Abstract
Background.
Anemia has been associated with elevated cerebral blood flow (CBF) in animal models and certain clinical conditions (eg, renal disease), but whether hemoglobin level variations across a relatively normal range are associated with local or diffuse CBF changes is unclear. We investigated whether lower hemoglobin is associated with regional increases in relative CBF in older individuals, and if these increases occur in watershed regions.
Methods.
Seventy-four older nondemented adults underwent serial 15O water positron emission tomography scans. Voxel-based analysis was used to investigate regional relative CBF patterns in association with hemoglobin level and in individuals with and without anemia. Analyses of cross-sectional relations between regional CBF and anemia were performed separately at two time points, 2 years apart, to identify replicable patterns of associations.
Results.
Restricting results to associations replicated across two cross-sectional analyses, lower hemoglobin was associated with higher relative CBF within the middle/inferior frontal, occipital, precuneus, and cerebellar regions. In addition, individuals with anemia (n = 15) showed higher relative CBF in superior frontal, middle temporal, hippocampal, and gyrus rectus regions than those without anemia. In some regions (right superior temporal gyrus, left inferior frontal gyrus, midline cuneus, and right precuneus); however, lower hemoglobin was associated with lower relative CBF.
Conclusions.
In nondemented individuals, lower hemoglobin is associated with elevated relative CBF in specific cortical areas but reduced CBF in other areas. Whether this association between anemia and CBF in the absence of chronic diseases and in a normal physiologic range is related to clinical endpoints warrants further study.
Keywords: Cerebral blood flow, Anemia, PET, Aging
Adequate cerebral oxygenation is dependent on enough blood volume reaching the brain as well as on sufficient concentration of hemoglobin. Anemia can result in decreased delivery of oxygen to the brain, which could potentially contribute to cerebral dysfunction or even ischemia. An increase in global cerebral blood flow (CBF) is often observed in patients with severe anemia of chronic diseases. However, whether such compensatory change in CBF is also active within the normal physiologic range of hemoglobin concentration in older adults or in patients with mild anemia is still controversial (1,2).
Compensatory changes in CBF have been shown in several patient populations with anemia. In positron emission tomography (PET) studies of patients with renal failure and anemia, an inverse relationship between hematocrit and CBF has been described (3). In addition, correction of the anemia results in a subsequent decrease in CBF, back to a normal range (4). Similar inverse associations between hematocrit and CBF have been found in the postcardiac surgery setting, using magnetic resonance imaging arterial spin labeling techniques (5) and in adults with unilateral steno-occlusive disease (6). It is still unclear, however, whether fluctuations in CBF within a normal physiologic range or inability to adequately compensate for anemia with an increase in CBF cause actual problems in cerebral function (or whether problems with cerebral function themselves lead to changes in CBF). There is some evidence supporting an association between anemia and impaired cognition (7) and in another study, individuals with dementia and atrophy or with old strokes had less of an increase in CBF than would be expected based on their hematocrit (3).
Although hemoglobin and CBF have been shown to be inversely associated based on animal studies (8), limited data exist describing the relationship between hemoglobin levels and CBF in older adults without significant medical comorbidities. The data that do exist (6) show global, not regional, associations between hemoglobin and CBF. It is unclear whether CBF increases occur throughout the brain or only in certain regions in which the need for blood flow may be greater—for instance, the watershed regions of the brain are generally more susceptible to low flow, and it might be hypothesized that these regions require disproportionately larger increases in CBF in response to low hemoglobin levels. The purpose of this study was to determine whether hemoglobin levels are inversely associated with CBF and to identify the regional distribution of these associations in nondemented older adults from the Baltimore Longitudinal Study of Aging (BLSA) neuroimaging substudy.
METHODS
Participants in this study were part of the neuroimaging substudy of the BLSA (9). These participants, all over 60 years of age at the time of scanning, underwent [15O] water PET scanning at baseline and at annual follow-ups for up to 8 years. In addition, they were evaluated every other year with multiple laboratory assays including hemoglobin (10). This article investigates 74 participants who had [15O] water PET and hemoglobin measurements. PET and hemoglobin data were available at two time points for 69 of these participants. All participants were free of stroke and dementia at baseline and at follow-up visits. Dementia diagnosis was based on standard neuropsychological measures and clinical history and was determined by consensus conference (11). Exclusion at initial assessment was made based on presence of central nervous system disease, severe cardiovascular or pulmonary disease, or metastatic cancer (9). The study was approved by the local Institutional Review Boards and the National Institute on Aging Intramural Research Program. All participants gave written informed consent at each visit.
