Abstract
Ketorolac tromethamine (Toradol®) is a non-steroidal anti-inflammatory drug that has potent analgesic and anti-inflammatory properties. It can be administered orally, intravenously, intramuscularly, or via a nasal route. Ketorolac injections have been used for several years in the National Football League (NFL), in both the oral and injectable forms, to treat musculoskeletal injuries and to prevent post-game soreness. In an attempt to determine the appropriate use of this medication in NFL players, the NFL Team Physician Society appointed a Task Force to consider the best available evidence as to how ketorolac should be used for pain management in professional football players. These treatment recommendations were established based on the available medical literature taking into consideration the pharmacokinetic properties of ketorolac, its accepted indications and contraindications, and the unique clinical challenges of the NFL. The Task Force recommended that 1) ketorolac should only be administered under the direct supervision and order of a team physician; 2) ketorolac should not be used prophylactically as a means of reducing anticipated pain either during or after participation in NFL games or practices and should be limited to those players diagnosed with an injury or condition and listed on the teams’ injury report; 3) ketorolac should be given in the lowest effective therapeutic dose and should not be used in any form for more than 5 days; 4) ketorolac should be given in its oral preparation under typical circumstances; 5) ketorolac should not be taken concurrently with other NSAIDs or by those players with a history of allergic reaction to ketorolac, other NSAIDs or aspirin; and 6) ketorolac should not be used by a player with a history of significant gastrointestinal bleeding, renal compromise, or a past history of complications related to NSAIDs.
Keywords: Toradol, Ketorolac, NFL, Football
Beginning with the introduction of ibuprofen in the 1950s, nonsteroidal anti-inflammatory drugs (NSAIDs) have been prescribed for athletic injuries in an attempt to blunt the body’s inflammatory response to injury, control pain, and aid in the return to sports.26 There are a variety of both prescription and nonprescription medications in this class of drugs. Among athletes using prescription medication, NSAIDs have been found to be among the most frequently prescribed (8.1%).1 Unfortunately, these agents are not without complications (see the following). Adverse events were reported in 20% of athletes using NSAIDs for a variety of musculoskeletal complaints.1
Athletes may even take NSAIDs as a preventive measure. For example, during the 2000 Olympic Games in Sydney, Canadian athletes used NSAIDs more than any other medication.17 Similarly, a survey of American football players showed that 1 out of 7 high school athletes took NSAIDs daily and that 29% of college athletes took them as a preventive measure on the day of a game.30 Warner et al31 found a similar occurrence in their study of athletes; independent of their analgesic effect, the athletes mentioned a potential performance improvement to justify taking these medications.
Ketorolac tromethamine (Toradol®) is an NSAID that has potent analgesic and anti-inflammatory properties. It can be administered orally, intravenously, intramuscularly, or via a nasal route. Ketorolac has been used principally for its analgesic properties following acute strains and sprains, overuse injuries, and as an adjunct to narcotic medication following surgery. Ketorolac injections have been used for several years in the National Football League (NFL), in both the oral and injectable forms, to treat musculoskeletal injuries and to prevent postgame soreness. The only study, to date, examining the prevalence of ketorolac use in professional sports was performed by Tokish et al in 2002.29 These authors investigated the use of injectable ketorolac in NFL teams during the 2001 season. Their study revealed that 28 of the 30 teams that responded to their survey used intramuscular ketorolac. Game-day usage was reported at 93% with pain relief of 1 to 2 days noted in 50% to 75% of players. There were 6 adverse reactions reported, including 4 muscle injuries, 1 gastrointestinal (GI) complaint, and 1 case of postinjection soreness. Anecdotally, some NFL medical staffs felt that ketorolac injections were considered more powerful than other NSAIDs because of the route of administration in that many players felt that “getting a shot” was an “intrinsic sign” that they were getting a more powerful medicine.29 Overall, these authors found ketorolac to be safe and effective when used in the pregame setting of the NFL. However, they were careful to recommend further study to develop standardized guidelines for ketorolac use in athletes to protect both players and medical staffs from potential complications. Since the publication of this study, it is widely believed by NFL team physicians that the use of ketorolac has increased in prevalence not only in the NFL but also in NCAA Division I football. However, we are unaware of any objective documentation proving this hypothesis.
