Abstract
Our aim was to record pancreaticobiliary endoscopic ultrasound (EUS) literature of the past 3 decades and evaluate its role based on a critical appraisal of published studies according to levels of evidence (LE). Original research articles (randomized controlled trials, prospective and retrospective studies), meta-analyses, reviews and surveys pertinent to gastrointestinal EUS were included. All articles published until September 2011 were retrieved from PubMed and classified according to specific disease entities, anatomical subdivisions and therapeutic applications of EUS. The North of England evidence-based guidelines were used to determine LE. A total of 1089 pertinent articles were reviewed. Published research focused primarily on solid pancreatic neoplasms, followed by disorders of the extrahepatic biliary tree, pancreatic cystic lesions, therapeutic-interventional EUS, chronic and acute pancreatitis. A uniform observation in all six categories of articles was the predominance of LE III studies followed by LE IV, IIb, IIa, Ib and Ia, in descending order. EUS remains the most accurate method for detecting small (< 3 cm) pancreatic tumors, ampullary neoplasms and small (< 4 mm) bile duct stones, and the best test to define vascular invasion in pancreatic and peri-ampullary neoplasms. Detailed EUS imaging, along with biochemical and molecular cyst fluid analysis, improve the differentiation of pancreatic cysts and help predict their malignant potential. Early diagnosis of chronic pancreatitis appears feasible and reliable. Novel imaging techniques (contrast-enhanced EUS, elastography) seem promising for the evaluation of pancreatic cancer and autoimmune pancreatitis. Therapeutic applications currently involve pancreaticobiliary drainage and targeted fine needle injection-guided antitumor therapy. Despite the ongoing development of extra-corporeal imaging modalities, such as computed tomography, magnetic resonance imaging, and positron emission tomography, EUS still holds a leading role in the investigation of the pancreaticobiliary area. The major challenge of EUS evolution is its expanding therapeutic potential towards an effective and minimally invasive management of complex pancreaticobiliary disorders.
Keywords: Endoscopic ultrasound, Fine needle aspiration, Contrast harmonic endoscopic ultrasound, Pancreatic tumors, Pancreatic cysts, Acute pancreatitis, Chronic pancreatitis, Bile duct stones, Duct drainage
INTRODUCTION
A unique property of endoscopic ultrasonography (EUS) is the detailed imaging of organs in close proximity to the digestive tract. This has been long documented in the investigation of the pancreaticobiliary area. Since its early days, EUS proved an accurate imaging modality for the pancreas and the extrahepatic biliary tree[1-3]. The introduction of curvilinear-array echoendoscopes and the potential of tissue sampling by EUS-guided fine needle aspiration (EUS-FNA) highly upgraded the diagnostic value of EUS and enabled its evolution to an advanced interventional technique with a wide range of applications.
A prolific trend of pancreaticobiliary EUS-related studies was recently reported, rendering pancreas and extrahepatic biliary tree the major fields of modern EUS research[4]. This review aimed to record the entire body of literature accumulated over the past 3 decades and to present a comprehensive perspective of current EUS indications, applications and test performance in the pancreatic and extrahepatic biliary tree pathology. Our objectives were then to perform a critical appraisal of published articles, based on the classification of studies according to levels of evidence (LE), in order to assess the scientific progress made in this field and to further inform policy regarding areas that need further research and improvements.
LITERATURE SEARCH
Based on our previous research on EUS literature of the period 1980-2010[4,5], all articles relevant to pancreaticobiliary diseases were extracted. The PubMed search was extended up to September 2011, to retrieve all additional publications. Moreover, the bibliographies of reviewed articles were scrutinized to obtain any other reference that eluded the primary search.
Original research articles [randomized controlled trials (RCTs), prospective and retrospective studies], meta-analyses, reviews and surveys pertinent to gastrointestinal EUS were included. Studies enrolling up to 15 patients were categorized as case series. Editorials, commentaries, letters, case reports, non-English language articles, abstracts, and articles in which EUS did not represent the principal study matter were not considered for review.
In regard to data collection, priority was assigned to the study subject, design and methods, the type and year of publication and the number of patients enrolled. All articles were classified in six major categories based on specific disease entities, anatomical subdivisions and therapeutic applications of EUS: (1) solid pancreatic tumors; (2) cystic pancreatic lesions; (3) chronic pancreatitis; (4) acute pancreatitis; (5) extrahepatic biliary tree; and (6) therapeutic EUS. The content of each study was further analyzed to identify relevant clinical issues such as the diagnostic and staging accuracy of the technique, technical properties of EUS procedures and the role of EUS-FNA.
Levels of evidence were stratified according to the North of England evidence-based guidelines[6,7]. LE Ia: Evidence obtained from meta-analysis of randomized controlled trials; LE Ib: Evidence obtained from at least one RCT; LE IIa: Evidence obtained from at least one well designed controlled study without randomization; LE IIb: Evidence obtained from at least one other type of well-designed quasi-experimental study; LE III: Evidence obtained from well-designed non-experimental descriptive studies such as comparative studies, correlation studies, and case studies; LE IV: Evidence obtained from expert committee reports or opinions, or clinical experiences of respected authorities.
A total of 1089 pertinent articles were retrieved (Figure 1). A detailed classification of the studies, according to the main subject-matters and subclasses, and the corresponding LE, is presented in Table 1. Published research focused primarily on solid pancreatic neoplasms (418 studies overall), followed by disorders of the extrahepatic biliary tree (230 studies), pancreatic cystic lesions (165 studies), therapeutic-interventional EUS (153 studies), chronic and acute pancreatitis (83 and 40 studies respectively). A uniform observation in all six categories of articles was the predominance of LE III studies followed by LE IV, IIb, IIa, Ib and Ia, in descending order. Strong evidence trials (LE Ib, IIa) and meta-analyses (Ia) were mainly recorded on pancreatic tumor diagnosis and staging, EUS-FNA of solid and cystic pancreatic lesions, common bile duct (CBD) stone detection and on the role of EUS-guided celiac plexus neurolysis (CPN). Novel therapeutic applications, like EUS-guided pancreaticobiliary drainage and pancreatic tumor therapy, are recently being increasingly addressed in well-designed studies (LE IIb, III), but data are still limited reflecting lack of maturation of these techniques. Due to the abundance of existing data, a focused description of well-evidenced issues is highlighted below, in a point-by-point form.
Figure 1.
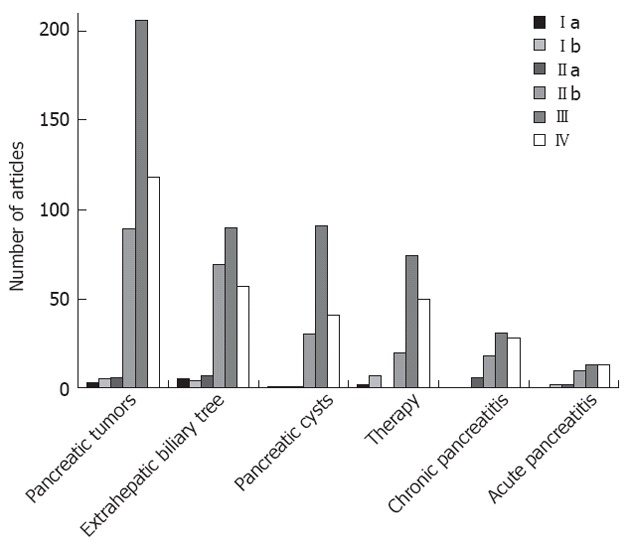
Distribution of papers in pancreaticobiliary endoscopic ultrasonography according to the levels of evidence.
Table 1.
