Abstract
The liver-expressed microRNA-122 (miR-122) is essential for hepatitis C virus (HCV) RNA accumulation in cultured liver cells, but its potential as a target for antiviral intervention has not been assessed. Here, we show that treatment of chronically infected chimpanzees with a locked nucleic acid (LNA)-modified oligonucleotide (SPC3649) complementary to miR-122 leads to long-lasting suppression of HCV viremia with no evidence for viral resistance or side effects in the treated animals. Furthermore, transcriptome and histological analyses of liver biopsies demonstrated derepression of target mRNAs with miR-122 seed sites, down-regulation of interferon-regulated genes (IRGs) and improvement of HCV-induced liver pathology. The prolonged virological response to SPC3649 treatment without HCV rebound holds promise of a new antiviral therapy with a high barrier to resistance.
Hepatitis C virus (HCV) infection is a leading cause of liver disease worldwide with over 170 million infected individuals who are at greatly increased risk of developing liver failure and hepatocellular carcinoma. The current standard anti-HCV therapy, which combines pegylated interferon-α (IFN-α) with ribavirin provides sustained clearance of HCV in only about 50% of patients and is often associated with serious side effects (1, 2). Therapies that target essential host functions for HCV may provide a high barrier to resistance and, thus, could present an effective approach for the development of new HCV antiviral drugs. MicroRNA-122 (miR-122) is a highly abundant, liver-expressed microRNA, which binds to two closely spaced target sites in the 5’ non-coding region (NCR) of the HCV genome, resulting in upregulation of viral RNA levels (3, 4). Interaction of miR-122 with the HCV genome is essential for accumulation of viral RNA in cultured liver cells and both target sites are required for modulation of HCV RNA abundance (3–5).
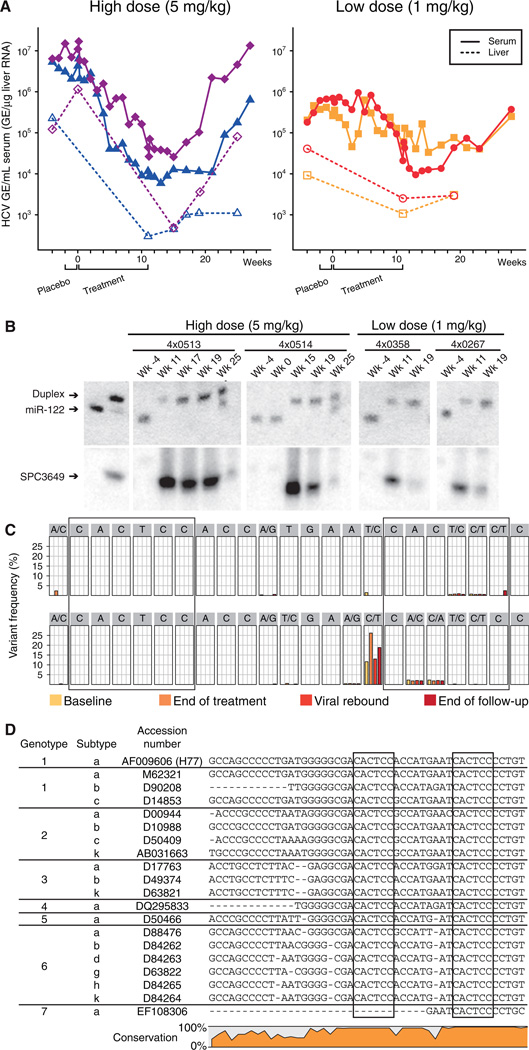
Previously, we reported on potent and specific miR-122 silencing in vivo using a LNA-modified phosphorothioate oligonucleotide (SPC3649) complementary to the 5’-end of miR-122 leading to long-lasting decrease of serum cholesterol in mice and African green monkeys (6). Here, we investigated the potential of miR-122 antagonism by SPC3649 as a new anti-HCV therapy in chronically infected chimpanzees (genotype 1). Baseline measurements were obtained from four chimpanzees for four weeks, the last two of which included an intravenous (i.v.) placebo dose of saline. Two animals each were assigned to the high and low dose groups, 5 and 1 mg kg−1, respectively, and were treated with i.v. injections of SPC3649 on a weekly basis for 12 weeks (Fig. 1A), followed by a treatment-free period of 17 weeks. In the high dose group, a significant decline of HCV RNA in the serum was detected three weeks after the onset of SPC3649 dosing with a maximum decrease of 2.6 orders of magnitude in HCV RNA levels two weeks after end of treatment (Fig. 1A). Analysis of HCV RNA levels in the liver showed a decrease of 2.3 orders of magnitude in the high dose animals. One low dose animal achieved a viral decline of 1.3 orders of magnitude, while the other experienced fluctuations in HCV RNA levels during dosing that made evaluation of the degree of suppression difficult (Fig. 1A).
