Abstract
The Recombination Activating Genes (RAGs) encode two enzymes that play key roles in the adaptive immune system. RAG1 and RAG2 mediate VDJ recombination, a process necessary for the maturation of B- and T-cells. Interestingly, RAG1 is also expressed in the brain, particularly in areas of high neural density such as the hippocampus, although its function is unknown. We tested evidence that RAG1 plays a role in brain function using a social recognition memory task, an assessment of the acquisition and retention of conspecific identity. In a first experiment, we found that RAG1-deficient mice show impaired social recognition memory compared to mice wildtype for the RAG1 allele. In a second experiment, by breeding to homogenize background genotype we found that RAG1-deficient mice show impaired social recognition memory relative to heterozygous or RAG2-deficient littermates. Because RAG1 and RAG2 null mice are both immunodeficient, the results suggest that the memory impairment is not an indirect effect of immunological dysfunction. RAG1-deficient mice show normal habituation to non-socially derived odors and habituation to an open-field, indicating that the observed effect is not likely a result of a general deficit in habituation to novelty. These data trace the origin of the impairment in social recognition memory in RAG1-deficient mice to the RAG1 gene locus and implicate RAG1 in memory formation.
Keywords: habituation learning, conspecific memory, open-field locomotor activity, olfactory behavior, immune system, somatic recombination
1. Introduction
The RAG genes have been the subject of intense study in the immune system, where they mediate the diversification of B- and T-cell receptors via somatic recombination. During somatic recombination, genetic ‘recombination signal sequences’ are targeted by RAG1 and RAG2 enzymes, which together cleave Variable, Diversity, and Joining (VDJ) gene segments located on several chromosomes. These are brought together during a process of DNA rearrangement by DNA repair mechanisms. The DNA rearrangement initiated by RAG occurs in a combinatorial fashion, greatly increasing the variety of B- and T-cell receptor subtypes. As a result of this and other processes, the immune system is able to recognize virtually any foreign pathogen. A number of researchers have speculated that similar processes of receptor diversification may occur in the Central Nervous System (CNS) (Pena De Ortiz and Arshavsky, 2001; Schatz and Chun, 1992; Yagi, 2003), in part because many of the same molecules involved in somatic recombination, such as those involved in DNA double-strand break repair, are necessary for neural development and function (Chun and Schatz, 1999a; Chun and Schatz, 1999b; Colon-Cesario et al., 2006). However, direct evidence of somatic recombination in the CNS remains to be demonstrated.
Intriguingly, RAG1 but not RAG2 is expressed in the CNS, the only tissue outside of the immune system shown to express RAG (Chun et al., 1991). RAG1 is expressed in both embryonic and postnatal brain, with expression highest in limbic areas, including in the hippocampus, and in the cerebellum (Chun et al., 1991; Sun et al., 2007). These are areas of high neural density, suggesting RAG1 may be present in neurons. The immunological consequence of RAG1 or RAG2 deficiency is an obvious severe combined immunodeficiency (Mombaerts et al., 1992; Shinkai et al., 1992). However, RAG1-deficient mice show no obvious alterations in CNS anatomy or physiology (Chun et al., 1991). The only other previous study to directly address functional alterations as a result of RAG1-deletion (Cushman et al., 2003) reported increased activity, but no effect of RAG1-deletion on water maze memory, pre-pulse inhibition, or acoustic startle response, standard measures of neurobehavioral function assessing limbic circuitry.
In the present study, we examined the performance of mice with RAG1-deletion (RAG1KO) on a social recognition memory task. The social recognition memory task assesses the ability of rodents to successfully identify previously encountered conspecifics, mainly via olfactory cues (Gheusi et al., 1994). In socially-housed mice, social recognition memory at delay intervals longer than 30 min following exposure to a conspecific depends upon intact hippocampal function (Kogan et al., 2000). A variety of social recognition memory paradigms have been developed with differing memory performance depending on the particular task parameters (Markham and Juraska, 2007). We sought to take advantage of the social recognition memory task’s rapid implementation, as an example of a so-called ‘one trial learning’ task and one that is amenable to pathogen-free testing conditions for use with immunocompromized subjects.
2. Results
2.1 Experiment 1: Social recognition memory among RAG1KO and RAG1 Wildtype (WT) mice
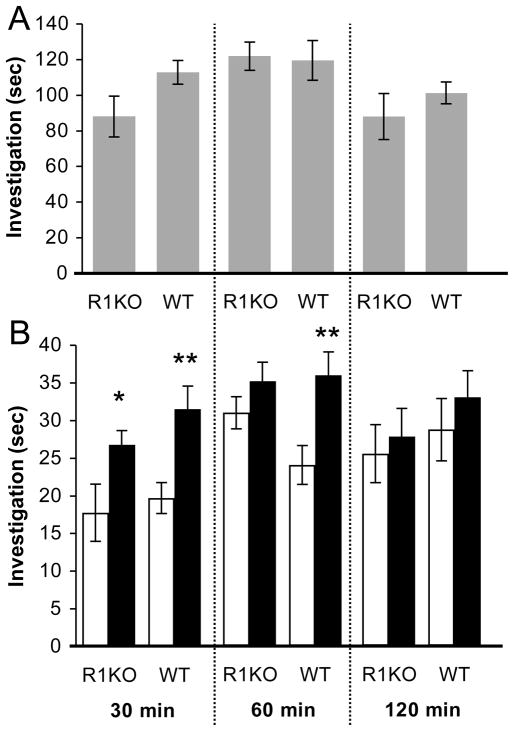
A social recognition memory task, consisting of an initial exposure to an ovariectomized ‘stimulus mouse’ and a subsequent discrimination trial where subjects were simultaneously exposed to the same stimulus mouse and a novel stimulus mouse, was employed to examine memory among RAG1KO and WT mice. Preliminary experiments during the dark phase of the cycle in our facility using WT mice indicated that social recognition memory was intact after a 30 min delay but was not evident after 120 min delay. We therefore assessed discrimination of the previously exposed stimulus mouse after delays of 30 min, 60 min, and 120 min. Separate cohorts of mice were used at the 30 min (n = 12 R1KO and n = 12 WT), 60 min (n = 16 R1KO and n = 16 WT) and 120 min trials (n = 12 R1KO and n = 12 WT). Subjects that repeatedly fought with or attempted to mount the non-receptive stimulus mouse were removed from analysis (30 min: n = 2 R1KO, n = 2 WT; 60 min: n = 2 R1KO, n = 1 WT; 120 min: n = 1 R1KO, n = 1 WT).