Levels of evidence per subject: Pancreatic disorders, extrahepatic biliary tree, endoscopic ultrasonography-guided therapy
| Ia | Ib | IIa | IIb | III | IV | Total | |
| Solid pancreatic tumors | |||||||
| Diagnosis, differential diagnosis | 1 | 0 | 1 | 10 | 34 | 45 | 86 |
| EUS - FNA, TCB, echobrush | 0 | 4 | 1 | 28 | 48 | 11 | 92 |
| Staging | 2 | 0 | 0 | 17 | 30 | 18 | 67 |
| Neuroendocrine tumors | 0 | 0 | 0 | 4 | 28 | 15 | 47 |
| Molecular markers | 0 | 0 | 1 | 5 | 16 | 3 | 25 |
| Screening | 0 | 0 | 1 | 5 | 6 | 7 | 19 |
| Elastography, CH-EUS | 0 | 0 | 1 | 13 | 7 | 4 | 25 |
| Other | 0 | 1 | 1 | 7 | 37 | 11 | 57 |
| Total | 3 | 5 | 6 | 89 | 206 | 115 | 418 |
| Pancreatic cysts | |||||||
| Diagnosis, differential diagnosis | 0 | 0 | 0 | 2 | 26 | 31 | 59 |
| EUS-FNA, TCB, brushing | 1 | 0 | 1 | 10 | 17 | 2 | 31 |
| IPMN | 0 | 1 | 0 | 5 | 27 | 6 | 39 |
| Molecular markers | 0 | 0 | 0 | 7 | 3 | 0 | 10 |
| EUS follow-up, clinical outcome | 0 | 0 | 0 | 0 | 5 | 0 | 5 |
| Other | 0 | 0 | 0 | 6 | 13 | 2 | 21 |
| Total | 1 | 1 | 1 | 30 | 91 | 41 | 165 |
| Chronic pancreatitis | |||||||
| Diagnosis | 0 | 0 | 6 | 14 | 20 | 21 | 61 |
| Autoimmune pancreatitis | 0 | 0 | 0 | 2 | 7 | 2 | 11 |
| Other | 0 | 0 | 0 | 2 | 4 | 5 | 11 |
| Total | 0 | 0 | 6 | 18 | 31 | 28 | 83 |
| Acute pancreatitis | |||||||
| Diagnosis | 0 | 0 | 1 | 4 | 7 | 10 | 22 |
| CBD stones | 0 | 0 | 0 | 4 | 1 | 1 | 6 |
| Other | 0 | 2 | 1 | 2 | 5 | 2 | 12 |
| Total | 0 | 2 | 2 | 10 | 13 | 13 | 40 |
| Extrahepatic biliary tree | |||||||
| Diagnosis (in general) | 1 | 1 | 0 | 12 | 8 | 9 | 29 |
| CBD stones | 3 | 3 | 7 | 21 | 6 | 16 | 56 |
| Cholangio-Ca | 0 | 0 | 0 | 3 | 9 | 11 | 23 |
| Gallbladder | 0 | 0 | 0 | 6 | 18 | 4 | 28 |
| IDUS | 0 | 0 | 0 | 12 | 11 | 7 | 30 |
| Periampullary neoplasms | 0 | 0 | 0 | 7 | 19 | 5 | 31 |
| Other | 1 | 0 | 0 | 8 | 19 | 5 | 33 |
| Total | 5 | 4 | 7 | 69 | 90 | 57 | 230 |
| EUS-guided therapy | |||||||
| Fluid collection drainage, necrosectomy | 0 | 2 | 0 | 8 | 31 | 14 | 55 |
| Billiary duct drainage | 0 | 0 | 0 | 2 | 23 | 15 | 40 |
| EUS-CPN, CPB | 2 | 3 | 0 | 4 | 4 | 11 | 24 |
| Pancreatic tumor therapy | 0 | 1 | 0 | 6 | 9 | 6 | 22 |
| Pancreatic duct drainage | 0 | 0 | 0 | 0 | 4 | 1 | 5 |
| Other | 0 | 1 | 0 | 0 | 3 | 3 | 7 |
| Total | 2 | 7 | 0 | 20 | 74 | 50 | 153 |
EUS-FNA: Endoscopic ultrasound-fine needle aspiration; TCB: Trucut biopsy; CH-EUS: Contrast harmonic EUS; IPMN: Intraductal papillary mucinous neoplasm; CBD: Common bile duct; IDUS: Intraductal ultrasound; CPN: Celiac plexus neurolysis; CPB: Celiac plexus block.
SOLID PANCREATIC TUMORS
Diagnosis
The only meta-analysis available, comparing the test performance of different diagnostic modalities, reports a sensitivity of combined positron emission tomography/computerized tomography (PET/CT) (90.1%) higher than PET (88.4%) and EUS (81.2%) alone, but a specificity of EUS (93.2%) higher than PET (83.1%) and PET/CT (80.1%) in diagnosing primary pancreatic carcinoma[8] (Ia).
In a systematic review of the period 1986-2004, EUS was found more sensitive (range: 93%-100%) than CT (50%-89%), especially for the detection of pancreatic tumors smaller than 3 cm[9] (Ia).
Early comparative studies indicate that the sensitivity and specificity of EUS (94%-99% and 100%, respectively) is higher than transabdominal ultrasound (US) (67% and 40%), CT (69%-77% and 53%-64%) and magnetic resonance imaging (MRI) (83% and 100%), for demonstrating the presence of a pancreatic neoplasm. This is even more obvious in small pancreatic tumors of 3 cm and less. The sensitivity of detecting tumors less than 3 cm was 93% for EUS, 53% for CT, and 67% for MRI imaging. However, as with other imaging procedures, EUS was not able to differentiate reliably malignant from inflammatory pancreatic masses (accuracy 76% for malignancy and 46% for focal inflammation)[10,11] (IIb).
The presence of a dilated pancreatic duct is related to a 65% prevalence of malignancy, compared to a prevalence of 17% in its absence[12] (IIa).
Data from retrospective studies demonstrates a negative predictive value (NPV) for EUS as high as 100%, suggesting that a normal EUS of the pancreas in the setting of subtle radiologic findings, serologic abnormalities, and/or nonspecific symptoms definitively rules out the presence of pancreatic cancer[13,14] (III).
Molecular markers
Broad panel microsatellite loss and K-ras point mutation analysis can reliably be performed on EUS-FNA samples from pancreatic masses, improving the diagnostic accuracy and differentiation between malignant and benign pancreatic masses[15] (IIa). When K-ras mutation analysis is combined with cytopathology, sensitivity, specificity, positive predictive value (PPV), NPV and overall accuracy reach 88%, 100%, 100%, 63% and 90%, respectively[16] (IIb).
Mucin expression pattern of EUS-FNA aspirates could serve as a potential biological marker for malignant lesions[17] (IIb), and combination of routine cytology with positive fluorescence in situ hybridization and K-ras analyses may help the discrimination of atypical FNA samples to benign and malignant[18] (III).
Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. None of these markers was identified as an independent factor of survival prognosis[19] (IIb).
Staging
Although EUS is the best test to define vascular invasion in pancreatic and peri-ampullary cancers, the specificity (90%) is high but the sensitivity (73%) is not as high as previously suggested[20] (Ia).
EUS is superior to angiography in the preoperative assessment of vascular involvement for patients with pancreatic carcinoma (sensitivity 86% vs 21%, respectively; specificity and accuracy 71% and 81% vs 71% and 38%, respectively)[21] (IIb).
No decisive comparative data between EUS and MRI exists. The most recent report shows that EUS and MRI have marginal correlation for staging, especially in the case of advanced tumors. Therefore, both tests should be performed for accurate staging[22] (IIb).
Comparison of EUS vs CT
Helical CT is more accurate in assessing the extent of primary tumor (73%), locoregional extension, vascular invasion, distant metastases, tumor node metastasis stage and tumor resectability, whereas EUS is more accurate in assessing tumor size and lymph node involvement[23] (IIb).
For portal vein and superior mesenteric vein invasion, multislice CT (MSCT) seems superior to EUS (sensitivity 88% vs 50%, respectively and specificity 92% vs 83%). For resectability, there is no significant difference and agreement is good among the two techniques. Therefore MSCT is the imaging method of choice and routine EUS should be reserved for tumors with borderline resectability on MSCT[24] (IIb).
Compared with MSCT, EUS is superior for tumor detection and staging but similar for nodal staging and resectability of preoperatively suspected non-metastatic pancreatic cancer[25] (IIb).
EUS-FNA
The sensitivity, specificity, PPV and NPV of EUS-FNA for solid pancreatic masses reach 95%, 100%, 100% and 85%, respectively. Patients with suspicious and atypical EUS-FNA aspirates deserve further clinical evaluation[26,27] (III).
In patients with negative CT-guided biopsies, EUS-FNA yields 90% sensitivity for malignancy, 50% specificity and 84% accuracy. Corresponding values for EUS-FNA in patients with negative endoscopic retrograde cholangiopancreatography (ERCP) tissue sampling are as high as 94%, 67% and 92%, respectively[28] (IIb). A more recent RCT comparing CT/US-FNA and EUS-FNA concludes that EUS-FNA is numerically (though not quite statistically) superior to CT/US-FNA for the diagnosis of pancreatic malignancy[29] (Ib).
