Abstract
An 87-year-old, Japanese woman was shown to have a submucosal tumor-like lesion with a deep, central ulceration covered with thick, whitish exudate in the stomach. Biopsy showed Candida tropicalis but not Helicobacter pylori (H. pylori). She had no predisposing factors or history of peptic ulcers nor had taken non-steroidal anti-inflammatory drugs (NSAIDs), diagnosed with Candida-associated gastric ulcer. Though cured of the lesion, she developed another ulcer in a different position, in which Candida was demonstrated but H. pylori was undetectable. This is the first case of recurrent Candida-associated gastric ulcer in the world. Detected in both the original and recurrent lesions in an H. pylori-negative patient with no antecedent ulcers who had not taken NSAIDs, Candida is considered, contrary to the prevailing opinion, to play an etiologic role in ulcer formation.
Keywords: Candida-associated gastric ulcer, Gastric candidiasis, Helicobacter pylori-negative gastric ulcer, Non-steroidal anti-inflammatory drugs-induced gastric ulcer, Recurrent gastric ulcer
INTRODUCTION
Candida is ubiquitously indigenous to the normal human gastrointestinal tract throughout[1] but infection with the fungus of the tract is more widespread than previously recognized[2,3]. The most frequently involved organ is the esophagus followed by the stomach[2]. Gastric candidiasis is classified into thrush, nodular and ulcerated types[4]. Though usually seen in immunocompromized hosts[2,3], Candida-associated gastric ulcer also occurs in apparently healthy individuals[5-9] and its frequency is widely different contingent on authors[8-12]. The clinical significance of the fungus in and the natural history of Candida-associated gastric ulcer remain to be clarified[2] so that the treatment of the disease has not yet been established. The fungus has been reported to be no longer detected once the ulcers were healed and no recurrence of the disease has been described, so far[9,11].
Presented is a hitherto unreported case of the disease relapsing in a different site with a different shape in a Helicobacter pylori (H. pylori)-negative patient with no antecedent peptic ulcers who had not taken non-steroidal anti-inflammatory drugs (NSAIDs).
CASE REPORT
Complaining of anorexia after succumbing to the summer heat, an 87-year-old, Japanese housewife was shown to have a medium-sized, submucosal tumor (SMT)-like elevation covered with the erythematous mucosa with an oval, deep, central ulceration covered with thick whitish exudate on the greater curvature of the upper gastric body (Figure 1). Biopsy showed no malignancy or H. pylori but a large number of hyphae in the ulcer slough (Figure 2A). No signs of candidiasis were detected in the oropharynx through esophagus or in the duodenal bulb through descending part of the duodenum.
Figure 1.
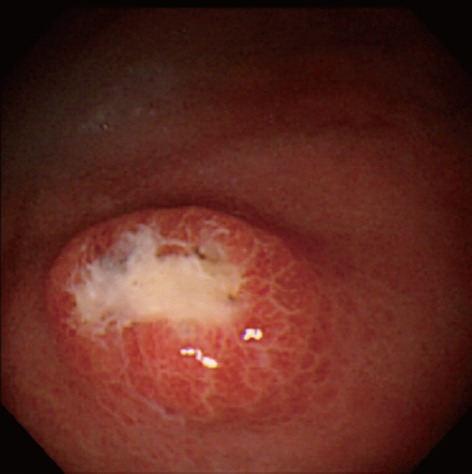
Endoscopic photograph showing the deep ulcer with the submucosal tumor-like margin on the greater curvature of the upper gastric body.
Figure 2.
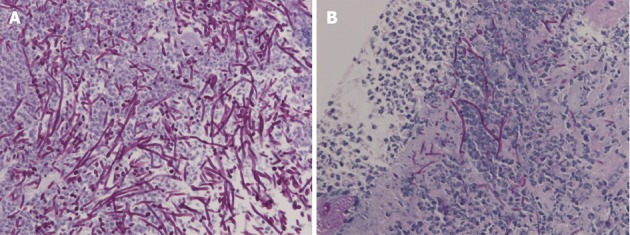
Biopsy demonstrated no malignancy but numerous hyphae. A: Light micrograph of the specimen biopsied from the original ulcer demonstrating hyphae in the ulcer slough [Periodic acid-Schiff (PAS)/diastase, original magnification × 400]; B: Biopsy from the recurrent ulcer illustrating hyphae of Candida on the ulcer edge (PAS/diastase, original magnification × 400).
