Abstract
Context:
Osteochondritis dissecans (OCD) of the capitellum is most often seen in adolescents who participate in sports that involve repetitive loading of the elbow. Unstable defects typically require surgical intervention that involves fragment fixation, debridement, or reconstruction with an osteochondral autograft transfer. Optimum surgical management of unstable defects remains controversial.
Type of Study:
Clinical review.
Evidence Acquisition:
Relevant articles published after 1992 were identified using MEDLINE, the EMBASE database, and the Cochrane Library.
Results:
Both debridement and osteochondral autograft transfer for treatment of capitellar OCD lesions result in good short- and midterm outcomes with a high rate of return to sports. Larger defects involving more than 50% of the articular surface or involving the lateral margin of the capitellum may have worse outcomes after debridement and may be better treated with fragment fixation or osteochondral autograft transfer.
Conclusions:
High-level evidence is lacking to determine the superiority of debridement or osteochondral autograft transfer for the treatment of capitellar OCD lesions. A prospective longitudinal multicenter study, using validated outcome measures, that enrolls a large number of patients is needed to establish optimal treatment for unstable capitellar OCD lesions.
Keywords: osteochondritis dissecans, elbow, capitellum
Osteochondritis dissecans (OCD) of the capitellum is most often seen in adolescents older than 12 years who participate in sports that involve repetitive loading of the elbow, such as baseball or gymnastics.23 In comparison, Panner’s disease, which may have radiographic and clinical features of capitellar OCD, is seen most commonly in boys between the ages of 5 and 12 years and is often successfully treated with activity modification until healing has occurred.23 The etiology of capitellar OCD is not entirely clear. Traumatic etiology has been proposed because of the association of capitellar OCD with sports that repetitively stress the radiocapitellar joint.24 The subchondral bone of the capitellum may be susceptible to injury from repetitive stress as it may be relatively hypovascular.32 Early recognition and intervention may protect these athletes from developing irreversible cartilage damage.
Clinical Presentation
OCD of the capitellum is commonly seen in baseball players and in gymnasts because of the repetitive loads that these sports place on the elbow.23 The onset of symptoms in patients with OCD of the capitellum is often insidious with many athletes reporting vague lateral elbow pain with loading of the elbow. Initially, symptoms may not bother them enough to seek medical attention. Over time the symptoms may worsen with activity. In any adolescent overhead athlete or gymnast who reports lateral elbow pain during athletic activity there should be a high index of suspicion for a capitellar OCD lesion. The presence of mechanical symptoms or loss of motion is often a sign of more advanced cartilage injury or loose body.
Radiographic Evaluation
The radiographic evaluation of OCD lesions of the capitellum should begin with anteroposterior (AP), lateral, and oblique radiographs of the elbow. A 45-degree flexion anterior-posterior radiograph may provide a clear profile of the capitellum.29 Comparison radiographs of the contralateral elbow are helpful to distinguish subtle changes from normal anatomic variants of the maturing epiphyseal ossification centers of the distal humerus. The Minami classification describes the appearance of the capitellum on plain radiographs.21 In Minami type 1 OCD lesions, there is flattening of the capitellum or cystic changes in the capitellum (Figure 1A).21 In Minami type 2 OCD lesions, there is clear subchondral detachment or fragment splitting in the capitellum (Minami Type 2) (Figure 1B).21
Figure 1.
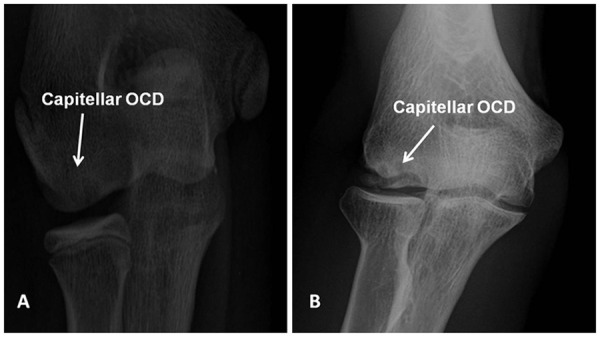
(A) Anterior-posterior view radiograph of the elbow demonstrating cystic changes in the capitellum (Minami type 1) suggestive of an osteochondritis dissecans lesion. (B) Anterior-posterior view radiograph of the elbow demonstrating fragmentation and splitting of the subchondral bone in the capitellum (Minami type 2) indicative of an osteochondritis dissecans lesion.