A lower sensitivity for EUS-FNA is observed in patients with chronic pancreatitis (CP) than in those without CP (73.9% vs 91.3%). While patients with CP had a higher NPV (88.9% vs 45.5%), no significant differences were observed for specificity (100% vs 93.8%), PPV (100% vs 99.5%), and accuracy (91.5% vs 91.4%) between those with and without CP[30] (IIb).
False positive EUS-FNA cytology was recently recorded in a large cohort trial, i.e., 1.1% when only “positive” cytology findings were interpreted as malignant and 3.8% when both suspicious and positive cytology findings were interpreted as malignant. False positive cases occurred primarily as a result of cytological misinterpretation in the setting of CP[31].
No statistically significant differences exist in the diagnostic yield of EUS-FNA between 22G vs 25G and 22G vs 19G needles, nor in the handling of different commercially available needle assemblies[32,33] (Ib).
EUS-FNA sampling with suction of solid masses increases the number of pathology slides, the sensitivity and negative predictive value, without increasing the overall bloodiness of samples[34] (Ib). Neither trained EUS performers nor cytotechnologists seem able to provide a reliable assessment of pancreatic FNA adequacy by using gross visual inspection of the specimen on a slide[35]. On the other hand, rapid on-site cytopathology performed by an attending cytopathologist shows excellent agreement with the final cytological evaluation[36] and may reduce the number of passes, ensure specimen adequacy and lower the number of inadequate samples[37] (IIb).
EUS-FNA of solid pancreatic masses is safe when performed by experienced endosonographers. The frequency of post EUS-FNA pancreatitis may be underestimated by retrospective analysis (0.64% when data was prospectively collected vs 0.26% in retrospective cohorts)[38] (IIb). A higher incidence of pancreatitis after pancreatic EUS-FNA (2%, with some more cases of silent hyperamylasemia) was recorded in a prospective controlled trial[39] (IIa).
Neuroendocrine tumors
EUS is reliable for the localization of pancreatic neuroendocrine tumors (NETs) (sensitivity, 82% and specificity, 95%) and highly accurate in estimating the actual size of these tumors (deviation within 2 mm between EUS and surgical pathology). Compared to angiography, EUS is significantly more sensitive for tumor localization (sensitivity, 82% vs 27%)[40] (IIb).
In patients with multiple endocrine neoplasia type 1 (MEN1) syndrome undergoing screening EUS, pancreatic NETs are identified in 17% of cases before the development of significant biochemical abnormalities[41] (IIb). The frequency of nonfunctioning pancreatic endocrine tumors is higher (55%) than previously thought and pancreatic EUS should be performed once MEN1 is diagnosed, to monitor disease progression[42] (IIb).
In patients with suspected pancreatic NETs undergoing EUS and MSCT, the sensitivity of EUS is greater than MSCT (92% vs 63%, respectively), particularly for insulinomas (84% vs 32%, respectively) and for lesions smaller than 2 cm. EUS may detect in up to 91% of cases missed by MSCT[43] (IIb).
Screening
A EUS-based strategy of screening for individuals at high risk for pancreatic cancer is feasible and safe. The incidence of clinically relevant findings at first screening is high, reaching 7%-10% for early asymptomatic cancer and 16% for premalignant intraductal papillary mucinous neoplasms (IPMN)-like lesions. Nevertheless, whether screening improves survival remains to be determined, as does the optimal screening interval with EUS. Moreover, without the ability to further quantify these patients’ risk, the most effective strategy in clinical and economical terms may be doing nothing[44-46] (IIa).
In familial pancreatic cancer a EUS/magnetic resonance cholangiopancreatography (MRCP) based screening program leads to the detection of potential precursor lesions of pancreatic cancer. However, the yield of an extensive screening program is low, especially since the tumorigenic value of low grade pancreatic intraepithelial neoplasia is not yet defined. Taking into account the enormous psychological stress for the tested individual and the high costs, a general pancreatic cancer screening in high-risk individuals is not justified[47] (IIb).
New imaging techniques
Detection of a hypoenhancing and inhomogeneous mass with contrast harmonic EUS (CH-EUS) accurately identifies patients with pancreatic adenocarcinoma. CH-EUS increases the detection of malignant lesions in difficult cases (patients with chronic pancreatitis or biliary stents) and helps to guide EUS-FNA. A hyper-enhancing pattern can be used to rule out adenocarcinoma[48] (IIb).
Hypoenhancement in CH-EUS yields higher sensitivity (89% vs 72%), lower specificity (88% vs 100%) and comparable accuracy (88.5% vs 86%) than EUS-FNA, for the diagnosis of pancreatic adenocarcinoma[49] (IIb).
Quantitative analysis of contrast-enhanced EUS seems to improve the efficacy of the technique. The diagnostic accuracy, based on contrast imaging pattern (84%) and time-intensity curves (TICs) (88%), was higher than that based on B-mode imaging (83%) and dynamic CT (81%). EUS in combination with TIC demonstrated increased sensitivity, specificity, and accuracy up to 96%, 93%, and 95%, respectively[50] (III).
EUS elastography is useful for the differential diagnosis of solid pancreatic masses and allows an objective evaluation of tissue stiffness[51] (IIb). The operating characteristics of the technique for detecting malignancy are: sensitivity 93%, specificity 66%, PPV 92%, NPV 69% and overall accuracy 85%[52,53] (IIb).
The sensitivity, specificity, and accuracy of combined contrast-enhanced power Doppler and real-time sono-elastography in differentiating hypovascular hard masses suggestive of pancreatic carcinoma were 76%, 95% and 83%, respectively, with a PPV and NPV of 96% and 71%, respectively[54] (IIb).
PANCREATIC CYSTS
Diagnosis
Certain morphologic features have been used to discriminate specific types of pancreatic cysts. A cystic lesion with accompanying parenchymal changes, in the absence of intracystic septation or mural nodule, is compatible with a pseudocyst. Multiple microcysts (< 3 mm) within a cystic lesion and a honeycomb appearance are typical of serous cystadenomas, but macrocystic types can also be found. Mucinous cystadenomas usually present with septations of variable thickness, a visible wall, and peripheral calcifications in up to 15% of cases. A communication between the cyst and the main pancreatic duct is strongly suggestive of IPMN[55-57] (III).
There is no universally accepted morphologic parameter to reliably predict the malignancy of pancreatic cysts by EUS. The size of cysts is generally considered suspicious for malignancy above the diameter of 3 cm; moreover, a cyst growth rate of more than 2 mm/year has been proposed to indicate a higher risk of malignancy (5-year risk 45.5% vs 1.8% for cysts with lower growth rate). The presence and size of mural nodules within the cysts is also predictive of malignancy in IPMNs[58-60] (III).
There is little more than chance interobserver agreement (IA) among experienced endosonographers for the diagnosis of neoplastic versus non-neoplastic (fair IA), specific type (moderately good IA for serous cystadenomas, but fair for other types of pancreatic cysts), and EUS features (IA ranged from slight to moderately good) of pancreatic cystic lesions. Accuracy rates of EUS for the diagnosis of neoplastic versus non-neoplastic lesions range from 40% to 93%[61] (IIb).
Interobserver agreement for the presence of mural nodules seems good among experts and fair in the semi-expert and novice groups. With respect to specific overall diagnosis, agreement is moderate in the expert group, poor among the semi-experts, and fair among novices[62] (IIb).
Preoperative assessment by intraductal ultrasound (IDUS) has an 85% diagnostic accuracy for tumor extension of IPMN compared with 50% for other imaging methods. Preoperative IDUS is useful in determining the type of surgery and the extent of resection, especially in main-duct IPMN[63] (Ib).
EUS-FNA
EUS-FNA based cytology has overall low sensitivity (63%) but good specificity (88%) in differentiating mucinous cystic lesions (MCLs) from non-mucinous lesions (NMCLs). Further research is required to improve the overall sensitivity of EUS-FNA-based cytology to diagnose MCLs[64] (Ia).
The decision to proceed with non-operative management should not be based on a negative or non-diagnostic FNA alone, as 67% of negative and 92% of non-diagnostic specimens may be associated with malignant or premalignant pathology at surgical pathology[65] (IIb).
Complications of EUS-FNA are encountered in 2.2% of patients overall, and comprise pancreatitis, abdominal pain, retroperitoneal bleed, infection and bradycardia. Type of cyst, size, presence of septations or mass, and same-day ERCP are not predictors of complications[66] (III).
