Abstract
AIM: To evaluate the effect of a novel alginate-based compound, Faringel, in modifying reflux characteristics and controlling symptoms.
METHODS: In this prospective, open-label study, 40 patients reporting heartburn and regurgitation with proven reflux disease (i.e., positive impedance-pH test/evidence of erosive esophagitis at upper endoscopy) underwent 2 h impedance-pH testing after eating a refluxogenic meal. They were studied for 1 h under basal conditions and 1 h after taking 10 mL Faringel. In both sessions, measurements were obtained in right lateral and supine decubitus positions. Patients also completed a validated questionnaire consisting of a 2-item 5-point (0-4) Likert scale and a 10-cm visual analogue scale (VAS) in order to evaluate the efficacy of Faringel in symptom relief. Tolerability of the treatment was assessed using a 6-point Likert scale ranging from very good (1) to very poor (6).
RESULTS: Faringel decreased significantly (P < 0.001), in both the right lateral and supine decubitus positions, esophageal acid exposure time [median 10 (25th-75th percentil 6-16) vs 5.8 (4-10) and 16 (11-19) vs 7.5 (5-11), respectively] and acid refluxes [5 (3-8) vs 1 (1-1) and 6 (4-8) vs 2 (1-2), respectively], but increased significantly (P < 0.01) the number of nonacid reflux events compared with baseline [2 (1-3) vs 3 (2-5) and 3 (2-4) vs 6 (3-8), respectively]. Percentage of proximal migration decreased in both decubitus positions (60% vs 32% and 64% vs 35%, respectively; P < 0.001). Faringel was significantly effective in controlling heartburn, based on both the Likert scale [3.1 (range 1-4) vs 0.9 (0-2); P < 0.001] and VAS score [7.1 (3-9.8) vs 2 (0.1-4.8); P < 0.001], but it had less success against regurgitation, based on both the Likert scale [2.6 (1-4) vs 2.2 (1-4); P = not significant (NS)] and VAS score [5.6 (2-9.6) vs 3.9 (1-8.8); P = NS]. Overall, the tolerability of Faringel was very good 5 (2-6), with only two patients reporting modest adverse events (i.e., nausea and bloating).
CONCLUSION: Our findings demonstrate that Faringel is well-tolerated and effective in reducing heartburn by modifying esophageal acid exposure time, number of acid refluxes and their proximal migration.
Keywords: Impedance pH-metry, Nonerosive reflux disease, Erosive esophagitis, Nonacid reflux, Proximal reflux
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common problem affecting about 20% of the population in western countries[1]. Nonerosive reflux disease (NERD) and erosive reflux disease (ERD) represent the most common phenotypic presentations of GERD, accounting for 90%-95% of the overall GERD patients[2]. Previous studies have documented that patients with ERD and NERD present the same clinical picture in terms of frequency and severity of reflux symptoms[2,3].
To date, the use of proton pump inhibitors (PPIs) has been considered the best therapeutic option for GERD patients, given their high efficacy in determining symptom relief and in inducing esophageal mucosal healing[4,5]. On the other hand, there is increasing evidence that not all patients respond satisfactorily to this kind of treatment and that about 30%-35% of the patients require additional intervention to control symptoms[6-8]. Thus, traditional antacids are frequently used as add-on therapy in order to neutralize gastric acidity and to help control of heartburn[7,9,10]. However, there are limited data regarding the mechanisms by which they are able to modify the determinants of reflux symptom perception[11]. Moreover, despite their utility, the majority of current antacid formulations are not well tolerated by patients and this limits their widespread use and efficacy.
Recently, a novel compound, Faringel (CADIGroup, Rome, Italy), containing sodium bicarbonate and alginate with the addition of herbal components (i.e., honey, chamomille or Matricaria recutita L., Calendula officinalis, Aloe vera, Propolis gel) has been introduced to the market. The first two elements are well known to have an antireflux effect due to their ability to neutralize gastric acidity and to create an alginate-based raft that remains in the upper part of the stomach as a physical barrier capable of preventing reflux episodes[12-15], while the latter components have been recently associated with mild anti-inflammatory and analgesic effects, and it has been suggested that they may favor the healing of human mucosa[16-22].
In recent years, multichannel intraluminal impedance combined with pH-metry (MII-pH) has been applied to assess the effectiveness of drugs or endoscopic devices proposed for the therapy of GERD, particularly if we want to know whether they can affect both acid and nonacid reflux or are able to reduce the proximal migration of the refluxate[23-26].
The aim of the present study was to evaluate the antireflux properties of an alginate antacid formulation (Faringel) on both acid and nonacid reflux episodes, and the height of the proximal extent of reflux events by means of MII-pH monitoring in patients with documented mild to moderate GERD. As a secondary aim, we assessed the therapeutic efficacy of this novel compound as well as its tolerability using validated questionnaires.
MATERIALS AND METHODS
Subjects
This was a prospective, open-label study, enrolling consecutive patients with typical reflux symptoms (i.e., heartburn and regurgitation) lasting for > 6 mo and occurring at least three times weekly, presenting to the University Hospital of Genova and to the University Hospital of Pisa, Italy. They were referred to our units because they were undergoing upper endoscopy, preoperative surgical evaluation, or being investigated for PPI refractoriness. Exclusion criteria were: history of thoracic, esophageal or gastric surgery; primary or secondary severe esophageal motility disorders (e.g., achalasia, scleroderma, diabetes mellitus, autonomic or peripheral neuropathy, myopathy); or history of alcohol or drug abuse. In women of childbearing age, pregnancy was excluded by urine analysis.