There were no statistically significant differences in investigation of the stimulus mouse during the initial encounter between RAG1KO and WT mice on either the 30 min, 60 min, or 120 min trials (Fig. 1A; P’s > 0.05), suggesting that differences in duration of exposure to the stimulus mouse were unlikely to influence subsequent recognition memory performance. After a delay of 30 min, both genotypes successfully recognized the previously exposed stimulus mouse (Fig. 1B, left panel; RAG1KO: t(9) = 2.39, P = 0.040, d = 1.11; WT: t(9) = 3.28, P = 0.009, d = 1.47). At the 60 min delay, RAG1KO mice showed impaired social recognition memory (Fig. 1B, middle panel; P > 0.05) whereas memory among WT mice was intact (t(14) = 4.93, P = 0.0001, d = 1.87). After a delay of 120 min, both genotypes did not show memory for the stimulus mouse (Fig. 1B, right panel; P’s > 0.05).
Figure 1.
Experiment 1: Social recognition memory in RAG1KO and RAG1WT mice. (A) Duration of investigation of the stimulus mouse on the initial encounter. (B) Duration of investigation on the discrimination trial towards the same previously encountered (white bars) and a novel (black bars) stimulus mouse after delay intervals of 30 min, 60 min, and 120 min. Data are mean +/− S.E.M.; * = P < 0.05, ** = P < 0.01.
2.2 Experiment 2: Social recognition memory among ‘intercross’ mice
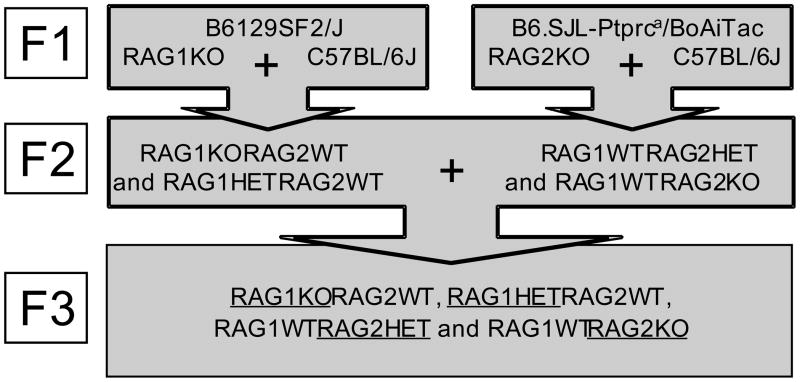
It is not clear from Experiment 1 whether the behavioral differences observed between RAG1KO and WT mice reflected impaired learning and memory per se or the effects of immunodeficiency (i.e., sickness). We therefore undertook extensive breeding that homogenized potential differences in background genetics, maternal factors, and secondary effects of immunodeficiency by generating ‘intercross’ mice from an F1 stock of RAG1- and RAG2-deficient animals (Fig. 2; also see section 4.1 of Experimental Procedures). RAG1KO, RAG1HET, RAG2KO, and RAG2HET littermates of homogenized background genetics were used in subsequent experiments. Whereas RAG2KO mice show exactly the same immunodeficient phenotype as RAG1KO, only RAG1 was shown to be expressed in the brain (Chun et al., 1991; Sun et al., 2007). Also, as heterozygocity for either RAG gene deletion is known to confer normal immunological function, heterogyzous animals served as a control for immunodeficiency. The use of two heterozygous genotypes (RAG1HET and RAG2HET) allowed for the examination of possible gene dosage effects among RAG1-deficient mice (i.e. a greater memory impairment among RAG1HET compared to RAG2HET could indicate effects of RAG1 hyposufficiency in the brain).
Figure 2.
Overview of the breeding strategy used to generate F3 ‘intercross’ mice for the behavioral experiments. Underlines denote nomenclatures of the adult male littermate genotypes used for experiments described in Figures 3–5.