Two small studies suggest that cytology brushings are more likely to provide an adequate mucinous epithelium specimen than standard FNA and could aid the diagnosis of cystic pancreatic lesions in a selective group of patients[67] (IIa). EUS brushing increases cellular diagnosis of pancreatic cystic lesions compared to fluid analysis, mainly in mucinous lesions. However, its use is not recommended in patients under anticoagulation therapy even after withdrawal of anticoagulants, as a fatal complication has been reported[68] (IIb). Whether this approach is superior to cyst fluid analysis has not been substantiated by data. The cost of the combined needle and the brush should also be taken into consideration.
Molecular markers
The accuracy of carcinoembryonic antigen (CEA) (79%) is significantly higher compared to EUS morphology (51%) or cytology (59%). No combination of tests provides greater accuracy than CEA alone. Of tested markers, cyst fluid CEA is the most accurate test available for the diagnosis of mucinous cystic lesions of the pancreas[69] (IIb).
Malignant cysts may be differentiated from premalignant cysts on the basis of fluid CEA level, DNA quality, number of mutations and on the sequence of mutations acquired. Early k-ras mutation followed by allelic loss was the most predictive of a malignant cyst (sensitivity, 91%; specificity, 93%)[70,71] (IIb).
There is poor agreement between CEA and molecular analysis (DNA quantity, k-ras mutation, and 2 or more allelic imbalance mutations) for the classification of mucinous cysts. Based on the final pathologic diagnosis, CEA has a sensitivity of 82% compared with 77% for molecular analysis. When CEA and molecular analysis are combined, 100% sensitivity can be achieved[72] (IIb).
Follow-up, clinical outcome
Most branch duct-IPMN asymptomatic patients who have no mural nodules on EUS can be managed without surgery. However, careful attention should be paid to disease progression and the development of pancreatic cancer during follow-up[73] (IIb).
Ductal adenocarcinoma of the pancreas distinct from IPMN may develop in patients with branch duct IPMNs during follow-up. The 5-year rate of ductal carcinoma has been reported to reach 6.9%, with annual incidence of 1.1%. In the same series, cancer developed in IPMN in 3% of branch duct IPMNs during follow-up[74] (IIb).
There is considerable variation in size estimates of pancreatic cysts by different imaging modalities. Median size differences between studies are: between EUS and CT (i.e., absolute value of size determined by EUS minus size determined by CT): 4 mm (range: 0-25 mm), between EUS and MRI: 4 mm (range: 0-17 mm), between CT and MRI: 3 mm (range: 2-20 mm). Median size differences for surgical pathology specimens compared to imaging estimates were as follows: EUS and pathology: 9.5 mm (range: 0-20 mm), CT and pathology: 5 mm (range: 0-21 mm), MRI and pathology: 5.5 mm (range: 2-44 mm). Clinicians should take into account these variations when making management decisions and prefer a single modality during follow-up[75] (IIb).
CHRONIC PANCREATITIS
Diagnosis
Various EUS features have been found to predict changes of chronic pancreatitis (CP). Parenchymal features comprise calcifications with shadowing, echogenic foci without shadowing, focal regions of reduced echogenicity (lobularity), hyperechoic strands and the presence of cysts within the gland. Ductal features include main pancreatic duct (MPD) calculi, dilation or irregular contour of the MPD, increased thickness/echogenicity of the MPD wall, and side branch dilation. EUS is highly sensitive and specific (> 85%) depending on the number of criteria present. CP is likely (PPV > 85%) when more than 2 criteria (for CP in general) and more than 6 criteria (for moderate to severe CP) are present. Moderate to severe CP is unlikely (NPV > 85%) when fewer than 3 criteria are present[76,77] (IIa).
An attempt to quantify the individual weight of widely accepted EUS features classifies them in major (hyperechoic foci with shadowing, MPD calculi and parenchymal lobularity with honeycombing) and minor criteria (cysts, dilated MPD or side branches, irregular MPD contour, hyperechoic MPD wall, strands, non-shadowing hyperechoic foci and non-contiguous lobularity). The diagnosis of CP is labeled as “most consistent with”, “suggestive of”, “indeterminate for” and “normal” depending on the number of major and minor criteria visualized[78] (IV).
EUS is as sensitive and effective as ERCP in the detection of CP, particularly when only mild disease is present. However, EUS findings have limited specificity (60%), particularly in patients with mild disease[79] (IIb).
Reports on the concordance of EUS and endoscopic pancreatic function test are inconclusive for patients with suspected early CP, but the combination of the two techniques can add complimentary information and reach a sensitivity of 100%[80,81] (IIb).
EUS may be more sensitive but equally specific, compared with MRCP, in diagnosing CP. The combination of EUS and MRCP has a sensitivity of 98% for either EUS or MRCP and a specificity of 100% for both EUS and MRCP[82] (IIb).
There is good intra-observer agreement in the interpretation of EUS features of CP. The intra-observer agreement seems better than the published inter-observer agreement for EUS features of CP and better than the published intra-observer agreement for ERCP[83] (IIb).
EUS-FNA
EUS-FNA with cytology is safe and improves the negative predictive value (from 75% to 100%) of EUS. Negative EUS-FNA findings rule out CP, but cytology alone does not improve the specificity of EUS findings (from 60% to 67%). Further improvements in tissue sampling and analysis are necessary to support routine use of FNA in patients with CP[78] (IIb).
Transgastric EUS-trucut biopsy of suspected non-focal CP infrequently demonstrates histologic CP in clinically suspected disease. Because of potential complications (acute pancreatitis) and limited diagnostic yield, this technique is not currently recommended for evaluation of these patients[84] (IIb).
Autoimmune pancreatitis
A diffusely hypoechoic, enlarged pancreas, together with chronic inflammatory cells in aspirated cytological specimens, is supportive of the diagnosis of autoimmune pancreatitis (AIP)[85] The presence of stromal fragments of high cellularity with a lymphoid infiltrate, in conjunction with clinical and radiology findings, could potentially establish the diagnosis of AIP and exclude carcinoma, thus preventing pancreatic resection[86] (IIb).
Diffuse or focal hypoechoic areas, diffuse or focal enlargement of pancreas, bile duct wall thickening, lymphadenopathy, and peri-pancreatic hypoechoic margins are significantly more frequent in AIP than in pancreatic cancer. All these features may resolve after steroid treatment[87] (III).
In AIP patients, CH-EUS demonstrates a unique vascularization pattern which enables discrimination between AIP and lesions caused by pancreatic cancer. Lesions caused by AIP and the surrounding pancreas typically demonstrate hyper-vascularization, whereas lesions caused by pancreatic cancer present hypo-vascularized[88] (III).
EUS elastography shows a typical and unique finding of homogenous stiffness of the whole organ, and this distinguishes AIP from the circumscribed mass lesion in ductal adenocarcinoma[89] (III).
ACUTE PANCREATITIS
In selected patients with acute pancreatitis (AP), EUS can safely replace diagnostic ERCP and select patients eligible for therapeutic ERCP with a higher success rate[90]. EUS may prevent ERCP in 71% of patients with AP and offers a complication-free alternative, whereas sphincterotomy is associated with bleeding in up to 22% of cases[91] (Ib).
EUS seems superior to MRCP (51% vs 20%) in the evaluation of AP. Cholelithiasis and biliary sludge (24%) are the most frequent EUS diagnoses, and pancreas divisum (8%) is the most frequent MRCP diagnosis. Only in 6% of cases does MRCP identify additional features in patients etiologically undiagnosed using EUS. The EUS yield is lower in patients with a previous cholecystectomy (11% vs 60%)[92] (IIa).
In univariate analysis, the presence of peri-pancreatic edema, parenchymal inhomogeneity, CBD dilation and ascites is associated with severe pancreatitis. In multivariate analysis, only the presence of peri-pancreatic edema in EUS correlates with the severity of AP according to the Atlanta criteria. EUS may be a new useful imaging modality for the prediction of severity of AP with prognostic significance in the early phase of AP[93] (III).
EXTRAHEPATIC BILIARY TREE
CBD stones
EUS has excellent overall sensitivity (94%) and specificity (95%) for the diagnosis of choledocholithiasis[94]. EUS performance is superior in detecting CBD stones compared to CBD malignancy (sensitivity 78%, specificity 84%)[95] (Ia).
