Abstract
Background and the purpose of the study
Epoxyeicosatrienoic acids (EETs), which are cytochrome P450 epoxygenase metabolites of arachidonic acid, have anti-inflammatory effects, modulate smooth muscle proliferation, and inhibit smooth muscle migration. This study was designed to determine whether exogenous EETs have any effect on the cell proliferation and apoptosis of carcinoma cell as well as the possible signaling pathways of EETs in this regulation.
Methods
The effects of EETs on the proliferation and anti-apoptosis of human carcinoma cells were measured by MTT assay and flowcytometric analysis, and the regulation of PPARγ, epithelial growth factor receptor (EGFR), extracellular signal-regulated kinase (ERK), phosphatidylinositol 3 (PI3)-Kinase/AKT pathways was investigated by reverse transcriptase polymerase chain reaction (RT-PCR) and western blot analysis.
Results
Results of this study suggested that 14, 15-EET may activate the expression of PPARγ in Tca-8113 cells. 14,15-EET may stimulate cell proliferation, and increase the percentage of cells during S-G2-M phase in Tca-8113 cells significantly. The levels of EGFR, ERK, and PI3 kinase/AKT proteins were significantly induced by treatment of 14, 15-EET and 14,15-EET/AUDA, but no significant changes were observed by addition of GW9662.
conclusion
These findings suggest that exogenous 14,15-EET has potent inhibitory effect on proliferation, and could induce apoptosis in Tca-8113 cell, and these changes are related to the expression of PPARγ, the activation of EGFR, ERK, and PI3 kinase/AKT proteins.
Keywords: Peroxisome proliferator-activated receptor gamma, Expression, Signaling pathways
INTRODUCTION
Peroxisome proliferator-activated receptor gamma (PPARγ), a steroid/retinoid nuclear receptor family of ligand activated transcription factors, is expressed in vascular and inflammatory cells. It may regulate the expression of genes networks encoding proteins involved in all aspects of adipogenesis and lipid metabolism (1). Evidences from cell culture models show that blocking PPARγ activity reduces EETs/ soluble epoxide hydrolase (sEH) and inhibitor-mediated anti-inflammatory effect, indicating PPARγ is an effector of EETs (2). Activation of PPARγ may inhibit tumour progression via induction of differentiation and apoptosis in several lung cancers and lung cancer-derived cell lines (3). In lung cancers, the expression of PPARγ is found to be significantly lower in tumour than in adjacent non-tumour tissues, and there is a correlation between a lower survival rate and decreased PPARγ expression (3, 4). These studies will help to understand the anti-proliferative functions of PPARγ in tumour cells. Epoxyeicosatrienoic acids (EETs), are synthesized from arachidonic acid via cytochrome P450 enzymemediated pathway to four regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (5). EETs are also reported to have diverse physiological and pathophysiological functions, such as vasodilation by activating membrane maxi-Ca2+-activated K+ (BKCa) channels, anti-inflammatory effects, ion channel activation, promotion of the proliferation of vascular cells and angiogenesis, modulation of the proliferation, and migration of vessel smooth muscle cells (6, 7). Recently, it has been found that EETs in particular 11,12- and 14,15-EET, activate several intracellular signaling molecules including tyrosine kinases and phosphatases, p38 MAP kinase, extracellular regulated protein kinases 1/2 (ERK1/2) and MAP kinase phosphatases (8, 9). Chen et al have reported that exogenous EET in cultured human-derived malignant hematologic cell lines may promote proliferation and attenuated apoptosis (10). These findings led us to further investigate the effects and mechanisms of EETs on proliferation, apoptosis and metastasis processes in tumour cells. In the present study, it was intended to verify the proliferative and anti-apoptotic effects of exogenous 14,15-EET, GW9662 and AUDA on carcinoma cancer cells. Furthermore, the expression of PPAR-γ, as well as regulation of EGFR, ERK1/2, PI3K and AKT proteins in carcinoma cancer cells was investigated.