In cases with suspicious radiographic changes, magnetic resonance imaging (MRI) is critical to confirm the diagnosis as well as to characterize the extent and stability of the OCD lesion. MRI can define lesion size, location, the status of the chondral surface and involvement of the subchondral bone, “high signal” at the lesion interface, and the presence of frank loose bodies within the joint. The earliest findings of a capitellar OCD lesion on MRI may be low signal changes in the superficial aspect of the capitellum on T1 images with normal appearance on T2 sequences.29
De Smet et al described 4 MRI criteria on T2-weighted images that predict the stability of OCD lesions in the knee.7 These findings include (1) a line of high signal intensity at least 5 mm in length between the OCD lesion and underlying bone, (2) an area of increased homogeneous signal at least 5 mm in diameter beneath the lesion, (3) a focal defect of 5 mm or more in the articular surface, and (4) a high signal line traversing the subchondral plate into the lesion. Kijowski and De Smet showed similar characteristics on MRI in a small series of patients with capitellar OCD lesions.16 A high signal line behind the fragment is most predictive of an unstable lesion.8 While the exact etiology of the high signal line remains controversial, it may represent a violation in the integrity of the articular cartilage surface that allows for communication of synovial fluid and granulation tissue formation below the OCD lesion. Additionally, magnetic resonance arthrogram may be beneficial in evaluating the OCD lesions if integrity of the cartilage surface is in question. If contrast can be seen behind the lesion, the cartilage is likely fragmented (Figure 2).23
Figure 2.
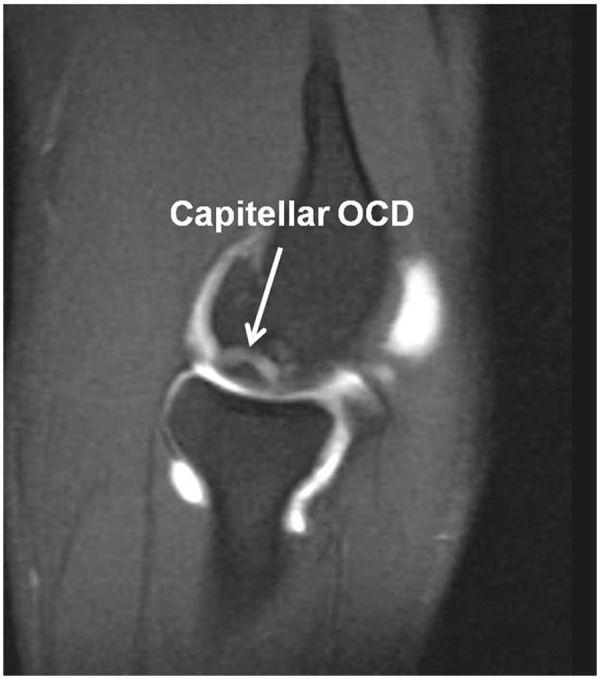
Sagittal magnetic resonance arthrogram demonstrating an unstable capitellar osteochondritis dissecans with contrast between the fragment and the underlying bone.
MRI may prove to be invaluable not only for diagnostic purposes but also to assess the maturation and efficacy of reparative or reconstructive surgical procedures for unstable OCD lesions. Iwasaki et al recently evaluated 10 young male athletes with advanced lesions of capitellar OCD treated with mosaicplasty with serial MRI.13 Fluid surrounding the graft was found in all patients at 3 months and 4 patients at 6 months. The grafts were all well seated within the recipient sites with no evidence of graft loosening at 12 months. Furthermore, T2 mapping and delayed gadolinium-enhanced MRI of cartilage may represent new, future adaptations of MRI to more rigorously evaluate the quality of repair tissue.
Understanding the Evidence
The primary controversy in the treatment of capitellar OCD is how to manage an unstable chondral lesion and how to interpret the existing literature. In general, the weakness of the literature is that outcome measures are limited because (1) there are no validated, patient-rated disease-specific outcome measures, (2) current validated outcome measures do not discriminate this particular disease state, (3) a clinically significant difference in outcomes (osteoarthrosis) may only be manifest 20 to 30 years after the injury.
The most commonly used outcome measures for capitellar OCD are the Andrews-Timmerman rating system31 or the Mayo Elbow Performance Score.20 Neither of these instruments has been validated. Also, radiographic observations have been used as indirect markers of outcome, such as radial head enlargement and capitellar flattening. There are not enough data to determine if these changes are or will be clinically meaningful. Moreover, return to sport has been used as a marker of outcome but is confounded by the level of play prior to injury and after surgery. In addition, if the teenager is competing at a high level, the time needed to recover from any intervention may affect return to play even if full recovery is achieved.
Another limitation is the difficulty of acquiring long-term follow-up. Patients who develop OCD in their early teens are often lost to follow-up once they are no longer dependents. Ultimately, there is little clinical data to distinguish whether debridement or reconstruction is superior. To find an answer, a validated, patient-rated outcome measure directed toward elbow osteoarthritis is needed for a multicenter study that enrolls a large number of patients with consistent longitudinal follow-up.