By performing EUS first, ERCP may be safely avoided in two-thirds of patients with CBD stones. A EUS-based selection of patients for therapeutic ERCP significantly reduces the complication rate[96] (Ia).
For patients with intermediate probability of CBD stones, EUS is more sensitive than ERCP in detecting stones smaller than 4 mm (90% vs 23%). A management strategy based on EUS (with selective ERCP in patients with confirmed stones) is safer and not associated with an excess of endoscopic procedures (can spare ERCP in up to 75% of patients) compared with a strategy based on ERCP alone[97-99] (Ib).
With respect to sensitivity, specificity and accuracy, there is no statistically significant difference between EUS and MRCP for the detection of choledocholithiasis[100,101] (Ia). However, the sensitivity of MRCP seems to diminish in the setting of small (< 6 mm) CBD stones, while EUS remains highly sensitive even for small stones[102] (Ia).
The diagnostic accuracy of catheter-probe extra-ductal ultrasonography is comparable to that of conventional EUS for the detection of CBD stones[103] (IIa).
Approximately half (57%) of patients with echogenic CBD material visible on IDUS actually have biliary crystals on bile microscopy. The size of the echogenic material is the only significant factor associated with bile microscopy positivity with an optimal size value of 1.4 mm (sensitivity and specificity 71% and 75%, respectively)[104] (IIb).
CBD neoplasms
EUS is significantly more sensitive than US or CT (100% vs 80% and 83%, respectively) in making a positive diagnosis of obstruction. EUS is also significantly more accurate than US and CT (97% vs 49% and 66%) in diagnosing the cause of the obstruction and in the loco-regional staging of malignant obstructions (75% vs 38% and 62%)[105] (IIb).
A comparison between ERCP and EUS for tissue diagnosis of biliary strictures depicts higher sensitivity for ERCP-based techniques in the subgroup biliary tumors (ERCP 75% vs EUS 25%), whereas EUS-guided biopsy is superior for pancreatic masses (EUS 60% vs ERCP 38%)[106] (IIB).
EUS-FNA is valuable for tissue diagnosis of undetermined hilar strictures. It is technically feasible without significant risks, when other diagnostic tests are inconclusive and is able to change preplanned management in about half of the patients. Accuracy, sensitivity, and specificity are 91%, 89% and 100%, respectively[107] (IIb).
EUS and EUS-FNA are sensitive (overall 73%) for the diagnosis of cholangiocarcinoma and very specific (97%) in predicting unresectability. The sensitivity of EUS-FNA is significantly higher in distal (81%) than in proximal (59%) lesions[108] (IIb).
IDUS is a valuable adjunct to ERCP-guided tissue sampling that increases the ability to distinguish malignant from benign strictures, but cannot assess the lymph node spread of malignant strictures[109] (IIb). When used in conjunction, IDUS increases the accuracy of ERCP for the characterization of biliary strictures from 58% to 90%[110] (IIb).
Ampullary lesions
Early observational studies reported high detection rates (96%-100%) and staging accuracy of EUS with respect to duodenal or CBD wall involvement, invasion of the pancreas and portal vein, and spread to regional lymph nodes. Accuracy rates for cancer extent was 78% for ampullary carcinoma and 81% for CBD carcinoma, when compared with surgical findings[111,112] (III).
In patients with biliary symptoms, EUS can reliably visualize and characterize a malignant lesion as the first diagnostic tool (detection rate 82%, overall sensitivity of 92% and specificity of 75%) and may be considered the basis for subsequent diagnostic steps[113] (III).
EUS is more accurate than CT and MRI in local tumor staging of ampullary neoplasms (EUS 78%, CT 24%, MRI 46%). No significant difference in nodal staging exists between the three imaging modalities (EUS 68%, CT 59%, MRI 77%). EUS T-staging accuracy decreases from 84% to 72% in the presence of a trans-papillary endo-biliary stent. This is most prominent in T2/T3 carcinomas and may result in underestimating the need for a Whipple resection because of tumor understaging[114] (IIb).
EUS is more sensitive and specific than CT for tumor and nodal staging of ampullary cancer, and the association of CT to EUS findings does not improve the final test performance characteristics of EUS[115] (IIb).
THERAPEUTIC EUS
EUS-guided celiac plexus neurolysis and block
Alcohol-based EUS-guided celiac plexus neurolysis (EUS-CPN) is a safe and effective technique for patients with pancreatic cancer and pain intractable to narcotic analgesics. The pooled proportion of patients that experience pain relief is 80%. On the other hand, in patients with pain due to CP the outcomes of EUS-CPN are inferior (59% clinical benefit) and better techniques or injected materials are needed to improve the response[116] (Ia).
Steroid-based EUS-guided celiac plexus block (EUS-CPB), although superior to the percutaneous fluoroscopy-guided approach[117], proves moderately adequate in managing abdominal pain in patients with chronic pancreatitis, and warrants improvement in patient selection and refinement of the technique[118] (Ia).
Pancreatic collection drainage and necrosectomy
Technical success is significantly greater for EUS-guided pancreatic pseudocyst drainage compared to the “blind” endoscopic approach, even after adjusting for luminal compression-bulging. Short-term clinical outcomes seem to favor the EUS-guided technique, yet long-term results are similar. EUS should be considered the first-line treatment modality for endoscopic drainage of non-bulging lesions[119,120] (Ib).
EUS-guided endoscopic trans-gastric necrosectomy of infected necrosis in acute pancreatitis appears to be a feasible and relatively safe treatment option in patients who are not critically ill. Emergency surgery as the initial treatment can be avoided in the majority of cases. Complications may include minor bleeding after balloon dilation and later development of recurrent pseudocysts because of the “disconnected-duct syndrome”[121,122] (III).
Biliary and pancreatic duct drainage
The overall success rate of EUS-guided cholangiography via an intrahepatic (trans-papillary, trans-gastric) or extrahepatic (trans-papillary, trans-enteric) route approaches 85%-90%, with complication rates in the range of 10%-16%. Based on intention-to-treat analysis, similar success rates of over 70% can be achieved by both types of approach[123,124] (III).
EUS-guided biliary drainage with one-step placement of a fully-covered self-expanding metal stent seems to be a feasible, safe, and effective alternative to percutaneous trans-hepatic biliary drainage in cases of malignant biliary obstruction when ERCP is unsuccessful[125] (IIb).
EUS-guided pancreaticogastrostomy or pancreaticobulbostomy appears to be an effective (technical success 90%, clinical success 70%) and relatively safe (major complications in 5.5%, including bleeding, severe AP and perigastric collection) procedure. It is indicated for the management of pain secondary to pancreatic ductal hypertension due to chronic pancreatitis or post-Whipple resection anastomotic strictures, in patients with inaccessible main pancreatic ducts by a trans-papillary route. Nevertheless, stent dysfunction may occur in up to 55% of patients after mid- to long-term follow-up, the procedure is technically demanding and careful pre-therapeutic evaluation is required[126,127] (III).
Pancreatic cyst ablation
EUS-guided ethanol lavage results in a greater decrease in pancreatic cyst size (43%) compared with saline solution lavage (11%), with a similar safety profile. Overall CT-defined complete pancreatic cyst ablation reaches 33%[128] (Ib).
EUS-guided ethanol injection and lavage, followed by injection of paclitaxel, appears to be a safe method for treating pancreatic cysts; 62% of patients may have complete resolution. Small cyst volume predicts complete resolution[129] (IIb).
Pancreatic cancer therapy
High technical and clinical success rates (90%) have been reported for EUS-guided fiducials placement in patients with locally advanced and recurrent pancreatic cancer. The complication rate (mild pancreatitis in 2%), as well as the rate of migration from the initial injection site, seem low[130,131] (IIb).
EUS-guided brachytherapy by implantation of radioactive seeds into unresectable pancreatic tumors could yield a “partial” objective tumor response in 27% of patients, “minimal” response in 20% patients, and “stable disease” in 33% of patients, during a median follow-up period of 10.6 mo. Up to 30% of patients may experience clinical benefit, mostly due to reduction in pain, but this lasts for a limited time. Local complications (pancreatitis and pseudocyst formation) occur in 20% of patients[132]. Worse clinical outcomes (partial remission in 13.6% and stable disease in 45.5%) were reported in more recent series[133] (IIb).
EUS-guided injection of the oncolytic virus ONYX-015 into unresectable pancreatic carcinomas by the trans-gastric route with prophylactic antibiotics is feasible and generally well tolerated either alone or in combination with gemcitabine[134] (IIb).
