is complete. |
| 3. Do you use IM Toradol in the treatment of athletes? |
| a) Yes b) No |
| If answered Yes to Question #3, please continue to Question #4. If No to Question #3, skip to #19. |
| 4. For which level athlete do you use IM Toradol? (Multiple answers allowed) |
| a) Professional b) Collegiate c) High School |
| 5. For which sports do you use IM Toradol? (Multiple answers allowed) |
| a) Baseball b) Basketball c) Football d) Ice Hockey e) Lacrosse f) Soccer g) Other ____________________ |
| 6. If you care for female athletes, do you use IM Toradol in their treatment? |
| a) Yes b) No c) Not applicable |
| 7. How long have you administered IM Toradol? |
| a) <1 year b) 1-5 years c) 5-10 years d) >10 years |
| 8. Please rate the frequency of which you administer IM Toradol. |
| a) Almost never b) Seldom c) Sometimes d) Often |
| 9. With what frequency do you feel comfortable administering IM Toradol to a single athlete during the competitive season? |
| a) Once daily b) Once weekly c) Once bi-weekly d) Once monthly e) Once annually |
| 10. Is there a minimum age below which you would not administer IM Toradol to an athlete? |
| a) 15 b) 20 c) 25 d) >25 e) No minimum age |
| 11. Is there a maximum age above which you would not administer IM Toradol to an athlete? |
| a) 30 b) 40 c) 50 d) >50 e) No maximum age |
| 12. What are your indications for IM Toradol use in athletes? (Multiple answers allowed) |
| a) Postinjury pain b) Postinjury swelling c) Accelerated recovery d) Postsurgical pain e) Postsurgical swelling f) Other______ |
| 13. What are your indications for administering IM Toradol to athletes? (Multiple answers allowed) |
| a) Improved function b) Decreased pain c) Decreased swelling d) Accelerated return to activities e) Other_______ |
| 14. How long does the above benefit last from a single injection? |
| a) <24 hours b) 24-48 hours c) 48-72 hours d) >72 hours |
| 15. Have your athletes suffered any adverse reactions from IM Toradol use? (Multiple answers allowed) |
| a) No complications b) Local skin reaction c) Bleeding d) Infection e) Kidney problems f) Systemic reactions e) Other ______________________ |
| 16. When do you typically administer IM Toradol? |
| a) >24 hours prior to athletic event b) 12-24 hours prior to athletic event c) 6-12 hours prior to athletic event d) <6 hours prior to athletic event e) During athletic event f) Postathletic event |
| 17. Into what anatomic area do you administer IM Toradol? |
| a) Thigh b) Buttock c) Shoulder d) Other ____________________ |
| 18. What dose do you most commonly administer? |
| a) 15 mg b) 30 mg c) 45 mg d) 60 mg e) Other _______________________ |
| 19. Please indicate the degree to which the following factors contribute to your decision to NOT administer IM Toradol to athletes? (1- Not Relevant, 2-Somewhat Relevant, 3-Very Relevant, 4-Most Relevant) |
| a) Lack of efficacy 1 2 3 4 |
| b) Fear of bleeding complications 1 2 3 4 |
| c) Fear of renal complications 1 2 3 4 |
| d) Fear of malpractice1 2 3 4 |
Survey responses were collected during an approximately 6-week period from April to June 2011. Data were collected and entered onto a spreadsheet, with descriptive results subsequently generated. This study was approved by the Institutional Review Board.
Results
The total number of respondents was 1100 (60% orthopaedics, 40% primary care–sports medicine). The response rate for orthopaedic surgeons was 13.6%, while primary care–sports medicine was 20.1%. Ninety-four percent of the respondents are involved in the direct care of athletes, and 48.9% use IM ketorolac in the treatment of athletes. Of the nonsurgical sports medicine physicians, 61.2% used IM ketorolac, while only 40.6% of the orthopaedic surgeons used IM ketorolac.
The most frequently recognized reasons for not using IM ketorolac in the care of athletes was fear of renal and bleeding complications. Lack of efficacy and fear of malpractice were less relevant in the decision not to administer ketorolac (Table 2).
Table 2.
