Abstract
Background:
The relationship between biological tissue healing following knee injury or surgery and long-term clinical outcome has come to the forefront of sports medicine practice. This has led many knee surgeons to incorporate biologically mediated healing factors into the management of knee injuries. In particular, the clinical use of mesenchymal stem cells has opened new horizons.
Evidence Acquisition:
Relevant studies were identified through a search of PubMed from January 2000 to April 2011, combining the term mesenchymal stem cells with articular cartilage, anterior cruciate ligament, and meniscus. Relevant citations from the reference lists of selected studies were also reviewed.
Results:
Knee injury treatment with mesenchymal stem cells shows potential. Most reports represent animal model studies; few advances have been translated to human clinical applications.
Conclusion:
Mesenchymal stem cell use to promote healing following knee injury is likely to increase. There are scientific methodological concerns and ethical and legal issues regarding mesenchymal stem cell use for treating knee injuries.
Keywords: biological tissue healing, cell-based therapy, cartilage repair
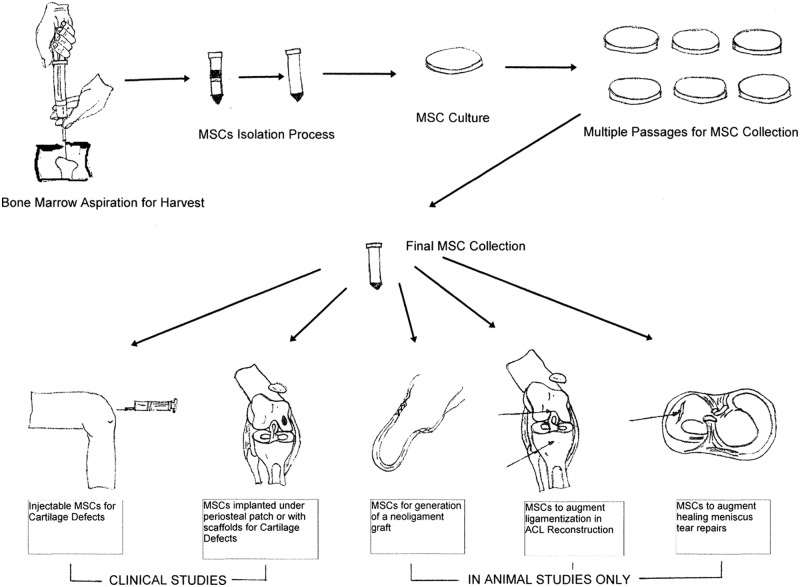
Many patients who have sustained knee injuries desire treatment options that will enable them to continue sports participation. Over the past several years, the sports medicine community has seen an increase in the use of biological agents, including cell-based therapies for this purpose.78 The introduction of stem cells, specifically mesenchymal stem cells (MSCs), into the clinical setting has opened new knee treatment horizons (Figure 1).12
Figure 1.
Mesenchymal stem cell (MSC) harvest, isolation, and culturing. Clinical studies have been performed with MSC injections under a periosteal patch or in combination with a scaffold to treat articular cartilage defects. Intra-articular MSC injections have also been performed clinically to treat knee articular cartilage defects. MSC use to create neoligaments, to augment graft ligamentization following anterior cruciate ligament reconstruction, and to enhance meniscal repair healing has been performed using only animal models.
What are mscs?