A single administration of cytoimplant (allogeneic mixed lymphocyte culture) immunotherapy by EUS-guided fine needle injection appears to be feasible and not associated with substantial toxicity[135] (III).
CONCLUSION
Despite the ongoing development of other cross-sectional imaging modalities, namely MSCT and MRI, EUS still holds a leading role in the investigation of pancreaticobiliary disorders. EUS remains the most accurate method for the detection of small (< 3 cm) pancreatic lesions, including NETs and ampullary neoplasms, and the best test to define vascular invasion in pancreatic and periampullary tumors. The ability of tissue acquisition by EUS-FNA is pivotal for clinical decision-making in patients with pancreatic cancer; it demonstrates excellent sensitivity and specificity and appears to be safe when performed by experienced endosonographers. The adjunct of molecular analysis of aspirates, particularly K-ras point mutation analysis, could guide the differential diagnosis of solid pancreatic lesions, once incorporated to routine clinical practice. Furthermore, early diagnosis of asymptomatic tumors in screening programs for familial pancreatic cancer and MEN1 syndrome seems feasible and reliable, but data regarding the management of these patients are still inconclusive. Novel imaging techniques such as CH-EUS and EUS-elastography are promising for the detection of pancreatic cancer, which exhibits a characteristic imaging pattern; quantitative analysis enables an objective evaluation of lesions and will potentially increase intra- and inter-observer agreement, but prospective comparative studies are still pending.
EUS provides detailed imaging of pancreatic cysts and helps their differentiation according to subtle structural features. Although no single criterion has been established to predict malignancy, the size of lesions, the presence and size of mural nodules and the cyst growth rate seem useful in estimating the risk of malignancy. This has been well documented in the case of branch-duct type IPMNs, where long term EUS follow-up has provided a deep insight to their natural history. The malignant potential can be further evaluated on the basis of biochemical (amylase, CEA) and molecular cyst fluid analysis (K-ras mutation). These markers also seem to compensate for the generally low yield of EUS-FNA based cytology, but refinement of the technique and the adjunct of EUS-FNA brushing may increase the cellularity of aspirates.
Early diagnosis of CP is challenging. EUS is equally sensitive, yet much safer than ERCP, and more sensitive than MRCP in detecting the subtle changes of mild disease. EUS-FNA seems to increase the specificity of the technique, but further improvement in tissue sampling and analysis is necessary to support the routine use of FNA for patients with CP. EUS could also add valuable information in cases of suspected AIP, by demonstrating characteristic morphologic features and a typical pattern on elastography and CH-EUS, compatible with the disease.
EUS is the most sensitive and specific modality for the diagnosis of small CBD stones (< 4 mm). This fact has a critical impact on the management of patients with biliary symptoms or AP; a strategy based on performing EUS as the primary diagnostic modality can prevent futile diagnostic ERCPs in more than two thirds of patients and select those who will benefit from a therapeutic ERCP with higher success and lower complication rates.
Probably the greatest challenge of EUS evolution is its expanding therapeutic potential. EUS-CPN for patients with pancreatic cancer and EUS-guided pseudocyst drainage are now established alternatives to more invasive and risky surgical or radiologic interventions, which are routinely performed by experienced endosonographers. EUS-guided pancreaticobiliary drainage and targeted fine needle injection therapy for pancreatic cysts and solid tumors are novel therapeutic applications with encouraging preliminary outcomes that await to be confirmed by further studies.
Footnotes
Peer reviewers: Fausto Catena, MD, PhD, Department of General, Emergency and Transplant Surgery, St Orsola-Malpighi University Hospital, Via Massarenti 9, 40139 Bologna, Italy; Evangelos Kalaitzakis, MD, PhD, Associate professor, Institute of Internal Medicine, Sahlgrenska Academy, University of Gothenburg, 41345 Gothenburg, Sweden
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Classen M, Strohm WD, Kurtz W. Pancreatic pseudocysts and tumors in endosonography. Scand J Gastroenterol Suppl. 1984;94:77–84. [PubMed] [Google Scholar]
- 2.Strohm WD, Kurtz W, Classen M. Detection of biliary stones by means of endosonography. Scand J Gastroenterol Suppl. 1984;94:60–64. [PubMed] [Google Scholar]
- 3.Yasuda K, Tanaka Y, Fujimoto S, Nakajima M, Kawai K. Use of endoscopic ultrasonography in small pancreatic cancer. Scand J Gastroenterol Suppl. 1984;102:9–17. [PubMed] [Google Scholar]
- 4.Fusaroli P, Kypreos D, Alma Petrini CA, Caletti G. Scientific publications in endoscopic ultrasonography: changing trends in the third millennium. J Clin Gastroenterol. 2011;45:400–404. doi: 10.1097/MCG.0b013e3181fbde42. [DOI] [PubMed] [Google Scholar]
- 5.Fusaroli P, Vallar R, Togliani T, Khodadadian E, Caletti G. Scientific publications in endoscopic ultrasonography: a 20-year global survey of the literature. Endoscopy. 2002;34:451–456. doi: 10.1055/s-2002-32006. [DOI] [PubMed] [Google Scholar]
- 6.Eccles M, Rousseau N, Freemantle N. Updating evidence-based clinical guidelines. J Health Serv Res Policy. 2002;7:98–103. doi: 10.1258/1355819021927746. [DOI] [PubMed] [Google Scholar]
- 7.Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. doi: 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 8.Tang S, Huang G, Liu J, Liu T, Treven L, Song S, Zhang C, Pan L, Zhang T. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol. 2011;78:142–150. doi: 10.1016/j.ejrad.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717–725; quiz 664. doi: 10.1016/j.cgh.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Rösch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–352. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 11.Müller MF, Meyenberger C, Bertschinger P, Schaer R, Marincek B. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology. 1994;190:745–751. doi: 10.1148/radiology.190.3.8115622. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez S, Faigel D. Absence of a dilated duct predicts benign disease in suspected pancreas cancer: a simple clinical rule. Dig Dis Sci. 2010;55:1161–1166. doi: 10.1007/s10620-009-0889-y. [DOI] [PubMed] [Google Scholar]
- 13.Klapman JB, Chang KJ, Lee JG, Nguyen P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658–2661. doi: 10.1111/j.1572-0241.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 14.Catanzaro A, Richardson S, Veloso H, Isenberg GA, Wong RC, Sivak MV, Chak A. Long-term follow-up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointest Endosc. 2003;58:836–840. doi: 10.1016/s0016-5107(03)02301-0. [DOI] [PubMed] [Google Scholar]
- 15.Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, McGrath KM. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–2500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Bournet B, Souque A, Senesse P, Assenat E, Barthet M, Lesavre N, Aubert A, O’Toole D, Hammel P, Levy P, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with KRAS mutation assay to distinguish pancreatic cancer from pseudotumoral chronic pancreatitis. Endoscopy. 2009;41:552–557. doi: 10.1055/s-0029-1214717. [DOI] [PubMed] [Google Scholar]
- 17.Carrara S, Cangi MG, Arcidiacono PG, Perri F, Petrone MC, Mezzi G, Boemo C, Talarico A, Cin ED, Grassini G, et al. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359–1363. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 18.Reicher S, Boyar FZ, Albitar M, Sulcova V, Agersborg S, Nga V, Zhou Y, Li G, Venegas R, French SW, et al. Fluorescence in situ hybridization and K-ras analyses improve diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses. Pancreas. 2011;40:1057–1062. doi: 10.1097/MPA.0b013e3182200201. [DOI] [PubMed] [Google Scholar]
- 19.Salek C, Minarikova P, Benesova L, Nosek V, Strnad R, Zavoral M, Minarik M. Mutation status of K-ras, p53 and allelic losses at 9p and 18q are not prognostic markers in patients with pancreatic cancer. Anticancer Res. 2009;29:1803–1810. [PubMed] [Google Scholar]
- 20.Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788–797. doi: 10.1016/j.gie.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad NA, Kochman ML, Lewis JD, Kadish S, Morris JB, Rosato EF, Ginsberg GG. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J Clin Gastroenterol. 2001;32:54–58. doi: 10.1097/00004836-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Shami VM, Mahajan A, Loch MM, Stella AC, Northup PG, White GE, Brock AS, Srinivasan I, de Lange EE, Kahaleh M. Comparison between endoscopic ultrasound and magnetic resonance imaging for the staging of pancreatic cancer. Pancreas. 2011;40:567–570. doi: 10.1097/MPA.0b013e3182153b8c. [DOI] [PubMed] [Google Scholar]
