Abstract
CD8+ T cell responses have been shown to be regulated by dendritic cells (DCs) and CD4+ T cells leading to the tenet that CD8+ T cells play a passive role in their own differentiation. In contrast, by using a DNA vaccination model, to separate the events of vaccination from those of CD8+ T cell priming, we demonstrate that CD8+ T cells, themselves, actively limit their own memory potential through CD8+ T cell-derived IFN-γ-dependent modification of the IL-12/IL-15Rα axis on DCs. Such CD8+ T cell-driven cytokine alterations result in increased T-bet and decreased Bcl-2 expression, and thus decreased memory progenitor formation. These results identify an unrecognized role for CD8+ T cells in the regulation of their own effector differentiation fate and a previously uncharacterized relationship between the balance of inflammation and memory formation.
INTRODUCTION
While the role for DCs and CD4+ T cells in regulating CD8+ T cell differentiation through modulating cytokine production and costimulation has been demonstrated, a direct role for CD8+ T cells in controlling their own effector differentiation remains unexplored. In response to cognate antigen, naïve T cells expand and differentiate into effector T cells. This initial encounter determines the amplitude and duration of the CD8+ T cell effector response, onset of contraction, and subsequent CD8+ T cell memory potential (1–3). CD8+ T cell effector differentiation is regulated in part by local exposure to cytokines (4). Specifically, the inflammatory cytokine IL-12 promotes the expansion, activation, and differentiation of cytotoxic CD8+T cells (CTLs) (5, 6). Moreover, IL-12 induces terminal differentiation of CD8+ T cells by augmenting T-bet expression (7–9). When transpresented by IL-15Rα, the common-γ chain cytokine IL-15 promotes CD8+ T cell transition from effector to memory by upregulating expression of Bcl-2 (10–12).
A major challenge remains in determining how pro-inflammatory (e.g., IL-12) and pro-survival (e.g., IL-15Rα) signals are integrated to balance terminal differentiation versus survival and memory pool entry of CD8+ T cells. Our study demonstrates that CD8+T cells limit their own memory potential by upregulating DC production of IL-12 and downregulating IL-15Rα expression through the production of IFN-γ during priming. These observations identify a previously unrecognized role for CD8+ T cells as direct cellular mediators in their own effector differentiation, as well as, a previously uncharacterized relationship between IFN-γ, IL-12, and IL-15Rα.
MATERIALS AND METHODS
Mice and cells
Six- to eight-week-old male C57BL/6 (B6), IFN-γ−/− (B6.129S7-Ifngtm1Ts/J), thymectomized C57BL/6, pmel-1 (B6.Cg-Thy1a/CyTg (TcraTcrb)8Rest/J) and IL-15Rα−/− (B6;129X1-Il15ratm1Ama/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Thymectomies were performed by Jackson Laboratories. OT-I (C57BL/6-Tg (TcraTcrb)1100Mjb/J) Thy1.1+TCR-transgenic mice were bred in-house. All mice were housed in a specific-pathogen-free facility at The University of Chicago and at Loyola University Chicago. Animal experiments were conducted in accordance with The University of Chicago and Loyola University Chicago Institutional Animal Care and Use Committee (IACUC) guidelines.
In vivo CTL; Adoptive transfer; Tumor challenge
Splenocytes were pulsed with 100ng/mL OVA257-264 (SIINFEKL) or 100ng/mL hgp10025-33 (KVPRNQDWL). Adoptive transfers were performed via retroorbital injection 5 days following the final depletion to allow for depletion antibody clearance. The in vivo CTL assay was performed as previously described (13). Mice were intradermally (right flank) challenged with B16 melanoma (105) and monitored as previously described (14).
DNA vaccination; CD8 depletion; IFN-γ and IL-12 blockade
DNA vaccination was performed using gene gun biolistic transfection on days 0 and 5, or days 0, 5, and 10, as indicated, for optimal responses as previously described (14, 15). CD8 antibody depletion (clone 2.43.1) as well as control IgG (LTF-2) was administered at 250μg or 100μg as indicated. IFN-γ blockade (clone XMG1.2) was administered at 500μg where indicated. IL-12 blockade (clone C17.8) was administered at 250μg where indicated.
Antibodies and flow cytometry
CD3-APC-Cy7 was purchased from BD Biosciences (San Diego, CA);Granzyme B-APC from Invitrogen (Grand Island, NY); CD8 depleting antibody (2.43.1) from The Fitch Monoclonal Antibody Facility (The University of Chicago); and IL-12 (C17.8), IFN-γ (XMG1.2), and control IgG (LTF-2) from BioXCell (West Lebanon, NH).OVA257-264 (SIINFEKL) tetramer was purchased from Becton Dickinson (Franklin Lakes, NJ). All other antibodies were purchased from eBioscience (San Diego, CA). Flow cytometry analysis was performed as previously described (14).
RT-PCR
RNA from lymphocytes was isolated using Trizol reagent and a protocol from Invitrogen. Conversion of RNA to cDNA was performed using the SuperScript III first-strand cDNA synthesis kit from Invitrogen. cDNA was amplified using PCR. Real-time PCR was performed and quantified on the cDNA product using SYBR Green Real Time PCR master mixes (Invitrogen).
DC cytokines; T cell co-culture; FITC paint
DCs were magnetically purified from inguinal lymph nodes using CD11c+ microbeads (Miltenyi, Auburn, CA) for positive isolation. CD8+ T cells were purified from splenocytes using a CD8+ T cell negative isolation kit (Miltenyi). To analyze cytokines, CD11c+ cells were pooled from 10 mice and stimulated with 100ng/mL IFN-γ and 1μg/mL LPS for 20 hours as previously described (16). After 8 hours, cells were treated for 12 hours with Golgi Stop (BD Biosciences). Cytokine production was assessed using flow cytometry. For the DC:T cell co-culture, purified CD8+ T cells were combined with purified DCs at a 10:1 ratio. Antibodies (IL-12 or control IgG) were added at the beginning of the co-culture at a concentration of 1μg/mL. Cells were washed in PBS and analyzed using flow cytometry after 4–12 hours, as indicated. Co-culture experiments were performed in triplicate. Mice were FITC painted at the site of vaccination (abdomen) as previously described (17, 18).
Statistical analyses
Two-tailed Student’s t test (for all figures unless otherwise specified) was used to calculate the p value. ANOVA test was performed for Figure S1. A p value of <0.05 was considered statistically significant.