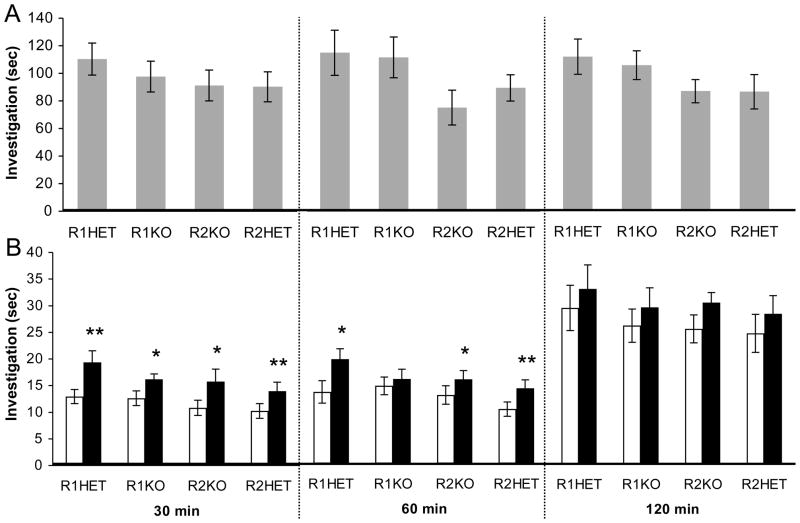
A single cohort of n = 12 animals of each genotype were used to assess social recognition memory performance at all delay intervals, where n = 1 RAG1HET, n = 2 RAG1KO, n = 4 RAG2HET and n = 1 RAG2KO mice were excluded from analysis due to repeated fighting with or attempting to mount the non-receptive stimulus female. As in Experiment 1 above, there were no significant differences between genotypes in the duration of investigation during the initial encounter with the stimulus mouse on either the 30 min, 60 min or 120 min trials (Fig 3A; P’s > 0.05). After a delay of 30 min, all genotypes successfully recognized the previously exposed stimulus mouse (Fig. 3B, left panel; RAG1HET: t(10) = 3.19, P = 0.004, d = 1.42; RAG1KO: t(9) = 2.69, P = 0.025, d = 1.23; RAG2HET: t(7) = 3.74, P = 0.007, d = 1.93; RAG2KO: t(10) = 2.32, P = 0.043, d = 1.03). After a delay of 60 min, RAG1KO mice showed impaired memory for the stimulus mouse (Fig. 3B, middle panel; P > 0.05), whereas memory was intact among the other genotypes (RAG1HET: t(10) = 1.81, P = 0.041, d = 1.00; RAG2HET: t(7) = 1.89, P = 0.009, d = 1.79; RAG2KO: t(10) = 2.56, P = 0.028, d = 1.10). After a delay of 120 min, none of the genotypes showed significant memory for the stimulus mouse (Fig. 3B, right panel; P’s > 0.05).
Figure 3.
Experiment 2: Social recognition memory in RAG1RAG2 intercross littermates. (A) Duration of investigation of the stimulus mouse on the initial encounter. (B) Duration of investigation on the discrimination trial towards the same previously encountered (white bars) and a novel (black bars) stimulus mouse after delay intervals of 30 min, 60 min, and 120 min. Data are mean +/− S.E.M.; * = P < 0.05, ** = P < 0.01.
2.3 Olfactory habituation
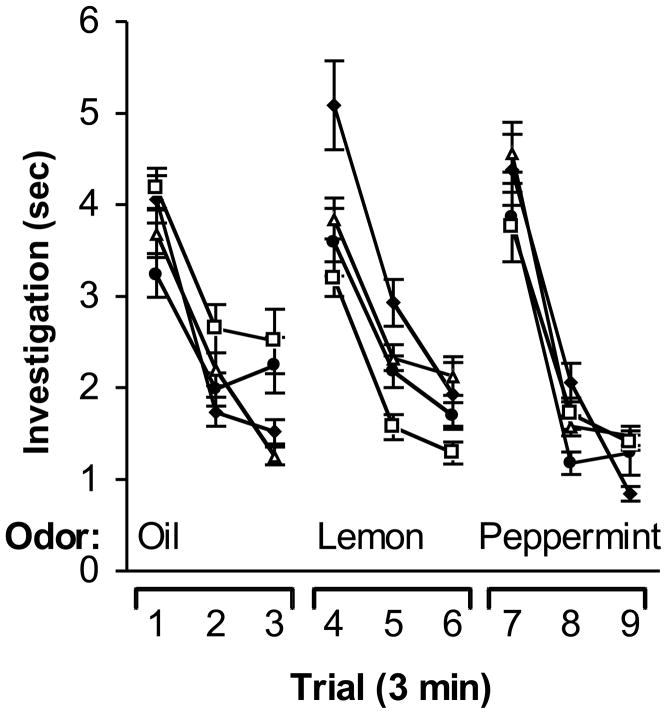
To assess whether RAG1KO mice show impaired olfactory function in the absence of socially-derived odor cues, intercross subjects were tested in an olfactory habituation task (Guan et al., 1993; Schellinck et al., 1992; Schellinck et al., 2001). The cohort of mice from experiment 2 above were used. All mice showed the ability to discriminate non-socially-derived odors (Fig. 4). There were no significant differences in investigation between genotypes, nor was there a significant interaction between genotype and trial (P’s > 0.05). However, there were significant differences in investigation duration among each of the 9 trials (F(2, 91) = 16.49, P < 0.0001). Post-hoc testing revealed that investigation on the first trial of each novel odor presentation (i.e., trials 1, 4, and 7) was significantly greater than investigation on the second and third trials for each odor, suggesting that all mice showed habituation to the continued presentation of the same odor (all P’s < 0.05). Investigation on trials 4 and 7 was also significantly higher than on the preceding trial (P’s < 0.05), indicating that all mice showed dishabituation to the presentation of each novel odor.