Fig. 1. Silencing of miR-122 by SPC3649 in chimpanzees with chronic hepatitis C virus infection.
(A) Analysis of HCV RNA levels in HCV-infected chimpanzees during the study. The HCV titers are shown as genomic equivalents (GE) for the high-dose animals (4×0513 blue triangles, 4×0514 magenta diamonds) and low-dose animals (4×0267 orange squares, 4×0358 red dots) in serum (GE/mL, solid lines) and liver (GE/µg liver RNA, dashed lines). The placebo and active treatment periods are indicated below. (B) Northern blot analysis of RNA from chimpanzee liver biopsies using LNA-modified probes detecting free and sequestered miR-122 (upper panel) and SPC3649 (lower panel). The first two lanes are positive controls for free miR-122 and preformed miR-122:SPC3649 heteroduplexes, respectively. (C) Detection of sequence variants in the miR-122 seed sites (boxed) by deep sequencing of the HCV 5’ NCR from the high dose animals at four time points: baseline, end of treatment, viral rebound and end of the follow-up period. (D) The two miR-122 seed sites (boxed) in the HCV 5’ NCR are conserved in all HCV genotypes and subtypes (see SOM for details).
We next assessed the in vivo antagonism of miR-122 in chimpanzee liver biopsies. Mature miR-122 was detected in the baseline samples (week −4) from all animals, while SPC3649 was detected in RNA samples obtained during treatment and up to 8 weeks after last dose in the high dose animals. This coincided with sequestration of miR-122 in a heteroduplex with SPC3649 as detected by a shifted band on the Northern blots (Fig. 1B) (6). Quantification of miR-122 levels by real-time RT-PCR showed a decrease of over 300-fold in free miR-122 levels for the high dose animals (fig. S1). Free SPC3649 was markedly reduced in the liver at week 25 in the high dose animals, accompanied with detection of free miR-122 alongside the miR-122:SPC3649 heteroduplex band (Fig. 1B). These findings demonstrate efficient delivery of SPC3649 to the chimpanzee liver resulting in potent and sustained antagonism of miR-122. The reason for the reduced response in the low dose group was not apparent from the Northern data, since no miR-122 was detected at the end of dosing. It is possible that low levels of miR-122 undetectable by Northern blot could still be sufficient to support HCV RNA accumulation in these animals. A recent report on markedly decreased miR-122 levels in primary nonresponder patients with chronic HCV infection supports the notion that even low levels of miR-122 could facilitate HCV abundance in vivo (7).
Two lines of evidence imply that no viral resistance to therapy occurred during treatment with SPC3649. First, no rebound in viremia was observed during the 12-week dosing phase with HCV RNA levels still being tenfold below baseline 16 weeks after dosing (4×0513, Fig. 1A). Second, deep sequencing of the HCV 5’ NCR from the two high dose animals which yielded between 73,000 and 214,000 reads each at four time points: baseline, end of treatment, viral rebound and end of the follow-up period, respectively, showed no enrichment of adaptive mutations in the miR-122 seed sites (Fig. 1C and fig.S2). This is consistent with the fact that both miR-122 sites are conserved in all HCV genotypes and subtypes (Fig. 1D and fig. S3–9). The lack of viral resistance during SPC3649 therapy is in stark contrast to what has been observed with direct acting antivirals in HCV-infected chimpanzees. Within two days of dosing with a non-nucleoside polymerase inhibitor 67% of the HCV clones already possessed known resistance mutations, with 10% of the clones having two resistance mutations, which triggered a rapid rebound in viremia (8).