For comparison, normal values were obtained from a group of 48 healthy volunteers [HVs; 22 male; mean age 44 years, range 22-77 years; mean body mass index (BMI) 23 kg/m2, range 16-34 kg/m2] without any type of digestive and systemic symptoms, and previously studied in our laboratory[27].
The study protocol was approved by the local Ethics Committee and performed according to the Declaration of Helsinki. All patients provided written informed consent to take part.
Study protocol
All subjects who agreed to undergo both upper gastrointestinal (GI) endoscopy and 24-h esophageal impedance pH, underwent physical and clinical examination and a detailed medical history was recorded. The medical history included information on their symptomatic response to previous PPI therapy taken for at least 8 wk at double dose. Patients reporting < 50% heartburn improvement were considered nonresponders to PPIs (i.e., heartburn more than twice weekly for at least 2 mo). Patients taking antisecretory or prokinetic drugs were asked to stop any medication at least 30 and 15 d before endoscopy, respectively. Antacids or alginate preparations were suggested in case of frequent symptoms. The frequency and intensity of symptoms and impact on quality of life were registered using a structured and validated questionnaire for the diagnosis of GERD[28].
Thereafter, within 1-5 d (median 3 d) from the upper GI endoscopy, every patient underwent esophageal impedance-pH testing off-therapy using an ambulatory multichannel intraluminal impedance and pH (MII-pH) monitoring system (Sleuth, Sandhill Scientific, Highland Ranch, CO, United States), according to our methodology[27]. During the test day, meal time and composition were standardized[29]. Stationary esophageal manometry was performed before MII-pH in order to locate with accuracy the lower esophageal sphincter (LES). Other features regarding the variables of reflux measurement by MII-pH and data analysis have been previously reported[30,31].
After the 24-h monitoring period, patients returned to our hospital service. Based on the results of endoscopy and impedance-pH testing, patients were classified as NERD, in case of absence of esophageal mucosal breaks in combination with an abnormal esophageal acid exposure time and/or a positive symptom association probability (> 95%) to acid and/or nonacid reflux during impedance-pH monitoring[3,27], and as ERD, in case of presence of esophageal mucosal injury according to international criteria[32]. Furthermore, patients with hypersensitive esophagus (i.e., normal upper endoscopy, normal MII-pH testing, and positivity for symptom association analysis) were ruled out from the whole group of NERD in order to include patients with well-documented GERD. Then, they were asked to ingest a refluxogenic meal consisting of a continental breakfast [one cappuccino, two brioches containing chocolate cream (450 kcal, 60%) fat and orange juice], at least 4 h after the breakfast, and completed two questionnaires including questions on the presence and intensity of heartburn and regurgitation after the meal, as well as a 10-cm visual analogue scale (VAS) scale for each one of the two symptoms (see below). Patients underwent an additional 2-h period of recording; 1 h under basal conditions and another 1 h after a single dose (10 mL) of Faringel. Studies were performed while patients laid in the right lateral decubitus position for 30 min and in the supine decubitus position for another 30 min; both under basal conditions and after Faringel treatment. In particular, the right lateral decubitus position was chosen because it has been shown to be associated with an increased esophageal acid exposure[33]. Afterward, data recording was concluded. Between the two sections and at the end of the test, patients filled out both symptomatic questionnaires.
Symptom assessment
The primary efficacy parameter was the change in the sum score of the validated Likert scale[34,35] filled in by the investigator. Intensity of heartburn (defined as a retrosternal burning sensation occurring in waves and tending to rise upward toward the neck) and regurgitation (return of partially digested food from the stomach to the mouth) during the test was recorded by interviewing the patient, using a 5-point Likert rating scale as follows: 0 = none (absence of symptoms); 1 = mild (minimal awareness of symptoms, which is easily tolerated); 2 = moderate (awareness of symptoms, which is bothersome but tolerable); 3 = severe (symptoms hard to tolerate); 4 = very severe (symptom impossible to tolerate). The score was used for the outcome measurement as a sum score, with its highest value of 8 points representing the most severe symptom intensity.
Patients were also asked to rate their satisfaction with symptom control on a global VAS of 0 (no relief at all) to 100 (complete symptom relief). The VAS score has been used as a self-assessment tool for symptom measure, which has been adopted in many other trials for evaluation of visceral symptoms[36,37].
The secondary target variable comprised the overall tolerability of the treatment, assessed by investigator and patient using a 6-point Likert scale ranging from very poor (1) to very good (6). Together with the exploratory target variables, the number of responders and patients free of symptoms were also studied. Responders were defined as patients for whom a 40% improvement in the Likert scale was achieved, whereas patients free of symptoms were defined as subjects showing an overall sum score of 0 or 1 point when treatment stopped.
Statistical analysis
Differences in proportions were compared using the χ2 or Fisher’s exact test, depending on the sample size. Unless otherwise specified, data were presented as median and percentile values (25th, 75th, 95th percentile). In case of non-normally distributed data, differences between patients were compared using the Kruskal-Wallis and/or Mann-Whitney tests. Differences were considered statistically significant at P < 0.05.
RESULTS
Patients
Forty patients with heartburn (20 female/20 male, mean age 48 years, range 18-76 years) reporting at least one symptom during the testing day were included in the study. Detailed demographic and clinical features of GERD patients and HVs are shown in Table 1. There was no difference among them and HVs in terms of sex and age. The prevalence of hiatal hernia as well as mean BMI was significantly higher in patients with GERD compared to the HVs (P < 0.01). All subjects tolerated well the examination and the test meal. No important technical failure occurred.