Figure 4.
Olfactory habituation. The duration of investigation is shown for RAG1KO (filed circles), RAG1HET (open triangles), RAG2KO (filled diamonds) and RAG2HET (open squares) intercross littermates when presented with sesame oil, 1% lemon scent in sesame oil, and 1% peppermint scent in sesame oil over nine consecutive trials of three minutes duration.
2.4 Open-field behavior
A previous report suggested that RAG1KO mice show impaired habituation to an open-field in comparison to WT controls (Cushman et al., 2003). To assess differences in habituation to a novel environment among RAG1KO mice and littermate controls, a naïve cohort of n = 19 RAG1HET and n = 17 RAG1KO ‘intercross’ adult male mice were used. In contrast to previously reported findings (Cushman et al., 2003), RAG1KO and RAG1HET mice showed similar rates of habituation in the open-field (Fig. 5). There was no significant main effect of genotype or interaction between time and genotype (P’s > 0.05), however the effect of time was highly significant (F(11, 374) = 32.15, P < 0.0001), suggesting that, like RAG1HET mice, RAG1KO mice show significant habituation in locomotor activity over time when exposed to a novel open-field. Similarly, while there was no significant main effect of genotype or interaction between time and genotype (P’s > 0.05), there was a highly significant main effect of time in the number of entries into the center of the open-field (F(11, 374) = 28.51, P < 0.0001). Post-hoc testing revealed no significant differences between genotypes at any of the time intervals examined (P’s > 0.05). As the number of center entries in a novel open-field is commonly used as a measure of anxiety-like behavior (Post et al., 2010), these data suggest that RAG1KO and RAG1HET exhibit similar levels of anxiety-like behavior in the open-field task.
Figure 5.
Open-field behavior. Locomotor activity among RAG1KO (filled circles) and RAG1HET mice (open triangles) intercross littermates. (A) The total number of movements is shown over each consecutive 5 min time block for the 60 min trial duration. (B) The total number of entries to the center of the open-field is shown over each consecutive 5 min time block for the 60 min trial duration.
3. Discussion
This study reports evidence that social recognition memory is impaired in RAG1KO mice. RAG1KO mice showed the ability to remember a previously exposed conspecific after a delay of 30 min, but were impaired relative to controls at a 60 min delay interval. This finding was replicated in separate cohorts of animals, reflecting the robustness of the effect (Figs. 1 and 3). Although not germane to the question of whether social recognition memory had occurred, absolute levels of investigation on the discrimination trials appeared somewhat lower in the 30 min and 60 min delay intervals compared to the 120 min intervals in experiment 2. Given that the length of the 120 min delay interval, it is possible that this difference reflected dishabituation to the testing procedure at the longest delay under conditions used in experiment 2, whereas higher levels of investigation at 30 min and 60 min delay intervals in experiment 1 reflected a ceiling effect of high levels of investigation.
The impairment in social recognition memory in RAG1KO mice relative to RAG1HET and RAG1WT mice was observed even among littermates, where background genotype and maternal factors were controlled (Fig. 3). An important finding in our study was that of intact social recognition memory in RAG2KO littermates, providing evidence that the observed effects do not likely result from secondary effects of immunodeficiency. We did not find evidence of a gene dosage effect, as RAG1HET mice showed similar social recognition memory performance as RAG2HET mice. These data support the view that RAG1-deficiency in the brain leads to impaired memory.
It is plausible that exploratory behavior by the familiar stimulus mouse on the discrimination trial may influence social recognition memory performance by virtue of the stimulus mouse’s memory for the subject. As an explanation for our findings, we cannot rule out the possibility that the subject’s olfactory signature was in some way altered as a result of RAG1-deletion, affecting the behavior of the stimulus mouse. However, we feel that this possibility is unlikely for at least three reasons. First, as virgin ovariectomized females, the stimulus mice were not sexually responsive and showed little investigative behavior of the male subjects, whereas each male subject showed robust investigation of the stimulus mice. Indeed, instances where either the novel or the familiar stimulus mouse engaged in active investigation of the subject were exceedingly rare in this study, precluding quantitative analysis, and such instances were not included in measures of the subject’s investigatory behavior. Second, whereas subjects were habituated to a novel cage for at least 20 min prior to the start of testing, reducing the subject’s locomotor responses to the novel home-cage environment, stimulus mice were introduced into the subjects’ cage at the start of each trial. In this respect, the task was similar to the resident-intruder task, where aggressive behavior of the subject towards a ‘stimulus mouse’ introduced into the subject’s home-cage is measured (Bartolomucci et al., 2009), though the use of group-housed animals in this task precluded the testing in the subject’s home-cage. Third, on a given testing day, the stimulus mice were alternately presented to the subjects during the ‘initial encounter’ trials. As a result, at a retention interval of 60 mins where differences between genotypes were detected, a given stimulus mouse had been presented to approximately 5–6 subjects during successive initial encounters (i.e. a new subject every 8–10 minutes throughout the delay interval) prior to re-encountering the same subject and the novel stimulus mouse on the discrimination trial. Thus, in order for the stimulus mouse to show a preference for the previously exposed subject, the stimulus mouse would have to maintain memory for the same subject during an increasing number of trials. Future studies are needed to examine the effects of such retroactive interference in this task.
