Abstract
Background and the purpose of the study
Encapsulation of human insulin in lipid vesicular systems such as niosomes was sought as a route to protect this protein against proteolytic enzymes and to improve its oral bioavailability. The purpose of this study was to assess the effect of insulin encapsulation in niosomes on oral bioavailability in diabetic rats.
Methods
Recombinant human insulin was entrapped in multilamellar niosomes composed of polyoxyethylene alkyl ether surfactants (Brij 52 and Brij 92) or sorbitan monostearate (Span 60) and cholesterol. The amount of insulin released in simulated intestinal fluid (SIF) and simulated gastric fluid (SGF) were measured at 37°C. The protection of entrapped insulin against pepsin, α-chymotrypsin and trypsin were evaluated in comparison with free insulin solution. Diabetes was induced by IP injection of streptozotocin (65 mg/kg) in male wistar rats and effects of orally administered niosomes and subcutaneously injected insulin on hypoglycemia and elevation of insulin levels in serum were compared.
Results and conclusion
The extent and rate of insulin release from Brij 92 and Span 60 vesicles were lower than that of Brij 52 niosomes (P<0.05). Vesicles protected insulin in comparison with free insulin solution against proteolytic enzymes (P<0.05) significantly. Animals treated with oral niosome-encapsulated insulin (100 IU/kg) showed decreased levels of blood glucose and elevated serum insulin, which in the case of Brij 92 niosomes, hypoglycemic effect was significant (P<0.05).
Niosomes were also stable in solubilizing bile salt solutions and could effectively prolong the release of insulin in both SGF and SIF. Results of this study showed that niosomes may be utilized as oral carriers of insulin; however, to increase bioavailability of insulin, further studies on the protease inhibitor co-encapsulation in niosomal formulations might be helpful.
Keywords: Protein delivery, Release, Vesicle size analysis
INTRODUCTION
For chronic therapies, oral delivery continues to be the preferred route of administration. This preference results form higher patient compliances and access to largest number of markets. Oral forms of peptide or protein drugs such as insulin eliminate or reduce the need to use injection as the mode of administration, but the gastrointestinal tract possesses a variety of morphological and physiological barriers, which limit its intestinal absorption. To overcome these barriers, a number of attempts have been made to deliver insulin orally by different drug delivery systems such as enteric-coated capsules (1), intestinal patches (2), liposomes (3, 4), hydrogel microparticles (5), microemulsions (6), hydrogels (7), pH-responsive nanoparticles (8), and superporous hydrogel polymers (9).
Non-ionic surfactant vesicles (niosomes) are quite new carriers for delivery of different therapeutic agents (10). Niosomes have been investigated for parenteral (11) and vaginal (12) delivery of insulin. The potential of niosomes to encapsulate and protect insulin from enzymatic degradation in vitro have been reported (13, 14). On the other hand, the surfactants or lipids from which the vesicles are prepared might act as penetration enhancers and increase the flux of peptide drugs across mucosal tissue. This approach has been reported for incorporation of influenza virus antigens in niosomes as a nasal immune vaccine (15).
In this study, the protection of insulin against proteolytic enzymes by entrapment in niosomes and its oral absorption and hypoglycemic effects in diabetic rats were investigated.
MATERIAL AND METHODS
Recombinant human insulin (27.5 IU/mg- Eli Lilly, France) was a kind gift from Exir Pharmaceutical Co. (Iran). The non-ionic surfactants used as vesicle-forming materials were Brij® 52 (polyoxyethylene 2 cetylether, C16EO2), Brij® 92 (polyoxyethylene 2 oleylether, C9=9EO2), and Span® 60 (sorbitan monostearte) which were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Pepsin (from porcine stomach mucosa, 3,200-4,500 U/mg protein), α-chymotrypsin (from bovine pancreas, 40-60 U/mg protein), trypsin (from bovine pancreas, 10,000 BAEE U/mg protein) and sodium desoxycholate were also obtained from Sigma. Triton x-100 and cholesterol (Chol) were bought from Fluka (Switzerland). Immunoradiometric assay kit was purchased from Biosource (Belgium). Streptozotocin (Zanosar®) was purchased from Upjohn (USA). All organic solvents and the other chemicals were of analytical grade and obtained from Merck (Germany).
Preparation of insulin niosomes
Multilamellar vesicles (MLVs) were prepared by film hydration method (14). Briefly, 300 µmol of surfactant/Chol (7:3 molar ratio) mixture was dissolved in 10 ml chloroform in a 100-ml round-bottom flask. The organic solvent was then removed at 50°C, under reduced pressure, in a rotary evaporator (Buchi, Switzerland). The flask was kept under vacuum-attached desiccator overnight for removal of chloroform completely. The dried lipid film was hydrated with 5 ml phosphate buffered saline (PBS) (pH 7.4) containing insulin (20 IU/ ml) by a gentle rotation in water bath at 55°C for 10 min. The resulting multilamellar nonionic surfactant vesicle dispersions were then left to cool slowly.
