Abstract
Age-associated influences on natural killer (NK) cell functions following cytokine stimulation were examined in splenocytes from C57BL/6 mice. NK cells of both young and aged mice exhibited significantly increased: interferon-γ production after interleukin (IL)-12 or IL-15 alone or any combination of IL-12, IL-18, and IL-2; cytotoxicity after IL-2 or IL-15; and granzyme B expression after IL-15. The only significant age-associated differences were observed in interferon-γ production after IL-15 or IL-12 + 18 + 2 and in granzyme B expression following IL-2 or IL-15. Perforin expression did not increase following stimulation; however, NK cells from aged mice expressed significantly higher levels than young mice. These results underscore the complexity of the cytokine-induced functional activities of NK cells and illustrate the differential response of NK cells from young and aged mice to cytokine stimulation.
Keywords: NK cells, Aging, Cytokines
Natural killer (NK) cells play a vital role in innate immunity, acting as first line of defense against foreign cells, tumors, and pathogens. NK cells also limit virus replication in host cells prior to the initiation of an adaptive immune response (1–4). The importance of NK cells in innate immunity has been the focus of several aging studies. Investigations of age-related changes in NK cells in humans and in animal models indicate that the absolute number or percentage of NK cells do not change substantially with advancing age (5–9). Age-related changes in both basal- and cytokine- or virus-induced NK cell activity have also been examined in humans and rodents. Basal in vitro NK cell activity measured in peripheral blood from elderly humans, in the absence of exogenous cytokines, does not decrease, and may increase, with age (10–15). In mice, however, there is a developmental change in NK activity, such that 6- to 8-week-old mice show significantly higher activity compared with aged (21- to 24-month-old) mice (7,16–22). NK cell activity declines between 2 and 6 months of age such that NK activity of 6- and 22-month-old mice do not differ significantly (7,13,16,17,21).
Studies have also examined age-related changes in inducible NK activity after exogenous stimulation with cytokines or after infection with viruses. Most reports support a decrease in inducible NK cell function with age (7,23,24). Both in vitro and in vivo treatment with interferon (IFN)-α/β increased NK activity of 6-month-old, but not of 22- to 26-month-old, mice over that produced at baseline (7). This age-associated decrease in IFN-α/β–induced NK cytotoxicity has also been seen in aged humans (25). Our laboratory has reported recently that there is an age-related decrease in NK cell function during primary infection with influenza virus (24). In addition, altered cytokine production was reported with aging (23,26).
It is now evident that many NK cell stimulatory factors are produced in response to virus infections, including influenza, which can induce NK cell functional activities. Although there is considerable information regarding the importance of cytokines that stimulate NK cells early during the course of viral infections (2,27–32), the link between these regulators and NK cell functions in aging is lacking. The major NK stimulatory factors that have been examined include interleukin (IL)-12, -18, -2, -15, and type I interferon (IFN-α/β). Reports have shown that an individual cytokine achieved induction of NK cell functional activities: induced IFN-γ production by IL-12 (33–35), IL-15 (34), or IFN-α/β (34) or enhanced cytotoxicity by IL-12 (12,36–40), IL-18 (36,38,39), IL-2 (11–14,37,40–43), IL-15 (11,44), or IFN-α/β (13,15,18). We have shown that influenza-induced NK cell cytotoxicity as well as IFN-γ production was reduced in aged compared with young mice during the early innate immune response to influenza (24). These data suggest that impaired NK cell function in aged mice may reflect an inability to respond to NK stimulatory cytokines produced during early infection.
The aim of the present study was to identify age-related defects in NK cell function using an in vitro system in which we could systematically identify the contribution of individual or combinations of known NK stimulatory cytokines on two primary effector functional activities of NK cells—IFN-γ production and cytotoxicity. Our data indicate that NK cells from young and aged mice respond similarly to stimulation with IL-12, IL-18, or IFN-α/β, yet exhibit a differential response to IL-2, or IL-15, or a combination of IL-12 + 18 + 2. These suggest that age-related defects in cytokine-induced NK cell function do not reflect a general decreased responsiveness to all cytokines present in the environment in which they become activated. This inability of NK cells to respond to specific cytokine stimuli may be important in determining the mechanisms for reduced inducible NK cell function with advanced age.
MATERIALS AND METHODS
Mice
Young (4–6 months) and aged (20–22 months) C57BL/6J mice were purchased from the National Institute of Aging colony at Charles River Laboratories (Wilmington, MA). Mice were housed in microisolator cages with food and water provided ad libitum in the AAALAC-accredited barrier facility at Drexel University. Mice were acclimated for at least 1 week prior to use. Mice with tumors were eliminated from the study. All protocols were approved by the Institutional Animal Care and Use Committee of Drexel University.