Degree to which the following choices contributed to the team physicians’ decision not to administer ketorolac to athletes (in percentages).
| Not | Somewhat | Very | Most | |
|---|---|---|---|---|
| Relevant | Relevant | Relevant | Relevant | |
| Lack of efficacy | 50.3 | 26.8 | 16.8 | 6.1 |
| Fear of bleeding complications | 32.3 | 42.3 | 20.6 | 4.8 |
| Fear of renal complications | 23.1 | 44.3 | 25.0 | 7.7 |
| Fear of malpractice | 50.1 | 34.5 | 11.5 | 3.9 |
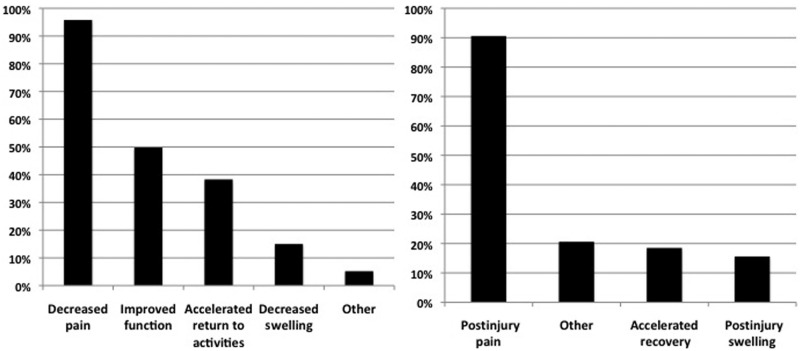
Seventy-nine percent of respondents used IM ketorolac in the collegiate population and 42.9% at the professional level. Football (87.7%), basketball (41.8%), and soccer (30.5%) are the sports in which IM ketorolac is most frequently used. Seventy-one percent of physicians used IM ketorolac in male and female athletes, with 28.4% using it in male athletes only. Most physicians only used IM ketorolac injections once weekly (64.3%), and the most recognized minimum age of injections was 15 years old (53.7%), followed by 20 years old (32.9%). Postinjury pain (90.6%) was the most recognized indication for IM ketorolac use; 95.8% thought that its administration decreased pain effectively in athletes (Figure 1). Fifty percent of respondents believed that it improved function, while 38.3% thought it accelerated the return to activities (Figure 1). Each injection was perceived to be efficacious for less than 24 hours (72.3%), and it was most frequently administered less than 6 hours prior to the start of an athletic event (75.8%). The most frequently administered dose was 30 mg (53.9%), followed by 60 mg (37.6%). Eighty-two percent administered IM ketorolac into the buttock. Very few adverse reactions were recognized: local skin reaction (5%), bleeding (2.9%), and kidney problems (1.9%). Eighty-eight percent of respondents reported no adverse reactions (Table 3). The survey was not designed to allow respondents to elaborate on the severity of their recognized adverse reactions.
Figure 1.
Comparison of results for perceived efficacy of ketorolac use (left) and indications for ketorolac use (right). Multiple answers were allowed.
Table 3.
Complete results for questions with multiple-answer choices (in percentages).