Another confounder is what secondary procedures are performed during a debridement. Some surgeons will remove the free fragment with minimal surface preparation. Some will perform varying degrees of chondroplasty, while others will perform a microfracture in conjunction with surface abrasion. It is unclear to what degree additional intervention contributes to clinical outcomes. In addition, the degree of chondroplasty is often not consistently described.
Surgical Options
In patients whose symptoms persist despite rest and activity modification or in patients in whom an OCD lesion becomes unstable, surgical intervention is indicated. There have been many surgical treatments described for capitellar OCD lesions. These include arthroscopic or open in situ fixation, debridement with or without drilling or microfracture, and osteochondral autograft transfer from the knee. The results of all these surgical interventions are generally good in short- and midterm follow-up.6 Outcomes of debridement appear to be less favorable in patients who have defects involving greater than 50% of the articular surface, defects involving more than 1 cm in diameter, or defects that violate the lateral margin of the capitellum.18,27 However, these data are far from conclusive and do not preclude debridement in these situations.
Fragment Fixation of Capitellar OCD Lesions
Fragment fixation can be attempted in patients with Minami type 1 cystic changes in the subchondral bone or in patients with grade 2 lesions with a split between the OCD fragment and the adjacent bone.21,30 In larger lesions with thicker bone within the OCD fragment, compression screws can be used for fragment fixation. Herbert screws have been used for capitellar OCD lesions.17 Bioabsorbable compression screws have been used for fixation of osteochondral fragments in the knee5 and may be useful in the elbow as well.
Fragment fixation can be performed arthroscopically through the direct lateral (“soft spot”) portal (Figure 3) or open using a lateral approach or an anconeus splitting approach. The anconeus split can be done by extending the direct lateral portal proximally and distally to expose the posterior capitellum (Figure 4). The elbow is hyperflexed to view the entire defect. The fragment can be fixed in situ. Alternatively, the fragment can be elevated to debride and bone graft the base of the lesion before fixation.
Figure 3.
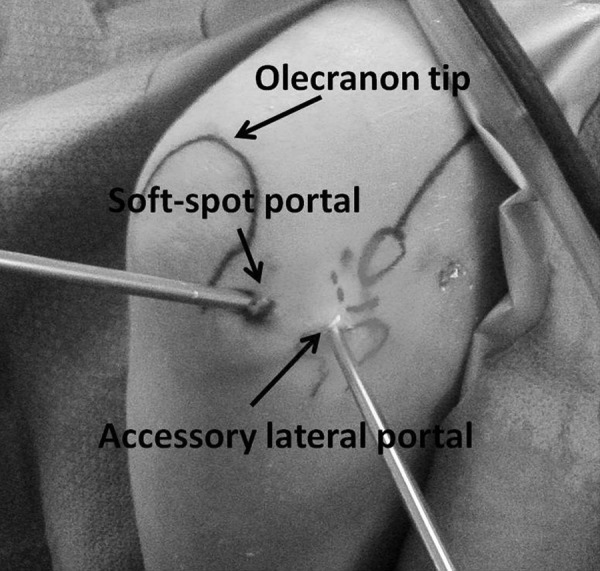
View of the posterolateral aspect of the elbow showing the soft-spot portal and an accessory lateral portal over the radiocapitellar joint.
Figure 4.
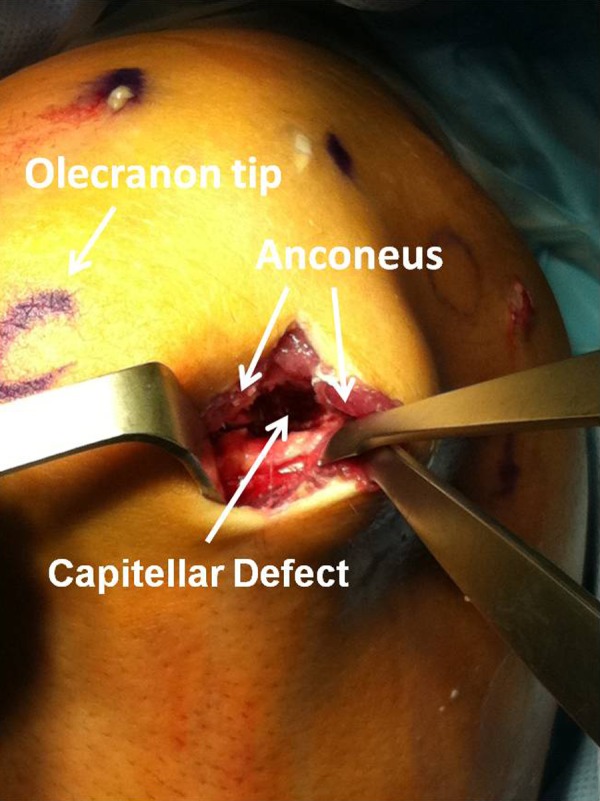
View of the posterolateral aspect of the elbow showing the soft-spot portal has been extended proximally and distally. The anconeus has been split exposing the posterior aspect of the capitellum. The osteochondritis dissecans lesion can be easily accessed through this approach with hyper flexion of the elbow.