Measurement of Hemoglobin Levels
Laboratory assays were performed biannually at each BLSA visit and included assessment of hemoglobin. Anemia was defined according to the World Health Organization criteria as hemoglobin below 12 g/dl, for women, and below 13 g/dl for men (12,13). For a secondary analysis, the 15 individuals in the cohort with anemia (by these definitions) were matched by age group and sex with 15 controls with hemoglobin levels in the normal range.
PET Scanning
Regional relative CBF was measured by PET using [15O] water. For each scan, 75 mCi of [15O] water were injected intravenously, in bolus form. Scans were performed at rest, while participants were instructed to keep their eyes open and focused on a screen covered by a black cloth. A custom thermoplastic mask was made for each participant at baseline, and used in subsequent years, to aid in head positioning. PET images were obtained on a GE 4096+ scanner, with 15 slices (axial resolution, 6.5-mm full width at half maximum). Images were acquired for 60 seconds from the time the total radioactivity counts in the brain reached the threshold level. A transmission scan in two-dimensional mode using a 68Ge rotating source was used for attenuation correction. Arterial blood sampling was not obtained for participants, so absolute quantitative measurements of CBF could not be determined.
Cognitive Assessment
Standard neuropsychological tests were administered to BLSA participants at each BLSA visit; these methods have been described previously (14). For the purposes of this analysis, cognitive performance at a single visit concurrent with PET scanning and hemoglobin assessment was used.
Data Analysis
PET scans were realigned and spatially normalized into standard stereotactic space. They were smoothed to full width at half maximum of 12, 12, and 12 mm, respectively, in the x, y, and z planes. To control for variability in global flow, regional CBF (rCBF) values at each voxel were ratio adjusted to the mean global gray matter blood flow and scaled to a mean of 50 ml/100 g/min for each image. All references to rCBF in this manuscript pertain to these measurements of relative flow.
Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, London, England) software was used for analysis of PET data. Two primary statistical approaches were used. First, replicable associations between hemoglobin and CBF were determined over time across the 69 participants with PET and hemoglobin measures at two time points. To do this, cross-sectional linear regressions were performed on a voxel-by-voxel basis to determine associations between hemoglobin and rCBF at two separate time points an average of 2.05 (SD 0.13) years apart (magnitude p < .005, spatial extent >50 voxels). A conjunction analysis was then used on the resultant regression maps to establish relative rCBF associations of elevated hemoglobin levels reproduced across the two time points (p < .05 significance level). Thus, the longitudinal data were used to replicate cross-sectional findings at two points in time, and only replicated findings are presented in this article. All models were adjusted for age at PET study and sex. This analysis of 69 individuals included the assessment of 138 [15O] water PET scans.
A case–control analysis was also performed using SPM5. For this analysis, rCBF patterns for 15 individuals with anemia (defined as described above) were compared with those for 15 individuals without anemia, and replicable group differences that occurred over a period of 2 (SD 0.27) years were determined. First, group differences in rCBF were determined at each time point separately (magnitude p < .005; spatial extent of >50 voxels). A conjunction analysis was then used on the resultant difference maps to establish anemia-related differences in patterns of rCBF that were present across the two time points (p < .05 significance level). This analysis of 30 individuals included the assessment of 50 [15O] water PET scans (five cases and five controls had only baseline data due to either unavailable hemoglobin or imaging data at follow-up).
Regions showing significant associations between hemoglobin and rCBF and significant differences between anemic and nonanemics were identified using a Talairach atlas and statistical parametric mapping anatomic overlays aided by anatomic atlases (15).
As a secondary analysis, we analyzed the association between hemoglobin level and cognitive performance; for the Mini-Mental State Examination, we used Poisson regression and evaluated hemoglobin level as a predictor, with age and sex as covariates. For all other cognitive measures, the cognitive scores were separate outcomes in ordinary least squares regression models, each with hemoglobin, age, and sex as covariates.
RESULTS
Characteristics of Participants
Imaging and hemoglobin data were available on 74 participants (Table 1). Hemoglobin values were lower for women than men (p < .0001), and hemoglobin levels were inversely associated with age, with a steeper slope in men (r = −0.49; p = .0014) than in women (r = −0.36; p = .034).
