Abstract
Context:
Tendinopathy is increasing in prevalence and accounts for a substantial part of all sports injuries and occupational disorders. Despite the magnitude of the disorder, high-quality scientific data on etiology and available treatments have been limited.
Evidence Acquisition:
The authors conducted a MEDLINE search on tendinopathy, or “tendonitis” or “tendinosis” or “epicondylitis” or “jumpers knee” from 1980 to 2011. The emphasis was placed on updates on epidemiology, etiology, and recent patient-oriented Level 1 literature.
Results:
Repetitive exposure in combination with recently discovered intrinsic factors, such as genetic variants of matrix proteins, and metabolic disorders is a risk factor for the development of tendinopathy. Recent findings demonstrate that tendinosis is characterized by a fibrotic, failed healing response associated with pathological vessel and sensory nerve ingrowth. This aberrant sensory nerve sprouting may partly explain increased pain signaling and partly, by release of neuronal mediators, contribute to the fibrotic alterations observed in tendinopathy. The initial nonoperative treatment should involve eccentric exercise, which should be the cornerstone (basis) of treatment of tendinopathy. Eccentric training combined with extracorporeal shockwave treatment has in some reports shown higher success rates compared to any therapies alone. Injection therapies (cortisone, sclerosing agents, blood products including platelet-rich plasma) may have short-term effects but have no proven long-term treatment effects or meta-analyses to support them. For epicondylitis, cortisone injections have demonstrated poorer long-time results than conservative physiotherapy. Today surgery is less indicated because of successful conservative therapies. New minioperative procedures that, via the endoscope, remove pathologic tissue or abnormal neoinnervation demonstrate promising results but need confirmation by Level 1 studies.
Conclusions:
Novel targeted therapies are emerging, but multicenter trials are needed to confirm the results of exercise and mini-invasive treatments.
Keywords: tendon, pain, tendinopathy, tendinosis
Tendinopathy is a clinical syndrome, often but not always implying overuse tendon injuries, characterized by a combination of pain, diffuse or localized swelling, and impaired performance.39 Tendinopathy can also occur without signs of overuse and is then mostly associated with medical conditions. Midportion and insertional tendinopathy (enthesopathy) should be distinguished as 2 different clinical diagnoses. The tendons most vulnerable to overuse are the Achilles and patellar tendons and, in the upper extremities, the rotator cuff and extensor carpi radialis brevis (tennis elbow) tendons.80
The common pathological conditions associated with tendinopathy are tendinosis and peritendinitis. Tendinosis is the histopathological finding of collagen disorganization and fiber separation, increase in mucoid ground substance, hypercellularity, and nerve and vessel ingrowth but mostly without signs of intratendinous inflammation (tendinitis).54 However, lately, the noninflammatory etiology of tendinopathy has been questioned, as inflammation may play a role in the initial phase of the disease.9
Tendinosis is per se not painful. Thus, histopathological alterations associated with degeneration, such as tendinosis, not correlated to pain must be separated from pain generating pathophysiology. Tendinitis, however, which is seen to a much lesser extent (< 3%) is associated with classic inflammation usually observed during the early reparative phase.53 Peritendinitis is an acute or chronic inflammation of the thin membrane, paratenon, surrounding the tendon, often induced by repetitive exercise and characterized by local swelling and infiltration of inflammatory cells.53
The tendon insertion and bursae surrounding the tendon are common sites of classical inflammation, as a response to repetitive stress, because of their greater density of blood vessels and nerves. The tendon proper meanwhile is mostly aneuronal and avascular and does not under normal conditions exhibit classical inflammatory responses.3,54
Epidemiology
Sports
The incidence of tendinopathy is rising in the developed world because of increased participation in recreational sports.53,80 Around 30% of all runners exhibit Achilles tendinopathy, with an annual incidence of 7% to 9%.51 Repetitive exposure seems to be associated with increased risk of injury. Long-distance runners compared with age-matched controls exhibit an increased incidence of tendinopathy, with an adjusted odds ratio of 31.2.46
Patellar tendinopathy is common in volleyball (14%), team handball (13%), basketball (12%), and track and field (7%) and is a fairly common condition in football/soccer (2.5%).104 Among top-level football/soccer players, high total exposure hours has been identified as a significant risk factor for patellar tendinopathy.30 Former male athletes participating in maximal overhead maneuvers such as the tennis serve or a baseball pitcher’s throw show 4 times increased risk (odds ratio) of shoulder tendinopathy before the age of 45 years compared with controls.38
Diseases
Although sports activity is the most common source of tendinopathy, 1 of 3 patients with Achilles tendinopathy is not active in sports. In the upper extremities of nonsports participants, tendinopathy is even more common and mostly work related. However, some patients may develop tendinopathies without being exposed to repetitive tendon loading. Recently, metabolic disorders such as disturbed glucose metabolism and atherosclerosis have been identified as underlying factors in tendinopathy.77 Thus, obesity, hypertension, diabetes mellitus, hypercholesterolemia, and other metabolic factors have been clearly associated with increased incidence of tendinopathy.1,10,26,43,77 The consequences of metabolic factors in healthy and pathologic tendons are currently an area of investigation.