Smaller lesions not amenable to screw fixation can be treated with a pullout wire technique.21,30 The pullout wire technique is usually done open through a lateral approach.20,29 The anterior aspect of the lateral collateral ligament is released to expose the anterior and posterior aspect of the lateral epicondyle.21,30 Parallel 1.5-mm Kirschner wires are drilled through the lateral epicondyle into the fragment. A soft wire is then passed through the drill holes with the loop over the cartilage surface. The soft wire is secured to the posterior aspect the lateral epicondyle to hold the fragment reduced until healing occurs. The advantage of the pullout wire technique is the ability to fix smaller lesions. The disadvantage is that the pullout wire technique requires wire removal after fragment healing. Once healing is confirmed on postoperative radiographs, activity can be increased as tolerated. Typical return to overhead athletic activities is between 4 and 6 months after surgery.
Outcomes
Kuwhata et al reported on 8 elbows with OCD of the capitellum treated with cancellous bone graft and internal fixation with a Herbert screw.17 An average of 32 months after surgery, all patients were pain-free. The cystic changes seen on radiographs completely resolved suggesting healing of the defect.
Nobuta et al reported on 28 patients with Minami grade 1 or 2 OCD lesions of the capitellum treated with drilling and fixation of the fragment with a double-wiring technique through a lateral arthrotomy.21 The capitellar osteochondral fragment was elevated and the fibrous tissue in the defect bed curetted, but bone graft was not added. Complete resolution of pain occurred in 25 of 28 patients. Complete radiographic healing was seen in 11 patients, while 12 patients had partial healing and 3 had no radiographic healing. Two patients developed loose bodies. Defect thickness greater than 9 mm on radiographs was associated with lower healing rates using this technique. Wire breakage was reported in three patients at the time of wire removal.
Takeda et al also reported on 11 male baseball players treated with the pullout wiring technique and bone grafting.30 All patients in this series had improvement of their pain. The wires were removed at an average of 17 weeks after surgery. Radiographs showed healing and no degenerative changes in the radiocapitellar joint at follow-up.
Return to sports
Kuwhata et al reported that all 8 patients treated with fragment fixation returned to sports.17 In the series of 28 patients treated with a pullout wire technique without bone grafting reported by Nobuta et al, 86% returned to sports.21 In the series reported by Takeda et al, 10 of 11 baseball players treated with a pullout wire technique with bone grafting returned to throwing.30
Debridement of Capitellar OCD Lesions
With advances in arthroscopic techniques, debridement has become the mainstay of surgical treatment for capitellar OCD lesions that are refractory to activity modification or in patients with unstable OCD lesions. Drilling or microfracture of the capitellar defect was an adjunct to debridement in several studies evaluating the outcomes of debridement.4,15,18,19,21,25 Comparisons of debridement alone versus debridement with drilling or microfracture have not been reported.
Many capitellar osteochondral defects are not visible or are only partially visible while viewing from the anterior compartment (Figure 5). A better view is obtained from a posterior approach (Figure 6). Even so, access to the radiocapitellar joint can be difficult from a posterior transtriceps portal or through a posterior lateral portal because of a thickened posterolateral plica. If access is difficult, this can be improved by using a small joint arthroscope in the direct lateral portal. An accessory portal 1 cm distal to the direct lateral portal or 1 cm lateral to the direct lateral portal over the radiocapitellar joint can be used as a working portal (Figure 3).
Figure 5.
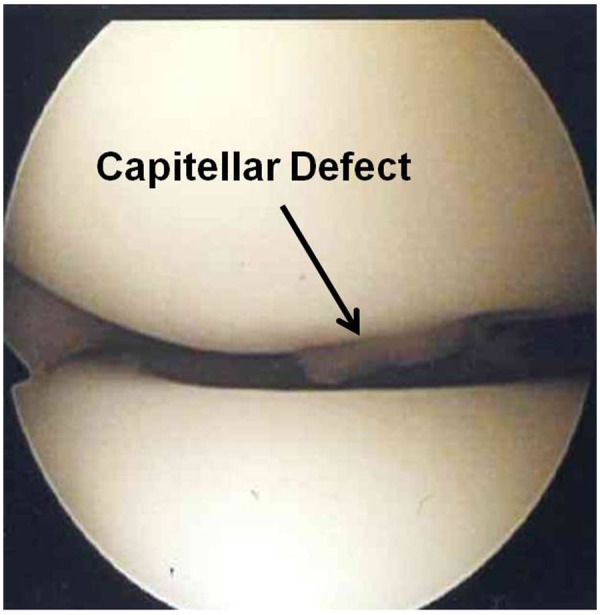
Viewing from the proximal anteromedial portal, the capitellar osteochondral defect is only partially visible.