Table 1.
Demographic and clinical characteristics of gastro-esophageal reflux disease patients and healthy volunteers n (%)
| Demographic and clinical parameters | GERD patients | HVs | P value |
| Patients | 40 | 48 | |
| Female/male | 20/20 | 27/21 | NS |
| Mean age, yr (range) | 48 (18-76) | 44 (22-77) | NS |
| Mean BMI, kg/m2 (range) | 26 (20-32) | 23 (16-34) | < 0.05 |
| NERD/ERD | 25 (63)/15 (38) | NA | |
| Patients with hiatal hernia | 22 (55) | 4 (10) | < 0.01 |
| Patients responding to PPI therapy | 34 (85) | NA |
NERD: Nonerosive reflux disease; ERD: Erosive reflux disease; GERD: Gastroesophageal reflux disease; PPI: Proton pump inhibitor; BMI: Body mass index; HV: Healthy volunteer.
24-h impedance-pH data
Detailed impedance-pH characteristics of our patients and HVs are reported in Table 2. Patients with GERD had significantly greater distal esophageal acid exposure time compared to HVs (5.5 vs 0.7, P < 0.0001). The median total number of reflux episodes was 65 and the median number of acid reflux events was 53, and both were significantly higher in GERD patients compared to HVs (32 and 17, respectively; P < 0.0001). Patients with GERD and HVs had a similar number of nonacid reflux episodes (20 vs 18, P < 0.0615). The percentage of total reflux episodes reaching the proximal measuring site (15 cm above the LES) was higher in GERD patients than in HVs (46% vs 33%, P < 0.0001).
Table 2.
Impedance-pH features in gastro-esophageal reflux disease patients and healthy volunteers
| Impedance-pH features | GERD patients | HVs | P value |
| % pH < 4 upright | 6.9 (5.5-12; 28) | 1 (0.2-1.9; 5) | < 0.0001 |
| % pH < 4 recumbent | 3.5 (1.2-7; 18) | 0 (0-0.1; 2.1) | < 0.0001 |
| % pH < 4 total | 5.5 (4.3-9; 22) | 0.7 (0.2-1.4; 4.2) | < 0.0001 |
| GER total | 65 (54-108; 177) | 32 (18-43; 54) | < 0.0001 |
| GER acid | 53 (34-72; 95) | 17 (8-31; 45) | < 0.0001 |
| GER nonacid | 20 (15-37; 116) | 18 (14-26; 45) | 0.0615 |
| Prox. extension | 31 (20-48; 86) | 9 (4-17; 30) | < 0.0001 |
GER: Gastroesophageal reflux; GERD: Gastroesophageal reflux disease; HV: Healthy volunteer.
2-h impedance-pH data before and after treatment
As shown in Figures 1 and 2A, Faringel decreased significantly (P < 0.001), in both the right lateral and supine decubitus positions, esophageal acid exposure time [10 (6-16; 23) vs 5.8 (4-10; 16) and 16 (11-19; 32) vs 7.5 (5-11; 15), respectively], acid reflux events [5 (3-8; 11) vs 1 (1-1; 2) and 6 (4-8; 11) vs 2 (1-2; 5), respectively], and proximal reflux episodes [4 (3-6; 11) vs 1 (1-2; 3) and 5 (4-7; 11) vs 3 (2-3; 4), respectively]. Also, the percentage of proximal migration of reflux events decreased significantly in both the right lateral and supine decubitus positions (60% vs 32% and 64% vs 35%) compared with baseline. In contrast, Faringel increased significantly (P < 0.01) the number of nonacid reflux events compared with baseline [2 (1-3; 5) vs 3 (2-5; 7) and 3 (2-4; 7) vs 6 (3-8; 13); Figure 2B]. The number of total reflux episodes slightly significantly decreased in the right lateral decubitus position, before and after Faringel treatment, while no difference was found in the supine decubitus position [7 (5-10; 15) vs 4 (3-5; 10) and 8.5 (7-11; 16) vs 7 (5-10; 17), P = 0.0001 and P = 0.1321, respectively].
Figure 1.
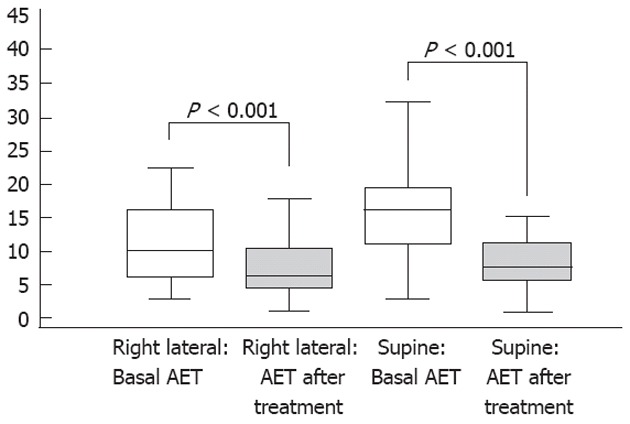
Median esophageal acid exposure under basal conditions and after Faringel intake in the two decubitus positions.
Figure 2.