Stem cells harvested from human embryos and adult tissues have the capacity for self-renewal.52,76 The utility of adult stem cells is generally restricted to the generation of more of the same tissue from which it was harvested, such as hematopoetic stem cells in blood.9 However, under certain conditions, some adult stem cells, such as those harvested from mesenchymal tissues, can differentiate into multilineages and become multipotent. MSCs are characterised by surface-specific antigen and the absence of hematopoetic stem cell markers, such as leukocyte common antigen.15Friedenstein et al26 demonstrated that bone marrow cells could differentiate into bone and cartilage. Subsequent research found that MSCs harvested from bone marrow could differentiate into bone, cartilage, tendon, ligament, fat, and other tissues of mesenchymal origin.42,68 MSCs can also be harvested from synovium,16,18 periosteum,27 skeletal muscle,1 adipose tissue,21,89,96 trabecular bone,71 and umbilical cord blood.54
Mesenchymal Stem Cells for Articular Cartilage Repair
Chondrogenesis
One of the primary concerns in articular cartilage repair is the integration of engineered calcified articular cartilage with underlying bone. MSC-mediated chondrogenesis may improve this integration.42-48,93 Since Ashton et al5 first reported MSC-mediated chondrogenesis, others have investigated chondrogenic potential when derived from tissues as diverse as human adipose19 and trabecular bone,71 as well as rat bone marrow, synovium, periosteum, adipose, and muscle tissues.11,94 Bone marrow–derived MSCs may allow better differentiation of the deep calcified articular cartilage zone adjacent to healthy bone.23,62,80 MSCs harvested from bone marrow have been the primary source in most animal model and human chondrogenesis research studies.13,14,33 MSCs derived from rat synovium may have greater proliferation and chondrogenesis potential than rat bone marrow, periosteum, adipose, or muscle sources. In humans, bone marrow has a high MSC density, providing similar culture expansion potential compared with other tissue sources.61,72,94 A rabbit study by Koga et al47 reported that MSCs harvested from bone marrow and synovium had a greater potential to repair articular cartilage defects than cells from skeletal muscle and adipose tissue.37
Enhancing Articular Cartilage Repair
Cytokines and growth factors are important for chondrogenesis.30,38,59,90 The cytokines and growth factors that promote chondrogenic differentiation also contribute to osteogenic differentiation.25,59 The most potent chondrogenic differentiation inducers are transforming growth factor β, bone morphogenic protein, fibroblast growth factor, and insulin-like growth factor 1.38,57,64,90 The induction process is enhanced in the presence of dexamethasone.15
Chondrogenesis can be confirmed using alcian blue staining to detect articular cartilage-specific proteoglycans and by microscopy to identify chondrocytes and type II collagen. Additionally, molecular testing can detect MSC chondrogenic-specific transcription factor Sox 9 and β1 integrin.52 With autologous chondrocyte implantation (ACI), the chondrocyte density required for treatment is 106 cells per milliliter or slightly less when embedded in a scaffold gel.63 The cell density required to achieve chondrogenesis with MSCs is greater than that for ACI.46-48 Similar concentrations eventually failed in animal models, while 107 cells per milliliter embedded in a collagen gel successfully repaired articular cartilage defects.46-48 Yokoyama et al92 reported that a MSC density of 5 × 107 or 5 × 108 cells per milliliter embedded in a collagen gel had more proteoglycans than lower cell densities, better facilitating chondral defect healing and supporting the need to identify a MSC source with a high proliferation potential.47
Johnstone et al42 first described a 3-dimensional micromass culture medium of bone marrow–derived MSCs for in vitro chondrogenesis to investigate regulatory factors and signaling events. In vitro high-density micromass culture enhances cell-cell interaction, aggregating into a high-density precartilaginous core. The extracellular matrix that develops resembles those seen in vivo compared with single-layer methods.91 The culture medium used for in vitro MSC expansion and clinical use has traditionally been fetal bovine serum.35,89-92 However, disease transmission and immune reaction issues exist with fetal bovine serum use in human clinical trials.58,76 Studies by Tateishi et al81 and Nimura et al61 have shown that human serum culture medium has the potential to increase MSC proliferation without the disease transmission or immune reaction risk of an animal source.