- 23.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 24.Mansfield SD, Scott J, Oppong K, Richardson DL, Sen G, Jaques BC, Manas DM, Charnley RM. Comparison of multislice computed tomography and endoscopic ultrasonography with operative and histological findings in suspected pancreatic and periampullary malignancy. Br J Surg. 2008;95:1512–1520. doi: 10.1002/bjs.6330. [DOI] [PubMed] [Google Scholar]
- 25.DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 26.Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–292. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587–1593. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 29.Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, Pappas T, Enns R, Robuck G, Stiffler H, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–975. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736; quiz 751, 753. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui AA, Kowalski TE, Shahid H, O’Donnell S, Tolin J, Loren DE, Infantolino A, Hong SK, Eloubeidi MA. False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc. 2011;74:535–540. doi: 10.1016/j.gie.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 32.Fritscher-Ravens A, Topalidis T, Bobrowski C, Krause C, Thonke E, Jäckle S, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration in focal pancreatic lesions: a prospective intraindividual comparison of two needle assemblies. Endoscopy. 2001;33:484–490. doi: 10.1055/s-2001-14970. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 34.Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen YP, Maple JT, Zhang Q, Ylagan LR, Zhai J, Kohlmeier C, Jonnalagadda S, Early DS, Edmundowicz SA, Azar RR. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264–1270. doi: 10.1016/j.gie.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Eloubeidi MA, Tamhane A, Jhala N, Chhieng D, Jhala D, Crowe DR, Eltoum IA. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–2847. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 37.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 38.Eloubeidi MA, Gress FG, Savides TJ, Wiersema MJ, Kochman ML, Ahmad NA, Ginsberg GG, Erickson RA, Dewitt J, Van Dam J, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385–389. doi: 10.1016/s0016-5107(04)01714-6. [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Esparrach G, Ginès A, García P, Pellisé M, Solé M, Cortés P, Gimeno-García AZ, Sendino O, Navarro S, Llach J, et al. Incidence and clinical significance of hyperamylasemia after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of pancreatic lesions: a prospective and controlled study. Endoscopy. 2007;39:720–724. doi: 10.1055/s-2007-966719. [DOI] [PubMed] [Google Scholar]
- 40.Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721–1726. doi: 10.1056/NEJM199206253262601. [DOI] [PubMed] [Google Scholar]
- 41.Wamsteker EJ, Gauger PG, Thompson NW, Scheiman JM. EUS detection of pancreatic endocrine tumors in asymptomatic patients with type 1 multiple endocrine neoplasia. Gastrointest Endosc. 2003;58:531–535. doi: 10.1067/s0016-5107(03)01965-5. [DOI] [PubMed] [Google Scholar]
- 42.Thomas-Marques L, Murat A, Delemer B, Penfornis A, Cardot-Bauters C, Baudin E, Niccoli-Sire P, Levoir D, Choplin Hdu B, Chabre O, et al. Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol. 2006;101:266–273. doi: 10.1111/j.1572-0241.2006.00367.x. [DOI] [PubMed] [Google Scholar]
- 43.Khashab MA, Yong E, Lennon AM, Shin EJ, Amateau S, Hruban RH, Olino K, Giday S, Fishman EK, Wolfgang CL, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc. 2011;73:691–696. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein JH, Scheiman JM, Anderson MA. A clinical and economic evaluation of endoscopic ultrasound for patients at risk for familial pancreatic adenocarcinoma. Pancreatology. 2007;7:514–525. doi: 10.1159/000108969. [DOI] [PubMed] [Google Scholar]
- 45.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781; quiz 665. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 47.Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 48.Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–634.e1-2. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Napoleon B, Alvarez-Sanchez MV, Gincoul R, Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet JC, Queneau PE, Scoazec JY, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–570. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 50.Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073–1079. doi: 10.1097/MPA.0b013e31821f57b7. [DOI] [PubMed] [Google Scholar]
- 51.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172–1180. doi: 10.1053/j.gastro.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 52.Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 53.Săftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535–542. doi: 10.1055/s-2006-927117. [DOI] [PubMed] [Google Scholar]
- 54.Săftoiu A, Iordache SA, Gheonea DI, Popescu C, Maloş A, Gorunescu F, Ciurea T, Iordache A, Popescu GL, Manea CT. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2010;72:739–747. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 55.Song MH, Lee SK, Kim MH, Lee HJ, Kim KP, Kim HJ, Lee SS, Seo DW, Min YI. EUS in the evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2003;57:891–896. doi: 10.1016/s0016-5107(03)70026-1. [DOI] [PubMed] [Google Scholar]
- 56.O’Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce-Dachez M, Fabre M, Dancour A, Aubert A, Sauvanet A, et al. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59:823–829. doi: 10.1016/s0016-5107(04)00346-3. [DOI] [PubMed] [Google Scholar]
- 57.Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997;122:617–625. doi: 10.1016/s0039-6060(97)90136-7. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79; quiz 309-310. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang MJ, Jang JY, Kim SJ, Lee KB, Ryu JK, Kim YT, Yoon YB, Kim SW. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429–1434. doi: 10.1111/j.1572-0241.2001.03794.x. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, Kimmey MB, Nickl NJ, Savides TJ, Wallace MB, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc. 2003;58:59–64. doi: 10.1067/mge.2003.298. [DOI] [PubMed] [Google Scholar]
- 62.de Jong K, Verlaan T, Dijkgraaf MG, Poley JW, van Dullemen H, Bruno MJ, Fockens P. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy. 2011;43:579–584. doi: 10.1055/s-0030-1256434. [DOI] [PubMed] [Google Scholar]
- 63.Cheon YK, Cho YD, Jeon SR, Moon JH, Jeong SW, Hur KY, Jin SY, Lee JS. Pancreatic resection guided by preoperative intraductal ultrasonography for intraductal papillary mucinous neoplasm. Am J Gastroenterol. 2010;105:1963–1969. doi: 10.1038/ajg.2010.169. [DOI] [PubMed] [Google Scholar]
- 64.Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–2766. doi: 10.1007/s10620-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–3192. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- 66.Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–236. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 67.Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, Skarvinko LN, Jamil LH, Hasan M, Wallace MB. Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study. Endoscopy. 2010;42:127–132. doi: 10.1055/s-0029-1215351. [DOI] [PubMed] [Google Scholar]
- 68.Sendino O, Fernández-Esparrach G, Solé M, Colomo L, Pellisé M, Llach J, Navarro S, Bordas JM, Ginès A. Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis. 2010;42:877–881. doi: 10.1016/j.dld.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Khalid A, McGrath KM, Zahid M, Wilson M, Brody D, Swalsky P, Moser AJ, Lee KK, Slivka A, Whitcomb DC, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–973. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 71.Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 72.Sawhney MS, Devarajan S, O’Farrel P, Cury MS, Kundu R, Vollmer CM, Brown A, Chuttani R, Pleskow DK. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106–1110. doi: 10.1016/j.gie.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364–370. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 74.Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–1565. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 75.Maimone S, Agrawal D, Pollack MJ, Wong RC, Willis J, Faulx AL, Isenberg GA, Chak A. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest Endosc. 2010;71:945–950. doi: 10.1016/j.gie.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 76.Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 77.Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 78.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 79.Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824–831. doi: 10.1055/s-2001-17337. [DOI] [PubMed] [Google Scholar]
- 80.Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol. 2010;105:2498–2503. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- 81.Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci. 2010;55:2681–2687. doi: 10.1007/s10620-009-1084-x. [DOI] [PubMed] [Google Scholar]
- 82.Pungpapong S, Wallace MB, Woodward TA, Noh KW, Raimondo M. Accuracy of endoscopic ultrasonography and magnetic resonance cholangiopancreatography for the diagnosis of chronic pancreatitis: a prospective comparison study. J Clin Gastroenterol. 2007;41:88–93. doi: 10.1097/MCG.0b013e31802dfde6. [DOI] [PubMed] [Google Scholar]