MATERIAL AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM), trypsin and Fetal Bovine Serum (FBS) were obtained from Hyclone (Hyclone, Logan, USA). Antibodies against Epidermal Growth Factor Receptor (EGFR), phosphorous EGFR (p-EGFR), phosphatidylinositol 3-kinase (PI3K), AKT, sEH and p-AKT were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody against β-actin was obtained from Neomarkers (Fremont, CA, USA). 2-Chloro-5-nitro-N-phenyl-benzamide (GW9662) was purchased from Sigma (St. Louis, USA). 14, 15-Epoxyeicosatrienoic acid (14,15- EET) and adamantyl-ureido-dodecanoic acid (AUDA) were obtained from Cayman Chemical Co. (Ann Arbor, USA), and ciglitazone was from Calbiochem (San Diego, CA, USA). All other reagents were purchased from standard commercial suppliers.
Assays of cell proliferation
Human lingual squamous cell carcinoma (Tca-8113) and umbilical vein endothelial (HUVEC, ECV304 cells) cell lines were obtained from Shanghai Institute of Cell Biology (introduced from American Type Culture Collection). The cells were seeded in triplicate 96-well plates at a density of 1×104cells. When the cells were grown to 60% confluence, they were incubated with FBS-free DMEM at 37 °C for 12 hrs to allow for synchronization. Then, AUDA and/or GW9662 (10 μM) were added into medium in the presence or absence of 14,15-EET (100 nM). After 12 or 24 hrs treatment, the medium was removed and replace with new medium containing 5 mg/ml MTT, and incubated for 4 hrs. The medium was then aspirated, and the product was dissolved in dimethyl sulfoxide. Absorbance was measured at 490 nm using a microplate reader (Bio-Tek. Instruments, Winooski, Vermont, USA).
Assay by flowcytometry
The cells (1.5×106 cells) were cultured in the presence of 14,15-EET, 14,15-EET/AUDA and/or GW9662 for 12 hrs in 24-wells plates, and then collected and fixed with 70% ethanol. The cells were resuspended and incubated in phosphate citrate buffer (4 mM citric acid and 192 mM Na2HPO4) for 30 min at room temperature. The cells were collected by centrifugation at 1000 g for 10 min, and were resuspended in PBS containing propidium iodide/RNase (10 μg/ml). The ratio of sub-G1 DNA content were detected and analyzed using CELLQuest program on a FACStar-Plus flow cytometer (BD Biosciences, San Jose, CA, USA).
Assays of transfection and luciferase
Luciferase assays were performed using a Dual- Glo luciferase assay system (Promega, Madison, WI, USA) with phRL-TK vector as an internal control for normalization of transfection efficiency. Plasmids were constructed as described previously (11). Transfection was performed in 24-well culture plates, and luciferase activities were measured using a luminometer (Bio-Lumat LB9507, Berthold, Wildbad, Germany). Briefly, cells were seeded into 24-well plates at a density of 8×104 cells/well, and cultured for 24 hrs. After removal of medium, the cells were co-transfected with 0.4 μg of PPRE-tk- Luc, 0.4 μg of pCMX3/PPARγ and 0.1 μg of control plasmid (phRL-TK) using Lipofectamine PLUS reagents (Invitrogen, Carlsbad, CA). After 4 hrs, 500 μl of full medium was added into each well and then after 24 hrs, the cells were incubated with 100 nM 14,15-EET, 10μM GW9662 and/or 20 μM ciglitazone for 12 hrs, and then luciferase activities were measured.
Quantification of mRNA levels by real-time PCR
Total RNA from cell cultures was isolated with TRIzol reagent, and the amount of RNA was measured spectrophotometrically. One microgram of total RNA from each sample were reversed transcribed. The obtained cDNAs were then used as the templates for quantitative RT-PCR with the use of the Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA, USA).
Assay of western blot
After treatments of 14,15-EET, 14,15-EET/AUDA and/or GW9662, the cells were lysed using lysate buffer, and cell lysate supernatants were harvested by centrifugation at l0000 rpm for 10 min at 4°C. Protein concentrations of the cell supernatants were evaluated. Proteins were separated in a SDS- polyacrylamide gel (10%) with 5% stacking gel in SDS-Tris-glycine running buffer. The proteins were transferred electrophoretically using a PVDF membrane. The membranes were incubated in TTBS buffer containing specific antibodies for 12 hrs, and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10000, Boehringer Mannheim) for 60 min. The immunodetection was accomplished by using an enhanced chemiluminescence detection system (Pierce Chemical Co., USA) and exposure to X-ray film.
