Abstract
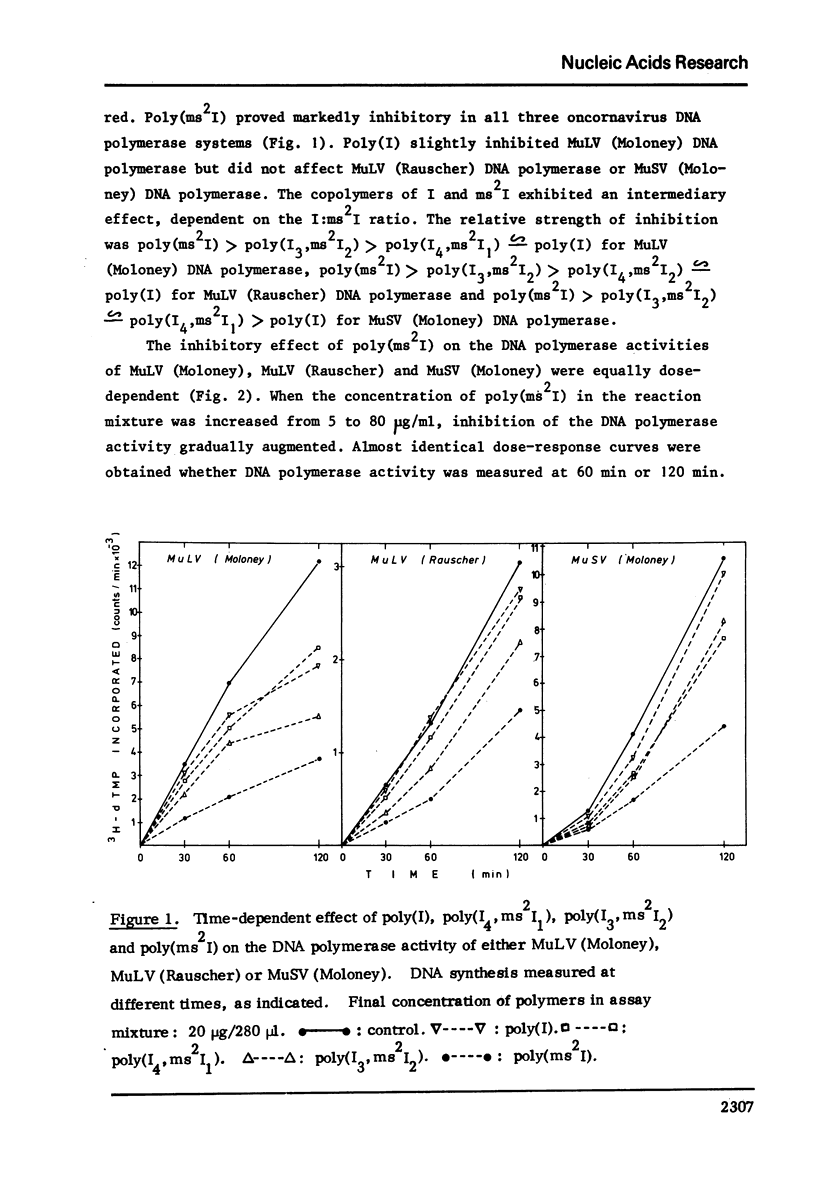
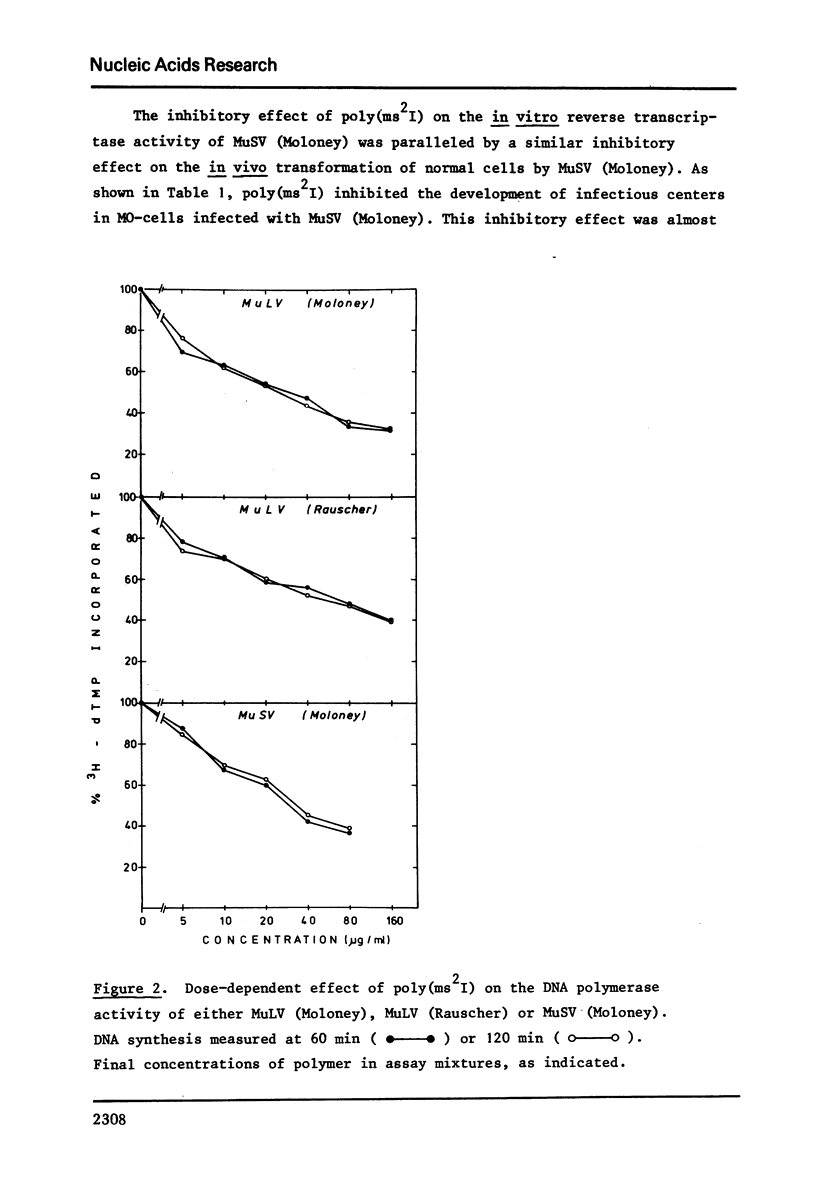
Poly (2-methylthioinosinic acid) [poly(ms2I)] was found to markedly inhibit the RNA directed DNA polymerase (reverse transcriptase) activity of murine (Moloney, Rauscher) leukemia virus and murine (Moloney) sarcoma virus, while under the same conditions the unsubstituted parent compound poly(I) showed little, if any, inhibitory effect. Copolymers of inosinic acid (I) and 2-methylthioinosinic acid2(ms2I) showed an intermediary effect, depending on the I:ms2I ratio. Poly(ms2I) also inhibited the transformation of normal cells by murine (Moloney) sarcoma virus, as assessed by an infectious center assay.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Smith R. G., Robert M. S., Gallo B. C. DNA polymerases from RNA tumor viruses and human cells: inhibition by polyuridylic acid. Science. 1972 Sep 22;177(4054):1111–1114. doi: 10.1126/science.177.4054.1111. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Carter W. A., Alderfer J. L., Ts'o P. O. Inhibition of RNA directed DNA polymerase of murine leukemia virus by 2'-O-alkylated polyadenylic acids. Biochem Biophys Res Commun. 1974 Jul 24;59(2):608–615. doi: 10.1016/s0006-291x(74)80023-9. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. R., Eckstein F., Hobbs J. B., Sternbach H., Merigan T. C. The antiviral activity of certain thiophosphate and 2'-chloro substituted polynucleotide homopolymer duplexes. Virology. 1972 May;48(2):537–545. doi: 10.1016/0042-6822(72)90064-5. [DOI] [PubMed] [Google Scholar]
- Chandra P., Bardos T. J. Inhibition of DNA polymerases from RNA tumor viruses by novel template analogues: partially thiolated polycytidylic acid. Res Commun Chem Pathol Pharmacol. 1972 Nov;4(3):615–622. [PubMed] [Google Scholar]
- Chirikjian J. G., Papas T. S. Inhibition of AMV DNA polymerase by polyriboadenylic acid containing epsilon-adenosine residues. Biochem Biophys Res Commun. 1974 Jul 24;59(2):489–495. doi: 10.1016/s0006-291x(74)80006-9. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Billiau A., Hobbs J., Torrence P. F., Witkop B. Inhibition of oncornavirus functions by 2'-azido polynucleotides. Proc Natl Acad Sci U S A. 1975 Jan;72(1):284–288. doi: 10.1073/pnas.72.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Claes P. J. A more sensitive assay system for the detection of RNA-dependent DNA polymerase in oncogenic RNA viruses. Biochim Biophys Acta. 1973 Dec 21;331(3):328–332. doi: 10.1016/0005-2787(73)90018-x. