Abstract
Thirty patients with diabetic polyneuropathy were recruited from the diabetic clinic in Hospital Universiti Sains Malaysia from 1996 to 1998. They were randomly assigned either sulbutiamine (Arcalion®) (15 patients) or no treatment (control group; 15 patients). Glycaemic control was assessed by blood glucose and HbA1. Severity of neuropathy was assessed by symptom and sign score, and electrophysiological parameters (nerve conduction velocity and compound muscle action potential) at entry to the study and after 6 weeks. There were improvements in the electrophysiological parameters in the treatment group when compared to the controls with significant improvement in the median nerve conduction velocity (p<0.001), median compound muscle action potential (p<0.001), peroneal nerve conduction velocity (p<0.001), and peroneal compound muscle action potential (p<0.001). No significant improvement in symptom and sign scores were noted between the groups but a significant improvement compared to base line was noted for the sulbutiamine treated group. (p< 0.05). The glycaemic control in both groups was not significantly different at base line and was stable throughout the study. Sulbutiamine objectively improved peripheral nerve function in diabetic polyneuropathy although the symptom score did not improve, possibly due to the short duration of the study.
Keywords: diabetic polyneuropathy, sulbutiamine, NCV, CMAP
Introduction
Diabetic neuropathy is an entity, either clinically evident or subclinical, that occurs in diabetes mellitus cases in the absence of other causes of peripheral neuropathy. Diabetic neuropathy remains the least understood and most difficult to treat late diabetic complication.
Attempts to prevent or avert the course of diabetic neuropathy have been made using a variety of substances. No specific treatment has been shown to be promising except for tight glycaemic control (1–2). Aldose reductase inhibitors (3–8), insulin therapy, myoinositol supplementation (9–12), prostaglandin analogues and essential fatty acids (13–16) have been investigated without any definite positive findings.
However, vitamin B mixture has been tried with varying response in diabetic neuropathy (17).
Thiamine deficiency has been documented in a number of patients with diabetes mellitus (19–20). It has been shown to aggravate the severity of diabetic polyneuropathy. Previous trials with thiamine had shown improvement in electrophysiological as well as clinical symptoms and signs in this group of patients (21–22). Thiamine contains pyrimidine and thiazole moieties linked by a methylene bridge. It acts as a coenzyme for several reactions that cleave carbon-carbon bonds and has a specific role in neuronal function.
Sulbutiamine (Arcalion 200 ®) is a thiamine derivative which has two different properties in comparison with vitamin B1 as a result of structural modification of free thiamine, namely opening of the thiazole ring, esterification of the alcohol groups and dimerization, with formation of a disulfide bridge.
Sulbutiamine crosses the blood brain barrier more easily than vitamin B1 because of its lipophilic properties (23). It leads to an increased formation of thiamine triphosphate (TTP) (24) that acts as a regulator of the synaptic transmission of many neurotransmission systems.
Behavioral studies in the rat have shown that sulbutiamine improves learning and memory (25) and vigilance (26). Moreover, it produces detectable effects on the EEG, suggesting that this molecule produces stimulation or enhancement of the noradrenergic transmission (27) in addition to its cholinergic action (Garattini et al, unpublished results).
In this study, we postulated that sulbutiamine, which has been shown to affect synaptic transmission, is able to improve diabetic polyneuropathy and this should manifest as an improvement in the electrophysiological parameters.
Patients and Methods
This was a randomised control study. Thirty patients were recruited from the Diabetic Clinic in Hospital Universiti Sains Malaysia (USM) from 1996 to 1998. After written consent had been obtained, they were randomly assigned to either oral sulbutiamine (Arcalion® manufactured by Les Laboratoires Servier) 400 mg daily or no treatment. The study was approved by the research and ethics committee of USM.
Inclusion criteria were, type 2 diabetes mellitus patients aged between 18 year old and 70 year old treated with diet or oral hypoglycaemic agents, with evidence of clinical symptomatic symmetric distal polyneuropathy. The diagnosis of peripheral neuropathy was based on symptoms and signs of peripheral neuropathy, which included reduced or absent ankle reflexes, reduced vibration, thermal, tactile, pinprick, and/or position sensation (28–29).