Figure 6.
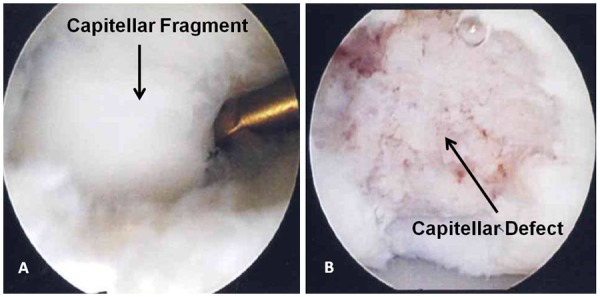
(A) Viewing through the direct lateral portal with a small joint arthroscope, the capitellar osteochondral defect can be seen in its entirety. (B) The cartilage and bone fragments have been removed from the capitellum to create a stable base.
Using the direct lateral portal, the osteochondral defect can be viewed in its entirety (Figure 6A). Arthroscopic shavers and biters can remove loose fragments leaving a stable base (Figure 6B). In addition to debridement of the loose osteochondral fragments, drilling of the defect or abrasion of the bone bed can be performed to stimulate fibrocartilage fill in the defect.
In the immediate postoperative period, patients are typically placed in a soft dressing until the wounds have healed. Passive and active assisted range of motion is allowed as soon as pain improves. Strengthening of the elbow, shoulder, and wrist can be started as soon as pain allows with restrictions on exercises that place an axial load on the elbow. Return to nonthrowing sports is allowed between 3 and 4 months depending on recovery of elbow strength and range of motion. For overhead athletes, an interval throwing program is typically started 4 months after surgery with the goal of returning to throwing by 6 months and pitching between 6 and 9 months.
Outcomes
The short- and midterm outcomes of arthroscopic debridement for capitellar OCD lesions are generally good with patients reporting pain relief, improved range of motion, and a high rate of return to athletic and work activities.6 Brownlow et al retrospectively reviewed 29 patients with capitellar OCD lesions treated with arthroscopic debridement.3 The average age at surgery was 22 years. With an average 6-year follow-up, all patients were able to perform activities of daily living and 28 of 29 patients reported good to excellent outcomes. However, 38% of patients reported persistent catching symptoms, none of which required further surgery. Mild pain was reported in 48% of patients and moderate pain in 10% of patients. Radiographic evaluation showed flattening of the capitellum in 12 of 18 patients, 6 had degenerative changes in the radiocapitellar joint, and 5 had loose bodies.
Miyake et al retrospectively reviewed 106 patients with an average age of 15 years who were treated with debridement for capitellar OCD lesions.19 At an average of 13 months after surgery, 84% of patients reported no elbow pain, 14% mild pain, and 2% moderate to severe pain. Poor radiographic and clinical outcomes were seen with large lesions with an open proximal radial physis. Three of the 4 patients who had large lesions with an open proximal radial physis developed osteoarthritis on follow-up radiographs. In separate series, 12 pediatric patients (average age of 14.5 years) who underwent arthroscopic debridement for capitellar OCD at a mean follow-up of 3.2 years had excellent pain relief and no activity limitations.22 The size of the lesion did not correlate with symptomatic relief postoperatively.22
With debridement of capitellar OCD lesions, the long-term results have been less favorable than the results in the short and midterm. In 39 patients treated with open debridement followed for 3 to 25 years, 46% had pain with daily activities.28 All patients with large lesions had poor outcomes, and 24% with moderate lesions had poor outcomes. In addition, Baur et al reported that 43% of patients treated with debridement for large lesions had mild symptoms but 61% had radiographic evidence of radiocapitellar osteoarthritis.2
Return to sports
After arthroscopic debridement, in a series by Brownlow et al, 81% were able to continue with their main sport.3 In a separate series, full return to sports was possible in 85% of patients treated with arthroscopic debridement, but only 1 patient with a large defect and an open radial physis was able to return.19 Six of 9 patients with a large defect and a closed radial physis returned to competitive sports. Ruch et al reported that 3 of 12 patients made it back to competitive athletics after arthroscopic debridement.22 In a series of 25 consecutive patients treated for capitellar OCD with either arthroscopic or open debridement and bone grafting, Jones et al noted that 86% of patients returned to sports.15 In yet another series reported by Schoch, 13 patients treated with an arthroscopic debridement had improvement in symptoms and outcome scores, but only 40% were able to return to their previous sports activities.25 In 10 baseball players treated with arthroscopic debridement, Byrd et al reported excellent average Timmerman-Andrews scores, yet only 4 of 10 returned to baseball.4
Osteochondral Autograft Transfer
Because of its success in the knee, osteochondral autograft transfer for the capitellum has gained popularity.10 The indications for osteochondral autograft transfer are still evolving, but patients with large defects and defects that extend into the lateral margin of the capitellum may benefit from this procedure. Osteochondral autograft transfer involves either a single or multiple bone and cartilage plugs (mosaicplasty). In 106 patients treated with fragment removal, fixation with bone graft, and reconstruction of the articular surface with mosaicplasty from the lateral femoral condyle of the knee, fragment fixation and mosaicplasty provided better results in defects larger than 50% of the articular surface.28 Osteochondral autograft transfer can also be considered for unstable defects more than 1 cm in diameter or defects that violate the lateral margin of the capitellum.18,27 It may also be indicated in patients who fail to improve after a debridement.