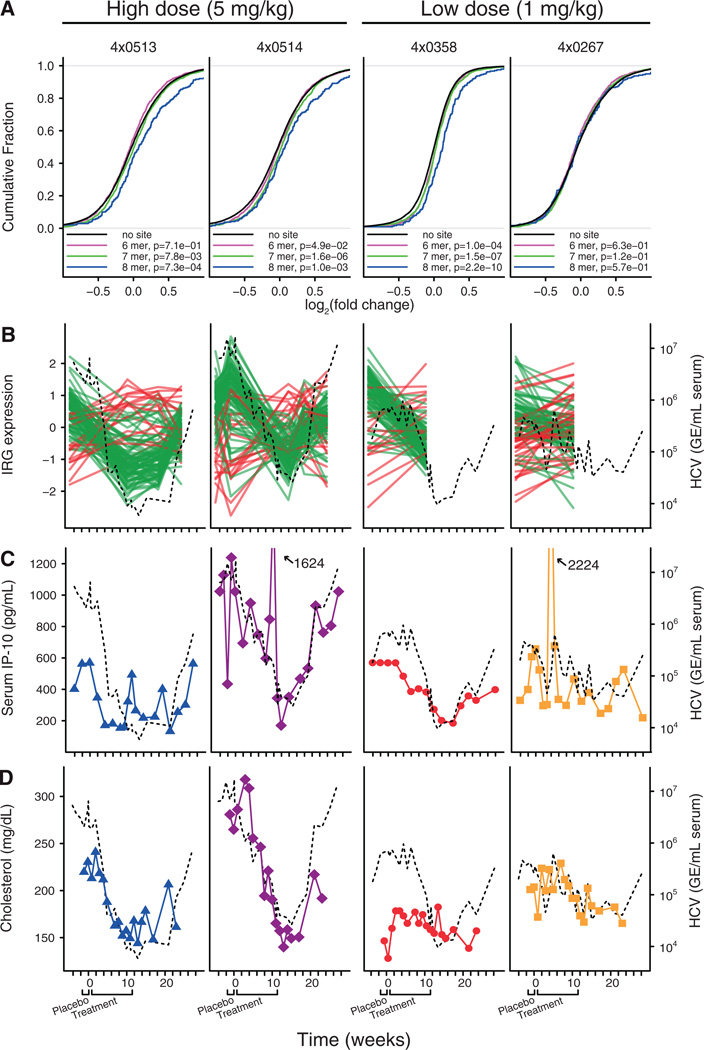
We next investigated the effect of miR-122 antagonism on the chimpanzee liver transcriptome by expression profiling of the liver biopsies performed after SPC3649 treatment relative to baseline samples. Liver mRNAs with miR-122 seed match sites in the 3’ UTRs showed a significant tendency to be derepressed in both high dose animals and the responding low dose animal compared to messages without miR-122 sites (Fig. 2A, P=7.3*10−4, 1.0*10−3 and 2.2*10−10, for 8mer seed sites, respectively, Kolmogorov-Smirnov test, and fig. S10). A total of 259 mRNAs with 8mer miR-122 seed sites were identified by this approach (table S1). By contrast, no significant target mRNA derepression was observed in the weakly responding low dose animal (Fig. 2A). We also examined the expression data for changes related to prolonged decrease in viral RNA during SPC3649 therapy. A supervised analysis of chimpanzee interferon-regulated genes (IRGs) (9, 10) revealed that the reduction in viremia was clearly associated with down-regulation of most IRGs in the high dose animals and the responding low dose animal (Fig. 2B and table S2). This correlated with the measured serum levels of the chemokine IP-10 (CXCL10), a highly induced IRG in HCV infections, which thereby provides a readily accessible biomarker of the hepatic IFN response during SPC3649 therapy (Fig. 2C). Together, these data imply that the endogenous IFN pathway in the liver is rapidly normalized in response to inhibition of HCV RNA accumulation even when therapy does not completely eradicate detectable viral RNA. Non-responders to IFNα-based HCV therapy have increased hepatic levels of IRG transcripts and serum IP-10 protein levels (11–17), reflecting a maximally induced and non-responsive hepatic IFN response. The chimpanzee appears to be an extreme representative of this phenotype in human HCV patients, designated as IFN null-responders (18). Thus, our findings that treatment with SPC3649 results in normalization of IRG levels suggest that this therapy could be used to convert IFN null-responders to responders by reducing the viral load, thereby permitting the endogenous IFN pathway to reset to responsiveness.
Fig. 2. Functional antagonism of miR-122 by SPC3649 in HCV-infected chimpanzees.
(A) Assessment of liver transcriptome changes after SPC3649 treatment in each animal by microarray expression profiling of liver biopsies. The liver mRNA 3’ UTRs were analysed for the presence of different types of canonical miR-122 seed match sites. The cumulative fraction plots show the distribution of log2 fold changes for each seed match type and a Kolmogorov-Smirnov test was used to compare the three miR-122 seed match types to mRNAs with no seed sites in the 3’ UTR. (B) Expression profiles of interferon-regulated genes (green lines correspond to IRGs with decreased expression from baseline to end of treatment, while red lines indicate IRGs showing an increase) and serum HCV RNA levels (black dashed line) in HCV-infected chimpanzees during the study. (C) Serum IP-10 levels (color coding and plot symbols as in Fig. 1A) and the serum HCV titer (dashed black line) in HCV-infected chimpanzees during the study. (D) Serum cholesterol levels (color coding and plot symbols as in Fig. 1A) and serum HCV RNA levels (dashed black line) in HCV-infected chimpanzees during the study.