Though suffered from adhesion ileus 2 mo ago, when no such lesions or any scars in the upper or any signs of candidiasis in the lower gastrointestinal tract were endoscopically detected, in the wake of ovariectomy and concomitant appendectomy she underwent when younger and had been given steroid inhaler for bronchial asthma for the past several years, she had no past history of peptic ulcers. Neither had she taken NSAIDs or antibiotics then. She was not diabetic. The ulceration was found much diminished in size spontaneously 2 wk after the first endoscopic examination and the organism was proven to be Candida tropicalis by culture. No circulating Candida antigens or β-D-glucan was detected, however. Though diagnosed as having Candida-associated gastric ulcer, she refused to undergo further endoscopic examination, not given any proton pump inhibitors (PPIs), H2 receptor antagonists, or antifungal agents.
She complained of heartburn 10 mo later, when the lesion was shown to have turned into a white scar (Figure 3A) but another large, oval, deep ulcer was detected covered with thick, whitish exudate surrounded by the markedly swollen margin on the lesser curvature of the lower gastric body (Figure 3B). Biopsy demonstrated no malignancy but numerous hyphae of Candida again (Figure 2B). No findings of candidiasis were recognized in the oropharynx through other parts of the upper gastrointestinal tract. H. pylori was not detected by histopathologic examination or rapid urease test. Though had not taken any NSAIDs or antibiotics then, either, she had been given risedronate by an orthopedist under the diagnosis of osteoporosis once a week for the past 9 mo. She was diagnosed with Candida-associated gastric ulcer recurrent in a different location with a different appearance.
Figure 3.
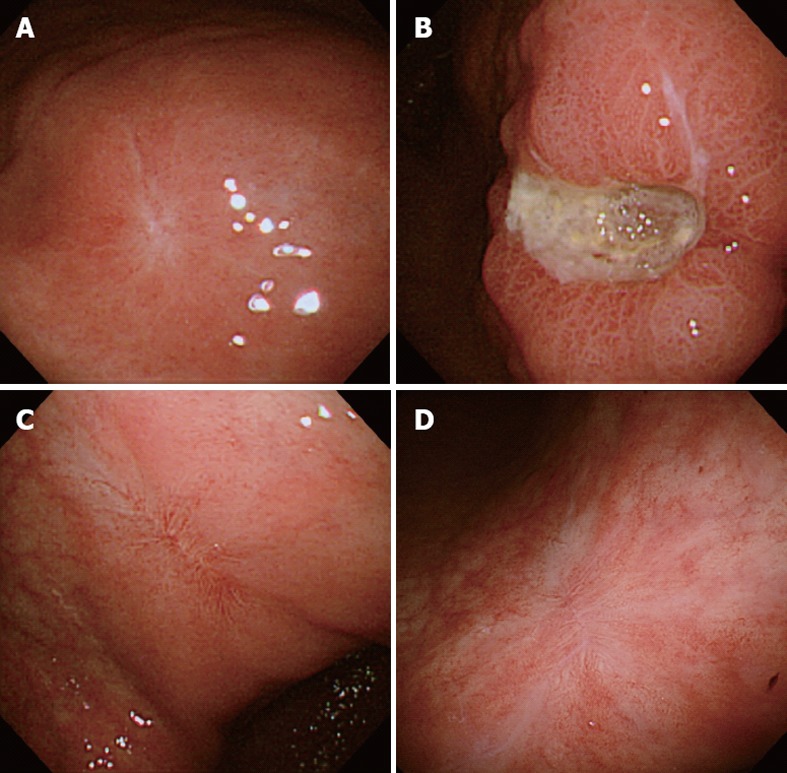
Endoscopic photographs. A: The white scar of the original ulcer; B: The recurrent ulcer on the lesser curvature of the lower gastric body; C: The red scar of the recurrent ulcer; D: The transition from the red to white scar in 3 mo.