Technique
Mosaicplasty or single-plug osteochondral autograft transfer can be done through a lateral arthrotomy or an anconeus splitting approach.26,30 The advantage of an anconeus split is that most capitellar defects are posterior and are easier to access. To perform an osteochondral autograft transfer through an anconeus split, the direct lateral portal is extended 1 cm proximal and 1 cm distal (Figure 4). Once through the skin and subcutaneous tissues, the fascia overlying anconeus is split in line with the portal defect. The posterior capsule is incised carefully to expose the capitellum. The elbow is then maximally flexed to expose the entire defect (Figure 7A). If the defect is 1 cm in diameter or less, a single osteochondral plug from the knee can be used to fill the defect (Figure 7B). Alternatively, multiple small plugs can be used which may better reconstitute the curvature of the capitellum.
Figure 7.
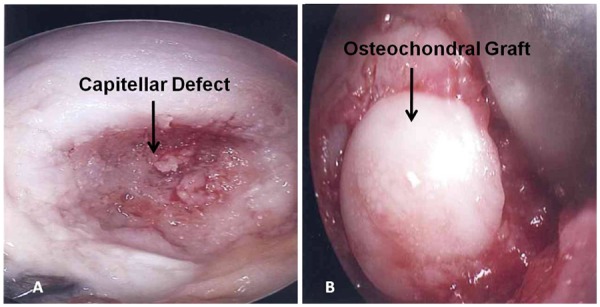
(A) Through the anconeus splitting approach, the posterior capsule has been incised and retracted to expose the capitellum. With the elbow maximally flexed, the entire capitellar defect can be seen. (B) A single osteochondral autograft plug from the lateral femoral condyle has been used to fill the capitellar defect.
In the immediate postoperative period, patients are typically placed in a soft dressing until the wounds have healed. Passive and active assisted range of motion is allowed as soon as pain improves. Strengthening of the elbow, shoulder, and wrist can be started as soon as pain allows with restrictions on exercises that place an axial load on the elbow. Return to nonthrowing sports is allowed when the graft has been completely incorporated into the capitellum on radiographs (Figure 8) and when the patient has recovered full strength and range of motion of the elbow. This usually occurs between 4 and 6 months after surgery. For overhead athletes, an interval throwing program is typically started once the graft has completely healed.
Figure 8.
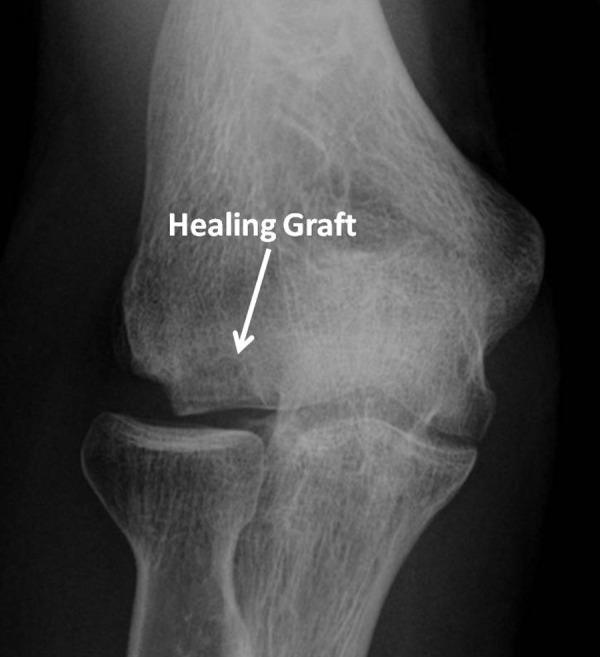
Anteroposterior radiograph of a single osteochondral autograft plug placed into a large capitellar osteochondritis dissecans defect. Note that this is the postoperative radiograph from Figure 1B.