Implantation Methods for Articular Cartilage Repair
The method used to deliver MSCs to a chondral defect is an important consideration. The goal is to create a 3-dimensional environment that optimizes cell proliferation and differentiation.16 First-generation MSCs for chondral repair involved direct implantation under a periosteal patch, like early ACI procedures.60 Second-generation techniques differentiate MSCs in vitro within a matrix or bioscaffold and implant the construct into a chondral defect at cellular maturity.91 This approach does not mimic natural articular cartilage formation.6,7,36,79 MSCs differentiated in vitro and transplanted subcutaneously fail to produce articular cartilage or become calicified.16,18,66 Creation of an appropriate in vivo microenvironment is essential for inducing articular cartilage formation of the desired phenotype when MSCs are used.16,66
A third-generation approach using a bioscaffold seeded with MSCs is similar to second-generation ACI techniques.40 Bioscaffolds can reduce cell leakage and complications from periosteal hypertrophy.20 MSCs can differentiate and adhere to scaffolds and matrices56,69,75 consisting of synthetic polymers, poly(L-lactide), poly(glycolide), poly(D,L-lactide-co-glycolide), alginate, or biomaterials such as collagen, fibrin, hydrogel, hyaluron, and chitosan.4,10,20,31,50,51,92 Scaffold-free MSC tissue-engineered constructs in porcine models and humans retain chondrogenic potential and display adequate mechanical properties without scaffold support.2,3 Intra-articular MSC injections have been investigated for treating chondral defects and knee osteoarthritis.53,74 In a porcine model, MSCs injected intra-articularly for medial femoral chondral defects resulted in better tissue morphology and histology scores compared with controls injected with saline or hyaluronic acid.53
Saw et al74 created 4-mm full-thickness chondral defects in goat stifle joints followed by microfracture. Group A did not receive intra-articular injections. Group B received weekly injections of 1 mL sodium hyaluronate for 3 weeks. Group C received the same injections as group B but with the addition of 2 mL of autologous bone marrow aspirate (carrying MSCs). At 24 weeks postsurgery, group C had a histologically superior articular cartilage repair.74 Electromagnetic fields may guide intra-articularly injected MSCs directly to the chondral defect.44
Articular Cartilage Repair: Human Clinical Studies
Although basic science MSC research is abundant, few studies have been translated directly into human clinical practice. Bone marrow MSCs were used to treat knee osteoarthritis in 24 patients with medial femoral condyle articular cartilage defects, including those treated previously with high tibial osteotomy and articular cartilage resurfacing.86 Patients were divided into 2 groups. Group 1 received MSCs suspended in a type I collagen gel scaffold covered with an autologous periosteal flap. Group 2 received a cell-free scaffold covered with an autologous periosteal flap.86 At 16 months, both groups had improved knee rating scores, and group differences were not evident. Nine of 12 patients in the MSC group (group 1) and 6 of 12 patients in the cell-free group (group 2) had second-look arthroscopy at a mean of 42 weeks postimplantation (range, 28-95). Histologic assessment revealed better scores for the MSC group. The main criticism of this study is that patients with preexisting knee osteoarthritis are generally not considered articular cartilage resurfacing candidates. Reports such as these have spurred additional MSC chondral defect studies.29,49,60,87,88 In 5 patients with isolated patellofemoral joint chondral defects treated with MSCs, decreased pain and improved walking ability at 6 months was reported lasting up to 4 years postsurgery.87,88 Clinical symptom improvement following bone marrow–derived MSC treatment of an isolated femoral chondral defect has been reported.49 At 6 and 12 months following bone marrow–derived MSCs delivered on a platelet-rich fibrin glue scaffold for femoral condyle articular cartilage defects, 5 patients had decreased knee pain and improved function.29 Two patients underwent second-look arthroscopy at 12 months postsurgery and had International Cartilage Repair Society evaluation scores of 8 and 11 (of 12; ie, nearly normal). Magnetic resonance imaging at 12 months postsurgery in 3 patients showed complete chondral defect filling and restored surface congruity. Two patients had incomplete chondral surface congruity; however, both had significantly decreased knee pain.
Despite these good results at 1 year postsurgery, it is unknown how they would compare with ACI or microfracture. A matched patient cohort of a MSC-derived chondrocyte group implanted with a periosteal patch (n = 36) were compared with an ACI group (n = 36) for the treatment of patella, trochlea, and femoral condyle chondral defects.60 Both groups had improved scores at 3, 6, 9, 12, 18, and 24 months postsurgery. MSCs cultured in human serum may provide cell proliferation and chondrogenesis that surpasses ACI articular cartilage–resurfacing capability.81 At 1 week after arthroscopic subchondral drilling of grade III and IV knee chondral lesions, 180 patients received a series of 5 weekly intra-articular injections (autologous peripheral blood-derived stem cells and hyaluronic acid).73,74 Five patients from this series underwent second-look arthroscopy between 10 and 26 months postsurgery. Histologic testing of the chondral core confirmed articular cartilage regeneration and hyaline cartilage formation.73
Several reports have described chondral drilling followed by bone marrow type I/III collagen patch use.41,56,65 At 24 months postsurgery, the median International Knee Documentation Committee Subjective Knee Evaluation score of 30 improved to 83 (176.7% improvement); the Lysholm Knee Scale score of 54 improved to 98 (81.5% improvement); and magnetic resonance imaging revealed decreased chondral defect size.65 At 8 months, an equine model using bone marrow aspirate concentrate and thrombin for femoral trochlea chondral defects treated with microfracture showed superior chondral defect filling and improved integration with the adjacent articular cartilage based on magnetic resonance imaging and histologic evaluations compared with the control group that underwent microfracture alone.24
Potential Benefits of MSC Use Compared With ACI
The advantage of bone marrow MSCs compared with ACI would be that of eliminating the need to harvest articular cartilage as a treatment source. A comparison of ACI and MSCs for chondral defect repair in a rabbit model showed ACI articular cartilage degeneration within 36 weeks postsurgery, while MSC-derived articular cartilage remained intact.34
Martin and Buckwalter58 suggested that autologous chondrocytes harvested from older patients would produce inferior results. In a rat study, bone marrow MSCs of older rats produced less extracellular matrix.17,95 Patients < 45 years of age had superior clinical outcomes (International Cartilage Repair Society, Short Form–36, International Knee Documentation Committee, Lysholm, and Tegner) than patients > 45 years of age for the ACI.60 Age did not influence the clinical outcome of patients that received bone marrow–derived MSCs.