In comparison to another recently published study (Noack et al., 2010) where social recognition memory was reported 24 hours after an initial encounter, in our procedure social recognition memory was not evident after a two-hour delay. As noted, preliminary studies in our facility yielded comparable results in additional wildtype mice. Although some reports indicate that mice are able to form long-lasting social recognition memory after a single exposure to a stimulus mouse (Kogan et al., 2000), our procedure differed in several ways. First, we conducted our studies in the dark ‘active’ phase of the circadian cycle. It should be noted that absolute duration of investigation on the initial encounter in our study is on the order of 2-fold higher than that reported by Noack et al. (2010) It is possible that such high levels of investigation, observed in all of the genotypes examined, obscured ‘memory’ for the previously exposed conspecific as a result of increased levels of active investigation rather than a deficit in memory per se. Also, in contrast to Noack et al. (2010), we used ovariectomized adult females as stimulus mice instead of juvenile conspecifics. Although ovariectomized females are routinely used in social recognition memory experiments (Ferguson et al., 2000; Gheusi et al., 1994), it is possible that these stimulus animals do not elicit the same levels of habituation with repeated exposure as do juveniles. Finally, unrecognized genetic contributions to social recognition memory may exist between C57Bl6/J, RAG1 mice and the C57BL/6JOlaHsd strain used in the procedures by Noack et al. (2010).
We did not observe differences between RAG1KO mice and other genotypes in rates of habituation to non-social odors or to an open-field, suggesting that the data do not likely reflect differences in general habituation to novelty. RAG1KO mice showed similar increases in investigation upon presentation of novel non-social odors, reflecting the ability to perceive and habituate to odors (Fig. 4). However, it is known that in addition to the contribution of the main olfactory system, an additional olfactory system mediated by the accessory olfactory bulb contributes to the processing of conspecific odors in rodents (Johnston, 1985). Although there was no difference in the initial investigation duration towards the stimulus mouse in RAG1KO mice compared to the other genotypes, differences in social recognition memory performance among RAG1KO mice may reflect effects on olfactory function specific to the detection of conspecific odors. Nevertheless, the results reported here cannot rule out the possibility that olfactory-mediated processes involving RAG1 contribute to social recognition memory performance. Future studies are needed to discern the impact of RAG1-deletion on memory in the absence of social-olfactory cues.
We also did not find differences in rates of habituation in exploratory behavior or in the number of center entries in the open-field – an index of anxiety-like behavior (Post et al., 2010) - upon exposure to a novel environment (Fig. 5). These data stand in contrast to those reported by Cushman et al. (2003) who reported no significant habituation to an open-field over a 60 min trial. However, it must be noted that the comparison between RAG1KO and RAG1WT mice by Cushman et al. (2003) is complicated by the fact that the mice used were derived from different breeding pairs. As maternal care exerts substantial effects on behavior in adult offspring, including anxiety-like behavior (Champagne and Curley, 2009), it is possible that our use of littermates was an important control for such differences. In addition, although the number of initial total movements reported by Cushman et al. (2003) is comparable to our findings, since our testing was done under conditions similar to those used for social recognition memory behavior (i.e. the dark phase of the circadian cycle), it is also possible that circadian effects played a role in the disparities between these two studies.
At this time, the molecular mechanisms involved in RAG1 function in the CNS are unknown, however there are two logical possibilities. First, RAG1 may participate in a somatic recombination-like process in the CNS similar to its role in the immune system. For example, candidate genes, such as the protocadherin superfamily of neural cell adhesion molecules that may specify neural circuitry during development, have been found to resemble the immunological loci in that they have multiple segments that are recombined to form various receptor mRNAs (Yagi, 2003). Olfactory receptors, taste receptors, and pheromone receptors - playing direct roles in the primary sensory modalities involved in conspecific recognition (Johnston, 1985) - have been proposed as candidates for DNA rearrangement in the brain due to their highly diversified repertoires (Yagi, 2003). However, there is as yet no evidence of altered DNA in mice cloned from individual olfactory receptor neurons (Eggan et al., 2004), nor does RAG1 appear to be involved in axonal targeting in olfactory sensory neurons or in amino acid detection (Feng et al., 2005). Nevertheless, the activities of several other molecular mechanisms, including DNA double strand break repair enzymes, are common to the immune system and the CNS (Chun and Schatz, 1999a; Chun and Schatz, 1999b). For example, pharmacological blockade of DNA ligases and polymerases with the nucleoside analog 1-beta-D-arabinofuranosylcytosine triphosphate (ara-CTP) during adulthood impairs hippocampally-mediated contextual fear memory (Colon-Cesario et al., 2006). Indeed, there is increasing evidence that a number of molecules involved in immunological function play a role in activity-dependent plasticity and brain development (Huh et al., 2000). Second, RAG1 may have a role in the CNS that is entirely distinct from somatic recombination. Evidence for this possibility comes from studies of the molecular structure of the recombinase enzymes themselves. Whereas the DNA cleavage and rearrangement of V(D)J recombination absolutely requires both RAG1 and RAG2, the RAG1 protein contains the catalytic DNA-binding core of the recombinase (Fugmann et al., 2000). Interestingly, this domain is similar to the active site of several transposases and integrases (Spanopoulou et al., 1996; Zhou et al., 2004). Kelch motifs that mediate the interaction of RAG2 with RAG1 have been observed in numerous proteins, and the discovery that a single kelch motif can mediate protein-protein interactions between RAG2 and RAG1 offers the possibility RAG1 may interact with as yet unidentified protein(s) in the CNS (Aidinis et al., 2000; Prag and Adams, 2003).