Determination of insulin encapsulation efficiency
To separate unentrapped insulin, the vesicle suspensions were centrifuged (90 XL Ultracentrifuge, Beckman, USA) at 40,000 × g for 30 min at 4oC and washed with PBS (pH 7.4). The amounts of insulin in the supernatant and also in the pellets were analyzed radioimmunometrically, after disrupting the niosomes by the use of isopropyl alcohol.
Particle size Measurement
The mean volume diameter and particle size distribution of niosomes were determined by laser-light scattering (Mastersizer 2000E, Malvern Instruments, UK) method. Measurement was carried out using a 100 mm focal length lens, which could measure vesicles in 0.1-100 µm size range. The source of light was a low power helium-neon laser.
Optical and transmission electron microscopy (TEM)
Some micrographs were prepared by a camera attached to the optical microscope (HFX-DX, Nikon, Japan) in 10×40 and 10×100 magnifications. Negative staining of prepared vesicles was carried out by the procedure which will be described subsequently. An electronic-microscope grid was covered with collodion as an electron-transparent support film and a drop of niosome suspension was placed on the grid and it was allowed that some vesicles to attach to the film. Then the excess of buffer and niosomes were drawn off with a piece of filter paper (Watman No.1) and quickly replaced with a drop of potassium phosphotungstate (1% solution in distilled water, pH 7.0). After a minute, the drop of stain was drawn off and the grid was allowed to dry thoroughly. Electron micrographs were taken by transmission electron microscope (Zeiss, LEO-910, Germany) in different magnification.
In vitro release of insulin from niosomes
The pellets obtained from centrifugation were washed twice and resuspended in PBS. The niosome suspensions were diluted to 10 fold in simulated intestinal fluid (SIF, pH 6.8) or in simulated gastric fluid (SGF, pH 1.2). A 1 ml sample of the diluted suspension was used for each time point; samples in screw cap Eppendorf tubes were incubated under gentle shaking conditions (10 Cycles/min) at 37°C (13). The release study was carried out for 4 hrs in SGF and for 24 hrs in SIF. Triplicate samples were taken for analyses according to planned schedule. Vesicle pellets were then separated by centrifugation in a microcentrifuge at 13,000 × g for 10 min. The supernatant was removed and the pellets were resuspended in PBS. After disruption by isopropyl alcohol, the amount of remained insulin in the pellets was determined radioimmunometrically.
Stability of vesicles in various media
Sodium Desoxycholate Solutions: Niosomes were centrifuged at 13,000 × g for 5 min, washed and resuspended in PBS solution. The stability of vesicles was assessed according to the turbidity of their 10-fold diluted suspensions in different concentrations of sodium desoxycholate (0-21 mM in PBS of pH 7.4) solutions at 37°C. Turbidities were defined as optical density, measured spectrophotometrically at 400 nm (16). The incubation time of vesicles in bile salt solutions was 60 min. Bile salt solution without any added vesicle was utilized as blank for each specific concentration. The initial absorbance was considered as 100 percent turbidity.
Triton X-100 solutions
The stability of vesicles in the presence of different concentrations of a micelle forming detergent, Triton x-100, solutions (0-3 mM in PBS pH 7.4) was assessed at 20°C, by a method similar to the evaluation of stability in bile salt solutions (16). Variations of optical densities (turbidities) were measured spectrophotometrically at 450 nm (13). The incubation time was 30 min. Detergent solution without any added vesicle was used as blank for each specific concentration.
Protection of Encapsulated Insulin
Diluted niosome suspensions were incubated with enzyme solutions at 37°C. Three enzyme solutions were used: the pepsin solution (5 IU/ml) in glycine buffer adjusted to pH 1.2, the trypsin solution (704 IU/ml), and the α-chymotrypsin solution (4.16 IU/ml) in phosphate buffer at pH 7.8. Pepsin solution (0.5 ml) was incubated for one hour at 37°C with 10 fold diluted niosome suspension (0.5 ml) or free insulin solution in PBS of pH 7.4 (0.5 ml, 2 IU/ml). For the other enzymatic solutions the same conditions were applied, except that the incubation time was 3 hrs (14). After appropriate incubation, samples (200 µl) were withdrawn, centrifuged at 13,000 × g for 5 min and the supernatant was removed. Then 200 µl of 0.05% trifluoroacetic acid (TFA) was added to the pellets to inactivate traces of enzyme present at the niosomes surfaces (14). Subsequently, 0.5 ml of isopropyl alcohol was added to dissolve the pellets and the concentration of remaining active insulin was determined.