Lymphocyte Isolation
Mice were euthanized via asphyxiation with CO2 followed by cervical dislocation. Spleens were aseptically removed, homogenized, and lymphocytes were isolated by red blood cell lysis using 0.83% ammonium chloride (Sigma).
In vitro Cytokine Stimulation
Spleen cell preparations were plated in U-bottom 96-well plates (BD Bioscience) with a cytokine or a combination of cytokines at concentrations per 106 cells of 20 ng IL-12 (R&D Systems), 20 ng IL-18 (MBL International Corporation; R&D Systems), 60 ng IL-2 (R&D Systems), 20 ng IL-15 (PeproTech), or 104U IFN-α/β for 4 or 24 hours. Protein transport inhibitor (BD Bioscience) was added to the each culture for the last 3 hours of stimulation.
NK Cell Cytotoxicity Assay
The standard 51Cr-release assay with YAC-1 target cells to assess splenic NK cytotoxicity has been described in detail previously (45). Radioactivity was quantitated with a gamma-counter (Packard Top Count) and reported as counts per minute (CPM). Percent killing activity was calculated using
Spontaneous release was determined in medium alone and maximum release in 5% Triton X-100 (Sigma). Spontaneous release was always less than 5% of maximum release.
Flow Cytometry
Following cytokine stimulation, cells were washed and then resuspended in Phosphate-buffered saline (Mediatech) containing fluorochrome-conjugated monoclonal antibodies (mAbs) for surface markers (anti-CD8, NK1.1; eBioscience) and incubated on ice in the dark for 30 minutes. Surface-stained cells were washed and permeabilized (BD Bioscience), then stained intracellularly with anti-IFN-γ, anti-perforin, or anti-granzyme B mAbs (eBioscience). Cells were washed and fixed with 1% paraformaldehyde (Sigma). Samples were acquired on a FACSCanto flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star).
Statistical Analyses
Statistical analyses were performed using GraphPad InStat 3 software (GraphPad Software Inc.). Comparisons between and within groups were analyzed by analysis of variance with Tukey–Kramer multiple comparisons. Mann–Whitney U tests were used when data were not normally distributed. Student’s t test was performed when comparing two parameters. Pearson’s correlation analyses assuming the Gaussian distributions were performed. Statistical significance was defined as p < .05.
RESULTS
Previously, we reported an increase in NK cytotoxicity following influenza infection of young, but not aged, mice (24). We hypothesized that the age-associated impairment in NK cytotoxicity could be due to decreased cytokine production induced by infection and/or by reduced responses of NK cells to the cytokine environment. In the present study, we used an in vitro system to test the direct effects of cytokine stimulation on NK cell function in splenocytes of young and aged mice.
Aged Mice Demonstrate Reduced IFN-γ Production by NK Cells Following Stimulation With Cytokines
In our initial studies, IL-12, IL-18, and IL-2 were each used in a 4-hour stimulation to activate NK cells and to assess IFN-γ production in response to each individual cytokine. Although the percentage of NK cells from young mice that produced IFN-γ was consistently higher than that of aged mice, these differences were not statistically significant (Figure 1A). These data suggested that a longer exposure to these individual cytokines may be necessary to induce maximum age-related differences in IFN-γ production.
Figure 1.

Percent of NK cells producing IFN-γ following cytokine stimulation. Splenocytes were cultured with cytokine for (A and C) 4 or (B) 24 hours. Following stimulation, intracellular staining was performed to assess the percent of NK (CD8−/NK1.1+ gated) cells producing IFN-γ. Bars represent mean ± SEM. Asterisks on top of the bar indicate significant increase from the baseline within the age group. A letter on top of the bar indicates significant difference between age groups. *: p < .05, **: p < .01,*** or c: p < .001, analyzed by analysis of variance with Tukey–Kramer multiple comparisons. Total n = 12 per age group in each treatment, with results from three separate experiments combined.
Figure 1B shows that a higher percentage of NK cells produced IFN-γ following 24-hour stimulation compared with the levels observed after 4-hour stimulation. However, the only significant increases in the percentage of NK cells producing IFN-γ in both young and aged mice resulted from stimulation with either IL-12 or IL-15. The percentage of NK cells from young mice producing IFN-γ after IL-15 simulation for 24 hours was significantly higher than that of aged mice (Figure 1B). In addition, although statistically not significant, the trend of efficiency in inducing IFN-γ production by NK cells, IL-12 first followed by IL-18, then IL-2, was preserved from 4- to 24-hour stimulation.