Concerns Regarding MSCS for Articular Cartilage Repair
There are 4 primary concerns regarding MSCs. First, MSC-derived chondrocytes may express hypertrophy-related genes, leading to cell death or calcification, followed by vascularization postimplantation.16 Second, the ultimate articular cartilage thickness is less than healthy tissue, and the tidemark may be violated.1,46-48,85-88 Third, MSCs may transform long after culturing (during the standard ex vivo expansion period, these transformations can be safely managed).8,70,82 Fourth, the mechanical integrity of the regenerated tissue is unknown.47,84 Based on mechanical tests, biochemical alterations may be needed before MSC chondrogenesis can produce results comparable with primary autologous chondrocytes.47,84
Stem Cells in Anterior Cruciate Ligament Reconstruction or Repair
Although MSC use for anterior cruciate ligament (ACL) reconstruction has been studied using animal models,43,55,77 we did not identify any human clinical studies. Surgically created partial ACL lesions in rat studies were treated with intra-articular stem cell injections to accelerate ACL healing.43 At 4 weeks postsurgery, the MSC group had superior histological scores and withstood greater mechanical loads at ultimate failure, compared with the control group.43 In a rabbit study, hamstring ACL autografts were coated with a fibrin glue laden with bone marrow–derived MSCs or with fibrin glue. The experimental graft withstood greater loads at failure.55 Similar results were obtained with fresh-frozen rabbit Achilles tendon allografts coated with bone marrow MSCs for ACL reconstruction.77
MSC might be used to create an ACL “neoligament”: The prerequisites are (1) a suitable cell source, (2) a biocompatible scaffold, and (3) a biomechanical environment that promotes safe healing and organized maturation.67 Adult fibroblasts retain many phenotypic qualities necessary for collagen synthesis but are relatively quiescent biologically and have limited potential for further differentiation.83 Comparison of goat bone marrow MSC, ACL, and skin fibroblasts found that bioscaffolds seeded with bone marrow–derived MSC had the highest DNA content and collagen production.83 In a rat study, MSC on a fascial wrap scaffold was compared with knotted poly-L-lactic-acid and poly-lactic-co-glycolic acid scaffolds.28 MSC–fascial wrap scaffold generated more type I and type III collagen essential to neoligament development. However, the increased collagen content did not translate into improved maximal tensile load or stiffness.
Msc Use for Augmenting Meniscus Repair
In a rat study, a surgically-created meniscal defect was treated with MSC embedded in fibrin glue, with fibrin glue alone, or was left untreated. At 12 weeks, an abundant extracellular matrix with meniscal-like tissue was observed in the MSC–fibrin glue group.39 In a porcine model, MSC and fibrin glue augmented meniscus suturing in avascular zone radial tears.22 When no fibrin or MSCs were used, healing did not occur. With MSCs and fibrin glue, 21 of 28 specimens (75%) had complete healing; 5 (17.9%) had incomplete healing; and 2 (7.1%) had no healing. With only fibrin glue augmentation, no healing occurred in 12 of 19 specimens (63.2%), and incomplete healing was observed in 7 (36.8%). Horie et al32 reported that synovium-derived MSC injected intra-articularly to heal massive meniscal tears adhered directly to the lesion and differentiated into meniscal cells.