Furthermore, in Achilles tendinopathy, approximately 2% of the disorders can be attributed to inflammatory joint disorders, such as rheumatoid arthritis.36 Notably, in diabetes mellitus and inflammatory disorders, the peripheral nervous system has been identified as a common cause of connective tissue healing malfunction and homeostasis.13,71,74
Drugs
Certain drugs should be avoided, especially among active sports participants. Drug treatment for hypercholesterolemia with statins56 and quinolone antibiotics58,94 have been reported to increase the risk for tendinopathy. Low molecular weight heparin and immunosuppressive drugs such as cortisone (especially intratendinous) and cyclosporine may exert detrimental effects on tendon metabolism and repair and should be used cautiously.42,98
Genetics
A study of twins revealed a heritability of 40% for tendinopathy at the lateral epicondyle.31 Recently, the genetic sequence variants that encode for several tendon extracellular matrix proteins associated with tendinopathy have been identified. Gene variants within the tenascin-C, collagen type I, alpha 1 chain (COL5A1) and matrix metalloproteinase-3 genes correlate to tendinopathy.63,64,76 Patients with this syndrome are prone to multiple problems, including rotator cuff, epicondylalgia, carpal tunnel syndrome, and trigger finger.79 The precise role of these genes in the development of tendinopathy is still unclear.17
Etiology: Pathophysiology
Mechanical loading of tendon tissue is anabolic by upregulating collagen gene expression and increasing synthesis of collagen proteins. This peaks around 24 hours after exercise and remains elevated for up to 70 to 80 hours.32,62 However, exercise also results in degradation of collagen proteins, although the timing of this catabolic peak occurs earlier than the anabolic peak. This results in a net loss of collagen around the first 24 to 36 hours after training, followed by a net gain in collagen.54 Thus, a certain restitution time interval in between exercise bouts is critical for the tissue to adapt and to avoid a net catabolic situation (Figure 1).
Figure 1.
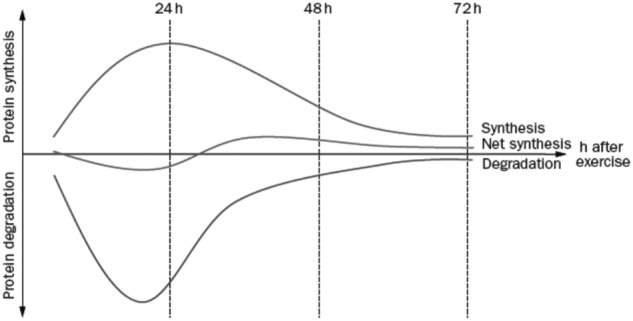
Diagram depicting collagen synthesis and degradation after acute exercise in humans. The first 24 to 36 hours after exercise results in a net loss of collagen. However, 36 to 72 hours after exercise, a net synthesis of collagen follows. Hence, repetitive training without enough resting time in between may result in a net catabolic situation with degradation of the matrix and lead to tendinopathy. Reproduced with permission from Magnusson et al.54
The tendon is able to adapt to load linked with the specific function of anatomic structures in and around the tendon—that is, the tendon cells, tenocytes, extracellular matrix, and nerve-ending receptors.
Repetitive strain causes tenocytes to produce inflammatory molecules and microruptures of collagen fibrils. Increased levels of inflammatory mediators (eg, prostaglandin E2 [PGE2]) are found in tendons after repetitive mechanical loading.100 Intratendinous injections of PGE2 cause intense degenerative changes, and peritendinous injections of PGE1 result in a histological pattern of tendinopathy.40,91 Today, several studies confirm a partly inflammatory background to tendinopathy with granulation alterations of capillary vessels and a significant inflammatory infiltrate consisting of macrophages, mast cells, B and T lymphocytes.60,86 These findings suggest a role for intrinsic immune pathways in the events that mediate early tendinopathy. Presumably, the inflammatory cells activate a cascade of proinflammatory cytokines (eg, IL-18, IL-15, and IL-6) found in tendinopathy.61
Tendon cells and fibroblasts, subjected to repetitive mechanical stress in combination with proinflammatory cytokines and transforming growth factor β (TGF-β) stimulation, can transform into myofibroblasts.93 Myofibroblasts are important cells for tendon healing, possibly also for tissue adaptation. After the healing process is completed and the mechanical stress is released on the myofibroblasts, these cells undergo programmed cell death (apoptosis).93 If this mechanism fails, the myofibroblasts will propagate a hyperproliferative process, fibrosis, seen as a prominent histological feature of tendinopathy (Figure 2).
Figure 2.
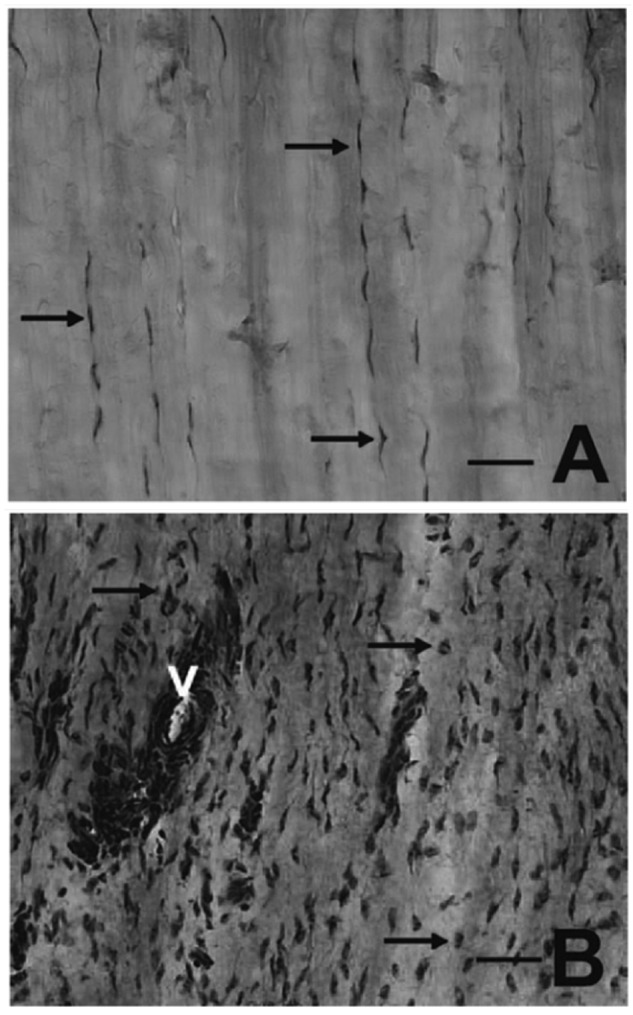
Patellar tendon of healthy control (A) and painful tendinopathy (B) after hematoxylin and eosin staining. Arrows denote tenocytes. The healthy tendon has homogeneous, organized parallel collagen structure and thin, elongated tenocytes (A). Tendinopathy, in contrast, is characterized by collagen disorganization, increased cell count, transformed tenocytes, and vascular ingrowth (V) in the tendon proper (B). Bar, 50 µm. Reproduced with permission from Lian et al.47
Another factor that may cause fibroblast hyperproliferation is hypoxia,23 which can upregulate matrix metalloproteinases, leading to altered material properties of the tendon.75 Hypoxia upregulates vascular endothelial growth factor, which increases microvessel ingrowth (angiogenesis) into the tendon—a major finding in tendinopathy.75
Angiogenesis has been speculated to be a causative factor for pain since sclerotherapy relieves pain in tendinopathy.69 However, Blood vessels per se are not painful, but ingrowth of sensory nerve fibers alongside of blood vessels can be painful (Figure 3).2,47,86 Healthy nonpainful tendons are almost aneuronal within the tendon proper.2,47 Chronic painful tendons, however, show ingrowth of sensory nerves from the paratenon with release of nociceptive substances. Restricting pathological nerve ingrowth by denervation (eg, mini-invasive surgery or release of the paratenon) can cause pain relief.97
Figure 3.