Outcomes
In a series of 19 male athletes with advanced capitellar OCD lesions using 3.5-mm plugs from the periphery of the lateral femoral condyle of the knee, 18 patients were pain-free at median follow-up of 45 months.11 One had mild occasional pain. The Timmerman-Andrews score improved from 131 to 191 points. Only 1 patient had mild anterior knee pain in the donor knee with stair climbing. Radiographic evidence of osteoarthritis was not seen at follow-up.
In another 10 patients treated with mosaicplasty for capitellar OCD, 8 had excellent results while 2 had poor results.26 The patients with poor results had lesions that involved the lateral aspect of the capitellum, which was attributed to grafts being poorly fixed because of the defect location. In yet another series of 8 patients with advanced capitellar OCD lesions treated with mosaicplasty, at 2-year follow-up, 7 were pain-free and 1 had mild pain.12 No degenerative changes were seen on follow-up radiographs. Similarly, 7 patients treated with mosaicplasty had significant improvements in outcome scores at a mean follow-up of 5 years.1 Follow-up radiographs did not show degenerative arthritis, and 6 of the 7 were asymptomatic.
Return to sport
In one report, 17 of 19 patients returned to their previous level of sport after a mosaicplasty while 2 switched sports.11 In a separate report, 6 of the 8 patients (including 3 pitchers) returned to a high level of baseball.12 In a series of 18 baseball players followed for a mean of 3.5 years after a mosaicplasty, 17 of 18 players could throw without pain 6 months after surgery.33 The one that could not had a grade 4 lesion greater than 15 mm in diameter. Six of 9 players returned to their previous sports level within 1 year of surgery. Eight of 9 patients with grade 4 lesions fully recovered and returned to sports.
Osteochondral Allograft Transfer
The advantages of fresh osteochondral allograft include the potential for improved contour of the capitellum.9 Although donor site morbidity has not been a significant problem after osteochondral autograft transfer, it still remains a theoretical concern for some surgeons.14 The disadvantages of osteochondral allograft transfer include the cost, donor availability, and potential for disease transmission.
Conclusions
Debridement of capitellar OCD results in good short- and midterm outcomes.6 Approximately 80% to 85% of patients are able to return to sports after debridement.3,19 Mild persistent pain is common after debridement. Larger defects that are greater than 50% of the articular surface, more than 1 cm in diameter, or violate the lateral margin of the capitellum are associated with worse outcomes after debridement.18,27 However, these data are far from conclusive and do not preclude debridement in these situations. Long-term follow-up studies demonstrate that arthritis in the radiocapitellar joint can occur after debridement.2,28
References
- 1. Ansah P, Vogt S, Ueblacker P, Martinek V, Woertler K, Imhoff AB. Osteochondral transplantation to treat osteochondral lesions in the elbow. J Bone Joint Surg Am. 2007;89(10):2188-2194 [DOI] [PubMed] [Google Scholar]
- 2. Bauer M, Jonsson K, Josefsson PO, Lindén B. Osteochondritis dissecans of the elbow: a long-term follow-up study. Clin Orthop Relat Res. 1992;(284):156-160 [PubMed] [Google Scholar]
- 3. Brownlow HC, O’Connor-Read LM, Perko M. Arthroscopic treatment of osteochondritis dissecans of the capitellum. Knee Surg Sports Traumatol Arthrosc. 2006;14(2):198-202 [DOI] [PubMed] [Google Scholar]
- 4. Byrd JW, Elrod BF, Jones KS. Elbow arthroscopy for neglected osteochondritis dissecans of the capitellum. J South Orthop Assoc. 2001;10(1):12-16 [PubMed] [Google Scholar]
- 5. Camathias C, Festring JD, Gaston MS. Bioabsorbable lag screw fixation of knee osteochondritis dissecans in the skeletally immature. J Pediatr Orthop B. 2011;20(2):74-80 [DOI] [PubMed] [Google Scholar]
- 6. Chen NC. Osteochondritis dissecans of the elbow. J Hand Surg Am. 2010;35(7):1188-1189 [DOI] [PubMed] [Google Scholar]
- 7. De Smet AA, Fisher DR, Graf BK, Lange RH. Osteochondritis dissecans of the knee: value of MR imaging in determining lesion stability and the presence of articular cartilage defects. AJR Am J Roentgenol. 1990;155(3):549-553 [DOI] [PubMed] [Google Scholar]