Statistical analyses
Data was expressed as mean S.E. Comparisons between groups were performed by a Student's paired two-tailed t test. One-way analysis of variance was used to examine differences in response to treatments and between groups. P values less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Effects of 14,15-EET on expression of PPARγ and sEH in human tumor and ECV304 cells
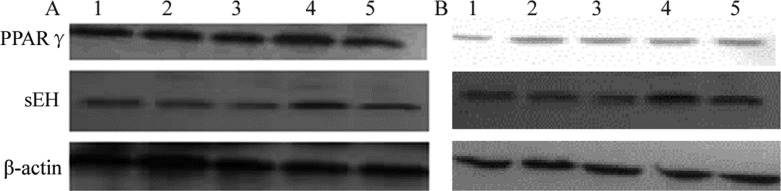
PPARγ has been proved to be a key transcription factor of adipocyte differentiation lipid and glucose homeostasis and an important target in type 2 diabetes and metabolic syndrome. Moreover, they also appear to be expressed in monocytes/macrophages, dendritic cells, eosinophils, T cells, B cells and endothelial cells, etc (12). To confirm whether PPARγ and sEH were expressed in human carcinoma and ECV304 cells, western blot analysis was performed. Results suggested that PPARγ and sEH proteins were expressed in two tested cell lines, and have different degrees of intensity when treated with different concentrations of 14,15-EET. The degree of intensity of PPARγ in Tca-8113 cell line was significantly higher than that of the ECV304 cells. Results also suggested that the expression of PPARγ reached a maximum level after 24 hrs treatment with 14,15-EET within the range of 25-200 nM (Fig. 1).
Figure 1.
Effects of 14,15-EET treatment on the expression of PPARγ and sEH in Tca-8113 (A) and HUVEC (B) cells. PPARγ protein levels in carcinoma and cells were measured by treatment with different concentrations (0-200 nM) of 14,15-EET after 24 hrs. Total protein was extracted and analyzed by Western blot as described in materials and methods. Data are representative of two independent experiments. Lanes from 1 to 5 were 0, 25, 50, 100 and 200 nM, respectively. β-actin is shown as an internal control.
Activation of PPARγ by 14,15-EET in tumor cells
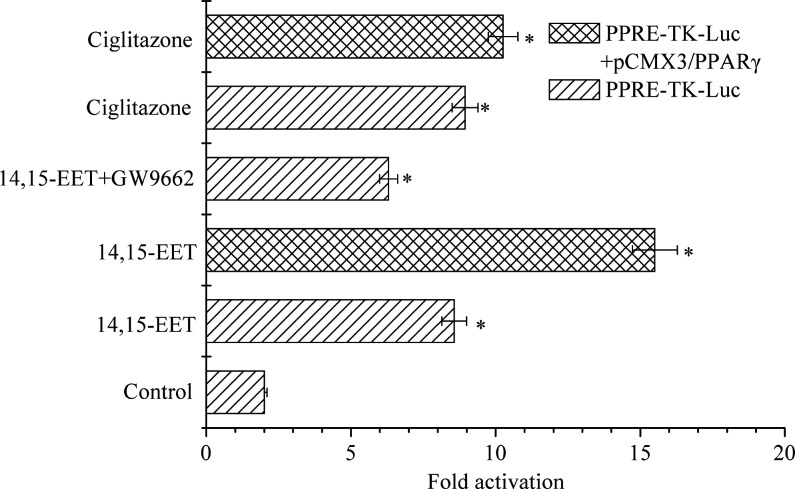
As a ligand-activated transcription factor, the role of PPARγ in cancer remains a subject of debate (4, 6, 13). Based on luciferase activity assay system, the activation of PPARγ in carcinoma cells by 100 nM of 14,15-EET was investigated. As shown in Figure 2, luciferase activities showed 4.28, 3.15 and 4.47- fold increase when the cells were incubated and transfected by using PPRE-TK-Luc and addition of 14,15-EET, 14,15-EET+GW9662 and ciglitazone, respectively. In addition, GW9662, as a PPARγ antagonist, may inhibit the luciferase activities compared to that which was obtained by addition of 14,15-EET. Under co-transfected PPRE-tk-Luc and pCMX3/PPARγ condition, activities resulting by the addition of 14,15-EET and ciglitazone compared to control increased significantly 7.75 and 5.13-fold, respectively.