The lesion was proven to have turned into a red scar in 6 wk with administration of a PPI without antifungal agents (Figure 3C). The original lesion was shown to have remained to be a white scar. The follow-up endoscopy performed 3 mo later showed the red scar to have turned into white (Figure 3D) and the original scar to have remained unchanged. She has kept an uneventful course ever since, even though she has kept taking risederonate. No further recurrence has been detected, so far.
DISCUSSION
Candida is a fungus indigenous to the entire human gastrointestinal tract[1] and the demonstration of infiltration of the tissue or ulcer slough by the hyphae is proposed as the diagnostic criterion of gastrointestinal candidiasis[5,8]. The present case was diagnosed according to the criterion. Candida-associated gastric ulcer is more widespread than previously considered, seen not only in patients with predisposing conditions but in apparently healthy individuals[5-9]. The present case was also lacking in conspicuous predisposing factors.
While endoscopic features of Candida-associated gastric ulcer have been asserted to be nonspecific[4,9,11], an SMT-like lesion with a deep, central ulceration has been regarded as specific by some Japanese authors[13,14]. In the present case, the original lesion did indeed assume such a form but the recurrent one appeared to be an ordinary, large ulcer. Since an SMT-like lesion with a central ulceration has been detected only in Japanese patients[13,14], it may be peculiar to the race and considered suggestive of Candida-associated gastric ulcer, though not all ulcers associated with the fungus take on such an appearance.
Whereas Candida-associated gastric ulcer has been reported to have low healing rate[6,11], some cases of the disease have been reported to have spontaneously healed[12]. In the present case, the original lesion was found to have spontaneously healed and the recurrent one was in no way intractable. The intractability of the disease may be affected by other factors than the fungus, such as H. pylori or NSAIDs because such factors were not inspected in the cases reported to be intractable[6,11].
Candida has been reported to be no longer detected once the ulcers were healed even without antifungal treatment and no recurrence of the lesions has been described, so far[9,11]. The natural history of Candida-associated gastric ulcer is yet to be elucidated and the disease was reported to engender no specific symptoms[8]. Presenting no specific symptoms, either, the present patient developed the disease without any antecedent peptic ulcers or their vestiges, which healed spontaneously at the first time and recurred 10 mo later. This is the first case of Candida-associated gastric ulcer in the world, which was demonstrated to have recurred not only in a different site but with a different appearance and followed up from before development of the original ulcer till complete cicatrization of the recurrent, disclosing the natural history of the disease.
Bisphosphonates are known to be ulcerogenic, considered to disrupt the protective hydrophobic phospholipid barrier of the gastric mucosa to trigger ulceration[15,16]. But they are reported to greatly differ in potential to damage the mucosa[15,16], risedronate having by far the weakest potential[15]: the drug has been shown to have a gastrointestinal safety profile similar to that of placebo[17-19]. It was not until 9 mo after the treatment with the drug was instituted when the recurrent ulcer was developed in the present case and, after it was healed, no recurrence has been detected even under the regimen. Sufficient ground is, therefore, lacking to suspect the drug to be the culprit in development of the recurrent ulcer. Though the clinical significance of the fungus has not yet been elucidated, it has not been regarded as directly etiologic in development of ulcer, possibly as the presence of the fungus aggravates or perpetuates ulceration[5,6,8]. Contrary to the prevailing opinion, the fungus is considered, by analogy with H. pylori, to play an etiologic role in ulcer formation in the present case on the basis of the circumstantial evidences, since it was detected not only in the original but also in recurrent lesion in an H. pylori-negative patient with no antecedent peptic ulcers who had not taken NSAIDs.
ACKNOWLEDGMENTS
The author wishes to express his gratitude to Dr. Masuda at the Anti-Cancer Institute, Miyagi and Dr. Iwama at Tohoku Hospital for Work-related Accident Cases for their very helpful instructions.