Antagonism of miR-122 in chimpanzees by SPC3649 led to markedly lowered serum cholesterol in the high dose group (Fig. 2D) similar to observations in mice and African green monkeys (6, 19). One of the high dose animals had a maximum decline of 44% at week 14, whereas the other animal showed a 29% decrease in cholesterol at the same time point. Pronounced decreases were observed in both low density lipoprotein (LDL) (25–54%) and apolipoprotein apo-B, its primary lipoprotein component (23–42%) (fig. S12). In contrast to our previous findings in monkeys where decreases in high-density lipoprotein (HDL) and its major apolipoprotein apo-A1 were more pronounced compared to LDL and apo-B (7), the observed changes in HDL or apo-A1 in chimpanzees were more variable (fig. S13). Thus, it is possible that the cholesterol lowering effect of miR-122 antagonism is different in chimpanzees and may better reflect the expected response in man.
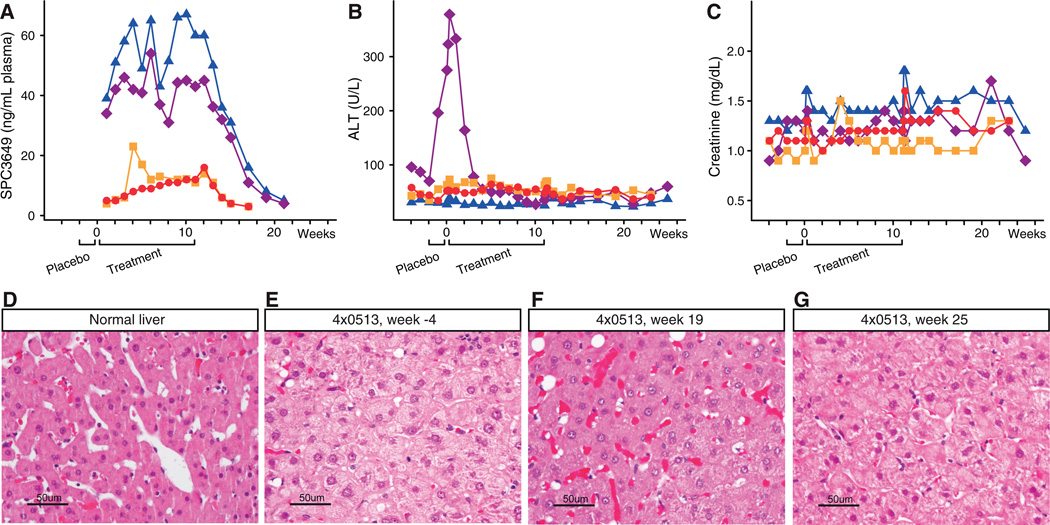
To assess the safety of miR-122 antagonism after prolonged treatment with SPC3649, an extensive set of clinical chemistries were monitored and correlated with plasma levels of the compound. The peak plasma concentrations (Cmax) were dose proportional and similar after first and last dose ranging from 5.7–7.3 µg/ml for the low dose animals and from 17.8–30.6 µg/ml for the high dose animals (Table 1). The terminal plasma half-life was about 20 days in the high dose animals. The plasma trough levels at the high dose ranged from 31 to 67 ng/ml and were maintained at this level for 4 weeks after the last dose (Fig. 3A). Complete blood counts, blood chemistries, coagulation markers, urinalysis, and complement activation were determined throughout the study, as were lymphocyte subsets, circulating cytokine-chemokine profiles and additional safety parameters (table S3). No SPC3649-related abnormalities were observed for any of the measurements (Fig. 3B and 3C, fig. S14 and fig. S15, table S3). A spike in alanine aminotransferase (ALT) was observed in one high dose animal (4×0514), but this commenced prior to the first dose and resolved in the early dosing phase (Fig. 3B). Notably, during therapy ALT was reduced to normal levels, likely due to reduction in the viral load, and was again elevated at the end of the follow-up period when viremia returned to baseline. Histology examinations of the baseline liver biopsies from the high dose animals revealed HCV-specific changes including mild hepatocellular swelling with disruption of hepatocellular sinuses and cords (Fig. 3D–G, fig. S16). Improved liver histology was observed in both high dose animals after treatment at week 19, indicating a response to prolonged suppression of viremia and normalization of the IFN pathway.