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Hattori M., Ikehara M. Antiviral activity of polynucleotides: copolymers of inosinic acid and N2-dimethylguanylic of 2-methylthioinosinic acid. Nucleic Acids Res. 1975 Jan;2(1):121–129. doi: 10.1093/nar/2.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Janik B. Antiviral activity of polynucleotides: poly(2'-fluoro-2'-deoxyuridylic acid). Biochim Biophys Acta. 1973 Sep 28;324(1):50–56. doi: 10.1016/0005-2787(73)90249-9. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Torrence P. F., Witkop B. Interferon induction by synthetic polynucleotides: importance of purine N-7 and strandwise rearrangement. Proc Natl Acad Sci U S A. 1974 Jan;71(1):182–186. doi: 10.1073/pnas.71.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Zmudzka B., Shugar D. Antiviral activity of polynucleotides: role of the 2'-hydroxyl and a pyrimidine 5-methyl. FEBS Lett. 1972 Jul 15;24(1):137–140. doi: 10.1016/0014-5793(72)80845-7. [DOI] [PubMed] [Google Scholar]
- Erickson R. J., Grosch J. C. The inhibition of avian myeloblastosis virus deoxyribonucleic acid polymerase by synthetic polynucleotides. Biochemistry. 1974 Apr 23;13(9):1987–1993. doi: 10.1021/bi00706a032. [DOI] [PubMed] [Google Scholar]
- Erickson R. J., Janik B., Sommer R. G. The inhibition of the avian myeloblastosis virus DNA polymerase by poly(U) fractions of varying chain length. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1475–1482. doi: 10.1016/0006-291x(73)90667-0. [DOI] [PubMed] [Google Scholar]
- Gerard G. F. Poly (2'O-methylcytidylate).oligodeoxyguanylate, a template-primer specific for reverse transcriptase, is not utilized by HeLa cell gamma DNA polymerases. Biochem Biophys Res Commun. 1975 Apr 7;63(3):706–711. doi: 10.1016/s0006-291x(75)80441-4. [DOI] [PubMed] [Google Scholar]
- Gerard G. F., Rottman F., Green M. Poly(2'-O-methylcytidylate)-oligodeoxyguanylate as a template for the ribonucleic acid directed deoxyribonucleic acid polymerase in ribonucleic acid tumor virus particles and a specific probe for the ribonucleic acid directed enzyme in transformed murine cells. Biochemistry. 1974 Apr 9;13(8):1632–1641. doi: 10.1021/bi00705a012. [DOI] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson D. W., Johnston M. D., Eaton M. A. Necessity for the 2'-hydroxyl group for the antiviral activity of synthetic polynucleotides. J Gen Virol. 1974 Jun;23(3):331–333. doi: 10.1099/0022-1317-23-3-331. [DOI] [PubMed] [Google Scholar]