Figure 2.
Activation of PPARγ by 100 nM 14,15-EET in carcinoma cells. Cells were seeded in 24 well plates and separated into six groups. The third and sixth groups were co-transfected using 0.4 μg PPRE-tk-Luc, 0.4 μg pCMX3/PPARγ and 0.1 μg control plasmid (phRL-TK). Other groups were transfected by using 0.4 μg PPRE-tk-Luc and 0.1 μg control plasmid (phRL-TK). The first group was incubated using DMSO as negative control. The fifth and sixth groups were administrated with Ciglitazone (20 μM) as positive control. The third and fourth groups were then incubated with 14,15-EET (100 nM) in the absence or presence of GW9662. Data points and error bars represent means±S.E. Significant difference is denoted as asterisk (*) between control and treatments.
Effects of 14,15-EET on carcinoma cell proliferation and cycle progression
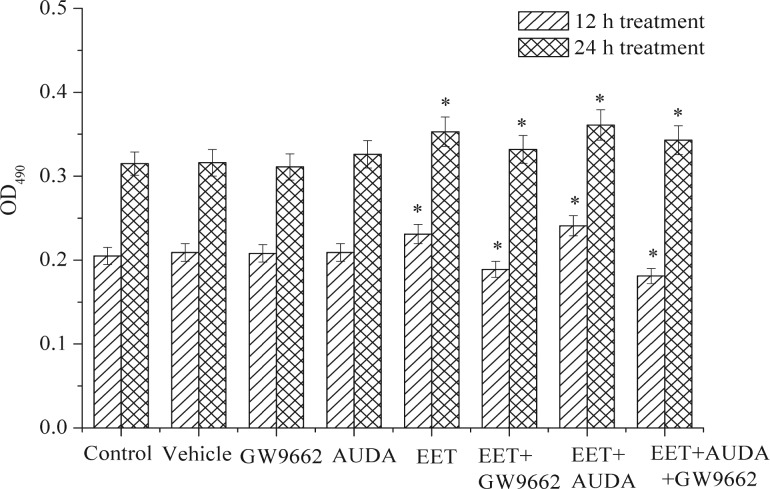
Previous studies have indicated that EET may induce cell growth and apoptosis in various cancer cells (5). However, the effects of 14,15-EET on the cell proliferation in human carcinoma cell is still unclear. In the present study, a human carcinoma cell was used as an in vitro model to evaluate whether exogenous EET-induced proliferative effects via PPARγ activation. As shown in Figure 3, results suggested that the addition of 14,15-EET (100 nM) in carcinoma cells cultured for 12 and 24 hrs may stimulate cell proliferation compared to the control. The addition of GW9662 and AUDA in the cells had no effects compared to the control, but cell proliferation was inhibited by the addition of 14,15- EET and GW9662. Recent data have shown that PPARγ activation by thiazolidinediones such as troglitazone induced inhibition of a wide variety of cancer cells, suggesting that activation of PPARγ down-regulates cell growth (3, 4). Results of this study suggested that 14,5-EET could up-regulate the expressions of PPARγ in the human carcinoma cells, and also showed that it may stimulate cell proliferation (Figs 2 and 3).
Figure 3.
Effects of exogenous 14,15-EET and 14,15-EET (100 nM)/AUDA carcinoma cell proliferation for 12 and 24 hrs. Data points and error bars represent means±S.E. Significant difference (p < 0.05) is denoted as asterisk (*) between control and treatments.