| For which level athlete do you use IM Toradol? (multiple answers allowed) | Collegiate | 79.1 |
| Professional | 42.9 | |
| High School | 15.1 | |
| For which sports do you use IM Toradol? (multiple answers allowed) | Football | 87.7 |
| Basketball | 41.8 | |
| Soccer | 30.5 | |
| Baseball | 25.5 | |
| Ice Hockey | 19.7 | |
| Other | 17.8 | |
| Lacrosse | 13.0 | |
| For which type of athlete do you consider using IM Toradol? | Males/Females | 71.2 |
| Males Only | 28.4 | |
| Females Only | 0.4 | |
| How long have you administered IM Toradol? | 1-5 years | 49.2 |
| 5-10 years | 27.6 | |
| >10 years | 14.1 | |
| <1 year | 9.1 | |
| Please rate the frequency of which you administer IM Toradol to all athletes in a single year | 5-10x/y | 29.3 |
| <5x/y | 25.4 | |
| >20x/y | 23.1 | |
| 10-20x/y | 22.2 | |
| With what frequency do you feel comfortable administering IM Toradol to a single athlete? | Once weekly | 64.3 |
| Once monthly | 12.4 | |
| Once daily | 10.5 | |
| Once biweekly | 8.6 | |
| Once annually | 4 | |
| Is there a minimum age below which you would not administer IM Toradol? | 15 | 53.7 |
| 20 | 32.9 | |
| No Minimum | 12.7 | |
| Is there a maximum age above which you would not administer IM Toradol? | No Maximum | 56.7 |
| 40 | 15.1 | |
| >50 | 11.4 | |
| 50 | 9.9 | |
| 30 | 6.9 | |
| What are your indications for IM Toradol use in athletes? (multiple answers allowed) | Post-injury pain | 90.6 |
| Other | 20.6 | |
| Accelerated Recovery | 18.5 | |
| Postinjury swelling | 15.6 | |
| What is your perceived efficacy of IM Toradol in athletes? | Decreased pain | 95.8 |
| Improved function | 49.8 | |
| Accelerated return to activities | 38.3 | |
| Decreased swelling | 15 | |
| Other | 5.2 | |
| How long do you perceive benefit from single injection? | <24 h | 72.3 |
| 24-48 h | 22.6 | |
| 48-72 h | 3.8 | |
| >72 h | 1.3 | |
| Have your athletes suffered any adverse reactions from IM Toradol? | None | 88.1 |
| Local skin reaction | 5.0 | |
| Other | 3.8 | |
| Bleeding | 2.9 | |
| Kidney problems | 1.9 | |
| Systemic Rxn | 0.4 | |
| Infection | 0 | |
| When do you administer IM Toradol? | <6 h prior to athletic event | 75.8 |
| Postathletic event | 12.6 | |
| During athletic event | 4.0 | |
| 6-12 h prior to athletic event | 2.5 | |
| 12-24 h prior to athletic event | 2.5 | |
| >24 h prior to athletic event | 2.5 | |
| Into what anatomic area do you administer IM Toradol? | Buttock | 81.9 |
| Shoulder | 13.5 | |
| Thigh | 2.5 | |
| Other | 2.1 | |
| What dose do you commonly administer? | 30 mg | 53.9 |
| 60 mg | 37.6 | |
| 15 mg | 6.2 | |
| 45 mg | 1.2 |
Discussion
The effect of ketorolac for the treatment of acute pain has been extensively evaluated over the past 20 years with varying conclusions.13,16,18,22,28,32 It serves as an alternative parenteral pain medication to opiates, which many patients cannot tolerate because of their side effect profile of sedation, constipation, urinary retention, and nausea/vomiting. Popp et al compared the effect of a combination of IM ketorolac and oral oxycodone with intravenous morphine in 90 postoperative anterior cruciate ligament reconstructions and found there to be no difference in pain control but a lower incidence of undesirable side effects in the combination therapy.22 Larkin et al concluded that 60 mg of IM ketorolac was significantly more effective at reducing acute renal colic than 100 to 150 mg of IM meperidine in the emergency room setting.13 Shrestha et al compared the analgesic effect of 60 mg of IM ketorolac to 50 mg of oral indomethacin in the treatment of acute gout attacks and found there to be no difference.28 Similarly, Turturro et al examined 82 emergency room patients with traumatic musculoskeletal pain secondary to fractures, sprains, strains, contusions, and dislocations. They randomized patients into 2 groups with the first receiving 60 mg of IM ketorolac and a placebo pill and with the second receiving 800 mg of oral ibuprofen and a placebo injection. There was no significant difference between the 2 groups for pain control over a 2-hour period.32 Neighbor and Puntillo found similar results in a comparison of IM ketorolac and oral ibuprofen in ER patients with moderate to severe pain.16 This study also identified that 40% of the patients had inadequate pain relief from either ketorolac or ibuprofen for moderate to severe pain.16 This lack of pain control was also described by Parke et al, who reported that ketorolac as a sole analgesic was inadequate for pain control for patients with moderate or severe pain following major orthopaedic surgery.18 In the population of physicians who completed this survey, 95.8% reported improved pain control in the athletes who receive IM ketorolac injections, while 50% reported improved athletic function.