Sports medicine specialists often use injections to deliver the intended medication directly to the site of injury (anesthetic or corticosteroid) or to provide a medication when oral dosing is not possible. Sterile preparation may reduce the risk of infection, but this can be challenging in the game-day training room or hotel setting. In addition, injections clearly pose a risk of bleeding and injury to adjacent structures. As with the administration of any other form of invasive treatment, informed consent has been recommended prior to administering injections to athletes.27
Injections are typically perceived as a more aggressive form of treatment that has recently garnered attention from the lay media as a result of ketorolac injections used prior to competition in the NFL. Injection of medication to treat sports-related conditions is not unheard of, but there is no evidence of increased effectiveness owing to this route of administration. Orchard24 published the results of a case series involving professional Australian Rules football and rugby players treated with local anesthetic injections to facilitate a quicker return to play. This study showed an 8% complication rate associated with the use of injections in these players and was an important first step in trying to adequately define the risks and complications associated with injections following athletic injuries. Public perception of team physicians administering various therapeutic (or “pain killing”) injections to allow athletes to return to competition likely varies depending on the level of competition and public “visibility” of the athlete. Moreover, the literature is deficient in terms of the ethical considerations implicit with the administration of injectable medications in the athletic setting solely for the athlete to return to competition.
Mechanism of Action of Ketorolac
The anti-inflammatory, antipyretic, and analgesic (pain-killing) properties of all NSAIDs are mediated through the inhibition of cyclooxygenase (COX), the pivotal enzyme responsible for the conversion of arachidonic acid to prostaglandins and thromboxanes.10,20,32 Prostaglandins are lipids that mediate a number of biological responses, some protective, others potentially harmful. It is now known that there are at least 2 cyclooxygenase subtypes: COX-1 and COX-2. COX-1 is often referred to as a constitutive subtype because it is found in the kidney, GI mucosa, uterus, and platelets and results in the maintenance of renal blood flow, production of mucosal and other GI cytoprotective factors, and platelet aggregation. COX-2 is normally found in the brain and kidney and, when induced by inflammatory mediators, becomes measurable in macrophages, gastric epithelium, vascular endothelium (particularly in the renal vasculature), and synoviocytes. Because it is largely detectable when upregulated, it is referred to as the inducible subtype.10,32
Ketorolac is a nonspecific COX inhibitor and, as such, inhibits both isoforms of COX, decreasing synthesis of prostaglandins. It has potent analgesic and anti-inflammatory properties that can be administered orally, intravenously, intramuscularly, and intranasally. It acts by blocking the synthesis of prostaglandins in the COX pathway. In addition to the anti-inflammatory effects of ketorolac, its effect decreases prostaglandin levels, which decreases the sensitivity of afferent nerve receptors, thereby ameliorating pain.8 Many studies have investigated the pain-relieving properties of intramuscular ketorolac in the acute postoperative period. These studies have shown ketorolac to be as effective and, in some cases, more effective than opioids for pain control in various dental pain models, pain conditions, and following anterior cruciate ligament reconstruction.3,19,21 Combination therapy with ketorolac and opioids has been shown to result in a 25% to 50% reduction in narcotic usage in the first 1 to 2 days postoperatively.13 In addition, the side effects of nausea and sedation are typically less common with ketorolac compared with meperidine or acetaminophen-codeine and may be decreased further when ketorolac is combined with these narcotic agents. As with all NSAIDs, whether pain relief and anti-inflammatory effects are linked or separate has not been determined; similarly, it is unknown whether there are different dose-response relationships between analgesic and anti-inflammatory effects. Studies with this class of drugs have been more oriented toward determining efficacy than sorting out mechanisms of effect. For ketorolac, specifically, most studies have been to support its approval and labeling for short-term analgesia. So far, less is known about its anti-inflammatory effects.