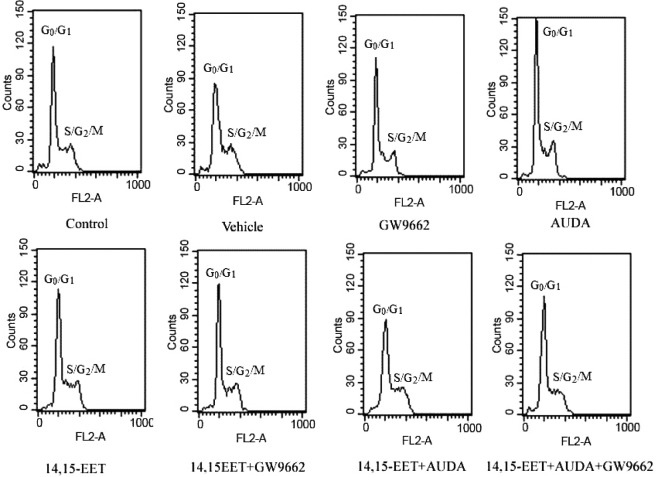
A reduction in cell proliferation, an increase in the rate of apoptosis, or both may explain the reduction of cell viability caused by PPARγ agonists. In a variety of cancer cells, PPARγ inhibits proliferation and cell cycle arrest at G0 /G1 restriction point (14). To study the proliferative effect of 14,15-EET, the fate of 14,15-EET-treated carcinoma cells within the cell cycle was investigated using flowcytometry analysis. As shown in Figure 4 and table 1, exogenous 14,15-EET significantly increased the percentage (47.08%) of cells during S-G2-M phase in Tca-8113 cells compared to the control and that of vehicle. However, the proportion of S-G2-M phase cell populations compared to the control was reduced significantly by treatment with GW9662. These results suggest that 14, 15-EEET may have the ability to promote the proliferation of carcinoma cells and contribute to the anti-apoptosis effects in human carcinoma cells.
Figure 4.
Effects of 14,15-EET on cell cycle progression in human carcinoma cell lines by treatment with 14,15-EET. Tca-8113 cells were pretreated with AUDA (1 μM) for 2 h and then incubated with 14,15-EET (100 nM) in the presence or absence of GW9662 (10 μM). After 12hrs, flow cytometry analysis was performed.
Table 1.
14,15-EET promotes cell cycle progression in human carcinoma cells.
| Group | G1 –G0 | S | G2 -M |
|---|---|---|---|
| Control | 57.22 ± 5.6 | 33.43 ± 3.5 | 9.35 ± 2.6 |
| Vehicle | 62.24 ± 8.6 | 31.05 ± 2.7 | 8.71 ± 4.3 |
| GW9662 | 55.14 ± 3.7 | 35.85 ± 3.1 | 9.01 ± 5.7 |
| AUDA | 56.74 ± 6.8 | 32.08 ± 2.3 | 11.18 ± 2.4 |
| 14,15-EET | 43.72 ± 5.6 | 47.66 ± 3.7* | 10.62 ± 5.6 |
| 14,15-EET+GW9662 | 54.46 ± 4.9 | 35.68 ± 6.4* | 9.86 ± 3.6 |
| 14,15-EET+AUDA | 36.86 ± 5.0 | 49.75 ± 3.7* | 13.39 ± 2.3 |
| 14,15-EET+AUDA+GW9662 | 60.13 ± 4.7 | 30.48 ± 5.4* | 9.39 ± 2.7 |
Data represent mean values ± SD (n = 3).