Table 1.
Demographics of Baltimore Longitudinal Study on Aging (BLSA) Cohort
| Variable | Mean (SD; range; unless otherwise specified) |
| Participants with baseline PET and hemoglobin data (n = 74) | |
| Sex (% female) | 47.3% |
| Age at initial PET | 74.1 (7.5; 60.5–89.1) |
| Race (Caucasian (N)/African American (N)) | 66/8 |
| Education (y) | 16.3 (2.5) |
| Subset with replicated PET and hemoglobin data included in the regression analysis (n = 69) | |
| Age at PET 1 | 73.9 (7.5; 60.5–89.1) |
| Age at PET 2 | 75.9 (7.5; 62.6–91.2) |
| Interval between PET 1 and PET 2 (y) (mean [SD]) | 2 (0.1) |
| Hemoglobin (g/dl) at PET 1 | 13.8 (1.4; 11.2–18.3) |
| Hemoglobin (g/dl) at PET 2 | 14.1 (1.4; 11.1–17.7) |
Note: PET = positron emission tomography; Data are presented as mean (SD; range). PET 1 and PET 2 in this article are the first and second scans at which hemoglobin data were available within 1.5–2.5 y.
Of the individuals with anemia included in the case–control analysis, 7 were women and 8 were men. There were no significant differences between cases and controls with respect to demographic or clinical characteristics (Table 2).
Table 2.
Demographic and Clinical Data for the Case–Control Study
| Anemics | Nonanemics | Difference (p value) | |
| N | 15 | 15 | |
| N follow-up | 10 | 10 | ns |
| Demographics | |||
| Age baseline | 78 (7; 68–88) | 78 (7; 66–89) | ns |
| Age at follow-up | 80 (7; 70–91) | 80 (7; 68–91) | ns |
| Sex (# females) | 7 | 7 | ns |
| Race (# Caucasian) | 12 | 13 | ns |
| Education | 15.9 (3.2; 8–20) | 16.9 (2.6; 12–20) | ns |
| Clinical data | |||
| SBP | 142 (19; 120–175) | 143 (25; 110–184) | ns |
| DBP | 73 (11; 58–90) | 77 (10; 60–92) | ns |
| Hypertension (#) | 9 | 9 | ns |
| Diabetes (#) | 1 | 3 | ns |
| BMI | 26.6 (2.9; 22.1–34.3) | 28.3 (3.5; 20.7–32.9) | ns |
| Smoking (# current) | 2 | 3 | ns |
Note: BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure. Data are presented as mean (SD; range) unless otherwise specified. Clinical data were assessed at the baseline study.
Regression Analysis: Cross-Sectional Hemoglobin Level and Relative rCBF
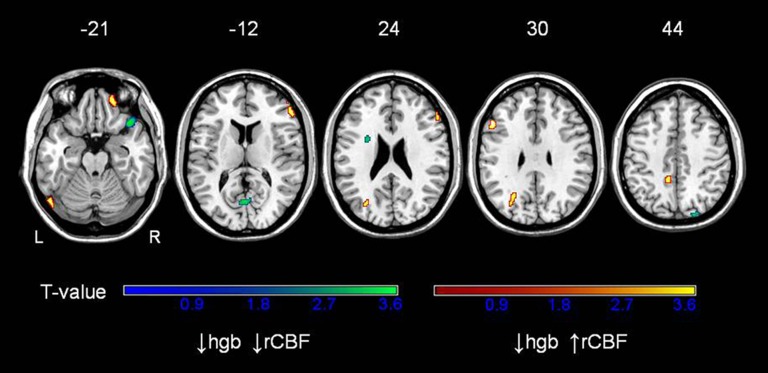
Regions of the brain in which hemoglobin was found to be associated with rCBF at both cross-sectional time points are displayed in Figure 1. Higher rCBF in association with lower hemoglobin was found in the frontal lobe bilaterally, including the middle (Brodmann area (BA) 9/11) and inferior (BA 46) frontal gyri as well as in the left precuneus (BA 7), left superior occipital gyrus (BA19), and cerebellum. The maxima of regions in which low hemoglobin is associated with higher rCBF are identified in Table 3.
Figure 1.
Correlations between hemoglobin and regional cerebral blood flow (rCBF) in older adults, replicated at two time points. Analysis is adjusted for age and sex. Red regions represent areas where increased rCBF is associated with decreased hemoglobin; blue regions represent areas where decreased rCBF is associated with decreased hemoglobin. Z levels indicate the axial level of the brain slices.