In vivo studies
Male Wistar strain rats (200-250 g) housed in a light dark cycle and temperature-controlled environment. Food was withheld 12 hrs before the experiment and water was accessed ad libitum. Diabetes mellitus was induced in rats by an intraperitoneal (IP) injection of streptozotocin (65 mg/kg) in citrate buffer of pH 4.5. Rats were left untreated for 10 days until they showed polyuria, polydipsia and polyphagia where acute disease phase was completed. Rats were considered diabetic and included in the study when fasted glycemia was higher than 250 mg/dl (17).
The study was performed on the day of 10 after streptozotocin injection and after an overnight fasting. Forty-two diabetic rats were divided into seven groups (n=6). Six groups were administered 1 ml dose of one of followings intragastrically) insulin solution, 2) PBS solution, 3) Brij 52/Chol niosomes, 4) Brij 92/Chol vesicles, 5) Span 60/Chol niosomal suspension or 6) empty Span 60/Chol vesicles plus insulin. The dose of insulin in each group was 100 IU/ kg. The seventh group received 2 IU/kg of insulin in PBS (2 IU/ml) subcutaneously (SC) (4, 18).
Prior and at specified time intervals over a 4 hrs period, blood samples (∼300µl) were collected by retroorbital blood sampling and collected in 1 ml micro-centrifuge tubes. The samples were centrifuged for 5 min at room temperature and the plasma were collected and frozen at –20°C for further analysis of glucose and insulin. Plasma glucose and insulin concentrations were measured by glucose oxygenase and radioimmunoassay methods, respectively.
The areas under the plasma insulin curve (AUC0-250 min) and above the blood glucose curve (AAC0-250 min) were calculated by linear trapezoidal method. The mean initial levels of blood glucose and insulin concentration was used as baseline for determination of AAC and AUC.
Statistics
Each value was expressed as the mean ± SD. One-way ANOVA tests were conducted for the obtained results, followed by Scheffe post-hoc tests in SPSS 15 for windows. P value less than 0.05 was considered significant.
RESULTS AND DISCUSSION
Niosomes characterization
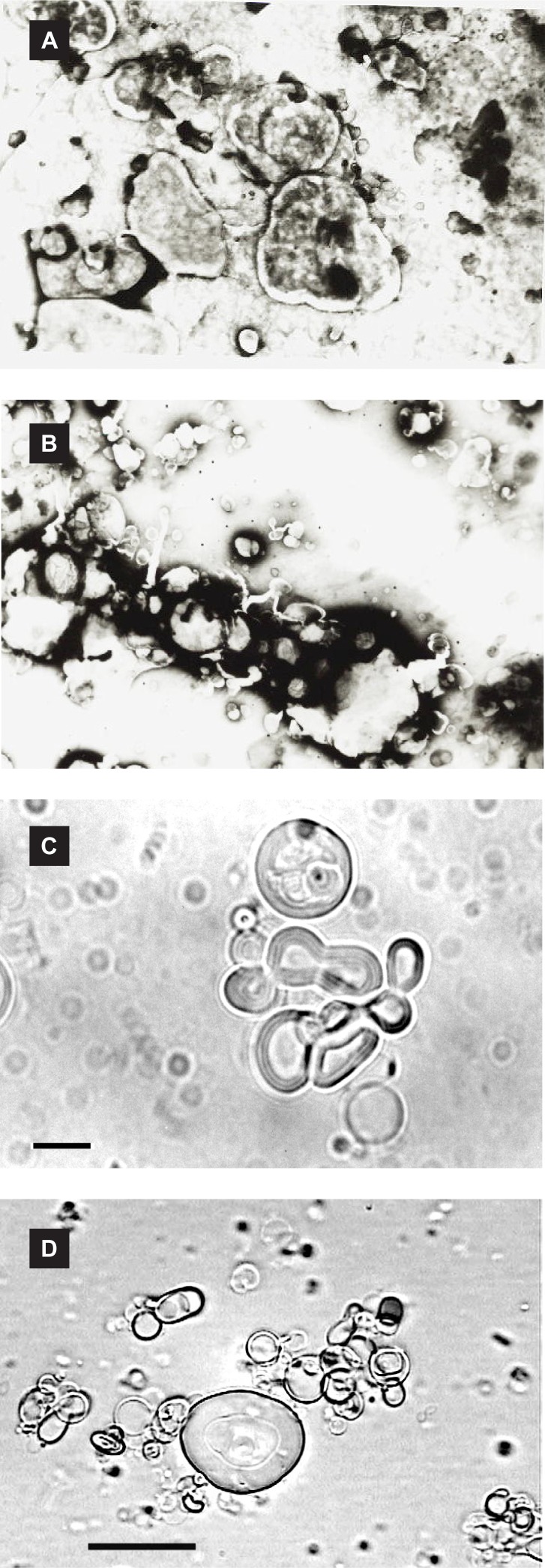
At room temperature, Span 60 is solid, Brij 52 is semisolid and Brij 92 is in liquid state. Therefore, three different physical states amphiphiles were employed which formed stable niosomal suspensions in the presence of Chol. The mean volume diameters (dv) of prepared vesicles and insulin entrapment efficiencies are presented in table 1. Some micrographs of vesicles are also shown in figure 1.