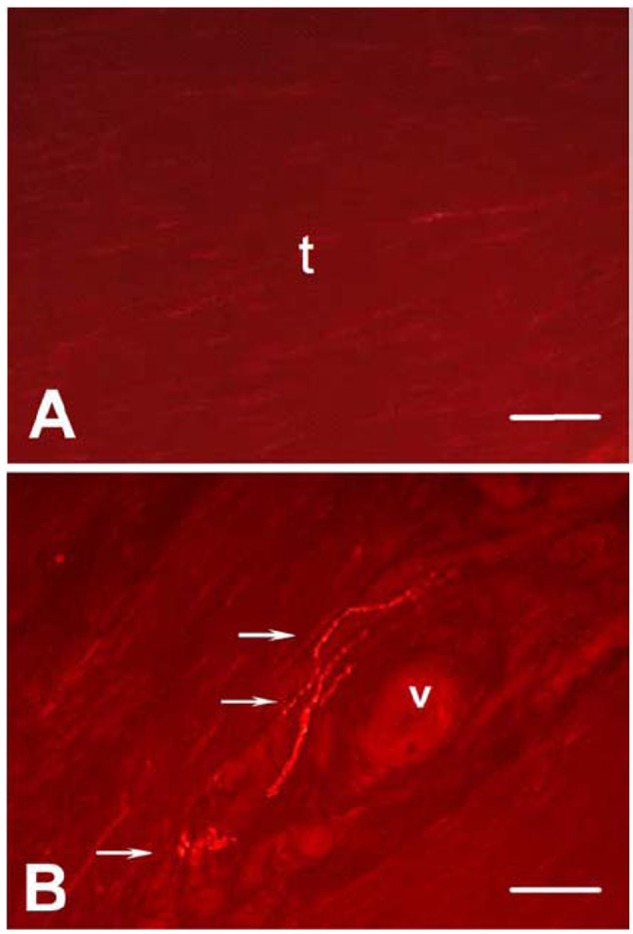
Achilles tendon of healthy control (A) and painful tendinopathy (B) after immunostaining for substance P (SP); picture taken with immunofluorescence microscopy. Arrows denote free nerve endings. The micrograph illustrates SP-positive nerve fibers in close vicinity to a proliferated vessel (B). v = blood vessel. Bar = 50 μm. Reproduced with permission from Ackermann et al.3
Interestingly, sensory nerve ingrowth in the tendon can be a reaction to repetitive loading and also a response to injury.59 In normal tendon repair, sensory nerve ingrowth is correlated with increased nociception, followed by autonomic nerve ingrowth, coinciding with decreased nociception and subsequent nerve retraction.2,3 In tendinopathy, the ingrown sensory nerves do not retract, as in normal healing. Thus, neuronal dysregulation in tendinopathy, characterized by aberrant sensory nerve sprouting may reflect a failed healing response (Figure 3), leading to increased pain signaling and possibly to the hyperproliferative changes associated with tendinopathy.47
In addition to pain transmission, peripheral nerve fibers react to mechanical stimuli and release several chemical substances, which are normally involved in healing and homeostasis but cause fibrosis during prolonged release.3 The presence of essential neuromediators in tendon was established more than 10 years ago3; recent studies have verified a diverse group of nerve mediators and receptors in tendon.3,87 Tendinopathic tendons exhibit increased sensory neuropeptide, substance P (SP),2,47,86 which may in addition to its role in nociception, reflect proinflammatory and trophic actions (Figure 3).3 SP regulates vasodilation, plasma extravasation, and release of cytokines by binding to its receptor, neurokinin 1, found in tendon and upregulated by loading.3,8,15,33,48,83 SP stimulates proliferation of fibroblasts and endothelial cells and possibly also transforms fibroblasts into myofibroblasts by increasing the production of TGF-β in fibroblasts.3,33 Hence, abnormal upregulation of SP may contribute to tendinosis (fibrosis), tenocyte transformation, hypercellularity, and hypervascularization observed in tendinopathic patients.
Other neuronal factors, highly upregulated in tendinopathy, are the neurotransmitter glutamate and its receptor, NMDAR1, which are implicated in various painful diseases.5,65,84 Furthermore, the localization of the increased glutamate signaling has just lately been established in tendinopathic patients, suggesting a pathological role.84 Thus, upregulated glutamate/NMDAR1 is observed in morphologically transformed tenocytes, in the endothelial and adventitial layers of neovessel walls, and in ingrown sprouting nerve fibers.84 Activated NMDA is seen in the tendon proper of tendinopathic biopsies but not in controls, suggesting a role in pathologic tenocyte transformation, neovessel formation, and pain signaling. These may be hypothetically mitigated by blocking of NMDAR1. Systematic investigation of the pathophysiological processes in tendinopathy may lead to novel and targeted therapies.
Nonoperative Treatment
The optimal management for tendinopathy is still debated. Nonoperative treatment is often effective and, compared with operative management, less costly and involving no risk of perioperative and postoperative complications. It is therefore still the first line of treatment.