We then used a combination of cytokines to induce a maximum NK cell response after short-term stimulation. A significant increase in the percent of NK cells producing IFN-γ was detected following 4-hour stimulation when combinations of IL-12, IL-18, and/or IL-2 were used (Figure 1C), but a difference between age groups was only found when splenocytes were stimulated with a combination of all three cytokines (Figure 1C). Although the 4-hour stimulation with IL-12, IL-18, or IL-2 alone in young resulted in 6.1%, 1.7%, and 1.6% of NK cells producing IFN-γ, respectively, 29%, 21%, and 16% of NK cells produced IFN-γ in response to IL-12 + 18, IL-12 + 2, or IL-18 + 2, respectively (Figure 1A and C). A similar enhancement of response was seen in NK cells of aged mice. Therefore, the combination of stimulatory factors led to synergistic effects, rather than additive effects, in both young and aged mice.
Enhancement of NK Cell Cytotoxic Activity by Cytokines
Cytotoxic activity of NK cells from young and aged mice was assessed following cytokine stimulation as a measure of another important index of NK cell function. NK cytotoxic activity was enhanced in response to 24-hour cytokine stimulation with IL-12, IL-18, IL-2, IL-15, or IFN-α/β. NK cells from young mice consistently exhibited higher cytotoxic activity compared with NK cells from aged mice; however, the only significant increase in cytotoxicity from the baseline was observed in NK cells of both young and aged mice after IL-2 or IL-15 stimulation (Figure 2). No significant differences between age groups were detected while assessing NK cytotoxicity following 24-hour cytokine stimulation (Figure 2).
Figure 2.
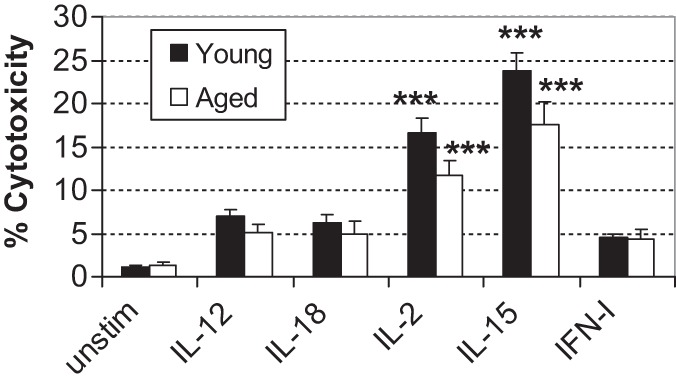
NK cell cytotoxicity following cytokine stimulation. Splenocytes were cultured with cytokine for 24 hours. Following stimulation, NK cytotoxicity was assessed at E:T ratio at 50:1. Bars represent mean ± S E M. Asterisks on top of the bar indicate significant increase from the baseline within the age group. ***p < .001 analyzed by analysis of variance with Tukey–Kramer multiple comparisons. Total n = 12 per age group in each treatment, with results from three separate experiments combined.
Enhanced Granzyme B and Consistently Lower Perforin Expression by Young NK Cells After Cytokine Stimulation
Intracellular cytolytic granules, such as perforin and granzyme B, are necessary for NK cytotoxicity. We, therefore, examined the ability of cytokines to enhance production of these cytolytic proteins. Perforin-expressing NK cells after 24-hour stimulation with any cytokine was not changed in both young and aged mice (Figure 3A). However, the percentage of NK cells from aged mice that produced perforin was consistently higher than that of young mice. In contrast, although there was no difference in the percent of NK cells expressing granzyme B between young and aged mice when unstimulated (Figure 3B), significant increases in the percent of NK cells expressing granzyme B were observed after NK cells of young mice when stimulated with IL-2 or IL-15 and NK cells of aged mice with IL-15 (Figure 3B). In addition, significantly fewer granzyme B producing NK cells was detected in aged mice compared with that of young mice following stimulation with IL-2 or IL-15 (Figure 3B).
Figure 3.
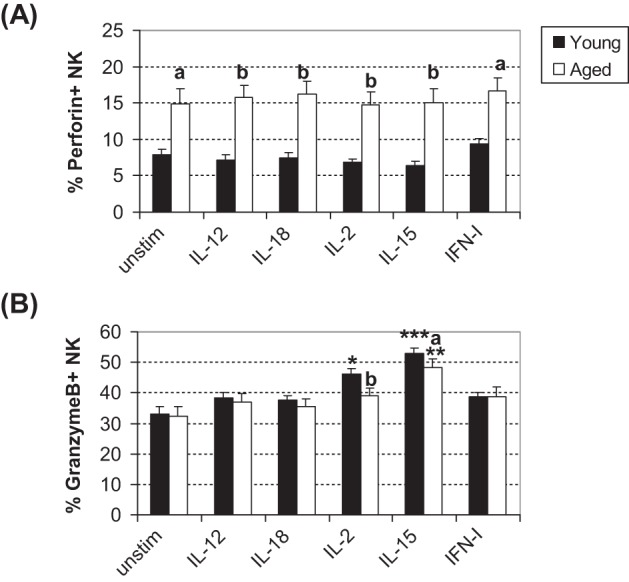
Percent of perforin or granzyme B positive NK cells following cytokine stimulation. Splenocytes were cultured with cytokine for 24 hours. Following stimulation, intracellular staining was performed to assess the percent of (A) perforin-expressing and (B) granzyme B–expressing NK (CD8−/NK1.1+) cells. Bars represent mean ± S E M. Asterisks on top of the bar indicate significant increase from the baseline within the age group. A letter on top of the bar indicates significant difference between age groups. * or a: p < .05, ** or b: p < .01, and ***: p < 0.001 analyzed by analysis of variance with Tukey–Kramer multiple comparisons. Total n = 12 per age group in each treatment, with results from three separate experiments combined.