Although the molecular mechanisms of RAG1 activity in CNS function remain to be identified, our data have implications that may extend to a broad class of neurological conditions. Mutations of RAG1 in humans lead to heterogeneous immune and clinical manifestations ranging from severe combined immunodeficiency to Omenn’s syndrome (Villa et al., 2001a; Villa et al., 2001b). In the brain, RAG1 expression is upregulated in cortical dysplasia, a well-recognized cause of intractable epilepsy (Kim et al., 2003). Our results suggest that these individuals also have altered CNS function that to date has been overlooked. Future studies geared towards expanding knowledge of the consequences of RAG1-deletion for behavior and neurophysiology, identifying proteins associated with RAG1 in the brain and RAG1’s role in DNA binding in neurons will be important in elucidating the function of RAG1 in the CNS.
4. Experimental Procedure
4.1 Mice
Male adult mice between 3 and 5 months of age were used as subjects in all experiments. For data shown in figure 1, subjects were purchased from Jackson laboratories (RAG1KO strain was B6,129-Rag1 tm1Mom, stock #002096, RAG1WT strain was B6129SF2/J, stock #101045). For data shown in figures 3–5, mice were purchased from Jackson laboratories as above (RAG1KO and RAG1WT) and also from Taconic laboratories (RAG2KO strain was B6.SJL-Ptprca/BoCrTac-Rag2tm1 stock #000461-M, RAG2WT strain was B6.SJL-Ptprca/BoAiTac, stock #004007) and maintained in the pathogen-free ‘barrier’ isolation facility of the Duke University vivarium. As shown in figure 2, male founders homozygous for disrupted RAG1null or RAG2 null alleles on the C57BL/6 genetic background were crossed with C57BL/6J females to generate (RAG1HETRAG2WT and RAG1KORAG2HET) F1 progeny. Intercrosses between unrelated F1 mice produced F2 offspring with genotypes RAG1HETRAG2HET, RAG1HETRAG2WT, RAG1WTRAG2HET, and RAG1WTRAG2WT in expected Mendelian ratios. Genotypes were determined by standard PCR methods using DNA from tail samples. F2 intercrosses of RAG1HETRAG2HET mice produced the F3 generation animals used to measure the longevity of social memory. This mating strategy homogenizes potential differences in the genetic backgrounds and maternal effects of the RAG1- and RAG2-deficient parental lines. RAG1KO, RAG2KO and phenotypically normal RAG1HET and RAG2HET F3 heterozygotes were provided blindly for behavioral experiments. Stimulus C57Bl6/J female mice used for all social recognition memory experiments were purchased from Jackson laboratories, ovariectomized at approximately 8 weeks of age, and allowed at least one week to recover before testing began. Mice were housed in groups of 3–5 mice/cage and were tested during the dark phase of the circadian cycle (23:00–05:00). Mice were tested and data were coded by an observer blind to genotype, and all procedures were conducted with the approval of the Duke University Institutional Animal Care and Use Committee.
4.2 Social recognition memory
Prior to social recognition testing, all subjects were exposed to the testing room and to novel stimulus mice (ovariectomized C57Bl6/J females) on three consecutive days to familiarize them with the room and procedure. During testing, subjects were removed from their home-cages and placed in a clean cage with fresh bedding prior to each encounter with stimulus mice. During an “initial encounter”, a stimulus mouse was introduced into a test cage with the subject for 4 min. The duration of investigation, consisting of direct anogenital and face contact, pawing, climbing over, and close following (within 2cm) was summed over the course of the trial (Gheusi et al., 1994; Kogan et al., 2000). After retention intervals of 30, 60, or 120 min, the familiar mouse and a novel stimulus mouse were introduced for a 4 min ‘discrimination’ test, during which the investigatory behaviour of the subject towards each stimulus mouse was measured. During both the initial encounter and the discrimination test, rare instances of investigation of the (male) subject by the (female) stimulus mice were excluded from measures of the duration of investigation. The order of presentation of the stimulus mouse on the initial encounter (one of the two stimulus mice used during the discrimination trial) was counterbalanced across trials. Experimentally naïve stimulus mice were used at each delay interval. Subjects that repeatedly fought with or attempted to mount the (non-receptive) stimulus mice were excluded from analysis. During testing, each subject’s cage was blocked from the view of the experimenter by opaque blinds and dividers, and behavior was monitored via a video camera. To control for the transfer of odor cues between stimulus mice and subjects, the experimenter either changed gloves or sprayed his gloves with disinfectant after handling each animal. Each subject was tested 3 times at each delay interval and the median duration of investigation was used for data analysis. For experiment 2, the order of the trials (30 min, 60 min, 120 min) was pseudorandomly assigned. Data were transcribed from the video record using the Noldus Observer software, which allowed accurate recording of the duration of investigation toward the stimulus mice (Noldus Information Technology Inc., Leesburg, VA, USA). Raw data were binned into 1-min intervals using software written in visual basic by the experimenter.