Conclusions
Numerous animal model studies have been performed to determine the ideal MSC source, culture medium, and implantation technique. In the right combination and with timely growth factor availability, articular cartilage healing may occur with MSCs, producing histologic differentiation and biomechanical characteristics of healthy tissue. Unfortunately, few animal model MSC study advances have been translated into the human clinical setting.
References
- 1. Adachi N, Sato K, Usas A, et al. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920-1930 [PubMed] [Google Scholar]
- 2. Ando W, Tateishi K, Hart DA, et al. Cartilage repair using in vitro generated scaffold-free tissue-engineered construct derived from porcine mesenchymal stem cells. Biomaterials. 2007;28:5462-5470 [DOI] [PubMed] [Google Scholar]
- 3. Ando W, Tateishi K, Katakai D, et al. In vitro generation of a scaffold-free tissue engineered construct (TEC) derived from human synovial mesenchymal stem cells: Biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14:2041-2049 [DOI] [PubMed] [Google Scholar]
- 4. Andriano KP, Tabata Y, Ikada Y, Heller J. In vitro and in vivo comparison of bulk and surface hydrolysis in absorbable polymer scaffolds for tissue engineering. J Biomed Mater Res. 1999;48:602-612 [DOI] [PubMed] [Google Scholar]
- 5. Ashton BA, Allen TD, Howlett CR, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294-307 [PubMed] [Google Scholar]
- 6. Barry FP. Mesenchymal stem cell therapy in joint disease. In: Bock G, Goode J, eds. Tissue Engineering of Cartilage and Bone. Vol 249 West Sussex, England: John Wiley & Sons; 2002:86-102 [Google Scholar]
- 7. Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from the bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;286:189-200 [DOI] [PubMed] [Google Scholar]
- 8. Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142-9149 [DOI] [PubMed] [Google Scholar]
- 9. Bongso A, Lee EH. Stem cells: their definition, classification and sources. In: Bongso A, Lee EH. eds. Stems Cells: From Bench to Bedside. Singapore: World Scientific Publishing, 2005 [Google Scholar]
- 10. Brun P, Abatangelo G, Radice M, et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337-346 [DOI] [PubMed] [Google Scholar]
- 11. Cao B, Zheng B, Jankowski RJ, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640-646 [DOI] [PubMed] [Google Scholar]
- 12. Caplan AI. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;1:6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Wang C, Lu S, et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005;319:429-438 [DOI] [PubMed] [Google Scholar]
- 14. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841-7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M. Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol. 2007;128:507-520 [DOI] [PubMed] [Google Scholar]
- 16. De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142-150 [DOI] [PubMed] [Google Scholar]
- 17. De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85-95 [DOI] [PubMed] [Google Scholar]
- 18. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942 [DOI] [PubMed] [Google Scholar]
- 19. Dicker A, Le Blanc K, Astrom G, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283-290 [DOI] [PubMed] [Google Scholar]
- 20. Dickhut A, Gottwald E, Steck E, Heisel C, Richter W. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci. 2008;13:4517-4528 [DOI] [PubMed] [Google Scholar]
- 21. Dragoo JL, Samimi B, Zhu M, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740-747 [PubMed] [Google Scholar]
- 22. Dutton A, Choong PF, Goh JCH, Lee EH, Hui JH. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg Br. 2010;92:169-175 [DOI] [PubMed] [Google Scholar]
- 23. Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696-2706 [DOI] [PubMed] [Google Scholar]
- 24. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-1937 [DOI] [PubMed] [Google Scholar]
- 25. Freyria AM, Cortial D, Ronziere MC, Guerret S, Herbage D. Influence of medium composition, static and stirred conditions on the proliferation of and matrix protein expression of bovine articular chondrocytes cultures in a 3D collagen scaffold. Biomaterials. 2004;25:687-697 [DOI] [PubMed] [Google Scholar]
- 26. Friedenstein AJ, Piaetezky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390 [PubMed] [Google Scholar]
- 27. Fukumoto T, Sperling JW, Sanyal A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:55-64 [DOI] [PubMed] [Google Scholar]
- 28. Ge Z, Goh CHJ, Lee EH. The effects of bone marrow-derived mesenchymal stem cells and fascia wrap application to anterior cruciate ligament tissue engineering. Cell Transplant. 2005;14:763-773 [DOI] [PubMed] [Google Scholar]