Exercises
The conservative treatment with the best documentation for successful management of tendinopathy is eccentric exercises (Figure 4). In a prospective study of 200 patients with a 6-week once-daily eccentric loading regime, Stanish et al found that 44% of patients had complete relief of pain and 43% reported a marked improvement.89
Figure 4.
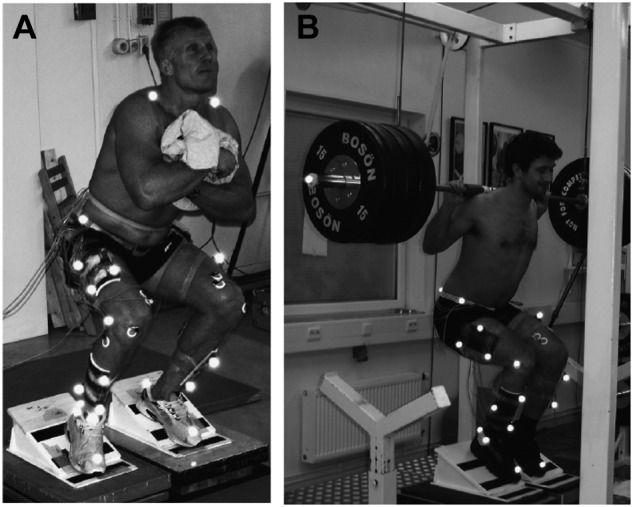
Eccentric squats exercises for the patellar tendon in an experimental setup for (A) free weight, decline board and (B) overload, decline board experiments. Reproduced with permission from Frohm et al.24-25
Several clinical studies investigating Achilles and patellar tendinopathy have verified a 40% to 60% good outcome after a home-based, twice-daily, 12-week regime of mainly eccentric training.7,28,41,44,50,99 Favorable outcomes after eccentric training are emerging on other tendinopathic locations, such as elbow and shoulder.11,55 Overall, the trend indicates positive effects of eccentric exercises without reported adverse effects.
Eccentric training produces around 20% more load on the tendon compared with concentric training.78 During eccentric work, the mean and peak patellar tendon force and angle at peak force were greater (25%-30%) for squats on a decline board compared with horizontal surface with free weight (Figure 4).24 Heavy slow eccentric overload strength training without decline board has also shown to be effective for patellar tendinopathy.25 A recent study also showed that eccentric training and static stretching exercises combined are superior to eccentric training alone to reduce pain and improve function in patients with patellar tendinopathy.21 Moreover, color Doppler sonography demonstrated decreased neovascularization following eccentric training intervention.68
Tendon loading, especially eccentrically, promotes collagen synthesis and collagen fiber cross-linking, thereby facilitating tendon remodeling (Figure 5A).28,54 Although the underlying mechanisms of successful eccentric exercises are not clear, the duration of such an exercise program is usually 3 months,99 the same time needed for the tendon to form new fibroblasts. Presumably, exercise stimulates the formation of new tendon cells, fibroblasts that are adapted to load.
Figure 5.
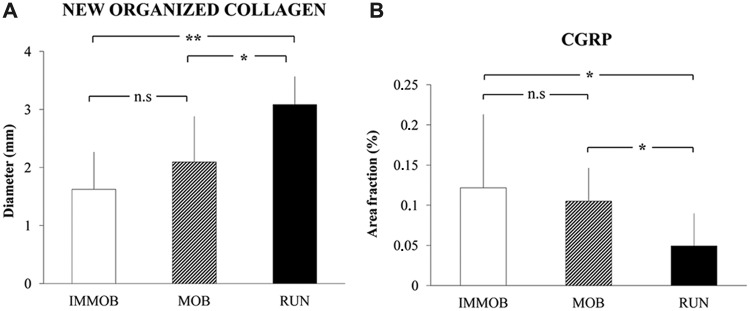
Achilles tendon subjected to 3 levels of physical activity during 4 weeks postrupture. Rats were post Achilles tendon rupture treated with a plaster cast (IMMOB), freely mobilized (MOB), or wheel-running (RUN). (A) Midtendon mediolateral diameter of new organized collagen in the healing area. (B) Area occupied by nerve fibers (%) immunoreactive to a sensory nerve marker, calcitonin gene related peptide (CGRP) in relation to total area, of the Achilles tendon proper. Increased physical activity seems to accelerate sensory nerve retraction from the tendon proper. Mean + SD. *P < 0.05; ns, P > 0.05. Reproduced with permission from Bring et al.14
Exercises exert mechanical effects on cells on nerve fibers and their receptors. Increased tendon training accelerates nerve retraction from the tendon proper, decreases load-induced pain, and modulates the expression of neuronal substances (Figure 5B).14,15 Thus, nerves may alter the chemical milieu in response to load, either by increased production and delivery of antinociceptive substances (eg, opioids) and their receptors or by decreased production of nociceptive substances (eg, substance P) and their receptors.3
Biophysical Procedures
In failed tendon healing (ie, tendinopathy), there is a lack of essential substances to initiate the repair process. There is a rationale to establish a new trauma to restart the healing process. Biophysical procedures, such as extracorporeal shockwave treatment (SWT) and low-intensity pulsed ultrasound (LIPUS), initiate tendon and tendon-to-bone healing.52 Thus, SWT and LIPUS have both demonstrated positive experimental effects on tendon healing; SWT selectively denervates sensory unmyelinated nerve fibers without affecting larger motor neurons.66 In symptomatic calcific tendinitis of the shoulder, LIPUS has helped resolve calcifications, followed by a short-term clinical improvement.22 However, in a double-blind study on patellar tendinopathy, LIPUS did not provide any additional benefit over placebo.101 SWT has shown encouraging clinical effects on tendinopathy.95 However, during the volleyball season players with jumper’s knee did not improve after SWT.105 A randomized clinical trial combining eccentric training and SWT demonstrated higher success rates for midportion Achilles tendinopathy, compared with eccentric loading or SWT alone.81,82 Thus, there are discrepancies in results of SWT for different tendons indicating that the proper indications for SWT are not yet fully known.