The Percentage of NK Cells Do Not Change Following Cytokine Stimulation
Enhanced NK cell cytotoxicity following cytokine stimulation was observed (Figure 2). There was no difference in the percent NK cells at baseline within the splenocyte preparations between young and aged mice (Table 1). Following 4- or 24-hour stimulation, the percent of NK cells within the preparation was unchanged (Table 1). This suggests that the changes in cytotoxicity levels following cytokine stimulation were due to increases in the cytotoxic activity of NK cells and not due to increases in numbers of NK cells within the splenocyte preparations.
Table 1.
Percent NK Cells Within Splenocyte Population Following 4- or 24-hour Stimulation With Cytokine(s)
| 4 h | 24 h | |||
| Young | Aged | Young | Aged | |
| Prestimulated | 2.77 ± 0.09 | 2.35 ± 0.13 | 1.99 ± 0.09 | 1.46 ± 0.13 |
| Unstimulated | 2.80 ± 0.09 | 2.40 ± 0.12 | 1.99 ± 0.08 | 1.63 ± 0.13 |
| IL-12 | 2.79 ± 0.10 | 2.36 ± 0.14 | 2.07 ± 0.08 | 1.61 ± 0.12 |
| IL-18 | 2.75 ± 0.08 | 2.26 ± 0.13 | 2.05 ± 0.09 | 1.45 ± 0.13 |
| IL-2 | 2.79 ± 0.08 | 2.35 ± 0.13 | 2.11 ± 0.09 | 1.59 ± 0.12 |
| IL-15 | NT | NT | 2.21 ± 0.09 | 1.65 ± 0.14 |
| IFN-I | NT | NT | 2.14 ± 0.10 | 1.67 ± 0.12 |
| IL-12 + 18 | 2.77 ± 0.08 | 2.35 ± 0.13 | NT | NT |
| IL-12 + 2 | 2.76 ± 0.09 | 2.40 ± 0.14 | NT | NT |
| IL-18 + 2 | 2.72 ± 0.08 | 2.34 ± 0.14 | NT | NT |
| All three | 2.78 ± 0.09 | 2.37 ± 0.14 | NT | NT |
Note: NK cells were identified as CD8−/NK1.1+. Values represent mean ± S E M. NT stands for not tested. Total n = 12 per age group in each treatment, with results of three separate experiments combined. No statistical significance was found. IFN = interferon; IL = interleukin.
Correlation Between NK Cell Cytotoxicity and Granzyme B Expression After Cytokine Stimulation
Significant enhancement of NK cytotoxicity (Figure 2) and of NK cells producing granzyme B (Figure 3) was demonstrated following IL-2 or IL-15 stimulation for 24 hours. Because granzyme B plays a role in cytotoxic function of NK cells by inducing DNA damage to the target cells (46), we hypothesized that changes in granzyme B expression may be correlated with NK cytotoxicity in young and aged mice in our study. Young mice exhibited a significant positive correlation between the percent NK cytotoxicity and the percentage of NK cells producing granzyme B following IL-2 or IL-15 stimulation (Figure 4A). However, no correlation was apparent from aged mice (Figure 4B).
Figure 4.