RESULTS
CD8+ T cells are Necessary for Vaccination-induced IL-12 Upregulation by DCs
Both innate and adaptive immune cells (DCs and CD4+ T cells, respectively) have been shown to regulate CD8+ T cell effector differentiation by providing inflammatory stimuli (IL-12, CD40:CD40L, IL-2) during priming (4). An inherent tenet of previous studies is that CD8+ T cells play a passive role in their own priming. However, emerging evidence suggests that CD8+ T cells can actively influence the activation of DCs (19, 20). This led us to hypothesize that by modifying DC function CD8+ T cells may intrinsically regulate their own effector differentiation. Therefore, to study the effects of CD8+ T cells on their own priming we determined: first, how the absence of CD8+ T cells during an active immune response would alter DC activation and function; second, how CD8+ T cells actively promote these changes in the priming environment; and third, whether these CD8+ T cell driven modulations of the priming environment negatively or positively affect CD8+ T cell effector differentiation. Specifically, CD8+ T cell responses to DNA vaccination (gene gun biolistic transfection) with plasmids encoding the well-known CD8+ T cell antigen chicken ovalbumin (OVA) were utilized to induce immune responses. To determine how CD8+ T cells modulate their priming environment, CD8+ T cells were antibody depleted during the course of vaccination and DC function was analyzed one day after the final depletion (Fig. 1A). By harvesting one day after CD8+ depletion the effects of the absence of CD8+ T cells on DCs was determined prior to CD8+ T cell recovery by recent thymic emigrants. To assess how the absence of CD8+ T cells would alter the priming environment and thus affect CD8+ T cell effector differentiation, CD8+ T cells (specifically, OT-ICD8+ T cells isolated from TCR transgenic mice reactive against OVA257-264) were reintroduced in to these environments using adoptive cell transfer (day 10) 5 days after the final depletion. Adoptive cell transfer at this time point allowed for the clearance of the depletion antibody (data not shown). After adoptive cell transfer, the effects of CD8+ T cells on their own effector differentiation were assessed by analyzing effector function (Fig. 1B) and memory function (Fig. 1C).
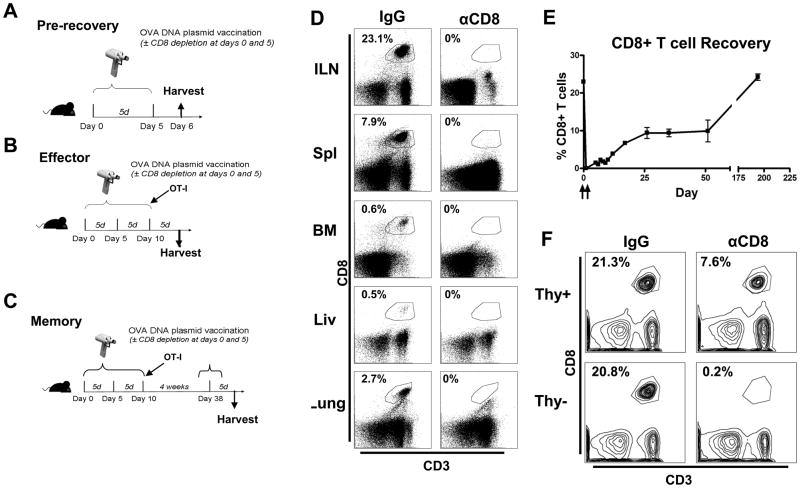
Figure 1.
CD8+ T cell depletion and recovery. (A–C) Depicted rationale and time points for experiments. (D) B6 mice were CD8 antibody depleted (250μg at day 0; 100μg at day 5) and tissues were harvested (day 6) (ILN=Inguinal Lymph Node, Spl=Spleen, BM=Bone Marrow, Liv=Liver). (E) B6 mice were OVA-vaccinated (days 0 and 5) and CD8 antibody depleted (250μg at day 0; 100μg at day 5) and recovery followed for 200 days in thymus-competent and (F) assessed (day 6) in thymus-competent mice (Thy+) and thymectomized (Thy−) mice. All data are representative of results from 2–3 independent experiments with n=3–5 per group.
CD8+ T cell depletion efficacy and specificity as well as post depletion recovery were verified in multiple tissues using flow cytometry (Fig. 1D). CD8+ T cells slowly recovered over 200 days in thymus-competent mice (Fig. 1E). No recovery was observed upon depletion of thymectomized mice (Fig. 1F). CD8+ T cell depletion specificity was demonstrated by the finding that CD8+ DCs were not depleted (data not shown). These results demonstrate that CD8+ T cells are specifically and globally depleted during CD8 antibody-mediated depletion.
To explore the role of CD8+ T cells in regulating DC function, CD8+ T cells were depleted in mice during vaccination and DC activation was analyzed by determining the expression of MHC-II and costimulatory molecules (CD80 and CD86) and production ofIL-12. No differences were observed in the expression of MHC-II, CD80, and CD86 on DCs (data not shown). DCs from non-CD8-depleted OVA-vaccinated mice produced significantly more IL-12 (~1.5-fold) than their CD8-depleted OVA-vaccinated counterparts (Fig. 2A). Interestingly, although vaccination induced IL-12 production in non-depleted hosts, CD8 depletion abrogated vaccination-induced DC production of IL-12 (Fig. 2A).Additionally, IL-12 mRNA was decreased in the draining inguinal lymph node in the absence of CD8+ T cells during vaccination (Fig. S1A,B). Further, no differences were observed in the number of DCs observed in the presence or absence of CD8+ T cells during vaccination (Fig. S1C). Importantly, vaccination-induced IL-12 production was not unique to DNA vaccination since in the context of another vaccination platform, using Complete Freund’s Adjuvant (CFA) and OVA peptide footpad injection, CD8 depletion during vaccination likewise reduced IL-12 production by DCs (Fig. S1D).These results show that CD8+ T cells augment IL-12 production from DCs during vaccination.
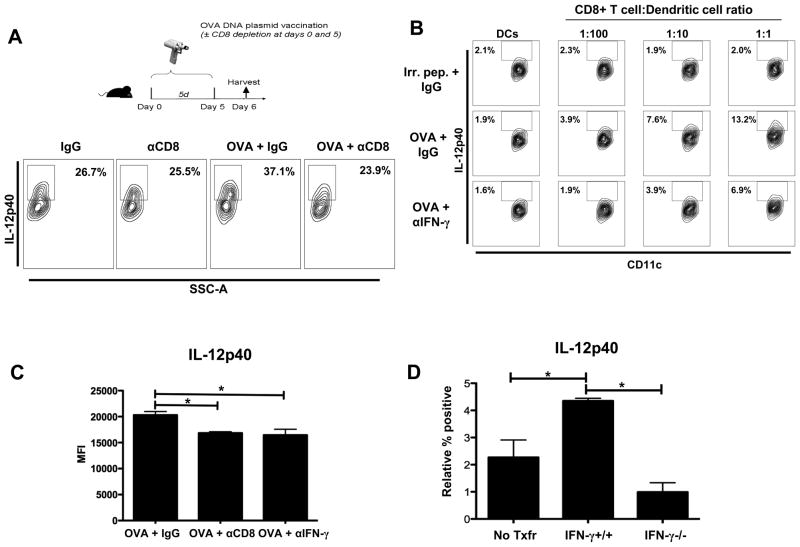
Figure 2.