Glyceryl Trinitrate Patches
Two recent meta-analyses of 7 randomized clinical trials concluded that topical nitroglycerin exerts short-term relief (maximum, 6 months) of pain in activities of daily living in chronic or acute tendinopathies.27,29 Side effects are common, including dizziness and headaches through vasodilation-induced hypotension. Longer term results show no benefit over placebo for lateral epicondylalgia with positive results in Achilles tendinopathy.57,72
Injection Therapies
Several local injection formulas have been tested to address the pathology and promote a healing response. Overall, there are no Level 1 studies or high-quality studies to support any of these therapies.
Corticosteroids
Corticosteroid injections are still used extensively in athletes but remain controversial.52,67,70,88 Reviews of corticosteroid injections in tendons showed little benefit.70,88 Moreover, there is good clinical evidence that cortisone produces a small positive short-term effect but also a long-term negative effect.70 Animal studies show that local corticosteroid injections reduce tendon strength. There are numerous case reports of tendon rupture after corticosteroid injections into humans.52,67
Sclerotherapy
In randomized controlled trial, sclerosing substances (Aethoxysclerol) have, under ultrasonography and color Doppler guidance, produced successful results in the peritendinous neovessel area.68,69 Presumably, the pathological nerve ingrowth in close proximity to the neovessels is reduced by this treatment.6,34,35,102 However, at 2 years follow-up, one-third of the patients seek additional treatment, and at 3 to 5 years follow-up, around 50% of the patients have received supplementary therapies.34,96 There is a need for large-scale randomized control studies with appropriate follow-up to determine the efficacy of injection therapies in tendinopathy.35 A comparative study of patients who received arthroscopic shaving of the neovessels and presumably nerves showed less pain than in those receiving sclerotherapy.102
Blood Products
Blood injections provide deficient healing areas devoid of bleeding (tendinopathies) with new growth factors. Autologous blood injections have been reported successful for different tendinopathies, although well-performed studies display less promising results.18,19,37
A recent popular development with autologous blood is to spin down the blood and inject the platelet-rich plasma. Platelets release a variety of growth factors (eg, PDGF, VEGF, IGF-1, TGF-β, FGF) that promote repair in various soft tissue models. In the only available randomized controlled study on Achilles tendinopathy, platelet-rich plasma treatment was not more effective than saline injections.19,20,37
Operative Treatment
The aims of operative treatment are to excise areas of failed healing, fibrosis, and pathological nerve ingrowth, initiate bleeding and a healing process, and thereby restore vascularity to initiate stem cell ingrowth and protein synthesis. The operative approach can be open, percutaneous, or endoscopic.52 The technique should be chosen as to best target the pain generating pathology. Targeting the pathological nerve and vessel ingrowth with ultrasound and color doppler-guided abrasion has shown good short-term results for midportion Achilles tendinosis.4 Arthroscopic surgery for patellar tendinopathy, refractory to nonoperative management, appears to provide significant improvements in symptoms and function maintained for at least 3 years.73 Endoscopy can access several tendons, such as the posterior tibial and peroneals, otherwise not easily visualized.16,85
The surgeon should address the pathological nerve ingrowth accompanied by pathological neovessels, to mitigate pain. The neonerves that follow the neovessels can be eliminated with electrocoagulation or mini-invasive stripping with color Doppler US visualization.12,49,92 A motorized shaver, diathermy, or radiofrequency can destroy neovessels and possibly the pathological pain-generating ingrown nerves.
Summary
The recommended treatment strategies for tendinopathy vary. Exercise enhances tendon repair and nerve withdrawal from the tendon proper. Eccentric loading may result in the tendon resistance to injury. Heavy, slow strength training, specifically eccentric, is effective for Achilles and patellar tendinopathy, with encouraging results for epicondylalgia. Physical therapy supervision increases compliance and quality since management of tendinopathy is difficult and time-consuming and may lead to frustration and reinjury. Exercise should be the cornerstone of tendinopathy treatment.
Adjuvant biophysical procedures, such as extracorporeal SWT, may initiate healing of the failed tendon repair by selective denervation of sensory nerves. Injection therapies with blood products, sclerosing agents, and cortisone may have good short-term effects, but all have limited long-term results. There are no Level 1 or high-quality studies to support any of the injection therapies. Thus, there is a limited role for injection treatments in the management of tendinopathy.
Surgery may occasionally be indicated in recalcitrant cases and may allow 60% to 85% of patients to return to preinjury activity levels. Rehabilitation after surgery, however, may take quite some time.73 Mini-invasive surgery addressing the pathological nerve and vessel ingrowth in tendinopathy shows good initial results.
New techniques addressing tendon repair, such as tissue engineering and regeneration, seem promising. These methods include molecular approaches by which genetically modified cells, including stem cells, synthesize growth factors or other mediators needed for healing. However, molecular procedures are not yet ready for routine clinical use. Novel mini-invasive procedures that target underlying pathology, such as abnormal neoinnervation, are being developed and are initially promising but necessitate high-quality randomized controlled trials before these can be recommended.