Footnotes
Peer reviewer: Khaled Jadallah, MD, Assistant Professor of Medicine, Consultant, Gastroenterologist and Hepatologist, Department of Internal Medicine, King Abdullah University Hospital, Jordan University of Science and Technology, Irbid 22110, Jordan
S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Cohen R, Roth FJ, Delgado E, Ahearn DG, Kalser MH. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969;280:638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- 2.Eras P, Goldstein MJ, Sherlock P. Candida infection of the gastrointestinal tract. Medicine (Baltimore) 1972;51:367–379. doi: 10.1097/00005792-197209000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Trier JS, Bjorkman DJ. Esophageal, gastric, and intestinal candidiasis. Am J Med. 1984;77:39–43. [PubMed] [Google Scholar]
- 4.Minoli G, Terruzzi V, Butti G, Frigerio G, Rossini A. Gastric candidiasis: an endoscopic and histological study in 26 patients. Gastrointest Endosc. 1982;28:59–61. doi: 10.1016/s0016-5107(82)72998-0. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein AL, Maksem J. Candidal infection of gastric ulcers. Histology, incidence, and clinical significance. Am J Clin Pathol. 1979;71:137–141. doi: 10.1093/ajcp/71.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Neeman A, Avidor I, Kadish U. Candidal infection of benign gastric ulcers in aged patients. Am J Gastroenterol. 1981;75:211–213. [PubMed] [Google Scholar]
- 7.Vilotte J, Toutoungi M, Coquillard A. [Candida infection of gastric ulcers. 6 cases (author’s transl)] Nouv Presse Med. 1981;10:1471–1474. [PubMed] [Google Scholar]
- 8.Scott BB, Jenkins D. Gastro-oesophageal candidiasis. Gut. 1982;23:137–139. doi: 10.1136/gut.23.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minoli G, Terruzzi V, Ferrara A, Casiraghi A, Rocca F, Rainer H, Porro A, Butti GC, Mandelli PG, Piffer R, et al. A prospective study of relationships between benign gastric ulcer, Candida, and medical treatment. Am J Gastroenterol. 1984;79:95–97. [PubMed] [Google Scholar]
- 10.Di Febo G, Miglioli M, Calò G, Biasco G, Luzza F, Gizzi G, Cipollini F, Rossi A, Barbara L. Candida albicans infection of gastric ulcer frequency and correlation with medical treatment. Results of a multicenter study. Dig Dis Sci. 1985;30:178–181. doi: 10.1007/BF01308206. [DOI] [PubMed] [Google Scholar]
- 11.Morishita T, Kamiya T, Munakata Y, Tsuchiya M. Radiologic and endoscopic studies of gastric ulcers associated with Candida infection. Acta Gastroenterol Latinoam. 1993;23:223–229. [PubMed] [Google Scholar]
- 12.Gotlieb-Jensen K, Andersen J. Occurrence of Candida in gastric ulcers. Significance for the healing process. Gastroenterology. 1983;85:535–537. [PubMed] [Google Scholar]
- 13.Hirasaki S, Koide N, Ogawa H, Tsuji T. Benign gastric ulcer associated with Canidida infection in a healthy adult. J Gastroenterol. 1999;34:688–693. doi: 10.1007/s005350050320. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura S, Nagata N, Kobayakawa M, Sako A, Nakashima R, Uemura N. A case of candidal infection of gastric ulcers with characteristic endoscopic findings. Nihon Shokakibyo Gakkai Zasshi. 2011;108:1393–1398. [PubMed] [Google Scholar]
- 15.Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology. 2000;119:631–638. doi: 10.1053/gast.2000.16517. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenberger LM, Romero JJ, Gibson GW, Blank MA. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig Dis Sci. 2000;45:1792–1801. doi: 10.1023/a:1005574009856. [DOI] [PubMed] [Google Scholar]
- 17.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, Brown J, Eriksen EF, Hoseyni MS, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–2318. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]