Table 3.
Regions in Which Regional Cerebral Blood Flow (rCBF) Is Associated With Hemoglobin Levels. This Analysis Was Replicated Across Two Separate Time Points 2.05 (SD 0.13) y Apart. Brodmann Area (BA) Is Noted in Parentheses
| Region | Side | Coordinates | p Value | T Value | Spatial Extent (voxels) | ||
| x | y | z | |||||
| ⇓Hgb ⇑CBF | |||||||
| Precuneus (BA7) | L | −14 | −46 | 50 | <.001 | 3.64 | 110 |
| Middle frontal gyrus (BA9) | L | −52 | 24 | 30 | <.001 | 3.60 | 58 |
| Superior occipital gyrus (BA19) | L | −26 | −64 | 34 | .001 | 3.23 | 116 |
| Middle frontal gyrus (BA11) | R | 22 | 50 | −20 | .001 | 3.23 | 67 |
| Inferior frontal gyrus (BA46) | R | 56 | 34 | 18 | <.001 | 3.48 | 170 |
| Cerebellum | L | −52 | −70 | −24 | <.001 | 3.59 | 72 |
| ⇓Hgb ⇓CBF | |||||||
| Superior temporal gyrus (BA38) | R | 40 | 24 | −24 | <.001 | 3.79 | 182 |
| Precuneus (BA19) | R | 20 | −86 | 40 | <.001 | 3.45 | 52 |
| Inferior frontal gyrus (BA44) | L | −26 | 4 | 22 | .001 | 3.08 | 60 |
| Cuneus (BA17) | 0 | −68 | 12 | .001 | 3.21 | 102 | |
Regions in which lower hemoglobin was associated with decreased rCBF, an opposite relationship to the original hypothesis, were also found. These regions include the right superior temporal gyrus (BA38), left inferior frontal gyrus region (BA 44), midline cuneus (BA 17), and right precuneus (BA 19).
Case–Control Analysis: Anemia and Relative rCBF
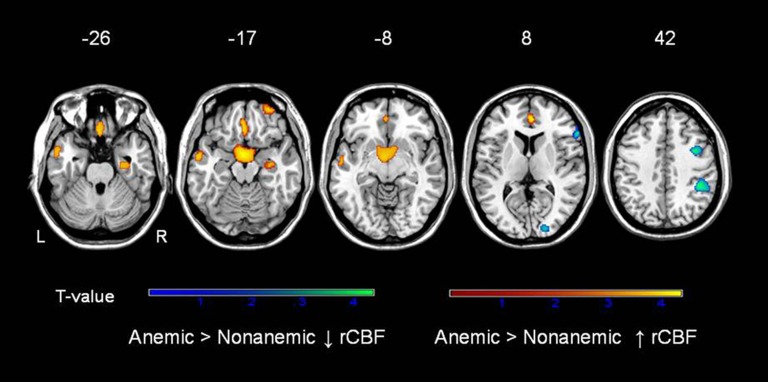
Figure 2 shows the regions in which anemic participants exhibited either higher or lower rCBF relative to nonanemics. Compared with nonanemics, anemics showed higher rCBF in the right entorhinal cortex (BA28) extending into the hippocampus, left gyrus rectus (BA 11), and right superior frontal gyrus (BA 11) as well as left middle temporal gyrus (BA 21; Table 4). The regions in which rCBF was lower in anemics than in nonanemics (opposite to the primary hypothesis) included the right inferior parietal lobule (BA 40), right middle (BA 6) and inferior (BA 43) frontal gyrus, and the right cuneus (BA 18; Table 4).
Figure 2.
Differences in regional cerebral blood flow (rCBF) in older adults with and without anemia, replicated at two time points. See figure 1 legend.
Table 4.