Table 1.
Mean volume diameter (dv) of vesicles and insulin encapsulation efficiency percent (EE%) in surfactant/ cholesterol (7:3 molar ratio) niosomes prepared by film hydration method (mean ± SD, n = 3).
| Surfactant | HLB | dv (µm) | EE% |
|---|---|---|---|
| Brij 52 | 5.3 | 13.39 ± 0.25 | 31.42 ± 3.68 |
| Brij 92 | 4.9 | 6.41 ± 0.09 | 35.20 ± 8.10 |
| Span 60 | 4.3 | 5.75 ± 0.07 | 39.35 ± 2.34 |
Figure 1.
Micrographs (×1000 magnification) of insulin-loaded niosomes (7:3 molar ratio of surfactant/cholesterol) prepared by classic film method: negative staining transmission electron microscopy micrographs of (a) Brij 52 vesicles, (b) Span 60 niosomes, and optical microscopy pictures of (c) Span 60 vesicles, (d) Brij 92 niosomes; Bar = 10 µm.
It has been reported that the mean size of niosomes increased by progressive increase in the HLB value of surfactants (19). This is in consistent with results of this study where increase in the HLB value from4.7 (Span 60) to 5.3 (Brij 52) significantly increased the mean volume diameter of vesicles (Table 1, P<0.05).
Maximum insulin encapsulation efficiency was found in Span 60 niosomes without significant differences with the other formulations (Table 1, P>0.05). This surfactant is less permeable to water soluble compounds such as 5(6)-carboxyfluorescein (19), salicylic acid and p-hydroxyl benzoic acid (20) or even poorly soluble molecules such as flurbiprofen (21).
Insulin release
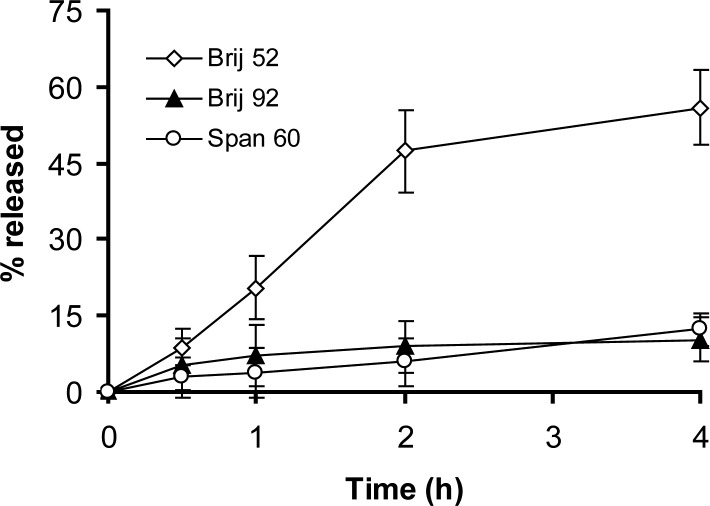
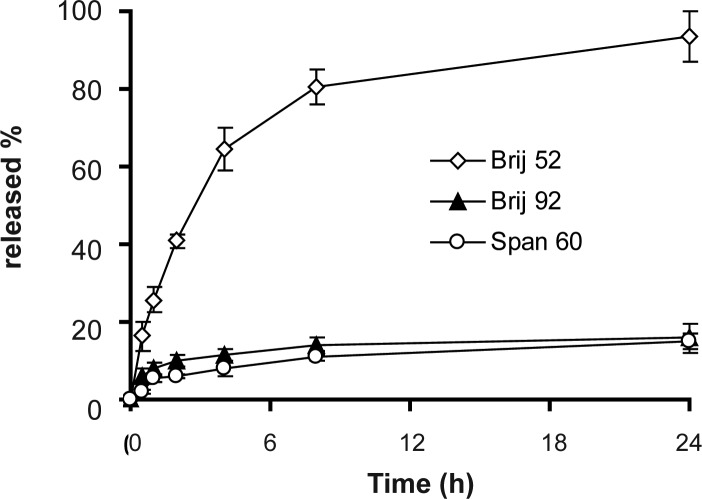
The release profiles of insulin from niosomes in SGF and SIF are presented in figures 2 and 3, respectively. Biphasic kinetic release of insulin is discernible in all formulations and includes a primary relatively fast release and an equilibrium state or a slower release phase achieved within approximately one hour in SGF and 2–4 hrs in SIF (Figs. 2 and 3). This kind of release profiles has been observed for 125I-labeled luteinizating hormone releasing hormone delivery in plasma and 5% muscle homogenate from niosomes (22).
Figure 2.
Release of insulin from niosomes in simulated gastric fluid at 37°C (mean of ± SD, n = 3).
Figure 3.