CD8+ T cells induce IL-12 production from DCs. (A) B6 mice were OVA vaccinated and CD8depleted as depicted. CD11c+ DCs were magnetically purified from inguinal DLNs and pooled (n=10).DCs were stimulated with LPS (1μg/mL) and IFN-γ (100 ng/mL) for 20 hours, and analyzed in triplicate. For analysis, DCs were defined as Thy1.2−/IgM−/CD3−/CD11c+/MHCII+/CD11b+. (B) Purified CD11c+ DCs (105) were loaded with OVA257-264 or hgp10025-33 (Irr. pep.) peptide and co-cultured with purified OT-I CD8+ T cells (103–105, as indicated) or DCs in the absence of OT-I CD8+ T cells (DCs). Representative flow plots showing intracellular IL-12p40 production by DCs following 24 hours of co-culture. The IL-12 gate was set by using MHCII− cells for comparison. For the blockade experiment, 1μg/ml of IFN-γ blocking antibody (XMG1.2) or control IgG antibody (LTF-2) was added to the culture. (C) CD11c+ DCs were magnetically purified from inguinal DLNs from B6 mice treated with either IgG, CD8 depletion, or anti-IFN-γ (IFN-γ blockade; 500μg at day 0 and day 5) and analyzed for IL-12p40 production as in (A). Cumulative bar graph of IL-12p40 production by DCs. (D)Thymectomized C57BL/6 mice were CD8+ T cell depleted (500μg) (day -14), rested for 14 days, and reconstituted with PBS(No Txfr; no CD8+ T cells), IFN-γ+/+ (C57BL/6), or IFN-γ−/−CD8+ T cells (4*106). One day following reconstitution mice were OVA vaccinated (days 1 and 6). IL-12p40 production was analyzed from DCs isolated from the draining inguinal lymph nodes (day 7). IL-12p40 production induced by vaccination was compared relative to non-vaccinated reconstituted mice receiving the same source of CD8+ T cells to account for intrinsic differences of DCs to produce IL-12 and thus, demonstrate IL-12 production induced by vaccination. Average percentages for IL-12p40 positive for non-vaccinated groups (No Txfr= 3.73%, IFN-γ+/+ = 2.52%,and IFN-γ−/− =6.53%) and for vaccinated groups (No Txfr= 6.00%, IFN-γ+/+ = 6.87%,and IFN-γ−/− = 7.52%).All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. *p < 0.05.
We have demonstrated that depletion of CD8+ T cells during vaccination results in the reduced production of IL-12; however, the mechanism by which CD8+ T cells regulate DC function remains unanswered. Therefore, we aimed to determine if CD8+ T cells regulate DC IL-12 production in an antigen-specific manner and if so, then by what mechanism. Increasing the number of OT-I CD8+ T cells co-cultured with OVA peptide-loaded DCs increased IL-12 production, in an antigen-specific manner (Fig. 2B).Studies have demonstrated that IFN-γ is secreted directionally and specifically, when the T cell receptor (TCR) is engaged (21, 22). Given these studies and our results demonstrating the dependence of IL-12 production on recognition of cognate antigen and on CD8+ T cell and DC interaction (Fig. 2B),we hypothesized that depleting CD8+ T cells prevented CD8+ T cells and DCs from interacting and thus decreased CD8+ T cell-derived IFN-γ production. To determine if CD8+ T cells regulate IL-12 production by DCs upon recognition of their cognate antigen is mediated by IFN-γ, we blocked IFN-γ during co-culture of purified DCs and OT-I CD8+ T cells. Such antibody blockade significantly reduced IL-12 production by DCs (Fig. 2B). Interestingly antibody blockade of GM-CSF, a known CD8+ T cell-derived regulator of DC IL-12 production (23), resulted in no significant changes in IL-12 production (data not shown). Based on these in vitro results indicating that CD8+ T cell number and IFN-γ production are key regulators of IL-12 production by DCs, we compared in vivo effects of IFN-γ blockade to CD8 depletion during vaccination on IL-12 production by DCs. Similar to the effect of CD8 depletion in vivo (Fig. 2A) and IFN-γ blockade in vitro (Fig. 2B), in vivo IFN-γ antibody blockade resulted in a similar reduction of IL-12 production by DCs (Fig. 2C). These data show that CD8+ T cell-derived IFN-γ is critical for the upregulation of IL-12 by DCs during vaccination.
However, CD4+ T cells and NK cells have been shown to produce IFN-γ during immune responses. Therefore, to demonstrate that IFN-γ specifically derived from CD8+ T cells is responsible for the upregulation of IL-12 by DCs during vaccination, we engineered a CD8+ T cell IFN-γ−/− mouse by CD8+ T cell depleting thymectomized mice and reconstituting them (14 days following depletion) with CD8+ T cells (4*106) from either IFN-γ+/+ (C57BL/6) or IFN-γ−/− mice, in a similar model utilized by Feau et al. to demonstrate the importance of autocrine IL-2 signaling for CD8+ T cells (24). Following reconstitution, mice reconstituted with IFN-γ−/− CD8+ T cells induced significantly less IL-12 production by DCs in response to the vaccination (Fig. 2D). Importantly, in a model in which all cells except CD8+ T cells can produce IFN-γ, these data demonstrate that IFN-γ specifically derived from CD8+ T cells is required for optimal production of IL-12 by DCs in response to vaccination.
CD8+ T cells Enhance DC-mediated Upregulation of T-bet in CD8+ T cells During DNA Vaccination
We have demonstrated that CD8+ T cells directly regulate IL-12 production by DCs during vaccination. Based on these observations, we asked whether these CD8+ T cell-driven changes in DC function would feedback on the programming of CD8+ T cells during antigen presentation. To answer this question, we adoptively transferred CFSE-labeled OT-I Thy1.1-marked CD8+ T cells (day 10) into OVA-vaccinated mice ± CD8 depletion at the effector phase and assessed intracellular T-bet expression 5 days following transfer. OT-I CD8+ T cells adoptively transferred into CD8-depleted OVA-vaccinated mice expressed significantly less T-bet compared to their counterparts in non-CD8-depleted OVA-vaccinated mice (Fig. 3A and Fig. S2A). To determine if DCs that interact with CD8+ T cells during vaccination were inherently better at upregulating T-bet expression in CD8+ T cells during vaccination, we purified DCs that had been minimally exposed to CD8+ T cells, by isolating DCs from inguinal DLNs on day 6 (1 day after the second vaccination and final depletion), pulsed them in vitro with OVA257-264 peptide, and co-cultured them with naive OT-I CD8+ T cells. OT-I CD8+ T cells expressed similar amounts of the activation marker CD44 when stimulated with DCs isolated from CD8-depleted OVA-vaccinated mice or from non-CD8-depleted vaccinated mice (Fig. S2B).Further, DCs from CD8-depleted OVA-vaccinated mice induced significantly lower levels of OT-I CD8+ T cell T-bet expression compared to DCs isolated from non-CD8-depleted OVA-vaccinated mice (Fig. 3B and S2C). These results demonstrate that the presence of CD8+ T cells during vaccination enhances the ability of DCs to induce high T-bet expression in CD8+ T cells.