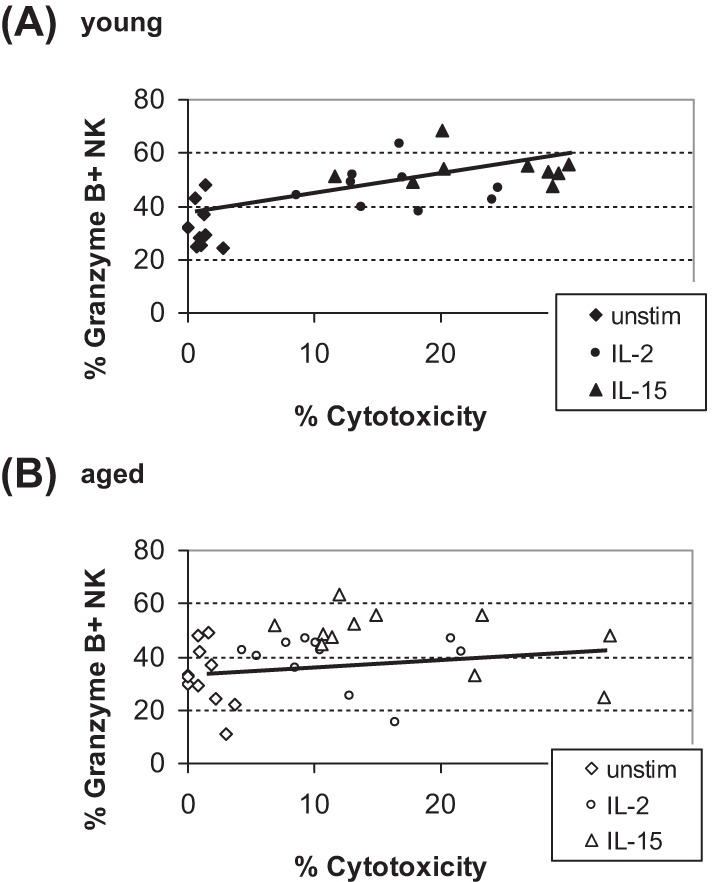
Correlation between NK cell cytotoxicity and NK cells expressing Granzyme B. Data from cytotoxicity and granzyme B expressing NK (CD8−/NK1.1+) cells in (A) young and (B) aged mice were examined. Enhancement in NK cytotoxicity was significantly correlated with percent granzyme B expression in young (r = .69, r2 = .48, p < .001), but not in aged (r = .18, r2 = .03, p > .1), by Pearson’s correlation analyses. Diamond represents unstimulated, circle IL-2, and triangle IL-15. Total n = 9 (young) or 12 (aged) in each treatment, with each point representing an individual mouse.
DISCUSSION
The initial immune response to primary viral infection is partially mediated by noncellular components, such as proinflammatory cytokines and chemokines. These soluble factors initiate inflammatory responses by activating NK cells or by recruiting immune cells to the site of infection (47,48). These rapidly produced agents of innate immunity include IL-12, IL-18, and IFN-α/β, which are capable of upregulating NK cell functions. Activated NK cells are efficient in eliminating infected cells as well as producing increased level of cytokines. Although studies exploring the relationship between changes in NK cytotoxic activity and individual cytokine levels have been conducted, limited data exist regarding this relationship in aging. Here, we report that each cytokine affects the functional activity of NK cells differently and that some cytokines result in age-related differences following stimulation.
In our study, each NK stimulatory cytokine was able to stimulate increased production of IFN-γ by NK cells from young and aged mice at 4 hours poststimulation; however, the only statistically significant increase observed was after IL-12 stimulation (Figure 1A). A significantly increased IFN-γ production by NK cells was observed compared with any single cytokine stimulation or other double combinations, such as IL-12 and IL-2 or IL-18 and IL-2. In fact, significantly higher IFN-γ production by NK cells from young mice compared with that of aged mice was only observed when IL-2 was added to the IL-12 and IL-18 combination. Thus, our findings are in accord with previous reports (35,49–51) indicating that stimulation of NK cells with a combination of IL-12 and IL-18 results in significantly increased IFN-γ production (Figure 1C).
An important observation in our study was with regard to exogenous stimulation with IL-15, which resulted in a significantly higher percentage of NK cells producing IFN-γ in young compared with that of aged mice (Figure 1B). It has been reported that the production of IL-15 is essential for NK cells in differentiation, survival, and maturation (49,52–55) as well as proliferation of NK cells (56,57), IFN-γ production by NK cells (34), and NK cell cytotoxic function (11,47). Thus, our data suggest that the age-related decline in influenza-induced NK cell function previously observed (24) may be due to reduced responsiveness of NK cells to IL-15 stimulation.
Although the exact mechanism of increased NK cytotoxicity in response to individual cytokines has not yet been elucidated, there is considerable information regarding the roles of IL-12, IL-18, IL-2, IL-15, and IFN-α/β as endogenous regulators of NK cell responses. In addition, IFN-α/β is required for the induction of cytotoxic activity (13,15,18), in part by stimulating the upregulation of NK cytotoxic factors, including perforin and granzyme (15). However, enhanced NK cytotoxicity was observed following stimulation with each cytokine for 24 hours but only significantly by IL-2 or IL-15 (Figure 2). The increase observed was due to existing NK cells acquiring the functional capacity in response to stimuli and was not due to an increase in the number of NK cells within the splenocyte population as evidenced by no change in the percent of NK cells in response to stimulation (Table 1). In addition to the enhanced NK cytotoxicity, stimulation of splenocytes with IL-2 or IL-15 resulted in an increase in NK cells expressing granzyme B, with the highest increase of granzyme B expression by NK cells after stimulation with IL-15, in accordance with a previous report (10). Taken together, these data imply that enhancement of NK cytotoxic activity may be associated with stimulation through the common gamma chain (γc) and/or the signal transduction receptor chain (Rβ) which are shared by both IL-2 and IL-15 (3).