Regions in Which Resting Regional Cerebral Blood Flow (rCBF) Is Higher in Individuals With Anemia Than in Nonanemic Controls. This Analysis Was Replicated Across 2 (SD 0.27) y. Brodmann Area (BA) Is Noted in Parentheses
| Region | Side | Coordinates | p Value | T Value | Spatial Extent (voxels) | ||
| x | y | z | |||||
| Anemic > Nonanemic ↑CBF | |||||||
| Entorhinal cortex (BA 28) | R | 30 | −12 | −22 | .001 | 3.23 | 156 |
| Superior frontal gyrus (BA 11) | R | 30 | 58 | −18 | <.001 | 3.83 | 83 |
| Middle temporal gyrus (BA 21) | L | −56 | 0 | −18 | <.001 | 3.54 | 238 |
| Gyrus rectus (BA 11) | L | −2 | 30 | −22 | <.001 | 4.1 | 535 |
| Hypothalamus | L | −2 | 0 | −16 | <.001 | 4.39 | 910 |
| Anterior cingulate gyrus (BA 32) | R | 4 | 44 | 8 | <.001 | 4.10 | 524 |
| Anemic > Nonanemic ↓CBF | |||||||
| Inferior parietal lobule (BA 40) | R | 42 | −38 | 42 | <.001 | 4.38 | 212 |
| Middle frontal gyrus (BA 6) | R | 38 | 4 | 44 | <.001 | 4.0 | 133 |
| Inferior frontal gyrus (BA 43) | R | 60 | 28 | 8 | <.001 | 3.93 | 98 |
| Cuneus (BA 18) | R | 18 | −90 | 12 | .001 | 3.27 | 80 |
Cognition and Hemoglobin
We evaluated the cross-sectional association between hemoglobin level and scores on a range of neuropsychological tests. Results are displayed in Table 5. None of the cognitive tests were significantly associated with hemoglobin level.
Table 5.
Beta Coefficients for the Analysis of Cognitive Score as Predicted by Hemoglobin (g/dl) Level, With Adjustment for Age and Sex
| Neuropsychological Test | Hemoglobin: Estimate | SE | p Value |
| California verbal learning, immediate recall | −0.057 | 0.95 | .95 |
| California verbal learning, long delay | −0.15 | 0.31 | .63 |
| California verbal learning, short delay | −0.25 | 0.32 | .43 |
| Benton visual retention test, errors | −0.40 | 0.31 | .20 |
| WAIS-R digit span: backwards | −0.17 | 0.18 | .34 |
| WAIS-R digit span: forwards | −0.21 | 0.18 | .25 |
| Mini-mental state examination | −0.061 | 0.88 | .48 |
| Category fluency | 0.068 | 0.27 | .80 |
| Letter fluency | 0.066 | 0.36 | .86 |
| Trailmaking test A | 0.93 | 0.78 | .24 |
| Trailmaking test B | −2.16 | 3.20 | .50 |
Note: WAIS-R = Wechsler Adult Intelligence Scale-Revised.
DISCUSSION
In our analysis of nondemented older individuals in the BLSA, we found that hemoglobin was inversely associated with relative rCBF in certain regions of the brain. Regions were also identified in which rCBF appeared to decrease, relative to other regions, in association with low hemoglobin levels. These findings are unique, as they explore the regional relationship between hemoglobin and CBF in relatively healthy individuals across what is primarily a normal range of hemoglobin values.
Despite our initial hypotheses that watershed regions would preferentially have higher rCBF in association with low hemoglobin, few of the regions identified by both the regression and case–control analyses are in the watershed regions of the brain. We expected that these regions, which are most vulnerable to ischemia in the setting of extreme hypoperfusion and might even experience ischemia in these regions with relatively minimal hypoperfusion injuries (such as from mild hypotension or reduced cardiac ejection fraction (16)), would be most in need of a compensatory response to low hemoglobin levels. It is therefore possible that these regions might even be at risk in individuals who are anemic, if anemia leads to a slight reduction in oxygenation, particularly in combination with other physiologic causes of hypoperfusion. The lack of clear watershed-type pattern of the associated regions suggests that other mechanisms may be at play contributing to these regional increases in CBF in the setting of low hemoglobin. One possibility is that the rCBF associations with hemoglobin occur in regions that are more vulnerable to variation in the level of oxygen. Similar to our findings of increased rCBF in anemics relative to nonanemics, rCBF changes in the hypothalamus (17), right anterior cingulate gyrus (BA 32), bilateral middle frontal gyrus (BA 9), left middle temporal gyrus (BA 21), and right parahippocampal gyrus (BA 28) are observed during hypoxemia (18). This suggests that the rCBF correlates of hemoglobin levels may reflect the regional needs for oxygen facilitated by erythrocyte redistribution to dilated microvessels.