Exclusion criteria were other causes of neuropathy such as nutritional deficiencies, collagen vascular disease, malignancies, tabes dorsalis, Parkinson’s disease, toxin exposure (alcohol, occupational toxins known to be associated with peripheral neuropathy), hypothyroidism, pernicious anaemia, dysproteinaemia, amyloidosis, AIDS, chronic idiopathic demyelinating neuropathy, spinal cord and cauda equina disease, and other mechanical conditions that damage peripheral nerve, use of medication likely to interfere with the interpretation of the results ( e.g. antidepressents, anticonvulsants, opiates, mexilitine, capsaicin, neuroleptics, vitamin B6 compounds, gamma linolenic, aldose reductase inhibitors, and antioxidants). Patients with peripheral vascular disease such as non-palpable pulses, intermittent claudication and severe concomitant diseases (e.g. malignancies, hepatic or renal diseases) were also excluded from the study.
Diabetic neuropathy was assessed in term of clinical symptoms, physical examination, and electrophysiological assessment. Systemic feedback, including family history of non-diabetic peripheral nerve disease and the presence of toxic, metabolic, mechanical, and vascular causes of nerve disease was obtained using a questionnaire. Symptoms of constricting sensation, paraesthesia, pain and weakness were reassessed to define the presence of clinically evident peripheral nerve disease. The positive response was confirmed by physical examination.
Patients were evaluated at baseline and at six weeks. At baseline and follow up, HbA1 and random blood sugar were measured and the following assessments were carried out :-
Isometric muscle strength on a Medical Research Council scale from 5 (normal) to 0 (complete paralysis). The following movements were assessed: wrist flexion, finger flexion and extension, finger and thumb spread, ankle dorsiflexion and plantar flexion, toe flexion and extension. For each limb the scores were added and divided by the number of movements assessed to give a single upper or lower limb score.
Tendon reflexes on a 3-point scale (0=absent, 1=present with reinforcement, 2= normal) for triceps, biceps and brachioradialis in the upper limb and quadriceps femoris and gastrocnemius in the lower limb. Again an average single score for upper or lower limb was calculated.
Sensation on a 3 point scale (0=absent, 1=reduced, 2=normal) for the following parameters in the hand and foot: Vibration perception were examined with tuning fork 128Hz, pain sensation by disposable pin, light touch with monofilament, joint position on finger and toe and thermal sensation with cold metal.
Glycohemoglobin was measured using Eagle Diagnostics.
Blood sugar was measured by Glucose Test strips (Boehringer Mannheim).
Motor nerve conduction velocities in the right median and right peroneal nerves.
Compound muscle action potential amplitudes (CMAP) in the thenar muscle and extensor digitorum brevis in the foot.
The following standardised measurement techniques: for CMAP were instituted
Amplitude – the CMAP amplitude is measured from the baseline to the peak of the negative wave. An initial positive deflection usually indicates an improper recording site and the active electrode is repositioned over the motor point.
Distal latency – Distal motor latency is measured to the onset of M wave
Distance – Distance is measured between the distal stimulation site and the midpoint of the active recording electrode (centimetre). Distance is also measured between stimulation sites when additional stimulation is performed.
Conduction velocity – The velocity of the fastest nerve fibres is calculated with CMAP onset latencies between two stimulation sites. The same gain and sweep speed is used for distal and proximal stimulation. At both sites, the configuration of CMAP should be similar.
Nerve conduction velocity and compound motor action potential were assessed using Teca MS 60 (Software Version E.04, Teca Corporation, USA).
Statistical Analysis
The continuous data were expressed by the arithmetic mean ± standard error of mean. The baseline variables were analysed using Mann-Whitney U test with p values of less that 0.05 were considered to be significant. The magnitudes of differences between the treatment and control groups were analysed using the Mann-Whitney U test. The differences between base-line and end of treatment values were analysed using Wilcoxan Signed Ranks test.
Results
Base-line parameters between the two groups (15 in each group) were comparable (no significant difference) except for peroneal compound muscle action potential (p = 0.045). The mean ages of the treatment and control groups were 55.2 ± 7.0 and 54.5 ± 7.4 years respectively (Table 1).