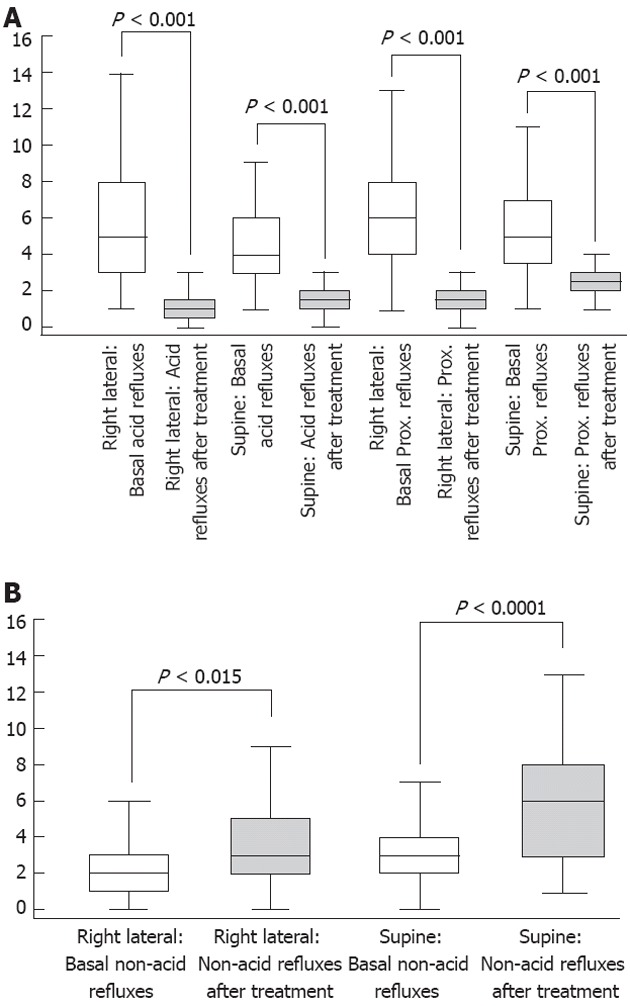
Number of acid reflux (A) and non-acid reflux (B) episodes under basal conditions and after Faringel intake in the two decubitus positions.
Symptom relief and drug tolerability
Patients reported a greater mean (range) number of symptoms before than after treatment and this included both heartburn [2.5 (1-9) vs 1 (0-2)] and regurgitation [2 (1-5) vs 1 (0-2)]. Faringel was found to be significantly effective in controlling heartburn, based on the both Likert scale [3.1 (range 1-4) vs 0.9 (0-2); P < 0.001] and VAS score [7.1 (3-9.8) vs 2 (0.1-4.8); P < 0.001), while it had less success against the symptom regurgitation based on both the Likert scale [2.6 (1-4) vs 2.2 (1-4); P = not significant (NS)] and VAS score [5.6 (2-9.6) vs 3.9 (1-8.8); P = NS]. Overall, the tolerability of Faringel was very good [5 (2-6)], with only two patients reporting modest adverse events (i.e., nausea and bloating).
DISCUSSION
Alginates are neutral polysaccharide polymers isolated from brown seaweed (Phacophycae) and are classified as dietary fiber. They are constituted by a proportion of D-mannuroic and L-glucuronic acids. In the presence of gastric acid, alginates precipitate and form a gel. One of the most interesting characteristics is due to the presence of sodium or potassium bicarbonate that, in the presence of gastric acid is converted to a dioxide which, when entrapped in the gel, converts it into a foam that floats on the surface of the gastric contents[12]. Thus, thanks to their unique mechanism, alginate-based raft-forming formulations have been marketed worldwide for > 40 years under various brand names for the symptomatic treatment of GERD, and many studies have reported their efficacy[12,38,39]. However, the majority of these studies have assessed only the control of symptoms without objective evaluation of the effect of these drugs on abnormal reflux by means of pH-monitoring, and and even less using impedance-pH testing that is available in the clinical setting since few years.
Therefore, in our prospective study we evaluated the effect of a new alginate raft-forming formulation, Faringel, in a group of 40 patients with GERD who underwent 24 h MII-pH testing after a reflux-provocative meal. Our results showed that this alginate-based formulation is able to reduce the number of acid refluxes and the esophageal exposure time below pH 4.0. Moreover, it is able to decrease significantly, in both the right lateral and supine decubitus position, the number of acid reflux events and their proximal migration. Finally, all patients also reported a lower number of symptoms after treatment, including both heartburn and regurgitation, although the effect on the latter was less evident.
The patients evaluated in the present study were truly representative of the GERD population. Indeed, they had typical reflux symptoms (i.e., heartburn and regurgitation), abnormal acid exposure time, and/or evidence of mucosal breaks at upper GI endoscopy. We opted to include patients with these characteristics in order to be sure of excluding those with functional heartburn. Moreover, we preferred not to enroll patients with normal acid exposure and positive symptom association (i.e., hypersensitive esophagus) for reducing possible confounding factors such as visceral hyperalgesia, overlap with functional disease, autonomic dysfunction and concomitant psychiatric illness that have been more associated with the above condition[40-43]. Finally, a recent report has suggested caution about overinterpretation of symptom indexes in reflux monitoring, thus supporting our decision to exclude patients with hypersensitive esophagus in order to avoid confusion[44].
Previously, Chatfield has reported a comparison of alginate preparation with placebo for the symptomatic relief of reflux esophagitis[39]. In this multicenter randomized double-blind study, alginate was superior to placebo in reducing symptom severity and increasing symptom-free days. Interestingly, the placebo group recorded a larger number of dropouts due to side effects. This means that alginate is safe and provides better relief of symptoms. An older study that simultaneously used pH-telemetry and X-rays demonstrated that pH within the raft is approximately neutral, while the pH of the gastric contents beneath the raft remains acidic (pH 1-2)[45]. These data are important because they explain why the alginate formulation is effective in controlling heartburn in the supine decubitus position, as observed in this study, and probably also during the night-time. More recently, using impedance-pH monitoring, we showed a reduction of acid reflux episodes and proximal migration of the refluxate and thereby a relevant decrease of GERD-related symptoms compared with baseline after sodium alginate administration[7]. However, the results of the latter study were less marked than those of the current investigation, probably because patients were enrolled only on the basis of symptoms without objective documentation of GERD, either by endoscopy or pH monitoring. Moreover, in the previous study, tolerability was not evaluated.