Release of insulin from niosomes in simulated intestinal fluid at 37°C (mean of ± SD, n = 3).
The extent and rate of insulin release in SGF and SIF were low for both Brij 92 and Span 60 formulations (P>0.05, Fig. 2 and 3). On the other hand, Brij 52 niosomes showed almost complete depletion of entrapped insulin in SGF and SIF which were different significantly with Span 60 and Brij 92 vesicles (P<0.05). This unusual release behavior may be related to specific structure of insulin and subsequent specific protein-lipid bilayers interactions.
Stability of vesicles in various media
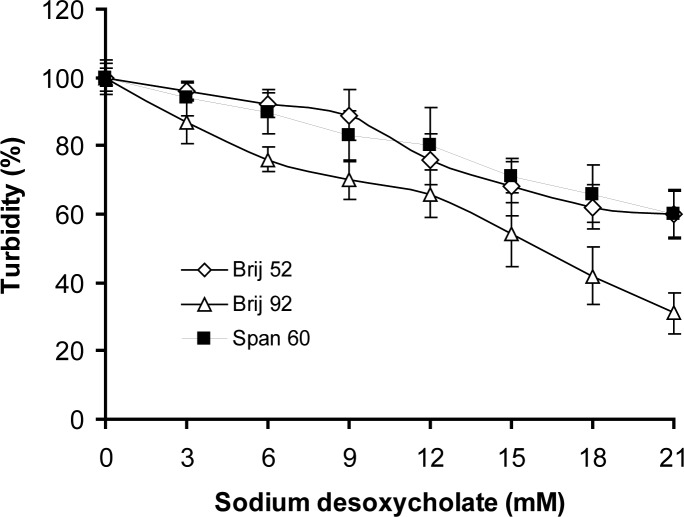
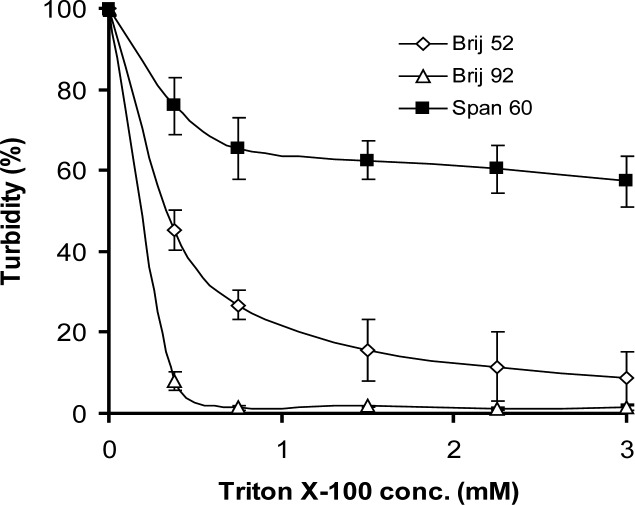
The changes in turbidities of niosome suspensions in sodium desoxycholate and Triton x-100 solutions are depicted in figures 4 and 5, respectively.
Figure 4.
Relative stability of niosome formulations prepared by film hydration method as a function of sodium desoxycholate concentration at pH 7.4 phosphate buffered saline at 37°C revealed as turbidities (mean±SD) at 400 nm after 60 min of incubation (n=3).
Figure 5.
Relative stability of niosome formulations prepared by film hydration method as a function of Triton x-100 concentration at pH 7.4 phosphate buffered saline at 20°C revealed as turbidities (mean±SD) at 450 nm after 60 min of incubation (n=3).
In the present study the three-stage model for solubilization of vesicles was relatively adjustable for MLVs. According to this model, vesicle-micelle transition includes I) the association of detergent with the bilayers and their saturations without any significant change in turbidity, II) outer bilayer solubilization and III) proceeding of solubilization through the remaining bilayers (23). The concentrations of bile salt solutions at the onset of phase II in the case of Brij 92 and Brij 52 were 3 and 9 mM, respectively. Span 60 niosomes did not display any abrupt change in turbidity at the studied detergent concentrations which is postulated to be due to high gel to liquid crystal transition temperature and rigidity of its bilayers. However, Brij 52 and Span 60 vesicles had particular resistance to lysis by bile salt in a manner that the final percent of turbidities of their dispersions were significantly higher than Brij 92 dispersion (P<0.05). This may be related to unsaturation of acyl chain and liquid state nature of Brij 92 surfactant, which allows an easy transfer from bilayer to micellar structure.
Low critical micelle concentration (cmc) of Triton x-100 and high solubilizing capability of this detergent led to rapid solubilization of vesicles even in very low concentrations (Fig. 5). Similarly in bile salt medium, gel-state surfactant, Span 60, had a higher resistance in comparison to both Brij 52 and Brij 92 vesicles (P<0.05).