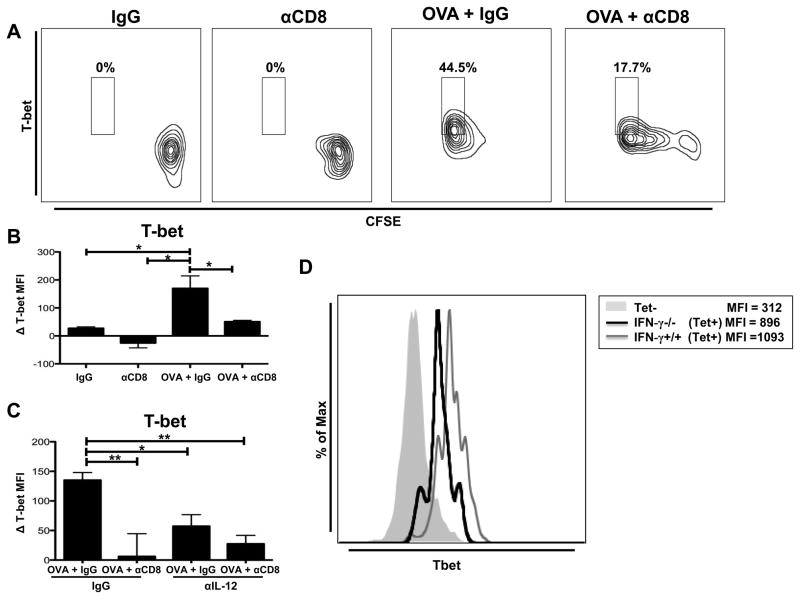
Figure 3.
CD8+ T cell-driven IL-12 production by DCs regulates expression of CD8+ T cell T-bet. (A) B6 mice were OVA vaccinated (days 0, 5, and 10) and treated with either IgG or anti-CD8 antibody (250μg at day 0; 100μg at day 5). CFSE-labeled OT-I CD8+ T cells were adoptively transferred at day 10.The draining inguinal lymph nodes were harvested 5 days after adoptive cell transfer. Representative flow plot showing intracellular T-bet expression on OT-I CD8+ T cells. (B) CD11c+ DCs were magnetically purified from inguinal DLNs of mice treated with either IgG or anti-CD8 antibody (250μg at day 0; 100μg at day 5), pooled (n=10), and run in triplicate, with some mice receiving OVA vaccination. Purified CD11c+ DCs (104) were loaded with OVA257-264 peptide and co-cultured for 6 hours with magnetically-purified naïve OT-I CD8+ T cells (105). Bar graph showing the difference in intracellular T-bet expression in OT-I Thy1.1+ CD8+ T cells compared to T-bet expression of unstimulated OT-I CD8+ T cells (MFI=550) cultured in the absence of DCs. (C)Co-culture was performed as in (B) with either control IgG (1μg/mL) or IL-12 blocking antibody (1μg/mL) at the beginning of the co-culture. T-bet expression was determined as in (B).Bar graph showing the difference in intracellular T-bet MFI in OT-I Thy1.1+ CD8+ T cells compared to T-bet expression of unstimulated OT-I CD8+ T cells (MFI = 720) cultured in the absence of DCs. (D) Thymectomized C57BL/6 mice were CD8+ T cell depleted (500μg ; day −14), rested for 14 days, and reconstituted with either IFN-γ+/+ (C57BL/6) or IFN-γ−/− CD8+ T cells (4*106). One day following reconstitution mice were OVA vaccinated (days 0, 5, and 10). T-bet expression was analyzed on OVA tetramer+ CD8+ T cells isolated from the draining inguinal lymph nodes (day 14).All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. *p < 0.05, **p < 0.01.
Our results show that CD8+ T cells co-cultured with DCs from non-CD8-depleted OVA-vaccinated mice upregulate T-bet (Fig. 3B and S2C). Additionally, we show that DCs from non-CD8-depleted OVA-vaccinated mice produce more IL-12 than DCs from CD8-depleted OVA-vaccinated mice (Fig. 2A). Thus, we determined if IL-12 from DCs purified from non-CD8-depleted OVA-vaccinated mice lead to the increased expression of T-bet in co-cultured OT-I CD8+ T cells. Blocking IL-12 from DCs purified from non-CD8-depleted OVA-vaccinated mice resulted in a significant reduction in the expression of T-bet in OT-I CD8+ T cells during co-culture (Fig. 3C and Fig. S2D). Importantly, the expression of T-bet in OT-I CD8+ T cells was similar when cultures were treated with IL-12 blocking antibody and when OT-I CD8+ T cells were co-cultured with DCs from in vivo CD8-depleted OVA-vaccinated mice (Fig. 3C). These results further demonstrate that CD8+ T cells mediate vaccination-induced IL-12 production from DCs and that this CD8+ T cell-induced IL-12 production leads to increased expression of T-bet in CD8+ T cells.
By utilizing reconstitution of CD8-depleted thymectomized mice with IFN-γ+/+ or IFN-γ−/− CD8+ T cells, we previously showed that IFN-γ derived specifically from CD8+ T cells induces IL-12 production by DCs (Fig. 2D).Therefore, we hypothesized that antigen-specific CD8+ T cells from these mice would express less T-bet. Our results show that OVA tetramer-reactive IFN-γ+/+ CD8+ T cells express higher levels of T-bet compared to IFNγ−/− CD8+ T cells (Fig. 3D). Importantly, these data demonstrate that IFN-γ from CD8+ T cells regulates IL-12 production by DCs and T-bet expression in CD8+ T cells.
CD8 Depletion During Vaccination Prevents IFN-γ/IL-12-mediated Downregulation of DC IL-15Rα Expression
We show in our model that CD8 depletion results in reduced proliferation and expansion of adoptively transferred CD8+ T cells in response to vaccination (Fig. 3A[CFSE dilution]and Fig. S3). Recent studies have shown that IL-15Rα expression on DCs and macrophages is important for CD8+ T cell proliferation, survival during the contraction phase, and on future memory maintenance (25, 26).Therefore, we assessed IL-15Rα expression on DCs from the inguinal DLNs. Surprisingly, we found that vaccination resulted in reduced IL-15Rα expression on DCs (Fig. 4A). Interestingly, CD8 depletion during vaccination rescued expression of IL-15Rαon these cells (Fig. 4A[day 6] and Fig. S4 [day 14]). Similar to the effect of CD8 depletion (Fig. 4A), IFN-γ antibody blockade resulted in increased expression of IL-15Rα on DCs during DNA vaccination (Fig. 4B). Further we analyzed the effects of IFN-γ specifically derived from CD8+ T cells on IL-15Rα expression on DCs as we have done previously with IL-12 (Fig. 2D),using thymectomized mice reconstituted with either IFN-γ+/+ or IFN-γ−/− CD8+ T cells. Our results show that IFN-γ from CD8+ T cells is necessary to downregulate IL-15Rα expression on DCs during vaccination (Fig. 4C). These differences in IL-15Rα are striking especially when comparing the expression of IL-15Rα to the isotype, and show that IFN-γ produced by CD8+ T cells downregulates IL-15Rα to a background level.