- Leis J., Schincariol A., Ishizaki R., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. V. Rous sarcoma virus single-stranded RNA-DNA covalent hybrids in infected chicken embryo fibroblast cells. J Virol. 1975 Mar;15(3):484–489. doi: 10.1128/jvi.15.3.484-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Rottman F. Influence of increasing 2'-O-methylation on the interferon stimulating capacity of poly(rl)-poly(rC). Virology. 1974 Jul;60(1):297–301. doi: 10.1016/0042-6822(74)90389-4. [DOI] [PubMed] [Google Scholar]
- Mohr S. J., Brown D. G., Coffey D. S. Size requirement of polyinosinic acid for DNA synthesis, viral resistance, and increased survival of leukaemic mice. Nat New Biol. 1972 Dec 20;240(103):250–252. doi: 10.1038/newbio240250a0. [DOI] [PubMed] [Google Scholar]
- O'Malley J. A., Ho Y. K., Chakrabarti P., DiBerardino L., Chandra P., Orinda D. A., Byrd D. M., Bardos T. J., Carter W. A. Antiviral activity of partially thiolated polynucleotides. Mol Pharmacol. 1975 Jan;11(1):61–69. [PubMed] [Google Scholar]
- Pitha P. M., Teich N. M., Lowy D. R., Pitha J. Inhibition of murine leukemia virus replication by poly(vinyluracil) and poly(vinyladenine). Proc Natl Acad Sci U S A. 1973 Apr;70(4):1204–1208. doi: 10.1073/pnas.70.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Farrelly J. G., Ihle J. N., Pal B. C., Kenney F. T., Brown A. Effects of polyadenylic acids on functions of murine RNA tumor viruses. J Virol. 1973 Dec;12(6):1216–1225. doi: 10.1128/jvi.12.6.1216-1225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Hanna M. G., Jr, Farrelly J. G. Effects of poly(2'-O-methyladenylic acid) on susceptibility and autogenous immunity to RNA tumor virus oncogenesis in vivo. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3167–3171. doi: 10.1073/pnas.71.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence P. F., De Clercq E., Waters J. A., Witkop B. A potent interferon inducer derived from poly (7-deazainosinic acid). Biochemistry. 1974 Oct 8;13(21):4400–4408. doi: 10.1021/bi00718a025. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Waters J. A., Buckler C. E., Witkop B. Effect of pyrimidine and ribose modifications on the antiviral activity of synthetic polynucleotides. Biochem Biophys Res Commun. 1973 Jun 8;52(3):890–898. doi: 10.1016/0006-291x(73)91021-8. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Kenney F. T. Inhibition of the DNA polymerase of Rauscher leukemia virus by single-stranded polyribonucleotides. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2198–2202. doi: 10.1073/pnas.68.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Mason W. S., Drost S. D., Baltimore D. DNA polymerase activity from two temperature-sensitive mutants of Rous sarcoma virus is thermolabile. Nature. 1974 Sep 6;251(5470):27–31. doi: 10.1038/251027a0. [DOI] [PubMed] [Google Scholar]