The good control of acid reflux confirms the results obtained in previous studies performed with pH-metry[7,14,46,47] or scintigraphic methods[48,49], and represents the main mechanism of the quick and effective relief of heartburn in reflux patients. In a recent study, the positive effect of sodium alginate in reducing acid refluxes has been confirmed using simultaneously stepwise pH pull-throughs, high-resolution manometry and fluoroscopy[15]. In fact, Kwiatek et al[15] have shown that alginate can also eliminate or displace the “acid-pocket”, which is a phenomenon seen in the proximity of the esophagogastric junction and is the likely origin of postprandial acid reflux in GERD patients.
Another interesting characteristic of sodium alginate has been emphasized by Manabe et al[50] in NERD patients, who are known to have a lower response rate to PPIs than patients with ERD when gauged by relief of heartburn. In this study, patients who received omeprazole combined with sodium alginate recorded longer symptom relief compared with those receiving omeprazole alone. They concluded that sodium alginate is useful in combination with PPI therapy and has to be considered for treating NERD patients who do not respond completely to PPIs. Also, in our investigation, we evaluated NERD patients and found similar results on symptom relief in this particular group of GERD patients, although we did not study Faringel as an add-on therapy.
It is likely that the positive effect of sodium alginate in controlling GERD-related symptoms is due to a whole equilibrium between raft-forming alginate and antacid substances. Faringel is constituted from sodium alginate and sodium carbonate. A previously published study has shown that, if two different antacid substances are present (e.g., Algicon Liquid), an effective reflux suppressing raft cannot form because a large amount of antacid prevents the raft formation by neutralizing the gastric acid required to react with alginate. Faringel and Gaviscon formulation consist of sodium alginate and sodium carbonate, and they have a lower acid-neutralizing capacity and a complete raft-forming gel reaction[13,47,49]. These studies have shown that a large amount of antacids is not required for strong raft formation and effective reflux suppression.
On the contrary, various findings suggest that alginate is less effective in reducing nonacid than acid reflux. In our study alginate increased significantly the number of nonacid reflux events compared with baseline. The number of total reflux episodes decreased slightly but significantly in the right lateral decubitus position, whereas no difference was found in the supine decubitus position. Similarly, Zentilin et al[7] have shown no action of alginate on nonacid reflux events. Surprisingly, in our study, the number of nonacid reflux episodes almost doubled after drug intake in 50% of patients. Probably the antacid effect of sodium alginate reduces acid reflux, but seems to increase nonacidic reflux.
Finally, our study shows that the percentage of proximal migration of reflux events decreased significantly both in the right lateral and supine decubitus positions compared with baseline, thus stopping one of the main determinants by which reflux causes symptoms[51-53]. These results confirm our previous findings with a different sodium alginate formulation, although the study was performed in a smaller sample of patients and without a clear documentation of GERD[7]. These investigations performed with impedance-pH monitoring technique permitted us to assess the ability of sodium alginate to reduce the proximal extension of refluxed material. The raft obtained with alginate represents a cork in the zone of the LES that prevents any gastric material migration into the esophagus independently of the patient decubitus. This beneficial effect could help in controlling not only typical, but especially extraesophageal symptoms. In particular, the Faringel formulation adds to alginate able to control GERD related typical symptoms a large number of vegetal extracts, which have the potential to promote healing of pharyngoesophageal mucosal lesions. The anti-flogistic properties of Faringel are due to herbs such as Propolis, A. vera, and Calendula. Eamlamnam et al[54] have observed that A. vera treatment induces a complete reduction in leukocyte adherence and tumor necrosis factor-α levels combined with elevated interleukin-10 levels, which are able to promote healing of gastric ulcers in male Sprague-Dawley rats. Propolis and A. vera have also demonstrated pain-killing effects[55]. Moreover, experimental studies have shown that C. officinalis has anti-inflammatory and antibacterial activities as well as angiogenic and fibroblastic properties acting in a positive way on the inflammatory and proliferative phase of the healing process[20]. Thus, we can speculate that all these data on the anti-inflammatory properties of the herbal components of Faringel may be relevant for extraesophageal reflux-related symptoms in which a flogistic component seems to be more evident[56].
In conclusion, our findings demonstrated that Faringel formulation is well tolerated and highly effective in controlling, or at least reducing, heartburn in GERD patients by modifying the number of acid reflux episodes and lowering the proximal migration of reflux events. It was less effective in controlling nonacid reflux and regurgitation. Its action in reducing the proximal extension of reflux events and the combined presence of natural substances (Calendula, Aloe, honey) that favor mucosal healing could be useful to improve GERD-related extraesophageal symptoms.
COMMENTS
Background
The use of proton pump inhibitors (PPIs) has been considered the best therapeutic option for gastroesophageal reflux disease (GERD), given their high efficacy in inducing symptom relief and esophageal mucosal healing. On the other hand, there is increasing evidence that not all patients (30%-35%) respond satisfactorily to this treatment. Thus, traditional antacids as alginate-based raft-forming formulations are used worldwide as add-on therapy to neutralize gastric acidity and help control heartburn. In recent years, multichannel intraluminal impedance combined with pH-metry (MII-pH) has been applied to assess the effectiveness of drugs or endoscopic devices proposed for the therapy of GERD, particularly if people want to know whether they can affect both acid and nonacid reflux or reduce the proximal migration of the refluxate.