4.3 Olfactory habituation
Subjects were allowed to habituate to a new home-cage with fresh bedding for 20 min, and then olfactory stimuli were presented by dipping a cotton-tipped applicator into the stimulus solution and passing the stimuli through the wire grids of the cage. The applicator was held in place by anchoring it to a plastic weigh boat, which ensured that the stimuli were presented at a level of approximately 4.4 cm above the cage floor (Wrenn et al., 2003). The odorants used were sesame oil (control), 1% lemon scent diluted in sesame oil, and 0.1% peppermint scent diluted in sesame oil. Scents and the dilutions used were selected based upon pilot experiments showing there was no preference for either scent. Each stimulus was presented for 3 min and then replaced with a new applicator three successive times for a total of nine presentations. Stimuli were presented in the following order: oil (3x), lemon (3x), and peppermint (3x). The experimenter viewed the interaction on a video monitor and recorded the time spent investigating the stimulus using the Noldus Observer software (Noldus Information Technology Inc., Leesburg, VA, USA). Raw data were binned into 3-min intervals using software written in visual basic by the experimenter. Investigation was defined as direct contact with the applicator, orienting towards the applicator with the head within 2cm of it, and rearing with the head oriented towards the applicator, within 2 cm of it. Occasional chewing was not considered olfactory investigation (Wrenn et al., 2003).
4.4 Open-field behavior
Open-field behavior was assessed in 43.2 cm × 43.2 cm Plexiglas boxes equipped with an array of 16 infrared photobeams controlled by software present on a computer (ENV-515 with SOF-811 open-field activity software, Med Associates, VT, USA). For each of three consecutive days prior to the start of testing, mice were brought into the experimental room and handled for at least 5 min to habituate them to the experimenter and room. On the day of testing, each subject was allowed to explore the empty open-field for 60 min. Locomotor activity in the open-field (total movements) was recorded for each min of the 60 min habituation trials. In addition, the number of entries into the center of the open-field, defined by movements more than 2.5-beams from the walls, was evaluated. Between each trial, the boxes were cleaned with 95% ethanol and allowed to air dry to attenuate and homogenize olfactory traces. Software written in visual basic by the experimenter was used to bin the data into 5 min blocks for statistical analysis.
4.5 Statistical analysis
Statistical analyses were done using Graphpad Prism 4.01 (Graphpad Software, San Diego, CA, USA) or the Analysis ToolPak in Microsoft Excel 2004 for Mac (Microsoft corporation, Redmond, WA, USA). For the social recognition memory task described in Experiment 1, differences between genotypes in the duration of investigation of the stimulus mouse on the initial encounter were examined by unpaired Student’s t-tests. For the social recognition memory tasks described in Experiment 2, differences between genotypes in the duration of investigation of the stimulus mouse on the initial encounter were examined by factorial ANOVA followed by Newman Keuls post-hoc testing. For the discrimination trials in Experiments 1 and 2, data were analyzed as previously described (Engelmann et al., 1995; Noack et al., 2010) to specifically determine whether or not recognition of the previously encountered stimulus mouse had occurred. As such, differences between Same versus Novel stimulus mouse were examined by paired Student’s t-test for each genotype, with increased investigation of the Novel stimulus mouse relative to the Same stimulus mouse taken to indicate that recognition had occurred. Standardized effect sizes for repeated measures (Cohen’s d) were calculated according to the following formula: d = (x1 − x2)/(s*(√(1-r)), where x1 − x2 = the mean difference between investigation of the novel and familiar stimulus mouse on the discrimination trial, s = the pooled standard deviation, and √(1-r) = the square root of 1 – the correlation coefficient (Cohen, 1977). Because, with the exception of the RAG1HET mice at the 30 min interval, there were no significant differences between investigation of the novel stimulus animal and the same stimulus animal during the last 2 min of the 4 min discrimination trial at all delay intervals (P’s > 0.05), investigation on only the first 2 min of each discrimination trial at each delay interval was subject to analysis. Data for the olfactory habituation task were analyzed as previously described (Schellinck et al., 1992) using ANOVA to assess effects of genotype and differences across trials in time spent investigating each odor. Newman Keuls post-hoc tests were used to determine which trials differed from each other. Open-field behavior was analyzed by ANOVA with genotype as the between groups measure and time as the within groups measure. Differences between genotypes across each time block were examined using Bonferroni-corrected post-hoc comparisons. All data are presented as mean (+/− S.E.M.) and data were considered statistically significant at P < 0.05.
Research Highlights.
The RAG1 gene initiates somatic recombination critical for adaptive immune function.
RAG1 is expressed in the brain, including hippocampus, but its function is unknown.
We find that RAG1-deficient mice show impaired memory for conspecifics.
This effect is not likely due to immunodeficiency or impaired habituation to novelty.