When hemoglobin levels decrease, cardiac output is augmented, afterload is reduced, and preload is increased. Oxygen extraction is increased at the tissue level, and vasodilation occurs in the brain (19). This has been shown in individuals (20) and animals (21) exposed to hemodilution, in whom CBF velocities increase in response to reductions in hematocrit and blood oxygen content. Our data suggest, however, that the increase in rCBF is not entirely due to this increase in cardiac output and is regionally regulated because we have identified particular regions in which rCBF is preferentially increased in the setting of anemia and low hemoglobin values. This regional regulation may occur via regulation of vasodilation (22), as vasodilatory changes occur in regions that are associated with greater rCBF in anemia.
Our finding that there are particular brain regions in which rCBF appears to “decrease,” relatively, in the setting of lower hemoglobin values (which may be also interpreted as higher rCBF in the setting of higher hemoglobin) is not consistent with our initial hypothesis but may also represent physiologic changes in response to anemia. Oxygen transport has been reported to be optimal at intermediate hemoglobin values, with reductions at both low and high values of hemoglobin concentrations. Because cardiac output increases in response to a drop in hemoglobin, and at higher hemoglobin values, there is an increase in viscosity in the setting of a relative reduction in cardiac output (23), oxygen transport appears to be reduced at higher hemoglobin concentrations. It is possible that higher rCBF in the face of high hemoglobin levels represents a localized vasodilatory or other brain-level response to this hyperviscosity, although in animal models, it has been suggested that the relationship between hemoglobin and CBF is independent of viscosity (24). Another possible explanation is that regions exposed to low hemoglobin may be chronically ischemic, and thus would not trigger the usual vasodilatory response that normally regulates local rCBF in response to anemia. These regions might be less metabolically active because of this low level of ischemia, and thus lower rCBF would be found in association with low hemoglobin. Our data do not specifically support this hypothesis, but this is a mechanism that could be explored in more detail in future studies.
Anemia and lower hemoglobin levels have been associated with lower cognitive performance across multiple domains (7,25). If the normal response to anemia, or to even a minimal decrease in hemoglobin level, is an increase in CBF, as supported by our findings, inadequate compensation might be associated with some cerebral (ie, cognitive) dysfunction. Inadequate compensation to reduced hemoglobin in particular brain regions (for instance, the vulnerable watershed regions of the brain) could result in cognitive dysfunction. Executive dysfunction, identified as one area of cognitive performance particularly involved in individuals with anemia (25), is also a domain typically involved in vascular cognitive impairment. We failed to find similar associations with cognition, which may be due to exclusion of individuals with dementia resulting in a relatively cognitively healthy cohort.
Our study has several limitations. While providing an initial exploration of consistent relationships between hemoglobin and global-adjusted rCBF, we did not quantify absolute values of CBF because repeated arterial blood sampling in a normal elderly sample is impractical. However, quantitative values of absolute CBF might help verify if the apparent reduced rCBF in response to low hemoglobin is actually a reduction in flow, as opposed to lack of increase in flow. In addition, detailed information about the presence or absence of intra- or extracranial large vessel stenosis may help explain some of the regional differences we observed, as would information involving brain perfusion. Nevertheless, our study has several strengths. First, the detailed longitudinal cognitive characterization of the participants provides for accurate diagnosis of dementia, limiting potential confounding effects of our findings. Second, although the sample size is relatively modest on an epidemiologic scale, using [15O] water PET data from multiple time points, allowed for replication of our findings over time in the same individuals.
Summary
Our finding that hemoglobin level, even across a relatively normal range of values, has a linear association with rCBF supplements important information in our understanding of a physiologic response to variability in hemoglobin. In this study, we have identified several brain regions, including some in the watershed areas, in which resting CBF is increased in association with lower hemoglobin values, either in the setting of anemia or even across a relatively normal range or hemoglobin values. Further studies are needed to explore the potential consequences of these changes in rCBF as well as the mechanisms by which this occurs in certain regions but not in others.
FUNDING
This work was supported in part by the Intramural Research Program of the National Institutes of Health , National Institute on Aging and by Research and Development Contract N01-AG-32124.
Acknowledgments
Disclosures: The authors have no disclosures.