Research frontiers
There are few data available regarding the mechanisms by which antacids as alginate-based raft-forming formulations are able to modify the determinants of reflux symptom perception, therefore, this study tried to evaluate the antireflux properties of an alginate antacid formulation (Faringel) on both acid and nonacid reflux episodes, and the height of proximal extent of reflux events by means of MII-pH monitoring in patients with documented mild to moderate GERD. As a secondary aim, authors assessed the therapeutic efficacy of this novel compound as well as its tolerability using validated questionnaires.
Innovations and breakthroughs
In this prospective, open-label study, 40 patients reporting heartburn and regurgitation with proven reflux disease (i.e., positive impedance-pH test/evidence of erosive esophagitis at upper endoscopy) underwent 2-h impedance-pH test after eating a refluxogenic meal. They were studied for 1 h under basal conditions and 1 h after taking 10 mL Faringel. Patients also completed validated questionnaires in order to evaluate the efficacy of Faringel for symptom relief. Tolerability of the treatment was also assessed.
Applications
The results suggest that Faringel is able to reduce the esophageal acid exposure time, the number of acid reflux events and their proximal migration, thus stopping two of the main determinants by which reflux causes symptoms (i.e., abnormal esophageal acid exposure time and proximal extension of the refluxate). Moreover, Faringel was very well-tolerated and effective in reducing heartburn and regurgitation, although the efficacy on the latter symptom was less evident.
Terminology
GERD is a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications; Faringel is an antacid formulation, containing sodium bicarbonate and alginate with the addition of herbal components (i.e., honey, Chamomille or Matricaria recutita L., Calendula officinalis, Aloe vera, Propolis gel) that have been recently associated with mild anti-inflammatory and analgesic effects; MII-pH is a novel technique for pH-independent detection of GER.
Peer review
The study is interesting, well conducted, with a clear statistical analysis and a comprehensive discussion.
Footnotes
Peer reviewers: Dr. William R Parker, PhD, Assistant Professor, Department of Surgery, Duke University Medical Center, Box 2605, Durham, NC 27710, United States; Fabio Pace, Professor, Division of Gastroenterology, “L. Sacco” University Hospital, University of Milan, Via G. B. Grassi, 74, 20157 Milano, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Li JY
References
- 1.Kahrilas PJ. Gastroesophageal reflux disease. JAMA. 1996;276:983–988. [PubMed] [Google Scholar]
- 2.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131–137. doi: 10.1097/01.mcg.0000225631.07039.6d. [DOI] [PubMed] [Google Scholar]
- 3.Savarino E, Tutuian R, Zentilin P, Dulbecco P, Pohl D, Marabotto E, Parodi A, Sammito G, Gemignani L, Bodini G, et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol. 2010;105:1053–1061. doi: 10.1038/ajg.2009.670. [DOI] [PubMed] [Google Scholar]
- 4.Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 5.Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, Skammer W, Levine JG. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575–583. doi: 10.1111/j.1572-0241.2002.05532.x. [DOI] [PubMed] [Google Scholar]
- 6.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58:295–309. doi: 10.1136/gut.2007.145581. [DOI] [PubMed] [Google Scholar]
- 7.Zentilin P, Dulbecco P, Savarino E, Parodi A, Iiritano E, Bilardi C, Reglioni S, Vigneri S, Savarino V. An evaluation of the antireflux properties of sodium alginate by means of combined multichannel intraluminal impedance and pH-metry. Aliment Pharmacol Ther. 2005;21:29–34. doi: 10.1111/j.1365-2036.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 8.Boeckxstaens GE, Beaumont H, Hatlebakk JG, Silberg DG, Björck K, Karlsson M, Denison H. A novel reflux inhibitor lesogaberan (AZD3355) as add-on treatment in patients with GORD with persistent reflux symptoms despite proton pump inhibitor therapy: a randomised placebo-controlled trial. Gut. 2011;60:1182–1188. doi: 10.1136/gut.2010.235630. [DOI] [PubMed] [Google Scholar]
- 9.Giannini EG, Zentilin P, Dulbecco P, Iiritano E, Bilardi C, Savarino E, Mansi C, Savarino V. A comparison between sodium alginate and magaldrate anhydrous in the treatment of patients with gastroesophageal reflux symptoms. Dig Dis Sci. 2006;51:1904–1909. doi: 10.1007/s10620-006-9284-0. [DOI] [PubMed] [Google Scholar]
- 10.Maton PN, Burton ME. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs. 1999;57:855–870. doi: 10.2165/00003495-199957060-00003. [DOI] [PubMed] [Google Scholar]
- 11.Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on Acid suppressive therapy. Am J Gastroenterol. 2008;103:1090–1096. doi: 10.1111/j.1572-0241.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 12.Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment Pharmacol Ther. 2000;14:669–690. doi: 10.1046/j.1365-2036.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 13.Lambert JR, Korman MG, Nicholson L, Chan JG. In-vivo anti-reflux and raft properties of alginates. Aliment Pharmacol Ther. 1990;4:615–622. doi: 10.1111/j.1365-2036.1990.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 14.Castell DO, Dalton CB, Becker D, Sinclair J, Castell JA. Alginic acid decreases postprandial upright gastroesophageal reflux. Comparison with equal-strength antacid. Dig Dis Sci. 1992;37:589–593. doi: 10.1007/BF01307584. [DOI] [PubMed] [Google Scholar]