However, there was no difference between young and aged mice in expression of IL-2/IL-15Rβ or γc chain on surface of NK cells or intracellular NK cells (data not shown). This lack of difference in receptor expression levels explains enhancement of NK cytotoxicity in both young and aged mice following IL-2 or IL-15 without an age-associated difference. Yet, this does not clearly elucidate the demonstrated age-associated difference in granzyme B expression following IL-2 or IL-15 stimulation nor another observation that stimulation with IL-15 resulted in increased IFN-γ production both in young and aged mice and exhibited an age-associated difference (Figure 1). These data indicate that the IL-15 has a greater effect on NK cells’ primary functional activities compared with IL-2, despite the similar expression of the shared receptors, and underscores the further investigation to determine the mechanism in which IL-15 and other related cytokines, by structurally or functionally, affect on NK cell activation. In addition, our results indicate that there is no difference in activating receptor expressions—NKp46 or NKG2D that is involved in cytotoxic function of NK cells (58–60) and expressed by both mouse and human NK cells—on NK cells of young and aged mice (data not shown). In human studies, increased proportion of CD56dim subset (cytotoxic) of NK cells while decrease in CD56bright subset (responsible for cytokine production) with advancing age has been reported (9,61,62). If similar would apply to the mice, this offers an explanation for the age-related decrease observed in IFN-γ production (Figure 1) but not in cytotoxicity (Figure 2) even with a functional impairment in per cell basis (63).
Enhancement in NK cytotoxicity by IL-2 or IL-15 was accompanied by an increase in granzyme B expressing NK cells, illustrated by a significant positive correlation between these two parameters (Figure 4). However, this correlation was only observed in young mice and not in aged mice. There was no significant age-associated difference in levels of enhanced NK cytotoxicity after stimulation with IL-2 or IL-15, but there was a lack of significant increase in NK cells expressing granzyme B in aged mice. The combination of these results could have contributed to lack of correlation in aged mice (Figure 4B). Granzyme B has been suggested to be a main contributor of DNA fragmentation upon initiating granule-dependent apoptosis of target cells by cytotoxic lymphocytes (46,64). Another intracellular cytolytic granule protein linked to NK cytotoxicity is perforin; mice deficient in this gene exhibit no NK cell killing (46,64,65). Consistently, higher perforin expression in NK cells from aged mice compared with young mice was exhibited in both the percent of positive cells (Figure 3A) and perforin expression by MFI compared with that of young mice (data not shown). These results suggest that the NK cells from aged mice are armed and ready to initiate the cytotoxicity function. However, in spite of the observation that more NK cells from aged mice expressing perforin (Figure 3A), aged mice did not exhibit a higher level of NK cytotoxic activity at baseline or following stimulation (Figure 2). No change in expression of perforin after cytokine stimulation was observed in NK cells from either young or aged mice (Figure 3A). The importance and involvement of granzyme B in cytolysis by NK cells, in early DNA fragmentation, are reported by Shresta and colleagues (64). This report considers granzymes to be more important in NK cytolytic function than perforin and offers an explanation that more NK cells of aged mice expressing perforin compared with young do not necessarily result in better cytolytic function without increase in granzyme expression.
In the present study, whole splenocytes population and not an enriched NK cell population was used in order to simulate a closer physiological system to that of in vivo. However, cytokines can strongly manipulate lymphocytes, and splenocytes include responders to or producers of NK stimulatory factor(s) other than NK cells, such as B cells, T cells, dendritic cells, or macrophages (3). We have excluded the CD8+ cells from analyses, by gating CD8−/NK1.1+ cells, however, the examined cell population included CD4+/NK1.1+ population that can produce large amount of IFN-γ following stimulation with IL-12 (66). This small but powerful IFN-γ producer may have contributed to the significant increase in IFN-γ production following IL-12 stimulation (Figure 1). In addition, CD8+ T cells possessing similar functional properties to NK cells respond to and produce some of NK stimulatory factors. For example, CD8+ cells produce Th-1–like cytokines including IL-2 in the presence of IL-12 (67) or respond to IL-15 and result in higher expression of IL-2Rβ (68), which in turn could compete with the available IL-2 and possibly IL-15.