The route of delivery of anti-inflammatory medications had previously been thought to play a role in patients’ perception of their pain control.27 A study by Schwartz et al revealed that route of medication administration played no role in pain control and contradicted the general belief that parenteral medications provide an additional placebo effect over oral medications.27
The most severe complications associated with the use of IM ketorolac are acute renal failure and increased risk of bleeding.4,8,9,20,29 However, when it is used appropriately, the risk of renal failure is no greater than other pain medications.8,13,28,32 Feldman et al reported that parenteral ketorolac, administered for 5 days or less, had the same rate of renal failure (1.1%) as parenteral opioids.8 Patient selection for NSAIDs is very important, as even a brief course of ibuprofen may result in acute renal failure in patients with asymptomatic, mild chronic renal failure.35 There are multiple documented cases of acute nephrotoxicity secondary to short-term IM ketorolac use; however, when it is used correctly in the appropriate patient population, the rate of acute renal failure is similar to most parenteral and oral pain medications.8,20,26,35 Patients should receive a maximum of 5 consecutive days of treatment, at a frequency no more than every 6 hours, and no more than 150 mg total on the first day and 120 mg on any subsequent days.8,12 Of the physicians who completed this survey, 1.9% reported renal complications in athletes following IM ketorolac administration. The survey did not ask respondents to specify whether these complications were mild or severe, but they may have been severe since they were reported. In the decision to not use ketorolac in the care of athletes, 44.3% of physicians felt that fear of renal complications was “somewhat relevant,” and 25% felt that it was “very relevant,” suggesting that the potential for renal complications is a limiting factor in ketorolac use.
The antiplatelet effect of NSAID medications has been well documented in the literature.5,23 As a member of this class of medications, ketorolac shares these same characteristics and bleeding risk. In a study of 20 young adults by Singer et al, bleeding time increased 50% after a single IM dose of 60 mg of ketorolac.29 There have been conflicting studies in the otolaryngology literature with regard to an increased risk of post-operative hemorrhage following tonsillectomy with the use of parenteral ketorolac.1,9 Agrawal et al identified no significant increased bleeding risk with ketorolac use, while Gallagher et al found a 5-time increased bleeding risk.1,9 For gastrointestinal bleeding, Strom et al found a small association between ketorolac and gastrointestinal bleeding (odds ratio, 1.3), especially when the medication was administered for more than 5 days, dosing greater than 105 mg daily, or in patients aged 65 years and older.30 In patients younger than 65 years, using the drug at most 5 days, with dosing at most 105 mg daily, the odds ratio for gastrointestinal bleeding was 1.03.30 Pediatric patients show no significant increased bleeding risk with ketorolac in postoperative patients.6,11,34 Of the physicians who completed this survey, 2.9% reported bleeding complications in athletes following IM ketorolac administration. The survey did not ask respondents to specify whether these bleeding complications were mild or severe.
The use of IM ketorolac in the treatment of athletes is a controversial topic. With known efficacy and proven side effects, it is being used by approximately half of our survey population. As expected for a controversial topic, there were some very insightful comments made by the physician respondents in this survey. Strong concerns were expressed regarding the medical-legal implications of supplying athletes with a medication that masks pain and allows them to continue to participate. If an athlete is unable to perform without the ketorolac injection, then the physician may be doing harm by providing a medication that would mask the pain by allowing the athlete to continue activity and jeopardizing long-term health. Some physicians indicated that they do not administer IM ketorolac to their own athletes but are willing to perform the injection on a visiting team athlete, if requested by the player and team staff. This provides an interesting dilemma for team physicians.
The primary limitation of this study is the relatively low response rate to the emailed questionnaire. The overall response rate of approximately 15.8% (1100 of 6950) is low and therefore not an adequate representation of the sports medicine physician. The standard goal in survey research is 50%, to lower the chance of the “nonresponse” bias.2 However, the total of 1100 respondents is a significant number and is the largest survey on the use of IM ketorolac in athletes. The response rate for the survey could be low for multiple reasons. The survey was e-mailed to approximately 6950 individuals who are members of the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, and the American Medical Society for Sports Medicine. However, not all of these individuals are physicians and not all participate in the care of athletes. Thus, after reading the introductory e-mail, this population may have chosen not to participate in the survey.