Protection of insulin
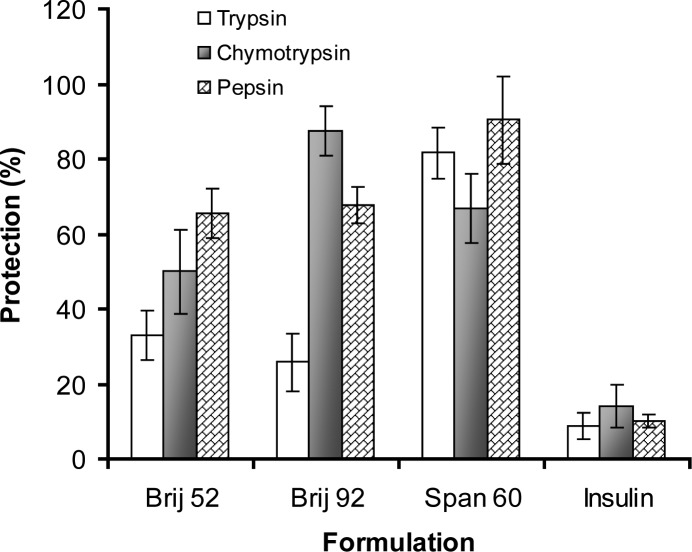
There are many studies indicating the stability enhancement of different compounds such as paclitaxel (24), desferrioxamine mesylate (25) and hemoglobin (26) following the entrapment in bilayer structures. The results of the present study also showed that insulin was protected against enzymatic degradation after entrapment inside niosomes, whereas free insulin solution used as control, were found to be extensively degraded after incubation under the same conditions (Fig. 6, P<0.05). Protection of insulin is possibly due to controlled release rate and suggested inactivation of enzymes following adsorption of insulin on the surface of vesicles (14).
Figure 6.
The insulin protection property (mean of±SD) of different niosomes against proteolytic effects of digestive enzymes in vitro. Vesicular preparations were incubated with α-chymotrypsin and trypsin solutions for 3 hrs and with pepsin solution for one hour at 37°C (n=3).
In vivo study
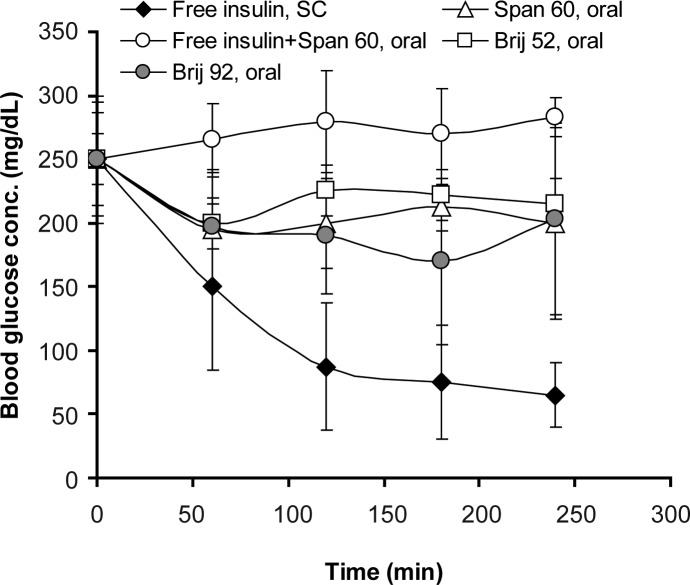
Serum glucose levels in diabetic rats receiving peroral niosomal formulations carrying insulin or SC injection of insulin solution are shown in figure 7. Brij 92 vesicular formulation showed the best hypoglycemic effect in comparison with Brij 52 and Span 60 niosomes (P<0.05, Fig. 7). Area above glucose concentration curves, AAC0-250min, and area under insulin concentration curve, AUC0-250min, are depicted in Table 2. AAC0-250 min in group receiving SC injection of insulin was not statistically different from AAC0-250 min in niosomes receiving groups (Table 2, P>0.05).
Figure 7.
Blood glucose levels in streptozotocin-induced diabetic rats receiving a single intragastric (niosome-encapsulated, oral, 100 IU/kg) or subcutaneous, SC, (free insulin solution, 2 IU/kg) insulin dose. Niosomes were composed of surfactant/cholesterol (7:3 molar ratio) and prepared by film hydration method (n = 6).
Table 2.