Acknowledgments
We thank Kamal Kolappa for technical assistance and Aya Sasaki for critical reading of earlier versions of the manuscript. This work was supported by NIH 5PO1AG009525 to C.L.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aidinis V, Dias DC, Gomez CA, Bhattacharyya D, Spanopoulou E, Santagata S. Definition of minimal domains of interaction within the recombination-activating genes 1 and 2 recombinase complex. J Immunol. 2000;164:5826–32. doi: 10.4049/jimmunol.164.11.5826. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Fuchs E, Koolhaas JM, Ohl F. Acute and chronic social defeat: stress protocols and behavioral testing. Vol. 42. Humana Press; 2009. [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Chun J, Schatz DG. Rearranging views on neurogenesis: neuronal death in the absence of DNA end-joining proteins. Neuron. 1999a;22:7–10. doi: 10.1016/s0896-6273(00)80671-6. [DOI] [PubMed] [Google Scholar]
- Chun J, Schatz DG. Developmental neurobiology: Alternative ends for a familiar story? Curr Biol. 1999b;9:R251–3. doi: 10.1016/s0960-9822(99)80156-0. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. Academic Press; New York: 1977. [Google Scholar]
- Colon-Cesario M, Wang J, Ramos X, Garcia HG, Davila JJ, Laguna J, Rosado C, Pena De Ortiz S. An Inhibitor of DNA Recombination Blocks Memory Consolidation, But Not Reconsolidation, in Context Fear Conditioning. Journal of Neuroscience. 2006;26:5524–5533. doi: 10.1523/JNEUROSCI.3050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10:13–8. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R. Mice cloned from olfactory sensory neurons. Nature. 2004;428:44–9. doi: 10.1038/nature02375. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social Discrimination Procedure: An Alternative Method to Investigate Juvenile Recognition Abilities in Rats. Physiology & Behavior. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Feng B, Bulchand S, Yaksi E, Friedrich RW, Jesuthasan S. The recombination activation gene 1 (Rag1) is expressed in a subset of zebrafish olfactory neurons but is not essential for axon targeting or amino acid detection. BMC Neurosci. 2005;6:46. doi: 10.1186/1471-2202-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe R-M, Goodall G, Dantzer R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behavioural Processes. 1994;33:59–88. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Guan X, Blank J, Dluzen D. Depletion of olfactory bulb norepinephrine by 6-OHDA disrupts chemical cue but not social recognition responses in male rats. Brain Res. 1993;622:51–7. doi: 10.1016/0006-8993(93)90800-3. [DOI] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–9. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Olfactory and vomeronasal mechanisms of communication. In: Pfaff DW, editor. Taste, Olfaction, and the Central Nervous System. The Rockefeller University Press; New York: 1985. pp. 322–346. [Google Scholar]
- Kim SK, Wang KC, Hong SJ, Chung CK, Lim SY, Kim YY, Chi JG, Kim CJ, Chung YN, Kim HJ, Cho BK. Gene expression profile analyses of cortical dysplasia by cDNA arrays. Epilepsy Res. 2003;56:175–83. doi: 10.1016/j.eplepsyres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–8. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Noack J, Richter K, Laube G, Haghgoo HA, Veh RW, Engelmann M. Different importance of the volatile and non-volatile fractions of an olfactory signature for individual social recognition in rats versus mice and short-term versus long-term memory. Neurobiol Learn Mem. 2010;94:568–75. doi: 10.1016/j.nlm.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Pena De Ortiz S, Arshavsky Y. DNA recombination as a possible mechanism in declarative memory: a hypothesis. J Neurosci Res. 2001;63:72–81. doi: 10.1002/1097-4547(20010101)63:1<72::AID-JNR9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Post AM, Weyers P, Holzer P, Painsipp E, Pauli P, Wultsch T, Reif A, Lesch KP. Gene-environment interaction influences anxiety-like behavior in ethologically based mouse models. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Prag S, Adams JC. Molecular phylogeny of the kelch-repeat superfamily reveals an expansion of BTB/kelch proteins in animals. BMC Bioinformatics. 2003;4:42. doi: 10.1186/1471-2105-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DG, Chun JJ. V(D)J recombination and the transgenic brain blues. New Biol. 1992;4:188–96. [PubMed] [Google Scholar]
- Schellinck HM, West AM, Brown RE. Rats can discriminate between the urine odors of genetically identical mice maintained on different diets. Physiol Behav. 1992;51:1079–82. doi: 10.1016/0031-9384(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Forestell CA, LoLordo VM. A simple and reliable test of olfactory learning and memory in mice. Chem Senses. 2001;26:663–72. doi: 10.1093/chemse/26.6.663. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Spanopoulou E, Zaitseva F, Wang FH, Santagata S, Baltimore D, Panayotou G. The homeodomain region of Rag-1 reveals the parallel mechanisms of bacterial and V(D)J recombination. Cell. 1996;87:263–76. doi: 10.1016/s0092-8674(00)81344-6. [DOI] [PubMed] [Google Scholar]
- Sun JG, Han S, Ji H, Zheng Y, Ling SC. [Expression of RAG-1 in brain during mouse development] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007;36:161–6. doi: 10.3785/j.issn.1008-9292.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, Arkwright PD, Baniyash M, Brooks EG, Conley ME, Cortes P, Duse M, Fasth A, Filipovich AM, Infante AJ, Jones A, Mazzolari E, Muller SM, Pasic S, Rechavi G, Sacco MG, Santagata S, Schroeder ML, Seger R, Strina D, Ugazio A, Valiaho J, Vihinen M, Vogler LB, Ochs H, Vezzoni P, Friedrich W, Schwarz K. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001a;97:81–8. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- Villa A, Sobacchi C, Vezzoni P. Recombination activating gene and its defects. Curr Opin Allergy Clin Immunol. 2001b;1:491–5. doi: 10.1097/00130832-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Yagi T. Diversity of the cadherin-related neuronal receptor/protocadherin family and possible DNA rearrangement in the brain. Genes Cells. 2003;8:1–8. doi: 10.1046/j.1365-2443.2003.00614.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Mitra R, Atkinson PW, Hickman AB, Dyda F, Craig NL. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature. 2004;432:995–1001. doi: 10.1038/nature03157. [DOI] [PubMed] [Google Scholar]