Table 1:
Baseline glycaemic control, clinical scores, and electrophysiological parameters of the patients for both groups.
| TREATMENT GROUP (n=15) | CONTROL GROUP (n=15) | p-value | |
|---|---|---|---|
|
| |||
| AGE (year) | 54.0 ± 7.0 | 54.0 ± 7.4 | n.s |
| DURATION (year) | 10.0 ± 3.7 | 7.0 ± 4.2 | n.s |
| BLOOD GLUCOSE (mmol/l) | 9.2 ± 1.3 | 9.7 ± 1.3 | n.s |
| HbA1 (%) | 8.2 ± 0.8 | 8.4 ± 0.7 | n.s |
| SYMPTOMS SCORE | 3.0 ± 0.7 | 3.0 ± 1.0 | n.s |
| SIGNS SCORE | 14.0 ± 2.0 | 14.0 ± 2.5 | n.s |
| MEDIAN NCV (m/s) | 44.0 ± 4.5 | 45.0 ± 5.2 | n.s |
| MEDIAN CMAP (mV) | 4.3 ± 0.7 | 4.5 ± 1.3 | n.s |
| PERONEAL NCV (m/s) | 36.8 ± 2.9 | 36.0 ± 4.4 | n.s |
| PERONEAL CMAP (mV) | 1.7 ± 0.3 | 1.4 ± 0.4 | 0.05 |
NCV nerve conduction velocity CMAP compound muscle action potential Median ± SD
As can be seen in Table 2, glycaemic control in both groups was stable with no significant change after the 6 weeks study period. Compared to the control group the sulbutiamine treated group showed significant improvement in all electrophysiological measurements. Median and peroneal nerve conduction velocity as well as compound muscle action potential was significantly improved in the sulbutiamine treated group. There was no significant improvement between the two groups for symptom and sign scores at week 0 and week 6 respectively but when compared to baseline, sign scores for the sulbutiamine treated group improved significantly (p = 0.014). There was however no difference in the symptom scores when compared between the two groups.
Table 2:
Glycaemic control, clinical scores, and electrophysiological parameters before and after 6 weeks treatment.
| Test | Group | Week 0 | Week 6 | pa | pb | pc |
|---|---|---|---|---|---|---|
|
| ||||||
| Median ± SEM | Median ± SEM | |||||
|
| ||||||
| Random Blood Glucose (mmol/l) | Control | 9.8 ± 0.4 | 10.3 ± 0.5 | N.S. | N.S. | N.S. |
| Sulbutiamine | 9.7 ± 0.3 | 10.3 ± 0.3 | N.S | |||
| HbA1 (%) | Control | 8.3 ± 0.2 | 8.3 ± 0.2 | N.S. | N.S. | N.S. |
| Sulbutiamine | 8.3 ± 0.2 | 8.2 ± 0.2 | N.S. | |||
| Symptom score | Control | 2.8 ± 0.3 | 2.9 ± 0.2 | N.S. | N.S. | N.S. |
| Sulbutiamine | 2.7 ± 0.2 | 2.7 ± 0.2 | N.S. | |||
| Signs score | Control | 13.5 ± 0.6 | 13.7 ± 0.6 | N.S. | N.S. | N.S. |
| Sulbutiamine | 14.0 ± 0.5 | 14.8 ± 0.6 | 0.01 | |||
| Median NCV (m/s) | Control | 46.0 ± 1.3 | 46.2 ± 1.3 | N.S. | 0.001 | N.S. |
| Sulbutiamine | 45.4 ± 1.2 | 48.3 ± 1.1 | 0.001 | |||
| Median CMAP (mV) | Control | 4.7 ± 0.3 | 4.7 ± 0.3 | N.S | 0.001 | NS |
| Sulbutiamine | 3.9 ± 0.2 | 4.1 ± 0.2 | 0.007 | |||
| Peroneal NCV (m/s) | Control | 36.6 ± 1.2 | 37.2 ± 1.2 | N.S | 0.001 | 0.002 |
| Sulbutiamine | 36.4 ± 0.8 | 39.2 ± 0.6 | 0.001 | |||
| Peroneal CMAP (mV) | Control | 1.6 ± 0.0 | 1.6 ± 0.0 | 0.05 | 0.001 | 0.05 |
| Sulbutiamine | 1.8 ± 0.0 | 1.9 ± 0.0 | 0.001 | |||
pa Mann-Whitney test at Week 0 between treatment groups
pb Mann-Whitney test for mean difference at Week 6 between treatment groups
pc Wilcoxan Signed Ranks Test (Week 6 - Week 0)
NCV nerve conduction velocity
CMAP compound muscle action potential
m/s meter per second
m/V millivolt
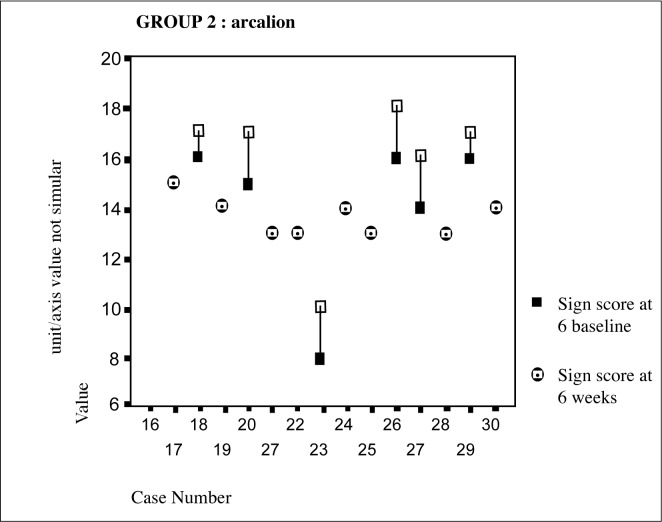