Some pharmacokinetic and pharmacodynamic parameters related to the hypoglycemic effects of human insulin in streptozotocin-induced diabetic rats following a single subcutaneous or oral administration of studied formulations (n = 6).
| Formulation | Insulin dose (U/kg) | Route of administration | [AUC0-250 min ±SD]×103(µU.ml-1.min)a | Cmax±SD (µU/ml)b | tmax (min) | F±SD (%)c | [AAC0-250 min±SD]×103 (percent.min)d | BGmax (mg/dL)e | tmax G (min)g | .f±SD (%)f |
|---|---|---|---|---|---|---|---|---|---|---|
| Free insulin | 2 | Subcutaneous (s.c.) | 24.98±6.22 | 143.5±15.8 | 50 | - | 14.22±3.32 | 233.7±29.48 | 250 | - |
| Brij 92 niosomes | 100 | Oral | 23.46±5.28 | 102.66±16.9 | 165 | 1.878±0.426 | 5.95±2.53 | 107.07±44.91 | 165 | 0.83±0.22 |
| Brij 52 niosomes | 100 | Oral | 13.98±4.46 | 63.32±23.61 | 110 | 1.119±0.573 | 2.938±1.7 | 59.67±33.90 | 50 | 0.41±0.32 |
| Span 60 niosomes | 100 | Oral | 18.21±7.67 | 72.66±21.36 | 110 | 1.457±0.326 | 4.44±2.12 | 66.55±27.57 | 50 | 0.62±0.29 |
Area under the insulin concentration
The maximum concentration of blood insulin
Percent of relative bioavailability
Area above blood glucose concentration depression
The maximum amount of blood glucose depression (base line glucose concentration-minimum blood glucose concentration)
Percent of relative pharmacological availability
Time for maximum blood glucose depression
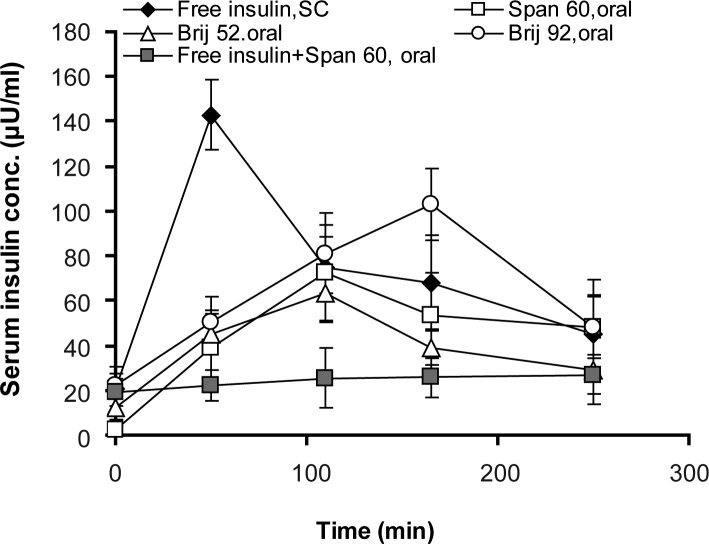
Serum insulin concentrations increased in all animals which received oral niosomal formulations, as depicted in figure 8. Within about 3 hrs, the insulin levels in rats receiving Brij 92 niosomal formulation rose to the highest level and good compatibility with hypoglycemic effect. AUC0-250 min in rats which received insulin (2 U/kg, SC) was significantly higher than rats receiving oral niosome-entrapped insulin dose (100 U/kg) as depicted in table 2 (P<0.05).
Figure 8.
Serum insulin concentrations in streptozotocin-induced diabetic rats receiving a single intragastric (niosome-encapsulated, oral, 100 IU/kg) or subcutaneous, SC, (free insulin solution, 2 IU/kg) insulin dose. Niosomes were composed of surfactant/ cholesterol (7:3 molar ratio) and prepared by film hydration method (n = 6).
The percents of relative bioavailability (F) and relative pharmacological availability (ƒ) were calculated using the following equations 1 and 2:
For oral Brij 92, Span 60 and Brij 52 niosomal formulations, F was 1.88 ± 0.43, 1.46 ± 0.43 and 1.12 ± 0.57 (%), respectively. The values of percent of relative pharmacological availabilities (ƒ%) for these formulations were also low (table 2).
CONCLUSIONS
The results of this study show that nonionic surfactants can be used for the preparation of insulin entrapping niosomes. Niosomes could effectively prolong the release of insulin in both SGF and SIF and protect this protein against different proteolytic enzymes including pepsin, trypsin and the α-chymotrypsin. Peroral administration of insulin encapsulated niosomes in diabetic rats led to a significant hypoglycemic effect and serum insulin elevation in comparison to free insulin solution. However, the relative bioavailability of oral niosomal insulin was low and more studies are required to develop new oral dosage form of insulin.
ACKNOWLEDGMENTS
This research was supported by the Health and Medical Education ministry of Iran. The authors thank Dr. M. Farzandi (Exir Pharmaceutical Co., Iran) for providing the insulin sample.