Pharmacologic Properties of Ketorolac
Ketorolac reaches its peak plasma concentration within 20 minutes when taken orally and within 45 minutes when administered via an intramuscular (IM) route.6 Its half-life is 6.5 hours in young, healthy adults, with oral bioavailability of 100%. The drug is 99% protein bound, metabolized in the liver through glucuronidation and oxidation, and excreted almost entirely by the kidneys as its metabolites.5 This relatively rapid turnover results in 90% of the drug clearing the plasma within 1 day.13 Somewhat unexpectedly, in patients with renal failure, the plasma clearance is decreased, with a mean half-life of 9 to 10 hours.13 This change in kinetics in patients with renal disease is due to accumulation of the glucuronide metabolite that then decomposes in plasma back to the parent drug—a phenomenon called “futile cycling.” Therefore, ketorolac needs downward dose adjustment in patients with renal insufficiency. However, ketorolac (like all NSAIDs) can decrease renal function in patients with renal disease; the more prudent alternative is to avoid the drug in such patients. No gender differences in pharmacokinetics have been reported. As expected, the elimination half-life and renal clearance are similar between oral and IM administration, though time to maximum concentration has been shown to be only 20 minutes for oral ketorolac compared with 45 minutes for the IM route.6 Buckley and Brogden6 have determined that there is direct proportionality between the maximum concentration (oral: 0.86 mg/L, IM: 2.99 mg/L) and area under the plasma concentration time-curve (oral: 2.84 mg/L × h, IM: 11.3 mg/L × h), for the 10-mg oral and 30-mg IM doses, respectively (Table 1). Jung et al,15 using a 3-way cross-over design, compared a single 10-mg dose of ketorolac via intravenous (IV), IM, and oral routes to 15 healthy subjects. They found that the mean plasma concentration-time curves for all 3 routes were nearly identical (Figure 1, Jung et al). In addition, the pharmacokinetics of this drug were found to be linear. Therefore, higher doses should provide higher plasma concentrations. Given this pharmacokinetic profile, the oral and IM dose equivalents are theoretically the same such that an oral dose of 30 mg has the same drug kinetics as an IM dose of 30 mg except that the oral route has approximately a 30-minute earlier peak concentration and presumably time of peak effect.6 Oral ketorolac was originally evaluated and approved by the Food and Drug Administration (FDA) only after IM or IV administration. However, the drug is now most commonly given alone in its oral form, which would be considered an off-label usage. Because of the established safety and efficacy of the oral version of ketorolac, it is unlikely that further evaluation by the manufacturer or FDA is warranted (Craig Brater, MD, personal communication, February 22, 2012).
Table 1.
Pharmacokinetic parameters of ketorolac after single doses.6
| Young Volunteers (n = 16) |
Elderly Volunteers (n = 12) |
Patients With Renal Impairment (n = 10) |
Patients With Hepatic Impairment (n = 7) |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | 10 mg PO | 30 mg IM | 10 mg PO | 30 mg IM | 10 mg PO | 30 mg IM | 10 mg PO | 30 mg IM |
| AUC, mg/L × h | 2.84 | 11.3 | 4.16* | 15.7 | 7.90* | 25.1* | 3.23 | 12.7 |
| Cmax, mg/L | 0.86 | 2.99 | 0.93 | 2.51 | 0.92 | 2.57 | 0.87 | 2.62 |
| tmax, h | 0.33 | 0.75 | 0.73* | 1.03 | 0.73* | 0.83 | 0.76* | 0.61 |
| t1/2, h | 4.69 | 4.45 | 6.21* | 7.01* | 9.91* | 9.62* | 4.46 | 5.43* |
| CL, L/h/kg | 0.033 | 0.027 | 0.023* | 0.019* | 0.019* | 0.016 | 0.032 | 0.029 |
PO, orally; IM, intramuscularly; AUC, area under the plasma concentration-time curve; Cmax, maximum plasma concentration; tmax, time to Cmax; t1/2, terminal elimination half-life; CL, total plasma clearance.
P ≤ 0.05, compared with young or healthy subjects.
Figure 1.