Each cytokine seems to possess a preferential effect toward either induction of IFN-γ production or enhancement of cytotoxicity by NK cells. IL-12 appears to be a more prominent IFN-γ inducer, in agreement with a previously reported result (27), while IL-15 and IL-2 are more effective in enhancing NK cytotoxicity and increasing cytolytic granule levels. IL-18 is most effective in tandem with other cytokines, resulting in synergistic effects on NK cell functional activities. NK cytotoxicity was enhanced the most by IL-15, followed by IL-2, and then IL-12, IFN-α/β, and IL-18 (Figure 2). A similar order of cytokine effect in young mice was reported (10). These findings indicate that each cytokine affects the functional activities of NK cells differently.
FUNDING
This work was supported by the National Institute on Aging (R15AG029637).
Acknowledgments
We thank Dr. David M. Duriancik and Jonathan F. Clinthorne for their helpful suggestions during the preparation of this manuscript.
References
- 1.Neff-LaFord HD, Vorderstrasse BA, Lawrence BP. Fewer CTL, not enhanced NK cells are sufficient for viral clearance from the lungs of immunocompromised mice. Cell Immunol. 2003;226:54–64. doi: 10.1016/j.cellimm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: The Immune System in Health and Disease. 5th ed. New York, NY: Garland Publishing; 2001. [Google Scholar]
- 4.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18(6):1613–1620. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 5.Albright JW, Bream JH, Bere EW, Young HA, Winkler-Pickett R, Ortaldo JR. Aging of innate immunity: functional comparisons of NK/LAK cells obtained from bulk cultures of young and aged mouse spleen cells in high concentrations of interleukin-2. Exp Gerontol. 2004;39:73–82. doi: 10.1016/j.exger.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Tarazona R, Casado JG, Delarosa O, et al. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naïve HIV-1-seropositive individuals. J Clin Immunol. 2002;22(3):176–183. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 7.Plett A, Murasko DM. Genetic differences in the age-associated decrease in inducibility of natural killer cells by interferon-alpha/beta. Mech Ageing Dev. 2000;112(3):197–215. doi: 10.1016/s0047-6374(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;122:197–215. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 9.Sansoni P, Cossarizza A, Brianti V, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82(9):2767–2773. [PubMed] [Google Scholar]
- 10.Fehniger TA, Cai SF, Cao X, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74:233–242. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- 12.Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 13.Provinciali M, Muzzioli M, Fabris N. Timing of appearance and disappearance of IFN and IL-2 induced natural immunity during ontogenetic development and aging. Exp Gerontol. 1989;24:227–236. doi: 10.1016/0531-5565(89)90014-4. [DOI] [PubMed] [Google Scholar]
- 14.Hefeneider SH, Conlon PJ, Henney CS, Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983;130(1):222–227. [PubMed] [Google Scholar]
- 15.Wright SC, Bona Vida B. Studies on the mechanism of natural killer cytotoxicity. III. Activation of NK cells by interferon augments the lytic activity of released natural killer cytotoxic factors (NKCF) J Immunol. 1983;130(6):2961–2964. [PubMed] [Google Scholar]
- 16.Gallucci RM, Meadows GG. Ethanol consumption suppress the IL2-induced proliferation of NK cells. Toxicol Appl Pharmacol. 1996;138:90–97. doi: 10.1006/taap.1996.0102. [DOI] [PubMed] [Google Scholar]
- 17.Lucini W, Boraschi D, Aleotti AA, Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981;43:663–668. [PMC free article] [PubMed] [Google Scholar]
- 18.Wright SC, Bona Vida B. Studies on the mechanism of natural killer cell-mediated cytotoxicity. IV. Interferon-induced inhibition of NK target cell susceptibility to lysis is due to a defect in their ability to stimulate release of natural killer cytotoxic factors (NKCF) J Immunol. 1983;160(6):2965–2968. [PubMed] [Google Scholar]
- 19.Haller O, Kiessling R, Orn A, Karre K, Nilsson K, Wigzell H. Natural cytotoxicity to human leukemia mediated by mouse non-T cells. Int J Cancer. 1977;20:93–103. doi: 10.1002/ijc.2910200116. [DOI] [PubMed] [Google Scholar]
- 20.Kiessling R, Hochman PS, Haller O, Shearer GM, Wigzell H, Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977;7:655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- 21.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Maloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 22.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 23.Murasko DM, Gardner EM. Immunology of aging. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, editors. Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill; 2003. pp. 35–52. [Google Scholar]
- 24.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129:223–230. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Misawa E, Sakurai T, Yamada M, Hayasawa H, Motoyoshi K. Effects of macrophage colony-stimulating factor and interleukin-2 administration on NK1.1+ cells in mice. Int J Immunopharmacol. 2000;22:967–977. doi: 10.1016/s0192-0561(00)00061-8. [DOI] [PubMed] [Google Scholar]
- 26.Speziali E, Aranha CHM, Teixeira-Carvalho A, et al. Aging down-modulates liver inflammatory immune responses to schistosome infection in mice. Scand J Immunol. 2010;71:240–248. doi: 10.1111/j.1365-3083.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen KB, Salazar-Mather TP, Dalod MY, et al. Coordinated and distinct roles for IFN-α/β, IL-12, and IL-15 regulation of NK cell response to viral infection. J Immunol. 2002;169(8):4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 28.Biron CA, Seb GC. Interferons and other cytokines. In: Knipe DM, Howley EM, Griffin DE, Martin MA, Lamb RA, Roizman B, Straus SE, editors. Fields Virology. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 321–351. [Google Scholar]