Conclusions
In the physician group that responded to this survey, IM ketorolac injections were used by approximately 49% in the care of athletes. These team physicians noted a high rate of improved pain control and a low incidence of adverse reactions; however, some of these may have been severe. The most frequently cited reasons for not administering ketorolac include fear of renal failure and bleeding complications.
References
- 1. Agrawal A, Roberts Gerson C, Seligman I, Dsida RM. Postoperative hemorrhage after tonsillectomy: use of ketorolac tromethamine. Otolaryngol Head Neck Surg. 1999;120:335-339 [DOI] [PubMed] [Google Scholar]
- 2. Alderman AK, Salem B. Survey research. Plast Reconstr Surg. 2010;126(4):1381-1388 [DOI] [PubMed] [Google Scholar]
- 3. Concannon MJ, Meng L, Welsh CF, Puckett CL. Inhibition of perioperative platelet aggregation using toradol (ketorolac). Ann Plast Surg. 1993;3:264-266 [DOI] [PubMed] [Google Scholar]
- 4. Dietzel DP, Hedlund EC. Injections and return to play. Curr Pain Headache Rep. 2005;9(11):11-16 [DOI] [PubMed] [Google Scholar]
- 5. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6)(suppl):299S-339S [DOI] [PubMed] [Google Scholar]
- 6. Eberson CP, Pacicca DM, Ehrlich MG. The role of ketorolac in decreasing length of stay and narcotic complications in the postoperative pediatric orthopaedic patient. J Pediatr Orthop. 1999;19:688-692 [PubMed] [Google Scholar]
- 7. Farquhar WB, Morgan AL, Zambraski EJ, Kenney WL. Effects of acetaminophen and ibuprofen on renal function in the stressed kidney. J Appl Physiol. 1999;86:598-604 [DOI] [PubMed] [Google Scholar]
- 8. Feldman HI, Kinman JL, Berlin JA, et al. Parenteral ketorolac: the risk for acute renal failure. Ann Intern Med. 1997;126:193-199 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher JE, Blauth J, Fornadley JA. Perioperative ketorolac tromethamine and postoperative hemorrhage in cases of tonsillectomy and adenoidectomy. Laryngoscope. 1995;105:606-609 [DOI] [PubMed] [Google Scholar]
- 10. Intravenous ibuprofen (Caldolor) Med Lett Drugs Ther. 2010;52:3-4 [PubMed] [Google Scholar]
- 11. Kay RM, Directo MP, Leathers M, Myung K, Skaggs DL. Complications of ketorolac use in children undergoing operative fracture care. J Pediatr Orthop. 2010;30(7):655-658 [DOI] [PubMed] [Google Scholar]
- 12. Ketorolac tromethamine Med Lett Drugs Ther. 1990;32:79-81 [PubMed] [Google Scholar]
- 13. Larkin GL, Peacock WF, Pearl SM, Blair GA, D’Amico F. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med. 1999;17:6-10 [DOI] [PubMed] [Google Scholar]
- 14. Leadbetter WB. Anti-inflammatory therapy in sports injury: the role of nonsteroidal drugs and corticosteroid injection. Clin Sports Med. 1995;14(2):353-410 [PubMed] [Google Scholar]
- 15. Mehallo CJ, Drezner JA, Bytomski JR. Practical management: nonsteroidal anti-inflammatory drug (NSAID) use in athletic injuries. Clin J Sport Med. 2006;16(2):170-174 [DOI] [PubMed] [Google Scholar]
- 16. Neighbor ML, Puntillo KA. Intramuscular ketorolac vs oral ibuprofen in emergency department patients with acute pain. Acad Emerg Med. 1998;5:118-122 [DOI] [PubMed] [Google Scholar]
- 17. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1:396-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parke TJ, Millett S, Old S, Goodwin APL, Rice ASC. Ketorolac for early postoperative analgesia. J Clin Anesth. 1995;7:465-469 [DOI] [PubMed] [Google Scholar]