- 15.Kwiatek MA, Roman S, Fareeduddin A, Pandolfino JE, Kahrilas PJ. An alginate-antacid formulation (Gaviscon Double Action Liquid) can eliminate or displace the postprandial ‘acid pocket’ in symptomatic GERD patients. Aliment Pharmacol Ther. 2011;34:59–66. doi: 10.1111/j.1365-2036.2011.04678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z Naturforsch C. 2003;58:580–589. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 17.Lotfy M, Badra G, Burham W, Alenzi FQ. Combined use of honey, bee propolis and myrrh in healing a deep, infected wound in a patient with diabetes mellitus. Br J Biomed Sci. 2006;63:171–173. doi: 10.1080/09674845.2006.11732742. [DOI] [PubMed] [Google Scholar]
- 18.Weichselgartner-Schröder C. [Healing naturally with propolis. With bee propolis to new health] Pflege Z. 1997;50:98–102. [PubMed] [Google Scholar]
- 19.Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378–382. doi: 10.1097/JCP.0b013e3181ac935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parente LM, Lino Júnior Rde S, Tresvenzol LM, Vinaud MC, de Paula JR, Paulo NM. Wound Healing and Anti-Inflammatory Effect in Animal Models of Calendula officinalis L. Growing in Brazil. Evid Based Complement Alternat Med. 2012;2012:375671. doi: 10.1155/2012/375671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preethi KC, Kuttan R. Wound healing activity of flower extract of Calendula officinalis. J Basic Clin Physiol Pharmacol. 2009;20:73–79. doi: 10.1515/jbcpp.2009.20.1.73. [DOI] [PubMed] [Google Scholar]
- 22.Ulbricht C, Armstrong J, Basch E, Basch S, Bent S, Dacey C, Dalton S, Foppa I, Giese N, Hammerness P, et al. An evidence-based systematic review of Aloe vera by the natural standard research collaboration. J Herb Pharmacother. 2007;7:279–323. doi: 10.1080/15228940802153339. [DOI] [PubMed] [Google Scholar]
- 23.Zentilin P, Dulbecco P, Savarino E, Giannini E, Savarino V. Combined multichannel intraluminal impedance and pH-metry: a novel technique to improve detection of gastro-oesophageal reflux literature review. Dig Liver Dis. 2004;36:565–569. doi: 10.1016/j.dld.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–1606. doi: 10.1053/gast.2001.24840. [DOI] [PubMed] [Google Scholar]
- 25.Vela MF, Tutuian R, Katz PO, Castell DO. Baclofen decreases acid and non-acid post-prandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther. 2003;17:243–251. doi: 10.1046/j.1365-2036.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- 26.Frazzoni M, Savarino E, Manno M, Melotti G, Mirante VG, Mussetto A, Bertani H, Manta R, Conigliaro R. Reflux patterns in patients with short-segment Barrett’s oesophagus: a study using impedance-pH monitoring off and on proton pump inhibitor therapy. Aliment Pharmacol Ther. 2009;30:508–515. doi: 10.1111/j.1365-2036.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 27.Savarino E, Zentilin P, Tutuian R, Pohl D, Casa DD, Frazzoni M, Cestari R, Savarino V. The role of nonacid reflux in NERD: lessons learned from impedance-pH monitoring in 150 patients off therapy. Am J Gastroenterol. 2008;103:2685–2693. doi: 10.1111/j.1572-0241.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson R, Dent J, Bolling-Sternevald E, Johnsson F, Junghard O, Lauritsen K, Riley S, Lundell L. The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol. 1998;33:1023–1029. doi: 10.1080/003655298750026697. [DOI] [PubMed] [Google Scholar]
- 29.Zentilin P, Iiritano E, Dulbecco P, Bilardi C, Savarino E, De Conca S, Parodi A, Reglioni S, Vigneri S, Savarino V. Normal values of 24-h ambulatory intraluminal impedance combined with pH-metry in subjects eating a Mediterranean diet. Dig Liver Dis. 2006;38:226–232. doi: 10.1016/j.dld.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredenoord AJ, Weusten BL, Smout AJ. Symptom association analysis in ambulatory gastro-oesophageal reflux monitoring. Gut. 2005;54:1810–1817. doi: 10.1136/gut.2005.072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Herwaarden MA, Katzka DA, Smout AJ, Samsom M, Gideon M, Castell DO. Effect of different recumbent positions on postprandial gastroesophageal reflux in normal subjects. Am J Gastroenterol. 2000;95:2731–2736. doi: 10.1111/j.1572-0241.2000.03180.x. [DOI] [PubMed] [Google Scholar]
- 34.Frazzoni M, Grisendi A, Lanzani A, Melotti G, De Micheli E. Laparoscopic fundoplication versus lansoprazole for gastro-oesophageal reflux disease. A pH-metric comparison. Dig Liver Dis. 2002;34:99–104. doi: 10.1016/s1590-8658(02)80237-7. [DOI] [PubMed] [Google Scholar]
- 35.Fass R, Johnson DA, Orr WC, Han C, Mody R, Stern KN, Pilmer BL, Perez MC. The effect of dexlansoprazole MR on nocturnal heartburn and GERD-related sleep disturbances in patients with symptomatic GERD. Am J Gastroenterol. 2011;106:421–431. doi: 10.1038/ajg.2010.458. [DOI] [PubMed] [Google Scholar]