- 29.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza A virus infected and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 30.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-γ responses during viral infection. J Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 31.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc Natl Acad Sci U S A. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godney EK, Gauntt CJ. Involvement of natural killer cells in coxsackie virus B3-induced murine myocarditis. J Immunol. 1986;137:1695–1702. [PubMed] [Google Scholar]
- 33.Chiax J, Tessmer MS, Hoebe K, et al. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-γ. J Immunol. 1999;162:5894–5901. [PubMed] [Google Scholar]
- 36.Prajeeth CK, Haeberlein S, Sebald H, Schleicher U, Bogdan C. Leishmania-infected macrophages are targets of NK cell-derived cytokines but not NK cell cytotoxicity. Infect Immun. 2011;79(7):2699–2708. doi: 10.1128/IAI.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CY, Wang X, Gadiana M, O’Shea JJ, Presky DH, Magram J. IL-12 receptor β2 (IL-12Rβ2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6211–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 38.Hyodo Y, Matsui K, Hayashi N, et al. IL-18 up-regulate perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 39.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 40.Wu CY, Ferrante J, Gately MK, Magram J. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)-deficient mice—IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- 41.Tomala J, Chmelova H, Strohalm J, et al. Antitumor activity of IL-2/anti-IL-2 mAb immunocomplexes synergizes with that of HPMA copolymer-bound doxorubicin conjugate due to its low immunosuppressive activity. Int J Cancer. 2010;129(8):2002–2012. doi: 10.1002/ijc.25859. [DOI] [PubMed] [Google Scholar]
- 42.Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 43.Weigent DA, Stanton GJ, Johnson HM. Interleukin 2 enhances natural killer cell activity through induction of gamma interferon. Infect Immun. 1983;41:992–997. doi: 10.1128/iai.41.3.992-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton K, Muthusamy N, Fischer C, et al. The Ets-1 transcription factor is required for the development of natural killer cells in mice. Immunity. 1998;9:555–563. doi: 10.1016/s1074-7613(00)80638-x. [DOI] [PubMed] [Google Scholar]
- 45.Plett PA, Gardner EM, Murasko DM. Age-related changes in interferon-α/β receptor expression, binding, and induction of apoptosis in natural killer cells from C57BL/6 mice. Mech Ageing Dev. 2000;118:129–144. doi: 10.1016/s0047-6374(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 46.Simon MM, Hausmann M, Tran T, et al. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186(10):1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conn CA, McDlellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ. Cytokines and the acute phase response to influenza virus in mice. Am J Physiol. 1995;268:78–84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- 48.Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149(3):932–939. [PubMed] [Google Scholar]
- 49.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haddad EA, Senger LK, Takei F. An accessory role for B cells in the IL-12-induced activation of resting mouse NK cells. J Immunol. 2009;183:3608–3615. doi: 10.4049/jimmunol.0901391. [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 52.Gill N, Paltser G, Ashkar AA. Interleukin-15 expression affects homeostasis and function of B cells through NK cell-derived interferon-γ. Cell Immunol. 2009;258:59–64. doi: 10.1016/j.cellimm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fehniger TA, Suzuki K, Ponnappan A, et al. Fetal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 56.French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35:229–234. doi: 10.1016/j.cyto.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Chakir H, Lemay AM, Webb JR. Cytokine expression by murine DX5+ cells in response to IL-12, IL-18, or the combination of IL-12 and IL-18. Cell Immunol. 2001;212:71–81. doi: 10.1006/cimm.2001.1844. [DOI] [PubMed] [Google Scholar]
- 58.Zompi S, Hamerman JA, Ogasawara K, et al. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 59.Pande D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 60.Sivori S, Vitale M, Morelli L, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida-Oliveira A, Smith-Carvalho M, Porto LC, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Gayoso I, Sanchez-Correa B, Campos C, et al. Immunosenescence of human natural killer cells. J Innate Immun. 2011;3(4):337–343. doi: 10.1159/000328005. [DOI] [PubMed] [Google Scholar]
- 63.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34(2):253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 64.Shresta S, MacIvor DM, Huesel JW, Russel JH, Ley TJ. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci U S A. 1995;92:5679–5683. doi: 10.1073/pnas.92.12.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Paul WE. Cultured NK1.1+CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by Anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 67.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8 T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2(3):271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]