Table 1.
Pharmacokinetic properties of SPC3649 in chimpanzee plasma*.
| Animal | Cmax (µg·mL−1) |
AUCinf (h · µg·mL−1) |
Terminal half- life (days) |
Vz (L·kg−1) |
Cl (mL·h−1·kg−1) |
|---|---|---|---|---|---|
| 4×0267 | 6.3 | 25.0 | 21.4 | 29.6 | 40.0 |
| 4×0358 | 6.1 | 22.2 | 21.0 | 32.8 | 45.0 |
| 4×0513 | 30.6 | 169 | 17.2 | 17.6 | 29.6 |
| 4×0514 | 17.7 | 106 | 22.6 | 37.0 | 47.3 |
The data are from week 11.
Fig. 3. Treatment of HCV-infected chimpanzees with SPC3649 was well tolerated.
(A) Plasma trough levels of SPC3649, (B) alanine aminotransferase (ALT) levels and (C) Creatinine levels in HCV-infected chimpanzees during the study. (D–F) Photomicrographs of hematoxylin and eosin stained sections from biopsies of (D) a normal chimpanzee liver, (E) the animal 4×0513 at week −4, (F) week 19, and (G) week 25, respectively.
Our results show that antagonism of miR-122 by the LNA oligonucleotide SPC3649 leads to marked suppression of viremia in chronically HCV-infected chimpanzees implying that miR-122 is essential for accumulation of HCV RNA in vivo. The good PK properties, safety profile and high stability of SPC3649 in vivo combined with the prolonged suppression of viremia beyond treatment suggest that less frequent dosing could be employed after viral suppression is attained. SPC3649 therapy provided a high barrier to resistance, as demonstrated by the lack of rebound in viremia during the 12-week treatment and the lack of adaptive mutations in the two miR-122 seed sites of HCV 5’ NCR. Conservation of both miR-122 seed sites in all HCV genotypes and subtypes suggest that such therapy will be genotype independent. Finally, this study demonstrates the feasibility and safety of prolonged administration of a LNA oligonucleotide drug that antagonizes the function of a specific microRNA in a highly relevant disease model.
Supplementary Material
Acknowledgments
The authors acknowledge the excellent technical assistance performed by Deborah Chavez, Bernadette Guerra and Helen Lee, the veterinarian support of Kathleen Brasky, the immunological analyses by Luis Giavedoni, the complement analyses by Patricia Giclas and the pathology examinations by Edward Dick. This study was supported by a grant from the Danish National Advanced Technology Foundation to S.K.. The primate studies performed at the Southwest National Primate Research Center are supported by the National Institutes of Health base grant P51 RR13986 and the Research Facilities Improvement Program Grant Number C06 RR 12087 from the National Center for Research Resources. The expression microarray data have been deposited in the ArrayExpress repository under accession number E-MEXP-2375.
References and Notes
- 1.Chisari F. Nature. 2005;436:930. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 2.Feld JJ, Hoofnagle JH. Nature. 2005;436:967. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 3.Jopling CL, Yi MK, Lancaster AM, Lemon SM, Sarnow P. Science. 2005;309:1577. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 4.Jopling CL, Schutz S, Sarnow P. Cell Host. Microbe. 2008;4:77. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randall G, et al. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12884. [Google Scholar]
- 6.Elmen J, et al. Nature. 2008;452:896. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 7.Sarasin-Filipowicz M, Krol J, Markiewicz I, Heim MH, Filipowicz W. Nat Med. 2009;15:31. doi: 10.1038/nm.1902. [DOI] [PubMed] [Google Scholar]
- 8.Chen CM, et al. Antimicrob.Agents Chemother. 2007;51:4290–4296. doi: 10.1128/AAC.00723-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanford RE, et al. Hepatology. 2007;46:999. doi: 10.1002/hep.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanford RE, et al. Hepatology. 2006;43:961. doi: 10.1002/hep.21167. [DOI] [PubMed] [Google Scholar]
- 11.Chen LM, et al. Gastroenterology. 2005;128:1437. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Lagging M, et al. Hepatology. 2006;44:1617. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- 13.Diago M, et al. Gut. 2006;55:374. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butera D, et al. Blood. 2005;106:1175. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero AI, et al. J. Infect. Dis. 2006;194:895. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 16.Sarasin-Filipowicz M, et al. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7034. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feld JJ, et al. Hepatology. 2007;46:1548. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigger CB, et al. J. Virol. 2004;78:13779. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmen J, et al. Nucleic Acids Res. 2008;36:1153. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.