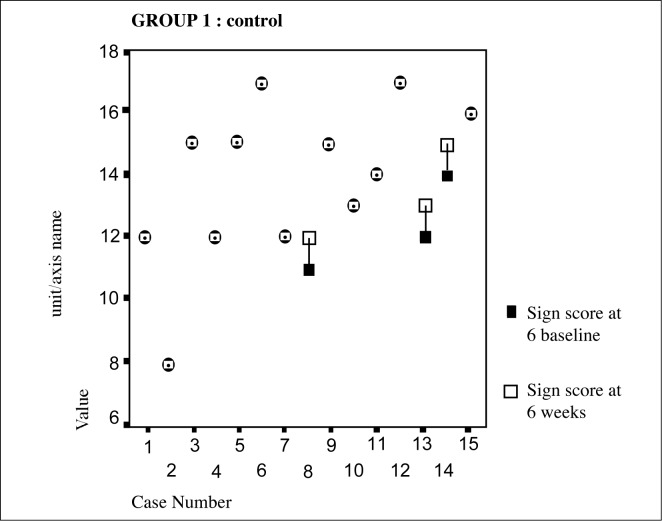
From the Figure 2 above it is evident that in the sulbutiamine group there was an improvement in sign scores in 47% of the patients with 53% showing no change/or deterioration. The control group on the other hand had only shown 20% improvement. (Figure 1)
Figure 2:
Sulbutiamine group individual sign scores over 6 week
Figure 1:
Control group individual sign scores over 6 week
Discussion
Distal symmetrical polyneuropathy is the commonest type of diabetic neuropathy. It has been the focus of most clinical trials because of its high prevalence and long-term impact on morbidity and mortality.
Previous observational and controlled trials have provided data, showing improvement in neurological symptoms and signs, and electrophysiological parameters in patients with diabetic polyneuropathy treated with thiamine combined with other vitamin B21,28,29.
The present study demonstrated that treatment with sulbutiamine at the dosage of 400mg daily over 6 weeks in type 2 diabetes mellitus patients was associated with significant improvement in nerve conduction velocity and compound motor action potential compared to the control group. However, there was no significant improvement in neurological symptoms and signs between groups but when compared to baseline the sulbutiamine treated group showed significant improvement in sign scores. This overall improvement was achieved without any significant difference in glycaemic control between both groups at baseline and 6 weeks.
In this study, statistically significant improvements in electrophysiological parameters were noted, but no improvement in clinical signs was documented between groups. This is consistent with the fact that electrophysiological methods are a more sensitive method in detecting small changes in nerve function. As there was no statistically significant difference in glycaemic control between treatment and control groups, at baseline and after 6 week, it is unlikely that the effects observed in this study were a result of improvement in glycaemic control.
This study however had a number of limitations. Firstly, it was an open controlled trial where examiner bias could not be ruled not and the placebo effect could play a role in the clinical improvement observed although unlikely effecting electrophysiological measurements. Secondly, the patients recruited in this study were not from a homogenous group; they had neuropathy of different severity, and this might have led to variable responses to the treatment. Thirdly, thiamine level was not measured in this study due to financial constraint. We were thus not able to correlate the level of thiamine to the severity of neuropathy. Fourthly, assessment of cutaneous sensation was done using crude clinical methods, which have poor sensitivity and reproducibility. Minor improvements in cutaneous sensation might have been missed. Fifthly, the duration of this study was very short. No follow up was carried out on the patients to assess the long-term effects of sulbutiamine supplement on peripheral nerve function and whether the improvement was sustained, over time.