14,15-EET stimulates cell proliferation via regulation of EGFR, PI3 kinase and AKT
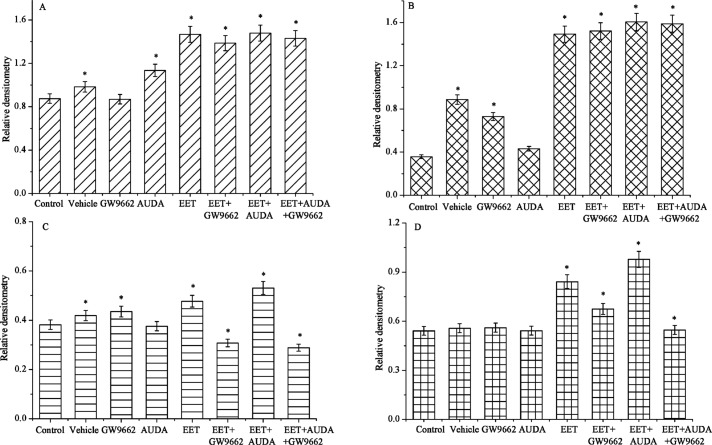
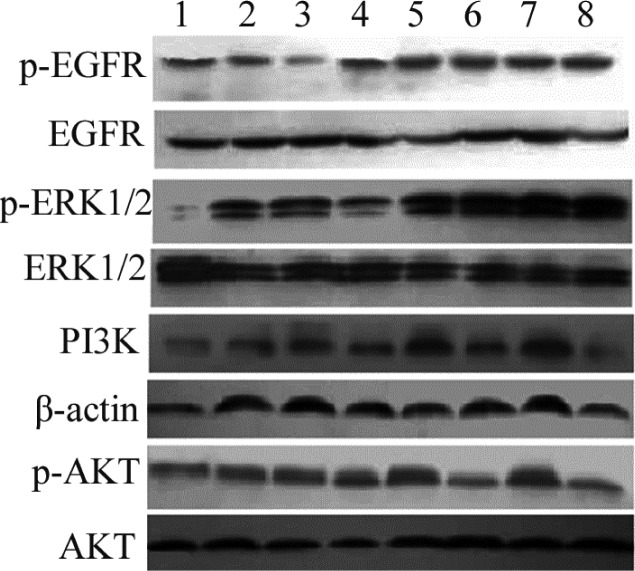
Previous studies have shown that EET may bind to putative binding protein or receptor, which may trans-activate EGFR and AKT in regulation of the cell motility, and have revealed that expression of by epoxygenase is very strong and selective in human cancer tissues. The levels of phosphorylated EGFR, PI3K/Akt, and ERK1/2 in tumor cells transfected with cytochrome epoxygenases were significantly upregulated. Studies have also shown the status of signal molecules, such as EGFR, PI3K, Akt, and ERK1/2, in tumor cells after transfection with CYP genes. These results suggest that PI3K/Akt and ERK1/2 pathways are involved in the promotion of tumor cells by CYP epoxygenases overexpression (15, 16). Results of this study suggest that the total amount of EGFR, ERK, and PI3 kinase/Akt proteins were quite refractory to 14, 15-EET and other additives, and their expression levels were correlated with these stimulators in human carcinoma cell (Figs 5 and 6). The phosphorylated ERK and PI3 kinase/Akt pathways are induced significantly by AUDA and 14,15-EET treatments. However, the levels of PI3 kinase-AKT pathway was reduced significantly by treatment with GW9662 compared to the control, suggesting that GW9662, an antagonist of PPARγ, may block PI3 kinases-AKT pathways slightly. The phosphorylated EGFR was also elevated significantly by treatment with 14,15-EET, but no significant changes were observed by treatment with GW9662. The changes in signal transduction pathways and transcriptional mechanisms induced by EET have been investigated, and recent studies are attempting to isolate an EET membrane receptor that mediates cell proliferation and anti-apoptosis in some tumor cell lines (6, 17). Findings of this study suggest that exogenous EETs significantly promote carcinoma cancer cell proliferation and inhibit apoptosis significantly via the activation of the EGFR, ERK and PI3 kinase/Akt signaling pathways.
Figure 5.
Effects of different treatments on the relative expression densitometry of EGFR (A), ERK1/2 (B), PI3K (C) and AKT (D) pathways in human carcinoma cells. Data points and error bars represent means ± S.E. Significant difference is denoted as asterisk (*) between control and treatments.
Figure 6.