- 29. Haleem AM, El Singergy AA, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: A pilot study and preliminary results. Cartilage. 2010;1:253-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heng BC, Cao T, Lee EH. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152-1167 [DOI] [PubMed] [Google Scholar]
- 31. Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64:441-445 [DOI] [PubMed] [Google Scholar]
- 32. Horie M, Sekiya I, Muneta T, et al. Intra-articular injected stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilisation to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878-887 [DOI] [PubMed] [Google Scholar]
- 33. Huang JI, Kazmi N, Durbhaskula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383-1389 [DOI] [PubMed] [Google Scholar]
- 34. Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatric Orthop. 2004;24:427-433 [DOI] [PubMed] [Google Scholar]
- 35. Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721-733 [DOI] [PubMed] [Google Scholar]
- 36. Ichinose S, Yamagata K, Sekiya I, Muneta T, Tagami M. Detailed examination of cartilage formation and endochondral ossification using human mesenchymal stem cells. Clin Exp Pharmacol Physiol. 2005;32:561-570 [DOI] [PubMed] [Google Scholar]
- 37. Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001;83:289-294 [DOI] [PubMed] [Google Scholar]
- 38. Iwasaki M, Nakata K, Nakahara H, et al. Transforming growth factor beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132:1603-1608 [DOI] [PubMed] [Google Scholar]
- 39. Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscus repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217-223 [DOI] [PubMed] [Google Scholar]
- 40. Jackson DW, Simon TM. Tissue engineering principles in orthopaedic surgery. Clin Orthop Relat Res. 1999;367:S31-S45 [DOI] [PubMed] [Google Scholar]
- 41. Jakob RP. AMIC technique for cartilage repair: a single-step surgical intervention as compared to other methods. Eur Cell Mater. 2006;12(suppl 1):2616941384 [Google Scholar]
- 42. Johnstone B, Hering TM, Caplan AL, Goldberg VM, Joo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265-272 [DOI] [PubMed] [Google Scholar]
- 43. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23:610-617 [DOI] [PubMed] [Google Scholar]
- 44. Kobayashi T, Ochi M, Yanada S, et al. A novel cell delivery system using magnetically labelled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24:69-76 [DOI] [PubMed] [Google Scholar]
- 45. Kobayashi T, Watanabe H, Yanagawa T, et al. Motility and growth of human bone-marrow mesenchymal stem cells during ex vivo expansion in autologous serum. J Bone Joint Surg Br. 2005;87:1426-1433 [DOI] [PubMed] [Google Scholar]
- 46. Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1289-1297 [DOI] [PubMed] [Google Scholar]
- 47. Koga H, Muneta T, Nagase T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207-215 [DOI] [PubMed] [Google Scholar]
- 48. Koga H, Shimaya M, Muneta T, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226-231 [DOI] [PubMed] [Google Scholar]
- 50. Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586-595 [DOI] [PubMed] [Google Scholar]
- 51. Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1-22 [DOI] [PubMed] [Google Scholar]
- 52. Lee EH, Hui JH. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006;88:841-851 [DOI] [PubMed] [Google Scholar]
- 53. Lee KBL, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects: a porcine model. Stem Cells. 2007;25:2964-2971 [DOI] [PubMed] [Google Scholar]
- 54. Lee OK, Kuo TK, Chen WM, Lee KD, Hseih SL, Chen TM. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675 [DOI] [PubMed] [Google Scholar]
- 55. Lim JK, Hui J, Li L, et al. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899-910 [DOI] [PubMed] [Google Scholar]
- 56. Loken S, Jakobsen RB, Aroen A, Thambyah A, Goh J, Lee EH. Bone marrow mesenchymal stem cells in a hyaluron scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2008;16:896-903 [DOI] [PubMed] [Google Scholar]
- 57. Makower AM, Wroblewski J, Pawlowski A. Effects of IGF-I, rGH, FGF, EGF and NCS on DNA-synthesis, cell proliferation and morphology of chondrocytes isolated from rat rib growth cartilage. Cell Biol Int Rep. 1989;13:259-270 [DOI] [PubMed] [Google Scholar]
- 58. Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257-264 [DOI] [PubMed] [Google Scholar]
- 59. Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone stromal cells. Osteoarthritis Cartilage. 2001;9(suppl A):S36-S40 [DOI] [PubMed] [Google Scholar]
- 60. Nejadnik H, Hui J, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110-1116 [DOI] [PubMed] [Google Scholar]