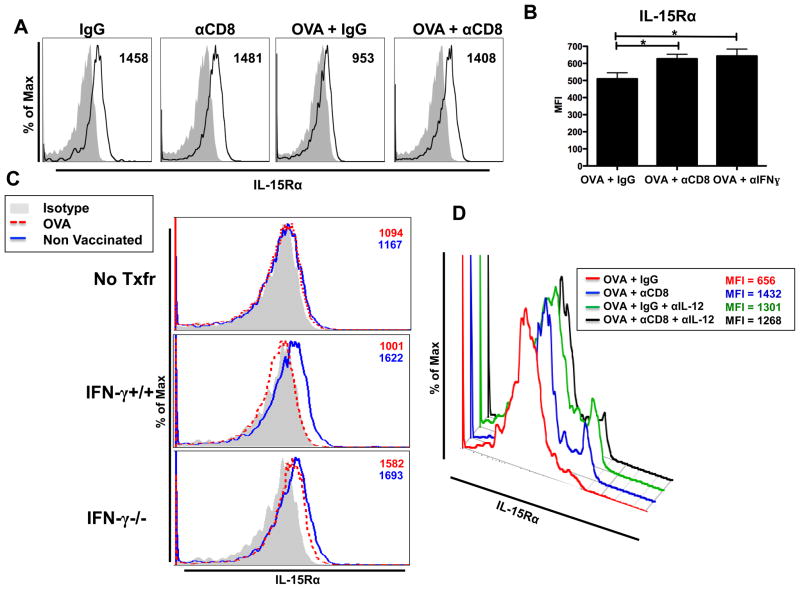
Figure 4.
CD8 depletion during vaccination rescues IL-15Rα expression by DCs. (A) B6 mice treated as shown in Fig 2A. Representative histograms showing IL-15Rα expression (black line) on DCs (Thy1.2-/IgM−/CD3−/CD11c+/MHCII+/CD11b+) and isotype staining (gray shaded area).Numbers listed indicate MFI. (B) CD11c+ DCs were magnetically purified from inguinal DLNs from B6 mice treated with IgG, CD8 depletion, or anti-IFN-γ (IFN-γ blockade; 500μg at day 0 and day 5) and analyzed for IL-15Rα expression as in (A). Cumulative bar graph of IL-15Rα expression by DCs. (C) Thymectomized C57BL/6 mice were CD8+ T cell depleted (500ug), rested for 14 days, and were reconstituted with PBS (No Txfr = No transfer), IFN-γ+/+ (C57BL/6), or IFN-γ−/− CD8+ T cells (4*106). One day following reconstitution mice were OVA vaccinated (days 1, 6, and 11). IL-15Rα expression was analyzed from DCs isolated from the draining inguinal lymph nodes (day 15). Numbers listed indicate MFI. (D) B6 mice were vaccinated and depleted as in (A). IL-12 blocking antibody (250μg) was administered via intraperitoneal injection at days 0, 2, 4, and 5. Representative flow plot showing IL-15Rα expression by DCs. The mean and standard error for the absolute values presented in the representative flow plot: OVA = 851.3 ± 103.0, OVA + αCD8 = 1350 ± 69.2, OVA + αIL-12 = 1129 ± 87.8, and OVA + αCD8 + αIL-12 = 1320 ± 70.2. Statistical significance was determined using the absolute MFI values. All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. *p < 0.05.
Based on the role of IFN-γ in downregulating IL-15Rα (Fig. 4C) and upregualting IL-12 (Fig. 2B–D), we investigated whether IL-12 in vivo regulates expression of IL-15Rα on DCs. Specifically, we blocked IL-12 during vaccination and demonstrate that, similar to the effect of CD8 depletion, IL-12 antibody blockade maintained the elevated expression of IL-15Rα on DCs (Fig. 4D). The combination of CD8 depletion and IL-12 blockade concomitant with vaccination did not have additive effects in the maintenance of IL-15Rα expression during vaccination (Fig. 4D). These data show that DC IL-12 production during vaccination reduces the expression of IL-15Rα in DCs. Additionally, the absence of additive effects on the maintained expression of IL-15Rα during vaccination with combined CD8 depletion and IL-12 blockade suggests that CD8+ T cells and IL-12 production regulate IL-15Rα expression through a shared pathway. Therefore, these data provide evidence that a pro-inflammatory signal, such as IL-12, can regulate a pro-survival signal, such as IL-15Rα.
CD8+ T cell Reduction of DC IL-15RαResults in the Downregulation of Bcl-2 in CD8+ T cells During DNA Vaccination
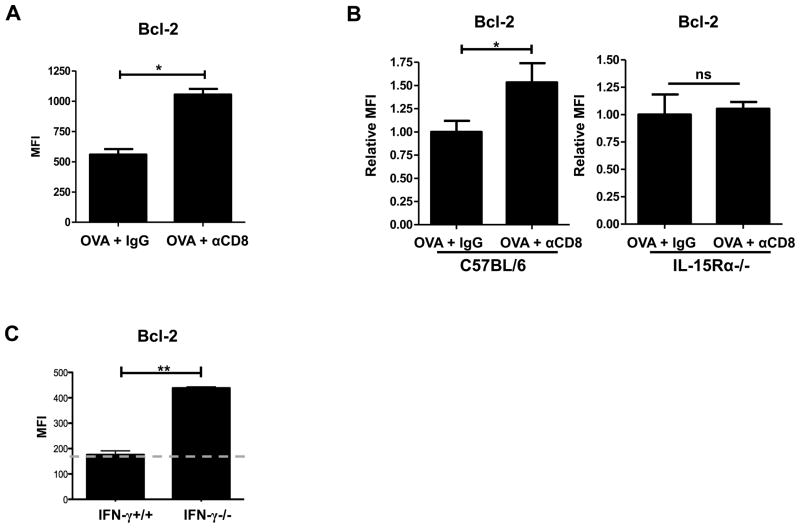
Transpresentation of IL-15 in complex with IL-15Rα leads to the upregulation of Bcl-2 in CD8+T cells, aiding in their survival (27).We hypothesized that the increased DC expression of IL-15Rα in CD8-depleted OVA-vaccinated mice would lead to increased expression of Bcl-2 in effector CD8+T cells. To test this hypothesis, we adoptively transferred OT-I Thy1.1-marked CD8+ T cells into OVA-vaccinated mice (day 10) ± CD8 depletion at the effector phase and assessed intracellular Bcl-2 expression 5 days following transfer. OT-I CD8+ T cells adoptively transferred into CD8-depleted OVA-vaccinated mice expressed significantly more Bcl-2 compared to their counterparts in non-CD8-depleted OVA-vaccinated mice (Fig. 5A). To determine if the differences in IL-15Rα expression resulted in the differences observed in Bcl-2 expression, we adoptively transferred OT-I CD8+T cells into IL-15Rα-deficient mice that received concomitant CD8 depletion. We compared the fold difference in Bcl-2 expression in OT-I CD8+ T cells transferred into C57BL/6 (B6; IL-15Rα+/+) and IL-15Rα-deficient (IL-15Rα−/−) mice. DNA vaccination with concomitant CD8 depletion resulted in a significant upregulation of Bcl-2 in OT-I CD8+ T cells adoptively transferred into C57BL/6 (IL-15Rα+/+) mice versus Bcl-2 expression from their non-CD8-depleted OVA-vaccinated counterparts (Fig. 5B). In contrast, OT-I CD8+ T cells transferred into IL-15Rα-deficient mice showed no differences in Bcl-2 expression with or without CD8 depletion (Fig. 5B). These data demonstrate that the increased expression of Bcl-2 in CD8+ T cells primed in CD8-depleted OVA-vaccinated mice is the result of increased expression of IL-15Rα on DCs.