References
- 1. Abboud JA, Beason DP, Soslowsky LJ. Emerging ideas: the effect of hypercholesterolemia on tendons. Clin Orthop Relat Res. 2012;470:317-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ackermann PW, Li J, Lundeberg T, Kreicbergs A. Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res. 2003;21(3):432-441 [DOI] [PubMed] [Google Scholar]
- 3. Ackermann PW, Salo PT, Hart DA. Neuronal pathways in tendon healing. Front Biosci. 2009;14:5165-5187 [DOI] [PubMed] [Google Scholar]
- 4. Alfredson H. Ultrasound and Doppler-guided mini-surgery to treat midportion Achilles tendinosis: results of a large material and a randomised study comparing two scraping techniques. Br J Sports Med. 2011;45(5):407-410 [DOI] [PubMed] [Google Scholar]
- 5. Alfredson H, Forsgren S, Thorsen K, Fahlstrom M, Johansson H, Lorentzon R. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons: Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001;9(2):123-126 [DOI] [PubMed] [Google Scholar]
- 6. Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: a double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):338-344 [DOI] [PubMed] [Google Scholar]
- 7. Alfredson H, Pietila T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26(3):360-366 [DOI] [PubMed] [Google Scholar]
- 8. Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept. 2008;150(1-3):81-87 [DOI] [PubMed] [Google Scholar]
- 9. Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc. 2011;19(3):213-217 [DOI] [PubMed] [Google Scholar]
- 10. Beeharry D, Coupe B, Benbow EW, et al. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis. 2006;65(3):312-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernhardsson S, Klintberg IH, Wendt GK. Evaluation of an exercise concept focusing on eccentric strength training of the rotator cuff for patients with subacromial impingement syndrome. Clin Rehabil. 2011;25(1):69-78 [DOI] [PubMed] [Google Scholar]
- 12. Boesen MI, Torp-Pedersen S, Koenig MJ, et al. Ultrasound guided electrocoagulation in patients with chronic non-insertional Achilles tendinopathy: a pilot study. Br J Sports Med. 2006;40(9):761-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bring DK, Heidgren ML, Kreicbergs A, Ackermann PW. Increase in sensory neuropeptides surrounding the Achilles tendon in rats with adjuvant arthritis. J Orthop Res. 2005;23(2):294-301 [DOI] [PubMed] [Google Scholar]
- 14. Bring DKI, Kreicbergs A, Renstrom PAFH, Ackermann PW. Physical activity modulates nerve plasticity and stimulates repair after Achilles tendon rupture. J Orthop Res. 2007;25(2):164-172 [DOI] [PubMed] [Google Scholar]
- 15. Bring DKI, Reno C, Renstrom P, Salo P, Hart DA, Ackermann PW. Joint immobilization reduces the expression of sensory neuropeptide receptors and impairs healing after tendon rupture in a rat model. J Orthop Res. 2009;27(2):274-280 [DOI] [PubMed] [Google Scholar]
- 16. Bulstra GH, Olsthoorn PG, Niek van Dijk C. Tendoscopy of the posterior tibial tendon. Foot Ankle Clin. 2006;11(2):421-427 [DOI] [PubMed] [Google Scholar]
- 17. Collins M, Raleigh SM. Genetic risk factors for musculoskeletal soft tissue injuries. Med Sport Sci. 2009;54:136-149 [DOI] [PubMed] [Google Scholar]
- 18. Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966-971 [DOI] [PubMed] [Google Scholar]
- 19. de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63-77 [DOI] [PubMed] [Google Scholar]
- 20. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149 [DOI] [PubMed] [Google Scholar]
- 21. Dimitrios S, Pantelis M, Kalliopi S. Comparing the effects of eccentric training with eccentric training and static stretching exercises in the treatment of patellar tendinopathy: a controlled clinical trial [published online ahead of print August 19, 2011]. Clin Rehabil. [DOI] [PubMed] [Google Scholar]
- 22. Ebenbichler GR, Erdogmus CB, Resch KL, et al. Ultrasound therapy for calcific tendinitis of the shoulder. N Engl J Med. 1999;340(20):1533-1538 [DOI] [PubMed] [Google Scholar]
- 23. Freeman TA, Parvizi J, Dela Valle CJ, Steinbeck MJ. Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty. Fibrogenesis Tissue Repair. 2010;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frohm A, Halvorsen K, Thorstensson A. Patellar tendon load in different types of eccentric squats. Clin Biomech (Bristol, Avon). 2007;22(6):704-711 [DOI] [PubMed] [Google Scholar]
- 25. Frohm A, Saartok T, Halvorsen K, Renstrom P. Eccentric treatment for patellar tendinopathy: a prospective randomised short-term pilot study of two rehabilitation protocols. Br J Sports Med. 2007;41(7):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaida JE, Ashe MC, Bass SL, Cook JL. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Rheum. 2009;61(6):840-849 [DOI] [PubMed] [Google Scholar]
- 27. Gambito ED, Gonzalez-Suarez CB, Oquinena TI, Agbayani RB. Evidence on the effectiveness of topical nitroglycerin in the treatment of tendinopathies: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2010;91(8):1291-1305 [DOI] [PubMed] [Google Scholar]
- 28. Gardin A, Movin T, Svensson L, Shalabi A. The long-term clinical and MRI results following eccentric calf muscle training in chronic Achilles tendinosis. Skeletal Radiol. 2010;39(5):435-442 [DOI] [PubMed] [Google Scholar]
- 29. Garrick JG. Topical nitroglycerin decreases pain intensity in daily activities: a review. Clin J Sport Med. 2011;21(6):539-540 [DOI] [PubMed] [Google Scholar]
- 30. Hagglund M, Zwerver J, Ekstrand J. Epidemiology of patellar tendinopathy in elite male soccer players. Am J Sports Med. 2011;39(9):1906-1911 [DOI] [PubMed] [Google Scholar]
- 31. Hakim AJ, Cherkas LF, Spector TD, MacGregor AJ. Genetic associations between frozen shoulder and tennis elbow: a female twin study. Rheumatology (Oxford). 2003;42(6):739-742 [DOI] [PubMed] [Google Scholar]
- 32. Heinemeier KM, Olesen JL, Haddad F, et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(pt 3):1303-1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann P, Hoeck K, Deters S, Werner-Martini I, Schmidt WE. Substance P and calcitonin gene related peptide induce TGF-alpha expression in epithelial cells via mast cells and fibroblasts. Regul Pept. 2010;161(1-3):33-37 [DOI] [PubMed] [Google Scholar]