- 36.Geeraerts B, Vandenberghe J, Van Oudenhove L, Gregory LJ, Aziz Q, Dupont P, Demyttenaere K, Janssens J, Tack J. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology. 2005;129:1437–1444. doi: 10.1053/j.gastro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Miwa H, Inoue K, Ashida K, Kogawa T, Nagahara A, Yoshida S, Tano N, Yamazaki Y, Wada T, Asaoka D, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–332. doi: 10.1111/j.1365-2036.2010.04517.x. [DOI] [PubMed] [Google Scholar]
- 38.Williams DL, Haigh GG, Redfern JN. The symptomatic treatment of heartburn and dyspepsia with Liquid Gaviscon: a multicentre general practitioner study. J Int Med Res. 1979;7:551–555. doi: 10.1177/030006057900700614. [DOI] [PubMed] [Google Scholar]
- 39.Chatfield S. A comparison of the efficacy of the alginate preparation, Gaviscon Advance, with placebo in the treatment of gastro-oesophageal reflux disease. Curr Med Res Opin. 1999;15:152–159. doi: 10.1185/03007999909114086. [DOI] [PubMed] [Google Scholar]
- 40.Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, Vigneri S, Camerini G, Tutuian R, Savarino V. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009;58:1185–1191. doi: 10.1136/gut.2008.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol. 2011;9:824–833. doi: 10.1016/j.cgh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Savarino E, Marabotto E, Zentilin P, Frazzoni M, Sammito G, Bonfanti D, Sconfienza L, Assandri L, Gemignani L, Malesci A, et al. The added value of impedance-pH monitoring to Rome III criteria in distinguishing functional heartburn from non-erosive reflux disease. Dig Liver Dis. 2011;43:542–547. doi: 10.1016/j.dld.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Savarino V, Savarino E, Parodi A, Dulbecco P. Functional heartburn and non-erosive reflux disease. Dig Dis. 2007;25:172–174. doi: 10.1159/000103879. [DOI] [PubMed] [Google Scholar]
- 44.Slaughter JC, Goutte M, Rymer JA, Oranu AC, Schneider JA, Garrett CG, Hagaman D, Vaezi MF. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2011;9:868–874. doi: 10.1016/j.cgh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Beckloff GL, Chapman JH, Shiverdecker P. Objective evaluation of an antacid with unusual properties. J Clin Pharmacol New Drugs. 1972;12:11–21. doi: 10.1002/j.1552-4604.1972.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 46.Johnson LF, DeMeester TR. Evaluation of elevation of the head of the bed, bethanechol, and antacid form tablets on gastroesophageal reflux. Dig Dis Sci. 1981;26:673–680. doi: 10.1007/BF01316854. [DOI] [PubMed] [Google Scholar]
- 47.Washington N, Steele RJ, Jackson SJ, Washington C, Bush D. Patterns of food and acid reflux in patients with low-grade oesophagitis--the role of an anti-reflux agent. Aliment Pharmacol Ther. 1998;12:53–58. doi: 10.1046/j.1365-2036.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- 48.Malmud LS, Charkes ND, Littlefield J, Reilley J, Stern H, Rosenberg R, Fisher RS. The mode of action alginic acid compound in the reduction of gastroesophageal reflux. J Nucl Med. 1979;20:1023–1028. [PubMed] [Google Scholar]
- 49.Washington N, Greaves JL, Iftikhar SY. A comparison of gastro-oesophageal reflux in volunteers assessed by ambulatory pH and gamma monitoring after treatment with either Liquid Gaviscon or Algicon Suspension. Aliment Pharmacol Ther. 1992;6:579–588. doi: 10.1111/j.1365-2036.1992.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 50.Manabe N, Haruma K, Ito M, Takahashi N, Takasugi H, Wada Y, Nakata H, Katoh T, Miyamoto M, Tanaka S. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus. 2012;25:373–380. doi: 10.1111/j.1442-2050.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 51.Savarino E, Zentilin P, Tutuian R, Pohl D, Gemignani L, Malesci A, Savarino V. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol. 2012;47:159–168. doi: 10.1007/s00535-011-0480-0. [DOI] [PubMed] [Google Scholar]
- 52.Cicala M, Habib FI, Emerenziani S. Proximal oesophagus: the added value in understanding GORD symptoms. Neurogastroenterol Motil. 2009;21:790–795. doi: 10.1111/j.1365-2982.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 53.Savarino E, Zentilin P, Frazzoni M, Cuoco DL, Pohl D, Dulbecco P, Marabotto E, Sammito G, Gemignani L, Tutuian R, et al. Characteristics of gastro-esophageal reflux episodes in Barrett’s esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterol Motil. 2010;22:1061–e280. doi: 10.1111/j.1365-2982.2010.01536.x. [DOI] [PubMed] [Google Scholar]
- 54.Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D. Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats. World J Gastroenterol. 2006;12:2034–2039. doi: 10.3748/wjg.v12.i13.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magro-Filho O, de Carvalho AC. Topical effect of propolis in the repair of sulcoplasties by the modified Kazanjian technique. Cytological and clinical evaluation. J Nihon Univ Sch Dent. 1994;36:102–111. doi: 10.2334/josnusd1959.36.102. [DOI] [PubMed] [Google Scholar]
- 56.Vaezi MF, Hagaman DD, Slaughter JC, Tanner SB, Duncavage JA, Allocco CT, Sparkman C, Clement LE, Wasden CM, Wirth D, et al. Proton pump inhibitor therapy improves symptoms in postnasal drainage. Gastroenterology. 2010;139:1887–1893.e1; quiz e11. doi: 10.1053/j.gastro.2010.08.039. [DOI] [PubMed] [Google Scholar]