Conclusion
Sulbutiamine has been shown in this study to improve peripheral nerve function in diabetic patients with peripheral neuropathy in terms of electrophysiological parameters. A trial of sulbutiamine supplement should be given to diabetic patients with symptomatic peripheral neuropathy.
References
- 1.DCCT Research Groups Factors in development of diabetic neuropathy. Diabetes. 1988;37:476–81. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837. [PubMed] [Google Scholar]
- 3.Sima AAF, Prashar A, Zhang W-X, Chakrabarti S, Greene DA. Preventive effect of long terms aldose reductace inhibition (Ponalrestat) on nerve conduction and sural nerve structure in the spontaneously diabetic BB rat. J Clin Invest. 1990;85:1410–20. doi: 10.1172/JCI114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giugliano D, Marfella R, Quatraro A, De Rosa N, Salvatore T, Cozzolino D, Ceriello A, Torella R. Tolrestat for mild diabetic neuropathy. A 52-week, randomized, placebo-controlled trial. Ann Intern Med. 1993;118:7–11. doi: 10.7326/0003-4819-118-1-199301010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Steele JW, Faulds D, Goa KL. Epalrestat. A review of its pharmacology, and therapeutic potential in late-onset complications of diabetes mellitus. Drugs Aging. 1993;3:532–55. doi: 10.2165/00002512-199303060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Van Gerven JM, Tjon-A-Tsien AM. The efficacy of aldose reductase inhibitors in the management of diabetic complications. Comparison with intensive insulin treatment and pancreatic transplantation. Drugs Aging. 1995;6:9–28. doi: 10.2165/00002512-199506010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nicolucci A, Carinci F, Cavaliere D, Scorpiglione N, Belfiglio M, Labbrozzi D, Mari E, Massi Benedetti M, Tognoni G, Liberati A. A meta-analysis of trials on aldose reductse inhibitors in diabetic peripheral neuropathy. Diabetic medicine. 1996;13:1017–26. doi: 10.1002/(SICI)1096-9136(199612)13:12<1017::AID-DIA252>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Laudadio C, Sima AA. Progression rates of diabetic neuropathy in placebo patients in an 18-month clinical trial. Ponalrestat Study Group. J Diabetes Complications. 1998;12:121–7. doi: 10.1016/s1056-8727(97)00074-3. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RE, Modert CW, Yip HK, Johnson EM., Jr Retrograde axonal transport of intravenoully administered 1251-nerve growth factor in rats with streptozotocin-induced diabetes. Diabetes. 1983;32:654–63. doi: 10.2337/diab.32.7.654. [DOI] [PubMed] [Google Scholar]
- 10.Service FJ, Rizza RA, Daube JR, O’Brien PC, Dyck PJ. Near normoglycaemia improved nerve conduction and vibration sensation in diabetic neuropathy. Diabetologia. 1985;28:722–7. doi: 10.1007/BF00265018. [DOI] [PubMed] [Google Scholar]
- 11.Reichard P. Are there any glycaemic thresholds for the serious microvascular diabetic complications? J Diabetes Complications. 1995;9:25–30. doi: 10.1016/1056-8727(94)00008-c. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE. Relation of glycaemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124(1 Pt 2):90–6. doi: 10.7326/0003-4819-124-1_part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda H, Sonobe M, Hatanaka I, Yamashita M, Miyamoto Y, Terada M, Amenomori M, Kikkawa R, Shigeta Y, Motoyama Y, Saito N. A new prostaglandin E1 analogue (TFC-612 prevents a decrease in motor nerve conduction velocity in streptozotocin-diabetic rats. Biochem Biophys Res Commun. 1988;150:225–30. doi: 10.1016/0006-291x(88)90509-8. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda H, Sonobe M, Hatanaka I, Yamasha M, Terada M, Shigeta Y, Huitian Z. Effect of prosglandin E1 analogue TFC 612 on diabetic neuropathy in streptozotocin-induced diabetic rats: comparison with aldose reductase inhibitor ONO 2235. Diabetes. 1989;38:832–8. doi: 10.2337/diab.38.7.832. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki K, Saito N, Sakata Y, Toyota T, Goto Y. A new prostaglandin E1 analogue (TFC-612) improves the reduction in motor nerve conduction velocity in spontaneously diabetic GK rats. Prostaglandins. 1990;40:463–71. doi: 10.1016/0090-6980(90)90109-9. [DOI] [PubMed] [Google Scholar]