- 61. Nimura A, Muneta T, Koga H, et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501-510 [DOI] [PubMed] [Google Scholar]
- 62. Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089-1097 [DOI] [PubMed] [Google Scholar]
- 63. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84:571-584 [DOI] [PubMed] [Google Scholar]
- 64. Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446-1465 [DOI] [PubMed] [Google Scholar]
- 65. Pascarella A, Ciatti R, Pascarella F, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18:509-513 [DOI] [PubMed] [Google Scholar]
- 66. Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254-3266 [DOI] [PubMed] [Google Scholar]
- 67. Petrigliano F, McAllister D, Wu B. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22:441-451 [DOI] [PubMed] [Google Scholar]
- 68. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147 [DOI] [PubMed] [Google Scholar]
- 69. Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246-255 [DOI] [PubMed] [Google Scholar]
- 70. Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035-3039 [DOI] [PubMed] [Google Scholar]
- 71. Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomaya K, Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from bone marrow aspirates. Blood. 2004;104:2728-2735 [DOI] [PubMed] [Google Scholar]
- 72. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529 [DOI] [PubMed] [Google Scholar]
- 73. Saw KY, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27:493-506 [DOI] [PubMed] [Google Scholar]
- 74. Saw KY, Hussin P, Loke SC, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391-1400 [DOI] [PubMed] [Google Scholar]
- 75. Solchaga LA, Yoo JU, Lundberg M, et al. Hyaluronan-based polymers in the treatment of osteochondral defects. J Orthop Res. 2000;18:773-780 [DOI] [PubMed] [Google Scholar]
- 76. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980-982 [DOI] [PubMed] [Google Scholar]
- 77. Soon MY, Hassan A, Hui JH, et al. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med. 2007;35:962-971 [DOI] [PubMed] [Google Scholar]
- 78. Starke C, Kopf S, Petersen W, Becker R. Meniscal repair. Arthroscopy. 2009;25:1033-1044 [DOI] [PubMed] [Google Scholar]
- 79. Steck E, Bettram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403-411 [DOI] [PubMed] [Google Scholar]
- 80. Steck E, Fischer J, Lorenz H, et al. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969-978 [DOI] [PubMed] [Google Scholar]
- 81. Tateishi K, Ando W, Higuchi C, et al. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical application. Cell Transplant. 2008;17:549-557 [DOI] [PubMed] [Google Scholar]
- 82. Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371-379 [DOI] [PubMed] [Google Scholar]
- 83. Van Ejik F, Saris DB, Riesle J, et al. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893-903 [DOI] [PubMed] [Google Scholar]
- 84. Vinardell T, Thorpe SD, Buckley CT, Kelly DJ. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng. 2009;37:2556-2565 [DOI] [PubMed] [Google Scholar]
- 85. Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579-592 [DOI] [PubMed] [Google Scholar]
- 86. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199-206 [DOI] [PubMed] [Google Scholar]
- 87. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595-600 [DOI] [PubMed] [Google Scholar]
- 88. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74-79 [DOI] [PubMed] [Google Scholar]
- 89. Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;412:196-212 [DOI] [PubMed] [Google Scholar]
- 90. Wozney JM. The bone morphogenetic protein family: multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci. 1998;106: 160-166 [DOI] [PubMed] [Google Scholar]
- 91. Yang JW, de Isla N, Huselstein C, et al. Evaluation of human MSCs cell cycle, viability and differentiation in micromass culture. Biorheology. 2006;43:489-496 [PubMed] [Google Scholar]
- 92. Yokoyama A, Sekiya I, Miyazaki K, Ichinose S, Hata Y, Muneta T. In vitro cartilage formation of composites of synovium-derived mesenchymal stem cells with collagen gel. Cell Tissue Res. 2005;332:289-298 [DOI] [PubMed] [Google Scholar]
- 93. Yoo JU, Barthel TS, Nishumura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745-1757 [DOI] [PubMed] [Google Scholar]
- 94. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue and muscle. Cell Tissue Res. 2007;327:449-462 [DOI] [PubMed] [Google Scholar]
- 95. Zheng H, Martin JA, Duwayri Y, Falcon G, Buckwalter JA. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol. 2007;62A:136-148 [DOI] [PubMed] [Google Scholar]
- 96. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295 [DOI] [PMC free article] [PubMed] [Google Scholar]