Figure 5.
CD8+ T cells regulate their own expression of Bcl-2. (A) B6 mice were OVA vaccinated (days 0, 5, and 10), and antibody treated (250μg at day 0; 100μg at day 5) with either IgG or anti-CD8. Thy1.1 marked OT-I CD8+ T cells were adoptively transferred at day 10. Draining inguinal lymph nodes were harvested 5 days after adoptive transfer. Cumulative bar graphs showing intracellular Bcl-2 expression on OT-I CD8+ T cells. (B) B6 mice and IL-15Rα−/− mice were OVA vaccinated, CD8 depleted, and received OT-I transfer as described in Fig. 3A. Cumulative bar graph (relatively compared to the non-CD8-depleted mice: CD8-depleted OVA-vaccinated/non-CD8-depleted OVA-vaccinated) of Bcl-2 expression in OT-I CD8+ T cells isolated from either B6 mice (non-CD8-depleted OVA-vaccinated MFI = 150; CD8-depleted OVA-vaccinated MFI = 247) or IL-15Rα-deficient mice (non-CD8-depleted OVA-vaccinated MFI = 363; CD8-depleted OVA-vaccinated MFI = 415). (C) Thymectomized C57BL/6 mice were CD8+ T cell depleted (500μg ; day −14), rested for 14 days, and were reconstituted with either IFN-γ+/+ (C57BL/6) or IFN-γ−/− CD8+ T cells (4*106). One day following reconstitution mice were OVA vaccinated (days 1, 6, and 11). Bcl-2 expression was analyzed on OVA tetramer+ CD8+ T cells isolated from the draining inguinal lymph nodes (day 15). The gray dotted line indicates MFI of tetramer negative CD8+ T cells. All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. *p < 0.05, **p < 0.01.
Utilizing reconstitution of CD8-depleted thymectomized mice adoptively transferred IFN-γ+/+ or IFN-γ−/− CD8+ T cells, we previously showed that IFN-γ specifically from CD8+ T cells downregulates IL-15Rα expression by DCs (Fig. 4C). Therefore, we hypothesized that antigen specific CD8+ T cells from these mice would express different levels of Bcl-2. Our results show that OVA tetramer-reactive IFN-γ+/+ CD8+ T cells express reduced levels of Bcl-2 compared to IFNγ−/− CD8+ T cells (Fig. 5C). These data demonstrate that IFN-γ from CD8+ T cells regulates IL-15Rα production by DCs and Bcl-2 expression in CD8+ T cells.
CD8+ T cells Limit Formation of Memory CD8+ T cell Recall Responses
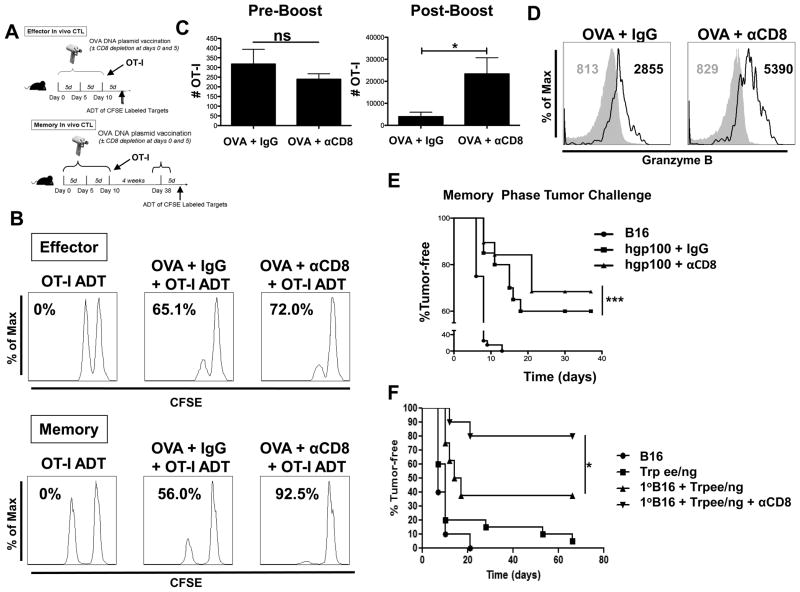
To assess the direct role of CD8+ T cells in regulating their own effector differentiation, mice that did or did not receive concomitant antibody-mediated CD8+ depletion during OVA vaccination, received adoptively transferred OT-I CD8+ T cells (Fig. 6A) after antibody clearance at day 5 (data not shown). CD8+ depletion during vaccination did not significantly alter OT-I CD8+ T cell in vivo effector cytolytic responses, but resulted in enhanced antigen-specific lysis by memory OT-I CD8+ T cells compared to the response by their non-CD8-depleted OVA-vaccinated counterparts (Fig. 6B). In addition to enhanced memory cytolytic function, a 4-fold increase in the number of memory OT-I CD8+ T cells in CD8-depleted OVA-vaccinated mice was observed compared to non-CD8-depleted OVA-vaccinated mice in response to a boost OVA vaccination (Fig. 6C).Notably, no differences were found in the number of OT-I CD8+ T cells prior to the boost vaccination (Fig. 6C).Based on the similar number prior to the boost vaccination, we observed significant differences in memory CD8+ T cell responses as demonstrated by the ~25-fold expansion of the OVA-vaccinated memory CD8+ T cells compared to the ~100 fold expansion seen from memory CD8+ T cells generated by CD8 depletion concomitant with vaccination (Fig. 6C). Next, we assessed the quality (cytolytic potential) of the memory CD8+ T cells from CD8-depleted OVA-vaccinated mice by determining their production of granzyme B. Memory OT-I CD8+ T cells primed in CD8-depleted OVA-vaccinated mice showed a significantly higher content of granzyme B on a per cell basis compared to memory OT-I CD8+ T cells primed in non-CD8-depleted OVA-vaccinated mice (Fig. 6D), thereby supporting the greater observed in vivo lysis (Fig. 6B).