- 83.Lieb JG, Palma DT, Garvan CW, Leblanc JK, Romagnuolo J, Farrell JJ, Savides TJ, Eloubeidi MA, Draganov PV, Forsmark CE, et al. Intraobserver agreement among endosonographers for endoscopic ultrasound features of chronic pancreatitis: a blinded multicenter study. Pancreas. 2011;40:177–180. doi: 10.1097/MPA.0b013e3182016a25. [DOI] [PubMed] [Google Scholar]
- 84.DeWitt J, McGreevy K, LeBlanc J, McHenry L, Cummings O, Sherman S. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76–84. doi: 10.1016/s0016-5107(05)00504-3. [DOI] [PubMed] [Google Scholar]
- 85.Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927–936. doi: 10.1016/s0016-5107(04)02230-8. [DOI] [PubMed] [Google Scholar]
- 86.Deshpande V, Mino-Kenudson M, Brugge WR, Pitman MB, Fernandez-del Castillo C, Warshaw AL, Lauwers GY. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464–1471. doi: 10.1097/01.pas.0000173656.49557.48. [DOI] [PubMed] [Google Scholar]
- 87.Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, Bhatia V, Yamao K. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44:154–159. doi: 10.1007/s00535-008-2294-2. [DOI] [PubMed] [Google Scholar]
- 88.Hocke M, Ignee A, Dietrich CF. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy. 2011;43:163–165. doi: 10.1055/s-0030-1256022. [DOI] [PubMed] [Google Scholar]
- 89.Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–720. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 90.Liu CL, Fan ST, Lo CM, Tso WK, Wong Y, Poon RT, Lam CM, Wong BC, Wong J. Comparison of early endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the management of acute biliary pancreatitis: a prospective randomized study. Clin Gastroenterol Hepatol. 2005;3:1238–1244. doi: 10.1016/s1542-3565(05)00619-1. [DOI] [PubMed] [Google Scholar]
- 91.De Lisi S, Leandro G, Buscarini E. Endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography in acute biliary pancreatitis: a systematic review. Eur J Gastroenterol Hepatol. 2011;23:367–374. doi: 10.1097/MEG.0b013e3283460129. [DOI] [PubMed] [Google Scholar]
- 92.Ortega AR, Gómez-Rodríguez R, Romero M, Fernández-Zapardiel S, Céspedes Mdel M, Carrobles JM. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of “idiopathic” acute pancreatitis. Pancreas. 2011;40:289–294. doi: 10.1097/MPA.0b013e318201654a. [DOI] [PubMed] [Google Scholar]
- 93.Sotoudehmanesh R, Hooshyar A, Kolahdoozan S, Zeinali F, Shahraeeni S, Keshtkar AA. Prognostic value of endoscopic ultrasound in acute pancreatitis. Pancreatology. 2010;10:702–706. doi: 10.1159/000320695. [DOI] [PubMed] [Google Scholar]
- 94.Tse F, Liu L, Barkun AN, Armstrong D, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67:235–244. doi: 10.1016/j.gie.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 95.Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616–623. doi: 10.1016/j.cgh.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 96.Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967–974. doi: 10.1002/bjs.6667. [DOI] [PubMed] [Google Scholar]
- 97.Karakan T, Cindoruk M, Alagozlu H, Ergun M, Dumlu S, Unal S. EUS versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a prospective randomized trial. Gastrointest Endosc. 2009;69:244–252. doi: 10.1016/j.gie.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 98.Lee YT, Chan FK, Leung WK, Chan HL, Wu JC, Yung MY, Ng EK, Lau JY, Sung JJ. Comparison of EUS and ERCP in the investigation with suspected biliary obstruction caused by choledocholithiasis: a randomized study. Gastrointest Endosc. 2008;67:660–668. doi: 10.1016/j.gie.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 99.Polkowski M, Regula J, Tilszer A, Butruk E. Endoscopic ultrasound versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a randomized trial comparing two management strategies. Endoscopy. 2007;39:296–303. doi: 10.1055/s-2007-966264. [DOI] [PubMed] [Google Scholar]
- 100.Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248–254. doi: 10.1016/j.gie.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 101.Ledro-Cano D. Suspected choledocholithiasis: endoscopic ultrasound or magnetic resonance cholangio-pancreatography? A systematic review. Eur J Gastroenterol Hepatol. 2007;19:1007–1011. doi: 10.1097/MEG.0b013e328133f30b. [DOI] [PubMed] [Google Scholar]
- 102.Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1–9. doi: 10.1016/j.gie.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 103.Wehrmann T, Martchenko K, Riphaus A. Catheter probe extraductal ultrasonography vs. conventional endoscopic ultrasonography for detection of bile duct stones. Endoscopy. 2009;41:133–137. doi: 10.1055/s-0028-1103491. [DOI] [PubMed] [Google Scholar]
- 104.Kim BJ, Kang P, Lee JK, Sinn DH, Lee KH, Lee KT, Rhee JC, Lim JH. Are the echogenicities on intraductal ultrasonography really biliary microlithiasis? Dig Dis Sci. 2010;55:836–841. doi: 10.1007/s10620-009-0770-z. [DOI] [PubMed] [Google Scholar]
- 105.Amouyal P, Palazzo L, Amouyal G, Ponsot P, Mompoint D, Vilgrain V, Gayet B, Fléjou JF, Paolaggi JA. Endosonography: promising method for diagnosis of extrahepatic cholestasis. Lancet. 1989;2:1195–1198. doi: 10.1016/s0140-6736(89)91801-1. [DOI] [PubMed] [Google Scholar]
- 106.Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–396. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 107.Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45–51. doi: 10.1046/j.1572-0241.2003.04006.x. [DOI] [PubMed] [Google Scholar]
- 108.Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG, Jones KJ, Fogel EL, McHenry L, Watkins JL, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 109.Farrell RJ, Agarwal B, Brandwein SL, Underhill J, Chuttani R, Pleskow DK. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest Endosc. 2002;56:681–687. doi: 10.1067/mge.2002.128918. [DOI] [PubMed] [Google Scholar]
- 110.Stavropoulos S, Larghi A, Verna E, Battezzati P, Stevens P. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy. 2005;37:715–721. doi: 10.1055/s-2005-870132. [DOI] [PubMed] [Google Scholar]
- 111.Yasuda K, Mukai H, Cho E, Nakajima M, Kawai K. The use of endoscopic ultrasonography in the diagnosis and staging of carcinoma of the papilla of Vater. Endoscopy. 1988;20 Suppl 1:218–222. doi: 10.1055/s-2007-1018179. [DOI] [PubMed] [Google Scholar]
- 112.Mukai H, Nakajima M, Yasuda K, Mizuno S, Kawai K. Evaluation of endoscopic ultrasonography in the pre-operative staging of carcinoma of the ampulla of Vater and common bile duct. Gastrointest Endosc. 1992;38:676–683. doi: 10.1016/s0016-5107(92)70563-x. [DOI] [PubMed] [Google Scholar]
- 113.Will U, Bosseckert H, Meyer F. Correlation of endoscopic ultrasonography (EUS) for differential diagnostics between inflammatory and neoplastic lesions of the papilla of Vater and the peripapillary region with results of histologic investigation. Ultraschall Med. 2008;29:275–280. doi: 10.1055/s-2008-1027327. [DOI] [PubMed] [Google Scholar]
- 114.Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, Stotland B, Rosato EF, Morris JB, Eckhauser F, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 115.Artifon EL, Couto D, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290–296. doi: 10.1016/j.gie.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 116.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 117.Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979–984. doi: 10.1111/j.1365-2036.2009.03963.x. [DOI] [PubMed] [Google Scholar]
- 118.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 119.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 120.Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 121.Seewald S, Groth S, Omar S, Imazu H, Seitz U, de Weerth A, Soetikno R, Zhong Y, Sriram PV, Ponnudurai R, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos) Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- 122.Schrover IM, Weusten BL, Besselink MG, Bollen TL, van Ramshorst B, Timmer R. EUS-guided endoscopic transgastric necrosectomy in patients with infected necrosis in acute pancreatitis. Pancreatology. 2008;8:271–276. doi: 10.1159/000134275. [DOI] [PubMed] [Google Scholar]
- 123.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 124.Brauer BC, Chen YK, Fukami N, Shah RJ. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video) Gastrointest Endosc. 2009;70:471–479. doi: 10.1016/j.gie.2008.12.233. [DOI] [PubMed] [Google Scholar]
- 125.Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 126.Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 127.Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518–525. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 128.DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 129.Oh HC, Seo DW, Song TJ, Moon SH, Park do H, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–179. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–1184. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 131.Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–518. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 132.Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 133.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 134.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 135.Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325–1335. doi: 10.1002/(sici)1097-0142(20000315)88:6<1325::aid-cncr8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]