References
- 1.Hare GM. Anaemia and the brain. Curr Opin Anesthesiol. 2004;17:363–369. doi: 10.1097/00001503-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hino A, Ueda S, Mizukawa N, Imahori Y, Tenjin H. Effect of hemodilution on cerebral hemodynamics and oxygen metabolism. Stroke. 1992;23:423–426. doi: 10.1161/01.str.23.3.423. [DOI] [PubMed] [Google Scholar]
- 3.Vorstrup S, Lass P, Waldemar G, et al. Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab. 1992;12:745–749. doi: 10.1038/jcbfm.1992.105. [DOI] [PubMed] [Google Scholar]
- 4.Metry G, Wikstrom B, Valind S, et al. Effect of normalization of hematocrit on brain circulation and metabolism in hemodialysis patients. J Am Soc Nephrol. 1999;10:854–863. doi: 10.1681/ASN.V104854. [DOI] [PubMed] [Google Scholar]
- 5.Floyd TF, McGarvey M, Ochroch EA, et al. Perioperative changes in cerebral blood flow after cardiac surgery: influence of anemia and aging. Ann Thorac Surg. 2003;76:2037–2042. doi: 10.1016/s0003-4975(03)01074-9. [DOI] [PubMed] [Google Scholar]
- 6.Ibaraki M, Shinohara Y, Nakamura K, Miura S, Kinoshita F, Kinoshita T. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J Cereb Blood Flow Metab. 2010:7, 1296–1305. doi: 10.1038/jcbfm.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaves PH, Carlson MC, Ferrucci L, Guralnik JM, Semba R, Fried LP. Association between mild anemia and executive function impairment in community-dwelling older women: the Women's Health and Aging Study II. J Am Geriatr Soc. 2006;54:1429–1435. doi: 10.1111/j.1532-5415.2006.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudak ML, Koehler RC, Rosenberg AA, Traytsman RJ, Jones MD., Jr Effect of hematocrit on cerebral blood flow. Am J Physiol. 1986;251:H63–H70. doi: 10.1152/ajpheart.1986.251.1.H63. [DOI] [PubMed] [Google Scholar]
- 9.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ershler WB, Sheng S, McKelvey J, et al. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53:1360–1365. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 12.Izaks GJ, Westendorp RGJ, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Nutritional Anemia: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1968. [Google Scholar]
- 14.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without cinical vascular disease. Stroke. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldszal AF, Davatzikos C, Pham DL, Yan MXH, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Roman GC. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004;26:454–458. doi: 10.1179/016164104225017686. [DOI] [PubMed] [Google Scholar]
- 17.Buck A, Schirlo C, Jasinsky V, et al. Changes of cerebral blood flow during short-term exposure to normobaric hypoxia. J Cereb Blood Flow Metab. 1998;18:906–910. doi: 10.1097/00004647-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Pagani M, Salmaso D, Sidiras GG, et al. Impact of acute hypobaric hypoxia on blood flow distribution in brain. Acta Physiol (Oxf) 2011;202:203–209. doi: 10.1111/j.1748-1716.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 19.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89. doi: 10.1186/cc7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhling J, Dehne MG, Sablotzki A, Hempelmann G. Cerebral blood flow velocity during isovolemic hemodilution and subsequent autologous blood retransfusion. Can J Anaesth. 1999;46:550–557. doi: 10.1007/BF03013545. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama Y, Jansen K, Brian JE, Jr, Todd MM. Hemodilution, cerebral O2 delivery, and cerebral blood flow: A study using hyperbaric oxygenation. Am J Physiol. 1999;276:H1190–H1196. doi: 10.1152/ajpheart.1999.276.4.H1190. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Yokoyama I, Iida H, et al. Regional differences in cerebral vascular response to PaCO2 changes in humans measured by positron emission tomography. J Cereb Blood Flow Metab. 2000;20:1264–1270. doi: 10.1097/00004647-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Richardson TQ, Guyton AC. Effects of polycythemia and anemia on cardiac output and other circulatory factors. Am J Physiol. 1959;197:1167–1170. [Google Scholar]
- 24.Ulatowski JA, Bucci E, Nishikawa T, et al. Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol. 1996;270:H466–H475. doi: 10.1152/ajpheart.1996.270.2.H466. [DOI] [PubMed] [Google Scholar]
- 25.Deal JA, Carlson MC, Xue QL, Fried LP, Chaves PH. Anemia and 9-year domain-specific cognitive decline in community-dwelling older women: the women's health and aging study II. J Am Geriatr Soc. 2009;57:1604–1611. doi: 10.1111/j.1532-5415.2009.02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]