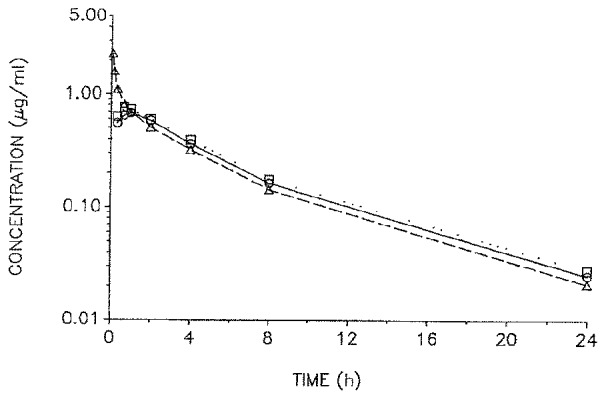
Mean plasma concertration-time profiles of ketorolac after intravenous (△—△), intramuscular (□—□) and oral (○—○) administration of 10 mg ketorolac tromethamine to 15 healthy subjects.
An intranasal (IN) formulation of ketorolac has been recently developed. In comparing the pharmacokinetics and safety of IN versus IM ketorolac, McAleer et al18 found that IN ketorolac was rapidly and well absorbed with a time to maximum concentration of 30 to 45 minutes and a half-life of 5 to 6 hours. Relative bioavailability of IN compared with IM dosing was found to be 75% and 67% at the 15-mg and 30-mg dose levels, respectively. Thus, the IN dosage of 30 mg produces a plasma level roughly equivalent to the 20-mg IM dose. Therefore, it appears that IN ketorolac is rapidly and well absorbed, with only a slightly reduced bioavailability compared with the IM route.
The oral form of ketorolac comes in 10-mg tablets. The usual oral dosage is 10 mg every 6 hours with a maximum of 40 mg per day in healthy adults. This maximum oral dosing was based on the premise that oral administration would follow IM/IV dosing in postoperative patients.16 Intramuscular and IV formulations are 15 mg/mL and 30 mg/mL. The typical dose is either 30 mg or 60 mg with a maximum daily parenteral dosage not to exceed 120 mg. The relatively new IN form of ketorolac is 15.75 mg per spray. The IN dosage is 1 spray in each nostril (31.5 mg) every 6 to 8 hours, not to exceed 4 doses per day (126 mg). The higher doses of ketorolac do not appear to confer increased pain relief over the lesser dose, though the risk of side effects are higher.15
Accepted Indications for Ketorolac
Ketorolac is indicated for the short-term (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level and only as continuation treatment following IV or IM dosing of ketorolac if necessary.15
Contraindications to the Use of Ketorolac
Ketorolac is contraindicated in patients with active peptic ulcer disease, in patients with recent GI bleeding or perforation, and in patients with a history of peptic ulcer disease or GI bleeding. Elderly patients are at greater risk for serious GI events. It is also contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft surgery, in patients with advanced renal impairment, and in patients at risk for renal failure due to volume depletion. Because ketorolac inhibits platelet function, it is therefore contraindicated in patients with suspected or confirmed cerebrovascular bleeding, hemorrhagic diathesis, incomplete hemostasis, and high risk of bleeding. Ketorolac is contraindicated as a prophylactic analgesic before any major surgery, in patients currently receiving aspirin or other NSAIDs (because of the cumulative risk of inducing serious NSAID-related side effects), and intraoperatively when hemostasis is critical (because of the increased risk of bleeding).15 Ketorolac is not indicated for use in pediatric patients and is not indicated for minor or chronic painful conditions.16
Side Effects of Ketorolac
Concerns over the high incidence of reported side effects with ketorolac has led to its withdrawal in several countries, while in others its permitted dosage and maximum duration of treatment have been reduced. From 1990 to 1993, 97 reactions with a fatal outcome were reported worldwide. Increasing the dose of ketorolac beyond a daily maximum of 40 mg in adults will not provide improved efficacy but will increase the risk of developing serious adverse events.16
Gastrointestinal Side Effects
Gastrointestinal symptoms such as heartburn, nausea, diarrhea, and occult fecal blood loss are among the most common side effects of NSAIDs.10,26 Ten to thirty percent of prescription NSAID users develop dyspepsia, 30% endoscopic abnormalities, 1% to 3% symptomatic gastroduodenal ulcers, and 1% to 3% GI bleeding requiring hospitalization. It is known that the risk of GI side effects increases in a linear fashion with the daily dose and duration of use.26 Risk also increases with age. These side effects occur via a systemic mechanism from the inhibition of prostaglandin synthesis, as well as through a local irritation of the GI mucosa. There are no published studies evaluating the effect of ketorolac on athletes in terms of GI side effects.26 Preventive strategies to reduce the risk of GI toxicity with NSAID use include short dosing intervals, taking the medication with food, and use of a concurrent proton-pump inhibitor or misoprostal.10,20
Renal Side Effects
In the kidney, prostaglandin E2 has a constitutive function to decrease sodium reabsorption, and prostaglandin I2 (prostacyclin) constitutively increases potassium excretion. Prostacyclin I2 also functions as a potent vasodilator and is critical to preservation of renal blood flow and glomerular filtration rate. Inhibition of COX activity in the kidney by NSAIDs, including ketorolac, has relatively mild consequences in healthy individuals but can lead to serious adverse events in patients whose renal function is prostaglandin dependent.4 Prostaglandin inhibition by NSAIDs may result in sodium retention, hypertension, edema, and hyperkalemia. No studies, to date, have been performed that allow meaningful comparisons of different NSAIDs at comparable doses.