Effects of 14,15-EET on the expression levels of PI3K-AKT pathways in human carcinoma cells. Tca-8113 cells were pretreated with AUDA (1 μM) for 2 hrs and then stimulated with 14,15-EET (100 nM) in the presence or absence of GW9662. After 8 hrs, the collected cell lysates were immunoblotted with anti-p-EGFR antibody, and p-ERK antibody. PI3K antibody and p-AKT antibodies. EGFR, ERK, β-actin, AKT were used as a loading control. Lanes from 1 to 8 were control, vehicle, GW9662, AUDA, 14,15-EET, 14,15-EET+GW9662, 14,15-EET+AUDA, 14,15-EET+ AUDA+GW9662, respectively.
CONCLUSION
Results of this study suggest that 14,15-EET may promote the proliferation of tumor cells through activation of PPARγ, up-regulation of PI3 kinase/Akt system in human carcinoma cells. These findings provide a novel clue regarding the role of 14,15- EET as a potential cancer therapeutic in tumor cells. Importantly, the detailed mechanisms of 14,15-EET on carcinoma cells will be of high importance for future study.
ACKNOWLEDGMENTS
This work was supported by The National Natural Science Foundation of China (No. 30540087) and National Key Basic Research Program (No. 2007CB512004 and No. 2002CB513107).
REFERENCES
- 1.Wray JA, Bishop-Bailey D. Epoxygenases and PPARs in vascular biology. Exp. Physiol. 2008;93:148–154. doi: 10.1113/expphysiol.2007.038612. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPAR gamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. U S A. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehrke M, Lazar MA. The many faces of PPAR gamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat. Rev. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 5.Kaspera R, Totah RA. Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology. Expert. Opin. Drug Metab. Toxicol. 2009;5:757–771. doi: 10.1517/17425250902932923. [DOI] [PubMed] [Google Scholar]
- 6.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J. Pharmacol. Exp. Ther. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 8.Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442:511–518. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- 9.Hoebel BG, Graier WF. 11,12-Epoxyeicosatrienoic acid stimulates tyrosine kinase activity in porcine aortic endothelial cells. Eur. J. Pharmacol. 1998;346:115–117. doi: 10.1016/s0014-2999(98)00118-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Wei X, Rao X, Wu J, Yang S, Chen F, Ma D, Zhou J, Dackor RT, Zeldin DC, Wang DW. CYP2J2 is highly expressed in hematologic malignant diseases and promotes tumor cell growth. J. Pharmacol. Exp. Ther. 2011;336:344–355. doi: 10.1124/jpet.110.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakashiro KI, Hayashi Y, Kita A, Tamatani T, Chlenski A, Usuda N, Hattori K, Reddy JK, Oyasu R. Role of peroxisome proliferator-activated receptor gamma and its ligands in non-neoplastic and neoplastic human urothelial cells. Am. J. Pathol. 2001;159:591–597. doi: 10.1016/s0002-9440(10)61730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Széles L, Töröcsik D, Nagy L. PPAR gamma in immunity and inflammation: cell types and diseases. Biochim. Biophys. Acta. 2007;1771:1014–1130. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Cho YS, Park JM, Yoon SO, Kim KW, Chung AS. Pro-MMP-2 activation by the PPARgamma agonist, ciglitazone, induces cell invasion through the generation of ROS and the activation of ERK. FEBS Lett. 2007;581:3303–3310. doi: 10.1016/j.febslet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Aiello A, Pandini G, Frasca F, Conte E, Murabito A, Sacco A, Genua M, Vigneri R, Belfiore A. Peroxisomal proliferator-activated receptor-gamma agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006;147:4463–4475. doi: 10.1210/en.2005-1610. [DOI] [PubMed] [Google Scholar]
- 15.Papageorgiou E, Pitulis N, Msaouel P, Lembessis P, Koutsilieris M. The non-genomic crosstalk between PPAR-gamma ligands and ERK1/2 in cancer cell lines. Expert. Opin. Ther. Targets. 2007;11:1071–1085. doi: 10.1517/14728222.11.8.1071. [DOI] [PubMed] [Google Scholar]
- 16.Gardner OS, Dewar BJ, Earp HS, Samet JM, Graves LM. Dependence of peroxisome proliferator- activated receptor ligand-induced mitogen-activated protein kinase signaling on epidermal growth factor receptor transactivation. J. Biol. Chem. 2003;278:46261–46269. doi: 10.1074/jbc.M307827200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]