- 8. De Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for stability of osteochondritis dissecans in the knee and ankle. Skeletal Radiol. 1996;25(2):159-163 [DOI] [PubMed] [Google Scholar]
- 9. Gross AE. Fresh osteochondral allografts for post-traumatic knee defects: surgical technique. Operative Techniques Orthop. 1997;7:334-339 [Google Scholar]
- 10. Hangody L, Kish G, Kárpáti Z, Udvarhelyi I, Szigeti I, Bély M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998;21:751-756 [DOI] [PubMed] [Google Scholar]
- 11. Iwasaki N, Kato H, Ishikawa J, Masuko T, Funakoshi T, Minami A. Autologous osteochondral mosaicplasty for osteochondritis dissecans of the elbow in teenage athletes. J Bone Joint Surg Am. 2009;91(10):2359-2366 [DOI] [PubMed] [Google Scholar]
- 12. Iwasaki N, Kato H, Ishikawa J, Saitoh S, Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34(8):1233-1239 [DOI] [PubMed] [Google Scholar]
- 13. Iwasaki N, Kato H, Kamishima T, Minami A. Sequential alterations in magnetic resonance imaging findings after autologous osteochondral mosaicplasty for young athletes with osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 2009;37(12):2349-2354 [DOI] [PubMed] [Google Scholar]
- 14. Iwasaki N, Kato H, Kamishima T, Suenaga N, Minami A. Donor site evaluation after autologous osteochondral mosaicplasty for cartilaginous lesions of the elbow joint. Am J Sports Med. 2007;35(12):2096-2100 [DOI] [PubMed] [Google Scholar]
- 15. Jones KJ, Wiesel BB, Sankar WN, Ganley TJ. Arthroscopic management of osteochondritis dissecans of the capitellum: mid-term results in adolescent athletes. J Pediatr Orthop. 2010;30(1):8-13 [DOI] [PubMed] [Google Scholar]
- 16. Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185(6):1453-1459 [DOI] [PubMed] [Google Scholar]
- 17. Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21(4):449-451 [DOI] [PubMed] [Google Scholar]
- 18. Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37 [DOI] [PubMed] [Google Scholar]
- 19. Miyake J, Masatomi T. Arthroscopic debridement of the humeral capitellum for osteochondritis dissecans: radiographic and clinical outcomes. J Hand Surg Am. 2011;36(8):1333-1338 [DOI] [PubMed] [Google Scholar]
- 20. Morrey BF, Adams RA. Semiconstrained arthroplasty for the treatment of rheumatoid arthritis of the elbow. J Bone Joint Surg Am. 1992;74(4):479-490 [PubMed] [Google Scholar]
- 21. Nobuta S, Ogawa K, Sato K, Nakagawa T, Hatori M, Itoi E. Clinical outcome of fragment fixation for osteochondritis dissecans of the elbow. Ups J Med Sci. 2008;113(2):201-208 [DOI] [PubMed] [Google Scholar]
- 22. Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14(8): 797-803 [DOI] [PubMed] [Google Scholar]
- 23. Ruchelsman DE, Hall MP, Youm T. Osteochondritis dissecans of the capitellum: current concepts. J Am Acad Orthop Surg. 2010;18(9):557-567 [DOI] [PubMed] [Google Scholar]
- 24. Schenck RC, Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78(3):439-456 [PubMed] [Google Scholar]
- 25. Schoch B, Wolf BR. Osteochondritis dissecans of the capitellum: minimum 1-year follow-up after arthroscopic debridement. Arthroscopy. 2010;26(11):1469-1473 [DOI] [PubMed] [Google Scholar]
- 26. Shimada K, Yoshida T, Nakata K, Hamada M, Akita S. Reconstruction with an osteochondral autograft for advanced osteochondritis dissecans of the elbow. Clin Orthop Relat Res. 2005;(435):140-147 [DOI] [PubMed] [Google Scholar]
- 27. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214 [DOI] [PubMed] [Google Scholar]
- 28. Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;(363):108-115 [PubMed] [Google Scholar]
- 29. Takahara M, Shundo M, Kondo M, Suzuki K, Nambu T, Ogino T. Early detection of osteochondritis dissecans of the capitellum in young baseball players: report of three cases. J Bone Joint Surg Am. 1998;80(6):892-897 [DOI] [PubMed] [Google Scholar]
- 30. Takeda H, Watarai K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30(5):713-717 [DOI] [PubMed] [Google Scholar]
- 31. Timmerman LA, Andrews JR. Arthroscopic treatment of posttraumatic elbow pain and stiffness. Am J Sports Med. 1994;22(2):230-235 [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi K, Sweet FA, Bindra R, Morrey BF, Gelberman RH. The extraosseous and intraosseous arterial anatomy of the adult elbow. J Bone Joint Surg Am. 1997;79(11):1653-1662 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720 [DOI] [PubMed] [Google Scholar]