In a multicenter trial of over 20 000 patients, Feldman et al9 has shown that the risk of renal failure was 1.1% with use of either ketorolac or opioids. However, multivariate adjusted rate ratios comparing ketorolac to opioids were 1.00 for less than 5 days and 2.08 for more than 5 days of therapy. Therefore, the FDA prohibits treatment with ketorolac beyond 5 continuous days irrespective of the route of administration. Patients who are at risk for adverse renal events should be monitored with caution when using ketorolac or other NSAIDs (selective or nonselective). They include patients with congestive heart failure, renal disease, or hepatic disease. They also include patients with a decrease in actual or effective circulating blood volume (eg, dehydrated athletes with or without sickle cell trait), hypertensives, or patients on renin-angiotensin-aldosterone-system inhibitors (formerly ACE inhibitor) or other agents that affect potassium homeostasis.4
Effects on Hemostasis
Ketorolac may increase the risk of internal bleeding related to its temporary inhibitory effects on platelet function. Several studies have examined this potentially detrimental effect of ketorolac on the increased risk of hemorrhage in the early perioperative period following anterior cruciate ligament reconstruction21 and plastic surgical procedures.7 Despite the theoretical association between ketorolac and the risk of hemorrhage, there have been no studies documenting a bleeding complication related to its use following contact or collision sports such as football. Mynster and Singer22 investigated the effects of IM ketorolac on the bleeding times of 20 healthy volunteers. The authors found that a single 60-mg injection of ketorolac was associated with a 50% prolongation of the bleeding time 4 hours following injection, but they were unable to determine if this finding was clinically relevant. Tokish et al commented that this effect warrants reconsideration of game-day injections for NFL players.29
Niemi et al23 compared IV ketoprofen, ketorolac, and diclofenac on platelet function. These authors found that all 3 NSAIDs caused reversible platelet dysfunction, but ketorolac had the most lasting effect with platelet dysfunction persisting 24 hours after use. The clinical significance of these findings are mixed, as Fragen et al11 found a statistically significant but clinically unimportant reduction in hematocrit in patients undergoing total knee arthroplasty. Garcia Rodriquez et al12 investigated the risk of hospitalization for upper GI bleeding associated with ketorolac against other NSAIDs. Ketorolac users had a relative risk of 5 compared with nonusers, and use was the most significant risk factor. Other NSAID users had a relative risk of 4.4 compared with nonusers. These authors concluded that ketorolac use carried an unfavorable risk-benefit profile compared to other NSAIDs and cautioned against the drug’s routine use. Strom et al28 found an increased risk of GI and operative site bleeding with ketorolac use postoperatively, but the risk was dose and duration dependent, with patients treated for more than 5 days showing the highest risk of significant bleeding. It should be noted that the Physician’s Desk Reference specifically states, “Ketorolac Tromethamine is contraindicated as a prophylactic analgesic before any major surgery, and is contraindicated intraoperatively when hemostasis is critical because of the increased risk of bleeding.”15
Cardiovascular Side Effects
Cardiovascular toxicity related to nonsalicylated NSAID use is generally not of concern in otherwise healthy young adults. Cardiovascular toxicity came to light with the emergence of the selective COX-2 inhibitors. It has been theorized that the disruption in the balance between prostacyclin and thromboxane formation by selective COX-2 inhibitors may increase this risk.2 The selective COX-2 inhibitors, via their inhibition of prostacyclin formation, favor increased thromboxane formation and subsequent platelet aggregation. Current data suggest that selective nonsalicylated NSAIDs may increase cardiovascular risk, but these data are conflicting.2 In 2005 the FDA requested the makers of prescription and over-the-counter nonsalicylated NSAIDs to include a boxed warning highlighting the increased risk for cardiovascular events as well as GI toxicity, including the risk for life-threatening GI bleeding.