- 34. Hoksrud A, Bahr R. Ultrasound-guided sclerosing treatment in patients with patellar tendinopathy (jumper’s knee): 44-month follow-up. Am J Sports Med. 2011;39:2377-2380 [DOI] [PubMed] [Google Scholar]
- 35. Hoksrud AF, Bahr R. Injectable agents derived from or targeting vascularity: has clinical acceptance in managing tendon disorders superseded scientific evidence? J Musculoskelet Neuronal Interact. 2011;11(2):174-184 [PubMed] [Google Scholar]
- 36. Jarvinen TA, Kannus P, Paavola M, Jarvinen TL, Jozsa L, Jarvinen M. Achilles tendon injuries. Curr Opin Rheumatol. 2001;13(2):150-155 [DOI] [PubMed] [Google Scholar]
- 37. Kampa RJ, Connell DA. Treatment of tendinopathy: is there a role for autologous whole blood and platelet rich plasma injection? Int J Clin Pract. 2010;64(13):1813-1823 [DOI] [PubMed] [Google Scholar]
- 38. Kettunen JA, Kujala U, Sarna S, Kaprio J. Cumulative incidence of shoulder region tendon injuries in male former elite athletes. Int J Sports Med. 2011;32(6):451-454 [DOI] [PubMed] [Google Scholar]
- 39. Khan KM, Maffulli N. Tendinopathy: an Achilles’ heel for athletes and clinicians. Clin J Sport Med. 1998;8(3):151-154 [PubMed] [Google Scholar]
- 40. Khan MH, Li Z, Wang JH. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin J Sport Med. 2005;15(1):27-33 [DOI] [PubMed] [Google Scholar]
- 41. Kingma JJ, de Knikker R, Wittink HM, Takken T. Eccentric overload training in patients with chronic Achilles tendinopathy: a systematic review. Br J Sports Med. 2007;41(6):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Knobloch K. Tendinopathy and drugs: potential implications for beneficial and detrimental effects on painful tendons. J Sci Med Sport. 2009;12(3):423. [DOI] [PubMed] [Google Scholar]
- 43. Knobloch K, Kraemer R, Vogt PM. Midportion Achilles tendinopathy: a cardiovascular disease? Med Sci Sports Exerc. 2010;42(1):213-214 [DOI] [PubMed] [Google Scholar]
- 44. Kramer R, Lorenzen J, Vogt PM, Knobloch K. [Systematic review about eccentric training in chronic achilles tendinopathy]. Sportverletz Sportschaden. 2010;24(4):204-211 [DOI] [PubMed] [Google Scholar]
- 45. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160 [DOI] [PubMed] [Google Scholar]
- 46. Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133-135 [DOI] [PubMed] [Google Scholar]
- 47. Lian O, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34(11):1801-1808 [DOI] [PubMed] [Google Scholar]
- 48. Ljung BO, Alfredson H, Forsgren S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus: studies on tennis elbow and medial epicondylalgia. J Orthop Res. 2004;22(2):321-327 [DOI] [PubMed] [Google Scholar]
- 49. Longo UG, Ramamurthy C, Denaro V, Maffulli N. Minimally invasive stripping for chronic Achilles tendinopathy. Disabil Rehabil. 2008;30(20-22):1709-1713 [DOI] [PubMed] [Google Scholar]
- 50. Lorenzen J, Kramer R, Vogt PM, Knobloch K. [Systematic review about eccentric training in chronic patella tendinopathy]. Sportverletz Sportschaden. 2010;24(4):198-203 [DOI] [PubMed] [Google Scholar]
- 51. Lysholm J, Wiklander J. Injuries in runners. Am J Sports Med. 1987;15(2):168-171 [DOI] [PubMed] [Google Scholar]
- 52. Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg Am. 2010;92(15):2604-2613 [DOI] [PubMed] [Google Scholar]
- 53. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675-692 [DOI] [PubMed] [Google Scholar]
- 54. Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262-268 [DOI] [PubMed] [Google Scholar]
- 55. Malliaras P, Maffulli N, Garau G. Eccentric training programmes in the management of lateral elbow tendinopathy. Disabil Rehabil. 2008;30(20-22):1590-1596 [DOI] [PubMed] [Google Scholar]
- 56. Marie I, Delafenetre H, Massy N, Thuillez C, Noblet C. Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990-2005 and review of the literature. Arthritis Rheum. 2008;59(3):367-372 [DOI] [PubMed] [Google Scholar]
- 57. McCallum SD, Paoloni JA, Murrell GA. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med. 2011;45(5):416-420 [DOI] [PubMed] [Google Scholar]
- 58. Melhus A. Fluoroquinolones and tendon disorders. Expert Opin Drug Saf. 2005;4(2):299-309 [DOI] [PubMed] [Google Scholar]
- 59. Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder: a pilot study. Cells Tissues Organs. 1999;165(1):30-39 [DOI] [PubMed] [Google Scholar]
- 60. Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085-2091 [DOI] [PubMed] [Google Scholar]
- 61. Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg Br. 2009;91(3):417-424 [DOI] [PubMed] [Google Scholar]
- 62. Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567(pt 3):1021-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mokone GG, Gajjar M, September AV, et al. The guanine-thymine dinucleotide repeat polymorphism within the tenascin-C gene is associated with achilles tendon injuries. Am J Sports Med. 2005;33(7):1016-1021 [DOI] [PubMed] [Google Scholar]
- 64. Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scand J Med Sci Sports. 2006;16(1):19-26 [DOI] [PubMed] [Google Scholar]
- 65. Molloy TJ, Kemp MW, Wang Y, Murrell GA. Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol. 2006;101(6):1702-1709 [DOI] [PubMed] [Google Scholar]
- 66. Murata R, Ohtori S, Ochiai N, et al. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci. 2006;128(1-2):96-100 [DOI] [PubMed] [Google Scholar]
- 67. Nichols AW. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15(5):370-375 [DOI] [PubMed] [Google Scholar]
- 68. Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004;12(5):465-470 [DOI] [PubMed] [Google Scholar]
- 69. Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36(3):173-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Orchard J, Kountouris A. The management of tennis elbow. BMJ. 2011;342:d2687. [DOI] [PubMed] [Google Scholar]