- 19.Pearsall AW, Kovaleski JE. Update on cox-2 inhibitors and non-selective NSAIDs: safety and patient risk. Sports Medicine Update. May-Jun 2007:2-7 [Google Scholar]
- 20. Perazella MA, Buller GK. NSAID nephrotoxicity revisited: acute renal failure due to parenteral ketorolac. South Med J. 1993;86(12):1421-1424 [DOI] [PubMed] [Google Scholar]
- 21. Pichard CP, LaPorte DM. Use of Ketorolac (toradol) in hand surgery. J Hand Surg Am. 2009;34:1549-1550 [DOI] [PubMed] [Google Scholar]
- 22. Popp JE, Sanko WA, Sinha AK, Kaeding CC. A comparison of ketorolac tromethamine/oxycodone versus patient-controlled analgesia with morphine in anterior cruciate ligament reconstruction patients. Arthroscopy. 1998;14(8):816-819 [DOI] [PubMed] [Google Scholar]
- 23. Risser A, Donovan D, Heintzman J, Page T. NSAID prescribing precautions. Am Fam Physician. 2009;80(12):1371-1378 [PubMed] [Google Scholar]
- 24. Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390-396 [DOI] [PubMed] [Google Scholar]
- 25. Sapakoff G. Painkiller injections common in college football. Charleston Post and Courier. October 4, 2009 [Google Scholar]
- 26. Schoch PH, Ranno A, North DS. Acute renal failure in an elderly woman following intramuscular ketorolac administration. Ann Pharmacother. 1992;26(10):1233-1236 [DOI] [PubMed] [Google Scholar]
- 27. Schwartz NA, Turturro MA, Istvan DJ, Larkin GL. Patients’ perceptions of route of nonsteroidal anti-inflammatory drug administration and its effect on analgesia. Acad Emerg Med. 2000;7(8):857-861 [DOI] [PubMed] [Google Scholar]
- 28. Shrestha M, Morgan DL, Moreden JM, Singh R, Nelson M, Hayes JE. Randomized double-blind comparison of the analgesic efficacy of intramuscular ketorolac and oral indomethacin in the treatment of acute gouty arthritis. Ann Emerg Med. 1995;26(6):682-686 [DOI] [PubMed] [Google Scholar]
- 29. Singer AJ, Mynser CJ, McMahon BJ. The effect of IM ketorolac tromethamine on bleeding time: a prospective, interventional, controlled study. Am J Emerg Med. 2003;21(5):441-443 [DOI] [PubMed] [Google Scholar]
- 30. Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding: a post marketing surveillance study. JAMA. 1996;275(5):376-382 [PubMed] [Google Scholar]
- 31. Tokish JM, Powell ET, Schlegel TF, Hawkins RJ. Ketorolac use in the National Football League: prevalence, efficacy, and adverse events. Phys Sportsmed. 2002;30(9):19-24 [DOI] [PubMed] [Google Scholar]
- 32. Turturro MA, Paris PM, Seaberg DC. Intramuscular ketorolac versus oral ibuprofen in acute musculoskeletal pain. Ann Emerg Med. 1995;26(2):117-120 [DOI] [PubMed] [Google Scholar]
- 33. Veenema KR, Leahey N, Schneider S. Ketorolac versus meperidine: ED treatment of severe musculoskeletal low back pain. Am J Emerg Med. 2000;18(4):404-407 [DOI] [PubMed] [Google Scholar]
- 34. Vitale MG, Choe JC, Hwang MW, et al. Use of ketorolac tromethamine in children undergoing scoliosis surgery: an analysis of complications. Spine J. 2003;3:55-62 [DOI] [PubMed] [Google Scholar]
- 35. Whelton A, Stout RL, Spilman PS, Klassen DK. Renal effects of ibuprofen, piroxicam, and sulindac in patients with asymptomatic renal failure. Ann Intern Med. 1990;112:568-576 [DOI] [PubMed] [Google Scholar]
- 36. Yarboro TL. Intramuscular ketorolac, gastrointestinal bleeding, and peptic ulcer perforation: a case report. J Natl Med Assoc. 1995;87(3): 225-227 [PMC free article] [PubMed] [Google Scholar]