Other Side Effects
Systemic side effects from the use of ketorolac include headache, vasodilatation, asthma, and weight gain related to fluid retention. Several animal and human clinical trials suggest that NSAIDs such as ketorolac negatively affect fracture healing.20 Prostaglandins, particularly PGE2, play a major role in bone formation and have been shown to be critical in bone repair. Because fracture healing relies on inflammation and formation of prostaglandins, any medication that blocks this process has the potential to impair fracture healing. Most of the published studies in humans are retrospective in design. Studies have shown an NSAID-associated delay in healing of tibial fractures, humeral shaft fractures, and other various long-bone fractures.20 In separate studies, Glassman et al14 and Reuben et al25 showed that postoperative ketorolac significantly increased the risk of nonunion after spinal fusion. No study has linked ketorolac use to delayed healing of athletically induced stress fractures. On the basis of these findings, avoidance of NSAIDs in the setting of acute traumatic fractures or stress fractures at higher risk for nonunion is recommended.20
Overview of Recommendations for Ketorolac Use in the NFL
Ketorolac has been safely used for several years in a variety of patient populations for the temporary relief of moderate to severe pain and inflammation. In general, NFL players are superbly fit and healthy with little risk of experiencing any of the known complications associated with the use of ketorolac. However, given the nature of professional football and the risk of injury associated with collision sports, it is the purpose of this task force to provide NFL physicians with therapeutic guidelines on the use of ketorolac to decrease the potential risk of severe complications associated with the nonsteroidal anti-inflammatory class of drugs—in particular, the increased risk of hemorrhage resulting from a significant collision or trauma. Despite the fact that, based on computerized literature searches, there have been no documented cases of severe complications associated with the use of ketorolac in NFL players, we are concerned with the potential for such an occurrence in this cohort of patients (players). First, given the admonition by the FDA that ketorolac not be used as a prophylactic medication prior to major surgery or where significant bleeding may occur,15 it is logical to also avoid prophylactic ketorolac use prior to collision sports such as football, where the risk of internal hemorrhage may be serious.
Second, the task force is concerned regarding the perceived increase in NFL players requesting IM ketorolac injections as a prophylactic medication to reduce the anticipated pain during, as well as after competition. The use of IM ketorolac, though safe from a medical perspective, has also been unfavorably viewed by the media and public as a necessary adjunct to pregame preparation. The perception of NFL players getting “shot up” before competition has shed an unfavorable light on the NFL as well as on team physicians who are perceived as being complicit with the players’ desire to play at all costs, irrespective of the medical consequences. Finally, given their relative safety, proven efficacy, and similar pharmacokinetic profiles of oral and IN ketorolac compared with the IM route of administration, it would appear preferable to use the lowest oral or IN forms of ketorolac instead of the IM or IV forms. The fact that oral ketorolac has a quicker onset of action than the IM form also favors the oral route of administration. Since ketorolac’s bioavailability depends on plasma volume rather than body mass, the same dose can be administered to a 300-lb lineman as would be given to a 200-lb wide receiver.
It is with these considerations in mind that this task force has proposed the following recommendations for ketorolac use in the NFL, keeping in mind that each team physician is ultimately free to practice medicine as he or she feels is in the best interest of the patient. These proposed recommendations are based on the available evidence from the medical literature, taking into consideration the pharmacokinetic properties of ketorolac, its accepted indications and contraindications, and the unique clinical challenges of the NFL.