REFERENCES
- 1.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-dried chitosan/poly ([gamma]-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–94. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead K, Shen Z, Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J Control Release. 2004;98:37–45. doi: 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, Ping Q, Wei Y, Lai J. Hypoglycemic efficacy of chitosan-coated insulin liposomes after oral administration in mice. Acta Pharmacol Sin. 2004;25:966–72. [PubMed] [Google Scholar]
- 4.Zhang N, Ping QN, Huang GH, Xu WF. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–59. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J Control Release. 2010;147:377–84. doi: 10.1016/j.jconrel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Sharma G, Wilson K, van der Walle C, Sattar N, Petrie J, Ravi Kumar M. Microemulsions for oral delivery of insulin: Design, development and evaluation in streptozotocin induced diabetic rats. Eur J Pharm Biopharm. 2010;76:159–69. doi: 10.1016/j.ejpb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Yamagata T, Morishita M, Kavimandan NJ, Nakamura K, Fukuoka Y, Takayama K, et al. Characterization of insulin protection properties of complexation hydrogels in gastric and intestinal enzyme fluids. J Control Release. 2006;112:343–9. doi: 10.1016/j.jconrel.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Sonaje K, Lin KJ, Wey SP, Lin CK, Yeh TH, Nguyen HN, et al. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: oral delivery using pH-Responsive nanoparticles vs. subcutaneous injection. Biomaterials. 2010;31:6849–58. doi: 10.1016/j.biomaterials.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 9.Dorkoosh F, Verhoef JC, Borchard G, Rafiee-Tehrani M, Verheijden J, Junginger H. Intestinal absorption of human insulin in pigs using delivery systems based on superporous hydrogel polymers. Int J Pharm. 2002;247:47–55. doi: 10.1016/s0378-5173(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 10.Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, et al. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010:1–10. doi: 10.3109/02652048.2010.506579. [DOI] [PubMed] [Google Scholar]
- 11.Khaksa G, D›Souza R, Lewis S, Udupa N. Pharmacokinetic study of niosome encapsulated insulin. Indian J Exp Biol. 2000;38:901. [PubMed] [Google Scholar]
- 12.Ning M, Guo Y, Pan H, Yu H, Gu Z. Niosomes with sorbitan monoester as a carrier for vaginal delivery of insulin: Studies in rats. Drug Deliv. 2005;12:399–407. doi: 10.1080/10717540590968891. [DOI] [PubMed] [Google Scholar]
- 13.Varshosaz J, Pardakhty A, Hajhashemi V, Najafabadi AR. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003;10:251–62. doi: 10.1080/drd_10_4_251. [DOI] [PubMed] [Google Scholar]
- 14.Pardakhty A, Varshosaz J, Rouholamini A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm. 2007;328:130–41. doi: 10.1016/j.ijpharm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Chattaraj S, Das S. Physicochemical characterization of influenza viral vaccine loaded surfactant vesicles. Drug Deliv. 2003;10:73–7. doi: 10.1080/713840363. [DOI] [PubMed] [Google Scholar]
- 16.Dong C, Rogers J. Quantitative determination of carboxymethyl chitin in polymer-coated liposomes. J Microencapsul. 1991;8:153–60. doi: 10.3109/02652049109071484. [DOI] [PubMed] [Google Scholar]
- 17.Kisel M, Kulik L, Tsybovsky I, Vlasov A, Vorob›Yov M, Kholodova E, et al. Liposomes with phosphatidylethanol as a carrier for oral delivery of insulin: studies in the rat. Int J Pharm. 2001;216:105–14. doi: 10.1016/s0378-5173(01)00579-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim A, Yun MO, Oh YK, Ahn WS, Kim CK. Pharmacodynamics of insulin in polyethylene glycol-coated liposomes. Int J Pharm. 1999;180:75–81. doi: 10.1016/s0378-5173(98)00408-6. [DOI] [PubMed] [Google Scholar]
- 19.Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85) Int J Pharm. 1994;105:1–6. [Google Scholar]
- 20.Hao YM, Li K. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403:245–53. doi: 10.1016/j.ijpharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Mokhtar M, Sammour OA, Hammad MA, Megrab NA. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm. 2008;361:104–11. doi: 10.1016/j.ijpharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Arunothayanun P, Turton JA, Uchegbu IF, Florence AT. Preparation and in vitro/in vivo evaluation of luteinizing hormone releasing hormone (LHRH) loaded polyhedral and spherical/tubular niosomes. J Pharm Sci. 1999;88:34–8. doi: 10.1021/js980286u. [DOI] [PubMed] [Google Scholar]
- 23.De la Maza A, Parra J, Sanchez Leal J. Alteration of permeability of neutral and electronegatively charged liposomes by alkyl sulfate surfactants. Langmuir. 1992;8:2422–6. [Google Scholar]
- 24.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99:2049–60. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 25.Fritzler M, behmanesh F, Fritzler M. Development of a depofoam technology for the sustained delivery of desferrioxamine mesylate. DARU. 2003;11:88–94. [Google Scholar]
- 26.Liu T, Guo R, Hua W, Qiu J. Structure behaviors of hemoglobin in PEG 6000/Tween 80/Span 80/H2O niosome system. Colloids Surf A. 2007;293:255–61. [Google Scholar]