Figure 6.
Enhanced memory responses in CD8-depleted OVA-vaccinated mice. (A and B) B6 mice were OVA-vaccinated and CD8 depleted (250μg at day 0 and 100μg at day 5) and adoptively transferred OT-I Thy1.1+ CD8+ T cells (105) as depicted. An in vivo CTL assay was performed following OVA boost for memory as indicated. (C and D) Cumulative bar graph and representative histograms showing OT-I CD8+ T cell numbers and granzyme B production from inguinal DLNs harvested 1 day prior to boost and 5 days following OVA vaccination boost. All data shown are representative of results from 2–3 independent experiments with n=3–5 per group. (E) B6 mice were hgp100 vaccinated (days 0, 5, and 10) and CD8 depleted (250μg at day 0 and 100μg at day 5) and received adoptively transferred pmel CD8+ T cells (day 10) (105). After 4 weeks mice received a hgp100 boost vaccination. Five days after boost, mice were intradermally challenged with B16 (105). For tumor challenge, mice (n=10) per experiment were inoculated and cumulative tumor incidence (n=30) from 3 independent experiments was plotted to achieve sufficient numbers for statistical analysis. (F) Indicated B6 mice were challenged with B16 (105) at day 0. Indicated B6 mice were vaccinated with Trp1ee/ng at days 5, 10, and 15. Indicated mice received CD8 depletion at days 5 and 10. Primary B16 (1∘) was resected at day 10 in indicated mice. The mice were rested for 28 days, received boost vaccination with Trp1ee/ng, and challenged with a secondary B16 (105).*p < 0.05, ***p < 0.001, ns = p > 0.05.
Further, we verified the effects of CD8 depletion on memory responses using a tumor challenge model. In order to avoid the confounding influence of CD8+ T cell responses against foreign antigen (i.e., OVA), we opted to for an aggressive, unmodified melanoma tumor model, B16 (14, 28). Specifically, we vaccinated against a B16 melanoma-associated antigen mouse gp100 (mgp100) by vaccinating with DNA encoding for human gp100 (hgp100). Vaccinating with hgp100 induces cross-reactive responses against the mgp100 expressed by B16(29). Additionally, as with the in vivo CTL experiments, we transferred antigen-specific TCR transgenic CD8+ T cells at day 10 (pmel-1, reactive against gp100), rested the mice for 28 days, boosted them with hgp100 and tumor challenged 5 days following boost vaccination. CD8+ depletion during vaccination resulted in an enhanced memory tumor response (Fig. 6E). Additionally we tested the effects of CD8 depletion using an additional, more stringent tumor model. As we have previously demonstrated, treatment provided during the perioperative period of tumor resection can enhance survival and protection from recurrent disease (14). Therefore, we utilized this perioperative model in which we challenge B6 mice with B16 at day 0. Mice were DNA vaccinated 3 times 5 days apart (days 5, 10, and 15) with CD8 depletion at days 5 and 10. DNA vaccinations consisted of DNA plasmid encoding a selectively mutated TRP1 termed Trp1ee/ng, which generates heteroclitic peptides that lack N-glycosylation sites for enhanced CD8+ T cell responses against TRP-1(28). At day 10 the primary B16 tumors were surgically resected. Following the final vaccination (day 15), mice were rested for 4 weeks, boosted with Trp1ee/ng and performed a secondary challenge with B16. These data demonstrate that depleting CD8+ T cells concomitant with vaccination during the perioperative period of tumor resection provides enhanced protection from recurrent B16 melanoma with a 40% increase in tumor survival in mice compared to non-depleted vaccinated mice (Fig. 6F).These results demonstrate that CD8+ T cells during vaccination limit both the quantity of the responding memory CD8+ T cells and the quality of their memory cytolytic responses.
DISCUSSION
Here, we demonstrate that CD8+ T cells actively determine their own effector differentiation by regulating DC activation during priming. Specifically, depletion of CD8+ T cells during vaccination increased the expansion of antigen-specific memory CD8+ T cells and enhanced their cytolytic function, as demonstrated by increased expression of granzyme B, more potent in vivo CTL response, and resistance to tumor challenge. Consistent with these superior memory CD8+ T cell responses, we show at the effector phase preferential formation of memory progenitor CD8+ T cells through CD8+ T cell-derived IFN-γ-dependent modification of the IL-12/IL-15Rα axis on DCs. These findings identify a novel and important role for CD8+ T cells in their own effector differentiation fate by establishing a previously unknown relationship between IL-12 (inflammation), and IL-15Rα (memory potential).
It is well accepted that multiple external contributors (CD4+ T cell help, costimulation, cytokine milieu, and growth factors) participate in the development of CD8+ T cell effector and memory responses (4, 30, 31). Studies have demonstrated that IL-15 transpresentation by DCs to effector CD8+ T cells upregulates anti-apoptotic protein Bcl-2 (26, 27). Bcl-2 enables antigen-experienced memory CD8+ T cells to avoid contraction and promotes their transition to memory CD8+ T cells (10, 26). Here, we show that during vaccination, CD8+ T cells downregulate the expression of IL-15Rα on DCs, resulting in reduced expression of Bcl-2 in effector CD8+ T cells. Interestingly, we observed differences in the overall expression of Bcl-2 in CD8+ T cells based on their transfer into IL-15Rα−/− mice, which may be the result of IL-15Rα’s role in CD8+ T cell proliferation (26, 27). This reduced expression of Bcl-2 directly correlates with a substantial decrease in the number of CD8+ memory T cells generated from vaccination. Based on these findings, we conclude that CD8+ T cells, themselves, limit the transition of effector CD8+ T cells to memory CD8+ T cells by reducing the expression of IL-15Rα by DCs.
Studies have shown that inflammation, as provided in part by IL-12, is necessary for CD8+ T cell effector function (5, 8). Accordingly, CD8+ T cells primed in IL-12-deficient mice undergo limited expansion, but have an augmented memory response (8, 9, 32). However, excessive levels and/or prolonged exposure to stimulation decreases CD8+ T cell memory potential (33–35). This has been presented as a “Goldilocks” analogy, where a sufficient amount of inflammation is necessary for CD8+ T cell priming, but too much inflammation leads to exhaustion and terminal differentiation (36). CD8+ T cells must experience the “just right” amount of inflammation to persist into memory (36). There is preliminary evidence showing that CD8+ T cells regulate inflammatory cytokines (20), and now we demonstrate that CD8+ T cell-derived regulation of their own inflammatory environment controls CD8+ T cell memory formation.