Task Force Recommendations
Ketorolac should be administered only under the direct supervision and order of a team physician.
Ketorolac should not be used prophylactically as a means of reducing anticipated pain either during or after participation in NFL games or practices.
Ketorolac use should be limited to those players diagnosed with an injury or condition and listed on the teams’ latest injury report or following a physician-diagnosed injury or condition that occurs after the last injury report has been submitted to the NFL prior to competition.
Ketorolac should be given in the lowest effective therapeutic dose and should not be used in any form for more than 5 days. There is no evidence that an increase in dosage is necessary for players with larger body mass.
Ketorolac should be given in its oral preparation under typical circumstances, as it is recognized that the oral preparation (1) has a faster onset of action than the IM preparation, (2) has a duration of action that is equivalent to the IM and IV forms, and (3) has a plasma concentration-time curve that is nearly identical to the IM and IV preparations.
IM and IV injection of ketorolac should not be used except following an acute, game-related injury where significant visceral or central nervous system bleeding is not expected and where other oral or IN pain medications are inadequate or not tolerated. If IM or IV ketorolac is felt to be appropriate by the treating physician, the lowest possible dosage should be used.
Ketorolac should not be taken concurrently with other NSAIDs.
Ketorolac should not be taken by those players with a history of allergic reaction to ketorolac, other NSAIDs, or aspirin. In addition, a player with a history of significant GI bleeding, renal compromise, or a past history of complications related to NSAIDs should not take ketorolac.
It is the goal of this task force to recommend standard procedures for the use of ketorolac in the NFL in an effort to prevent or lessen the risk any of the known and theoretical side effects of this medication. The Toradol® task force believes that these recommendations for the use of ketorolac in the NFL not only reduces the potential occurrence of side effects but also maintains competitive balance taking into consideration the perceived, yet unproven, psychological benefits associated with its use.
References
- 1. Alaranta A, Alaranta H, Helenius I. Use of prescription drugs in athletes. Sports Med. 2008;38(6):449-463 [DOI] [PubMed] [Google Scholar]
- 2. American College of Rheumatology Ad Hoc Group on Use of Selective and Nonselective Nonsteroidal Anti-inflammatory Drugs Recommendations for use of selective and nonselective nonsteroidal anti-inflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59(8):1058-1073 [DOI] [PubMed] [Google Scholar]
- 3. Barber FA, Gladu DE. Comparison of oral ketorolac and hydrocodone for pain relief after anterior cruciate ligament reconstruction. Arthroscopy. 1998;14:605-612 [DOI] [PubMed] [Google Scholar]
- 4. Brater DC. Anti-inflammatory agents and renal function. Semin Arthritis Rheum. 2002;32(3):33-42 [DOI] [PubMed] [Google Scholar]
- 5. Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23(6):415-427 [DOI] [PubMed] [Google Scholar]
- 6. Buckley MMT, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39(1):86-109 [DOI] [PubMed] [Google Scholar]
- 7. Concannon MJ, Meng L, Welsh CF, Puckett CL. Inhibition of perioperative platelet aggregation using toradol (Ketorolac). Ann Plastic Surg 1993;3:264-266 [DOI] [PubMed] [Google Scholar]
- 8. Dietzel DP, Hedlund EC. Injections and return to play. Curr Sports Med Rep. 2004;3:310-315 [DOI] [PubMed] [Google Scholar]
- 9. Feldman HI, Kinman JL, Berlin JA, et al. Parenteral ketorolac: the risk for acute renal failure. Ann Intern Med. 1997;126:193-199 [DOI] [PubMed] [Google Scholar]
- 10. Feucht CL, Patel DR. Analgesics and anti-inflammatory medications in sports: use and abuse. Pediatr Clin N Am. 2010;57:751-774 [DOI] [PubMed] [Google Scholar]
- 11. Fragen RJ, Stulberg SD, Wixson R, et al. Effect of ketorolac tromethamine on bleeding and on requirements for analgesia after total knee arthroplasty. J Bone Joint Surg Am. 1995;77:998-1002 [DOI] [PubMed] [Google Scholar]
- 12. Garcia Rodriquez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39 [DOI] [PubMed] [Google Scholar]
- 13. Gillis JC, Brogden RN. Keterolac: a reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997;53(1):139-188 [DOI] [PubMed] [Google Scholar]
- 14. Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838 [DOI] [PubMed] [Google Scholar]
- 15. Jung D, Mroszczak E, Bynum L. Pharmacokinetics of ketorolac tromethamine in humans after intravenous, intramuscular, and oral administration. Eur J Pharmacol. 1988;35:423-425 [DOI] [PubMed] [Google Scholar]
- 16.Ketorolac. http://www.pdr.net/drugpages/concisemonographprint.aspx?concise=2632.