- 71. Origuchi T, Iwamoto N, Kawashiri SY, et al. Reduction in serum levels of substance P in patients with rheumatoid arthritis by etanercept, a tumor necrosis factor inhibitor. Mod Rheumatol. 2011;21(3):244-250 [DOI] [PubMed] [Google Scholar]
- 72. Paoloni JA, Murrell GA. Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28(10):1064-1068 [DOI] [PubMed] [Google Scholar]
- 73. Pascarella A, Alam M, Pascarella F, Latte C, Di Salvatore MG, Maffulli N. Arthroscopic management of chronic patellar tendinopathy. Am J Sports Med. 2011;39(9):1975-1983 [DOI] [PubMed] [Google Scholar]
- 74. Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15(4):211-222 [DOI] [PubMed] [Google Scholar]
- 76. Raleigh SM, van der Merwe L, Ribbans WJ, Smith RK, Schwellnus MP, Collins M. Variants within the MMP3 gene are associated with Achilles tendinopathy: possible interaction with the COL5A1 gene. Br J Sports Med. 2009;43(7):514-520 [DOI] [PubMed] [Google Scholar]
- 77. Rechardt M, Shiri R, Karppinen J, Jula A, Heliovaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord. 2010;11:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology (Oxford). 2008;47(10):1493-1497 [DOI] [PubMed] [Google Scholar]
- 79. Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford). 2006;45(5):508-521 [DOI] [PubMed] [Google Scholar]
- 80. Renstrom PAHF, Woo S-Y. Tendinopathy: a major medical problem in sport. In: Woo S, Renström P, Arnoczky S, eds. Tendinopathy in Athletes. London, UK: Wiley-Blackwell; 2008:1-9 [Google Scholar]
- 81. Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy: a randomized, controlled trial. J Bone Joint Surg Am. 2008;90(1):52-61 [DOI] [PubMed] [Google Scholar]
- 82. Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37(3):463-470 [DOI] [PubMed] [Google Scholar]
- 83. Schizas N, Andersson T, Fahlgren A, Aspenberg P, Ahmed M, Ackermann P. Compression therapy promotes proliferative repair during rat achilles tendon immobilization. J Orthop Res. 2010;28(7):852-858 [DOI] [PubMed] [Google Scholar]
- 84. Schizas N, Lian Ø, Frihagen F, Engebretsen L, Bahr R, Ackermann PW. Coexistence of up-regulated NMDA receptor 1 and glutamate on nerves, vessels and transformed tenocytes in tendinopathy. Scand J Med Sci Sports. 2010;20:208-215 [DOI] [PubMed] [Google Scholar]
- 85. Scholten PE, van Dijk CN. Tendoscopy of the peroneal tendons. Foot Ankle Clin. 2006;11(2):415-420 [DOI] [PubMed] [Google Scholar]
- 86. Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64(7):1083-1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scott A, Bahr R. Neuropeptides in tendinopathy. Front Biosci. 2009;14:2203-2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Speed CA. Fortnightly review: corticosteroid injections in tendon lesions. BMJ. 2001;323(7309):382-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986(208):65-68 [PubMed] [Google Scholar]
- 90. Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol. 2008;35(10):2038-2046 [PubMed] [Google Scholar]
- 91. Sullo A, Maffulli N, Capasso G, Testa V. The effects of prolonged peritendinous administration of PGE1 to the rat Achilles tendon: a possible animal model of chronic Achilles tendinopathy. J Orthop Sci. 2001;6(4):349-357 [DOI] [PubMed] [Google Scholar]
- 92. Thermann H, Benetos IS, Panelli C, Gavriilidis I, Feil S. Endoscopic treatment of chronic mid-portion Achilles tendinopathy: novel technique with short-term results. Knee Surg Sports Traumatol Arthrosc. 2009;17(10):1264-1269 [DOI] [PubMed] [Google Scholar]
- 93. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349-363 [DOI] [PubMed] [Google Scholar]
- 94. van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ. 2002;324(7349):1306-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van Leeuwen MT, Zwerver J, van den Akker-Scheek I. Extracorporeal shockwave therapy for patellar tendinopathy: a review of the literature. Br J Sports Med. 2009;43(3):163-168 [DOI] [PubMed] [Google Scholar]
- 96. van Sterkenburg MN, de Jonge MC, Sierevelt IN, van Dijk CN. Less promising results with sclerosing ethoxysclerol injections for midportion achilles tendinopathy: a retrospective study. Am J Sports Med. 2010;38(11):2226-2232 [DOI] [PubMed] [Google Scholar]
- 97. van Sterkenburg MN, van Dijk CN. Mid-portion Achilles tendinopathy: why painful? An evidence-based philosophy. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1367-1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Virchenko O, Aspenberg P, Lindahl TL. Low molecular weight heparin impairs tendon repair. J Bone Joint Surg Br. 2008;90(3):388-392 [DOI] [PubMed] [Google Scholar]
- 99. Visnes H, Bahr R. The evolution of eccentric training as treatment for patellar tendinopathy (jumper’s knee): a critical review of exercise programmes. Br J Sports Med. 2007;41(4):217-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang JH, Jia F, Yang G, et al. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44(3-4):128-133 [DOI] [PubMed] [Google Scholar]
- 101. Warden SJ, Metcalf BR, Kiss ZS, et al. Low-intensity pulsed ultrasound for chronic patellar tendinopathy: a randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2008;47(4):467-471 [DOI] [PubMed] [Google Scholar]
- 102. Willberg L, Sunding K, Forssblad M, Fahlstrom M, Alfredson H. Sclerosing polidocanol injections or arthroscopic shaving to treat patellar tendinopathy/jumper’s knee? A randomised controlled study. Br J Sports Med. 2011;45(5):411-415 [DOI] [PubMed] [Google Scholar]
- 103. Woodley BL, Newsham-West RJ, Baxter GD. Chronic tendinopathy: effectiveness of eccentric exercise. Br J Sports Med. 2007;41(4):188-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zwerver J, Bredeweg SW, van den Akker-Scheek I. Prevalence of jumper’s knee among nonelite athletes from different sports: a cross-sectional survey. Am J Sports Med. 2011;39(9):1984-1988 [DOI] [PubMed] [Google Scholar]
- 105. Zwerver J, Hartgens F, Verhagen E, van der Worp H, van den Akker-Scheek I, Diercks RL. No effect of extracorporeal shockwave therapy on patellar tendinopathy in jumping athletes during the competitive season: a randomized clinical trial. Am J Sports Med. 2011;39(6):1191-1199 [DOI] [PubMed] [Google Scholar]