Our findings highlight how CD8+ T cells actively promote terminal differentiation. It has been demonstrated that the number of CD8+ T cells adoptively transferred and primed in vivo alters their memory phenotype (37–39). These changes in memory phenotype based on the number of CD8+ T cells primed have been attributed to clonal competition (37–39). By demonstrating that CD8+ T cells negatively impact memory formation and alter effector memory progenitor phenotype by directly regulating DC function, we provide an additional explanation for intraclonal competition raised in previous studies.
Joshi et al. have demonstrated that graded expression of T-bet determines effector CD8+ T cell memory potential, with increased T-bet expression promoting terminal differentiation and decreased memory formation for effector CD8+ T cells (7). Based on this finding of T-bet being a functional determinant of effector CD8+ T cell memory potential, we demonstrate that CD8+ T cells during vaccination promote an environment that is conducive to terminal differentiation by upregulating T-bet in effector CD8+ T cells. Studies show that increased IL-12, as a result of increased inflammation, is responsible for increased T-bet expression in effector CD8+ T cells, which limits memory potential (5, 7, 9, 40).We further demonstrate that increased IL-12 production from DCs isolated from non-CD8-depleted OVA-vaccinated mice leads to the upregulation of T-bet in effector CD8+ T cells, thus promoting terminal differentiation. Our data also demonstrate that IFN-γ, derived specifically from CD8+ T cells, is necessary for IL-12 upregulation by DCs. While other cells such as NK cells and CD4+ T cells produce IFN-γ, these cells are unable to compensate for CD8+ T cell-derived IFN-γ. Previous studies have shown that IL-12 is directionally secreted through the immunological synapse, and that CD8+ T cell IFN-γ production is rapidly turned on or off based on TCR ligation (22, 41). These studies, combined with our data, establish evidence for a cell-directed positive feedback regulation of cytokine production, whereby i) IFN-γ from CD8+ T cells is critical for the induction of IL-12 in DCs during vaccination; and ii) DC-derived IL-12 upregulates T-bet in CD8+ T cells, resulting in the production of IFN-γ. This positive inflammatory feedback loop between DC production of IL-12 and T cell production of IFN-γ promotes CD8+ T cell terminal differentiation, thereby limiting potential memory responses.
In this study, we identify CD8+ T cells as key regulators of effector differentiation, which limit their own memory formation by controlling the IL-12/IL-15Rα axis in an IFN-γ-dependent manner. Our results provide novel evidence that CD8+ T cells sabotage their own memory potential by polluting the environment in the promotion of inflammation during priming which strongly reduces the quality and quantity of the memory CD8+ T cell response. Moreover, our findings have clinical relevance in providing an attractive alternative to current non-myeloablative preconditioning regiments, such as total body irradiation and chemotherapy for adoptive cell transfer in the treatment of malignant disease. While much has been written about the role that CD4+ T cell help, costimulation, and cytokine milieu play in CD8+ T cell differentiation, our study reveals the important and previously unrecognized specific role of CD8+ T cells in mediating their own fate.
Supplementary Material
Acknowledgments
We thank The University of Chicago F. W. Fitch Monoclonal Antibody Facility (supported by NCI #5P30CA014599-35) for production of the CD8 depletion antibody. We thank the flow cytometry facilities at The University of Chicago and Loyola University Chicago for invaluable support. We thank M. J. Turk (Dartmouth University) and M. Nishimura (MUSC) for critically reviewing the manuscript and C.R.L. for constructive discussions.
This work was supported in part by the American Cancer Society (ACS; ACSLIB112496-RSG, to J.A.G.), ACS–Illinois Division (Young Investigator Award #07-20, to J.A.G.), the NIH (R21CA127037-01A1 to J.A.G. and 1P01CA154778-01A1 to J.A.G. and D.J.C.), CRF (Young Investigator Award, to J.A.G.), and the NIH T32 (Immunology Training Grant, The University of Chicago, AI007090 to A.Z., J.A.O., and F.J.K.).
References
- 1.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 4.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 7.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 10.D’Cruz LM, Rubinstein MP, Goldrath AW. Surviving the crash: transitioning from effector to memory CD8+ T cell. Semin Immunol. 2009;21:92–98. doi: 10.1016/j.smim.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 12.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byers AM, Kemball CC, Moser JM, Lukacher AE. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Bellavance EC, Kohlhapp FJ, Zloza A, O’Sullivan JA, McCracken J, Jagoda MC, Lacek AT, Posner MC, Guevara-Patino JA. Development of tumor-infiltrating CD8+ T cell memory precursor effector cells and antimelanoma memory responses are the result of vaccination and TGF-beta blockade during the perioperative period of tumor resection. J Immunol. 186:3309–3316. doi: 10.4049/jimmunol.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan JA, Zloza A, Kohlhapp FJ, Moore TV, Lacek AT, Dulin NO, Guevara-Patino JA. Priming with very low-affinity peptide ligands gives rise to CD8(+) T-cell effectors with enhanced function but with greater susceptibility to transforming growth factor (TGF) beta-mediated suppression. Cancer Immunol Immunother. 60:1543–1551. doi: 10.1007/s00262-011-1043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahara T, Uchi H, Lesokhin AM, Avogadri F, Rizzuto GA, Hirschhorn-Cymerman D, Panageas KS, Merghoub T, Wolchok JD, Houghton AN. Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood. 115:4384–4392. doi: 10.1182/blood-2009-11-251231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 18.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Ruedl C, Kopf M, Bachmann MF. CD8(+) T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 22.Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 23.Min L, Mohammad Isa SA, Shuai W, Piang CB, Nih FW, Kotaka M, Ruedl C. Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol. 184:4625–4629. doi: 10.4049/jimmunol.0903873. [DOI] [PubMed] [Google Scholar]
- 24.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 27.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Guevara-Patino JA, Engelhorn ME, Turk MJ, Liu C, Duan F, Rizzuto G, Cohen AD, Merghoub T, Wolchok JD, Houghton AN. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins WG, Gold JS, Dyall R, Wolchok JD, Hoos A, Bowne WB, Srinivasan R, Houghton AN, Lewis JJ. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery. 2000;128:273–280. doi: 10.1067/msy.2000.107421. [DOI] [PubMed] [Google Scholar]
- 30.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 31.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 36.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 38.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 41.Pulecio J, Petrovic J, Prete F, Chiaruttini G, Lennon-Dumenil AM, Desdouets C, Gasman S, Burrone OR, Benvenuti F. Cdc42-mediated MTOC polarization in dendritic cells controls targeted delivery of cytokines at the immune synapse. J Exp Med. 207:2719–2732. doi: 10.1084/jem.20100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.