- 17. Lippi G, Franchini M, Guidi GC. Nonsteroidal anti-inflammatory drugs (NSAIDs) in athletes. Br J Sports Med. 2006;40:661-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McAleer SD, Majid O, Venables E, Polack T, Sheikh MS. Pharmacokinetics and safety of ketorolac following single intranasal and intramuscular administration in healthy volunteers. J Clin Pharmacol. 2007;47:13. [DOI] [PubMed] [Google Scholar]
- 19. McGuire DA, Sanders K, Hendricks SD. Comparison of ketorolac and opioid analgesics in postoperative ACL reconstruction outpatient pain control. Arthroscopy. 1993;9:653-661 [DOI] [PubMed] [Google Scholar]
- 20. Mehallo CJ, Drezner JA, Bytomski JR. Practical management: nonsteroidal anti-inflammatory drug (NSAID) use in athletic injuries. Clin J Sport Med. 2006;16(2):170-174 [DOI] [PubMed] [Google Scholar]
- 21. Milne JC, Russell JA, Woods GW, Dalton MD. Effect of ketorolac tromethamine (Toradol) on Ecchymosis following anterior cruciate ligament reconstruction. Am J Knee Surg 1995;1:24-27 [PubMed] [Google Scholar]
- 22. Mynster CI, Singer AJ. Effect of intramuscular toradol on bleeding times (abstract). Acad Emerg Med. 2001;8:429 [Google Scholar]
- 23. Niemi TT, Taxell C, Rosenberg PH. Comparison of intravenous ketoprofen, ketorolac, and diclofenac on platelet function in volunteers. Acta Anaesthesiol Scand. 1997;41:1353-1358 [DOI] [PubMed] [Google Scholar]
- 24. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med 2002;36:209-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth. 2005;52:506-512 [DOI] [PubMed] [Google Scholar]
- 26. Sawyer GA, Anderson BC, Raukar NP, Fadale PD: Intramuscular Ketorolac Injections in the Athlete. Sports Health. 2012;4:319-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoor S. Athletes, nonsteroidal anti-inflammatory drugs, coxibs, and the gastrointestinal tract. Curr Sports Med Rep. 2002;1:107-115 [DOI] [PubMed] [Google Scholar]
- 28. Smith BJ, Collina SJ. Pain medications in the locker room: to dispense or not. Curr Sports Med Rep. 2007;6:367-370 [DOI] [PubMed] [Google Scholar]
- 29. Strom BL, Berlin JA, Kinman JL, et al. Parenteral ketorolac and risk of gastrointestinal and operative site bleeding: a postmarketing surveillance study. JAMA. 1996;275:376-382 [PubMed] [Google Scholar]
- 30. Tokish JM, Powell ET, Schlegel TF, Hawkins RJ. Ketorolac use in the National Football League: prevalence, efficacy, and adverse effects. Phys Sportsmed. 2002;30(9):19-24 [DOI] [PubMed] [Google Scholar]
- 31. Tricker R. Painkilling drugs in collegiate athletics. J Drug Educ. 2000;30:313-324 [DOI] [PubMed] [Google Scholar]
- 32. Warner DC, Schnepf G, Barret MS, et al. Prevalence, attitudes and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J Adolesc Health. 2002;30:150-153 [DOI] [PubMed] [Google Scholar]
- 33. Ziltener JL, Leal S, Fournier PE. Non-steroidal anti-inflammatory drugs for athletes: an update. Ann Phys Rehabil Med. 2010;53:278-288 [DOI] [PubMed] [Google Scholar]