- 16.Shindo H, Tawata M, Aida K, Onaya T. Clinical efficacy of a stable prostacyclin analog, iloprost, in diabetic neuropathy. Prostaglandins. 1991;41:85–96. doi: 10.1016/0090-6980(91)90108-r. [DOI] [PubMed] [Google Scholar]
- 17.Sakitama K, Saito K, Aikawa M, Nago M, Ishikawa M. Effect of viamin B mixture on neuropathy in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol (Tokyo) 1989;35:95–9. doi: 10.3177/jnsv.35.95. [DOI] [PubMed] [Google Scholar]
- 19.Saito N, Kimura M, Kuchiba A, Itokkawa Y. The relationship between blood thiamine levels and dietary thiamine content in diabetic outpatients and healthy subjects. J. Nutr. Sci. Vitaminol. 1987;33:431–8. doi: 10.3177/jnsv.33.431. [DOI] [PubMed] [Google Scholar]
- 20.Saito N, Kimura M, Kuchiba A, Itokkawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J. Nutri. Sci. Vitaminol. 1987;33:421–430. doi: 10.3177/jnsv.33.421. [DOI] [PubMed] [Google Scholar]
- 21.Tong HI. Influence of neurotropic vitamins on the nerve conduction velocity in diabetic neuropathy. Ann Acad Med Singapore. 1980;9:65–70. [PubMed] [Google Scholar]
- 22.Stracke H, Lindemann A, Federlin K. Abenfotiaminevitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endoclinol Diabetes. 1996;104:311–6. doi: 10.1055/s-0029-1211460. [DOI] [PubMed] [Google Scholar]
- 23.Du Boitesselin R, Hun M. Etude histochimique de l’impregnation des formations cerebrales apres administration de sulbutiamine. Synthese Medicale. 1985;309:11–12. [Google Scholar]
- 24.Bettendorff L, Weekers L, Wins P, Schoffeniels E. Injection of sulbutiamine induces an increase in thiamine triphospholate in rat tissues. Biochem. Pharmacol. 1990;40, 11:2557–2560. doi: 10.1016/0006-2952(90)90099-7. [DOI] [PubMed] [Google Scholar]
- 25.Michea J, Durkin T, Destrade C, Rolland Y, Jaffard J. chronic administration of sulbutiamine improves long-term memory formation in mice, possible cholinergic mediation. Pharmacol Biochem Behad. 1985;23–2:195–198. doi: 10.1016/0091-3057(85)90555-6. [DOI] [PubMed] [Google Scholar]
- 26.Balzamo E, Vuillon – Cacciutollo G. Facilitation de l’etat de veille d’un traitement semi-chronique par la sulbutiamine (Arcalion) chez le Macaca Mulatta EEG Neuro. 1982;12:373–378. doi: 10.1016/s0370-4475(82)80029-4. [DOI] [PubMed] [Google Scholar]
- 27.Sebban C. Effets electroencephalographiques d’Arcalion 200. J. Med. Prat. 1989;3(suppl. Oct):11–18. [Google Scholar]
- 28.Dyck PJ, Karnes JL, O’Brien P, Okazaki H, Lais A, Eugelstad J. The spatial distribution of fibre loss in diabetic polyneuropathy suggests ischaemia. Ann Neurol. 1986;19:440–9. doi: 10.1002/ana.410190504. [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ, Lais A, Karnes JL, O’Brien P, Rizza R. Fibre loss is primary and multifocal in sural nerve in diabetic polyneuropathy. Ann Neurol. 1986;19:425–39. doi: 10.1002/ana.410190503. [DOI] [PubMed] [Google Scholar]
- 30.Simeonov S, Pavlova M, Mincheva L, Troev D. Therapeutic efficacy of “Milgamma” in patients with painful diabetic neuropathy. Folia Med. 1997;39:5–10. [PubMed] [Google Scholar]
- 31.Abbas ZG, Swai ABM. Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East African Medical Journal. 1997;74:803–8. [PubMed] [Google Scholar]