Abstract
CD4-unhelped CD8+ T cells are functionally-defective cells primed in the absence of CD4+ T cell help and present a critical problem. Based on the co-stimulatory and non-canonical roles of NKG2D on CD8+ T cells, we investigated its ability to rescue these immunologically-impotent cells. We demonstrate that augmented co-stimulation through NKG2D during priming paradoxically rescues memory, but not effector, responses. NKG2D-mediated rescue is characterized by reversal of elevated T-bet expression and recovery of IL-2 and IFN-γ production and cytolytic responses. Rescue is abrogated in CD8+ T cells lacking NKG2D. Augmented co-stimulation through NKG2D confers a high rate of survival to mice lacking CD4+ T cells in a CD4-dependent influenza model and rescues HIV-specific CD8+ T cell responses from CD4-deficient HIV-positive donors. These findings demonstrate that augmented co-stimulation through NKG2D is effective in rescuing CD4-unhelped CD8+ T cells from their pathophysiological fate and may provide therapeutic benefits.
INTRODUCTION
Memory CD8+ T cells confer efficient and long-lasting immunity against secondary pathogen exposure1, 2. Events during primary exposure (priming) impact the quality of the initial effector and subsequent memory cytotoxic T lymphocyte (CTL) responses. Unless environmental cues (e.g., CD4+ T cell help or inflammation) are present, T cell receptor (TCR) signaling does not result in effective activation of CD8+ T cells3–7. Furthermore, in the absence of CD4 help, these resultant “CD4-unhelped” CD8+ T cells do not differentiate into sustainable memory cells4.
NKG2D on natural killer (NK) cells and on activatedef CD8+ T cells binds to retinoic acid early inducible-1 gene ε (Rae-1ε), MULT-1, and H60 in mice8–13, and MICA/B and ULBP in humans14, 15. NKG2D engagement on CD8+ T cells contributes to TCR signaling co-stimulation and amplification of T cell signals and recognition of stress-associated ligands16–18. Besides this canonical function, NKG2D is implicated in CD8+ T cell-mediated autoimmune pathophysiology19. Therefore, strategies augmenting NKG2D engagement on CD8+ T cells and harnessing its non-canonical functions may result in the rescue of CD4-unhelped CD8+ T cell responses.
RESULTS
NKG2D co-stimulation regimen rescues CD4-unhelped CD8+ T cell memory recall expansion
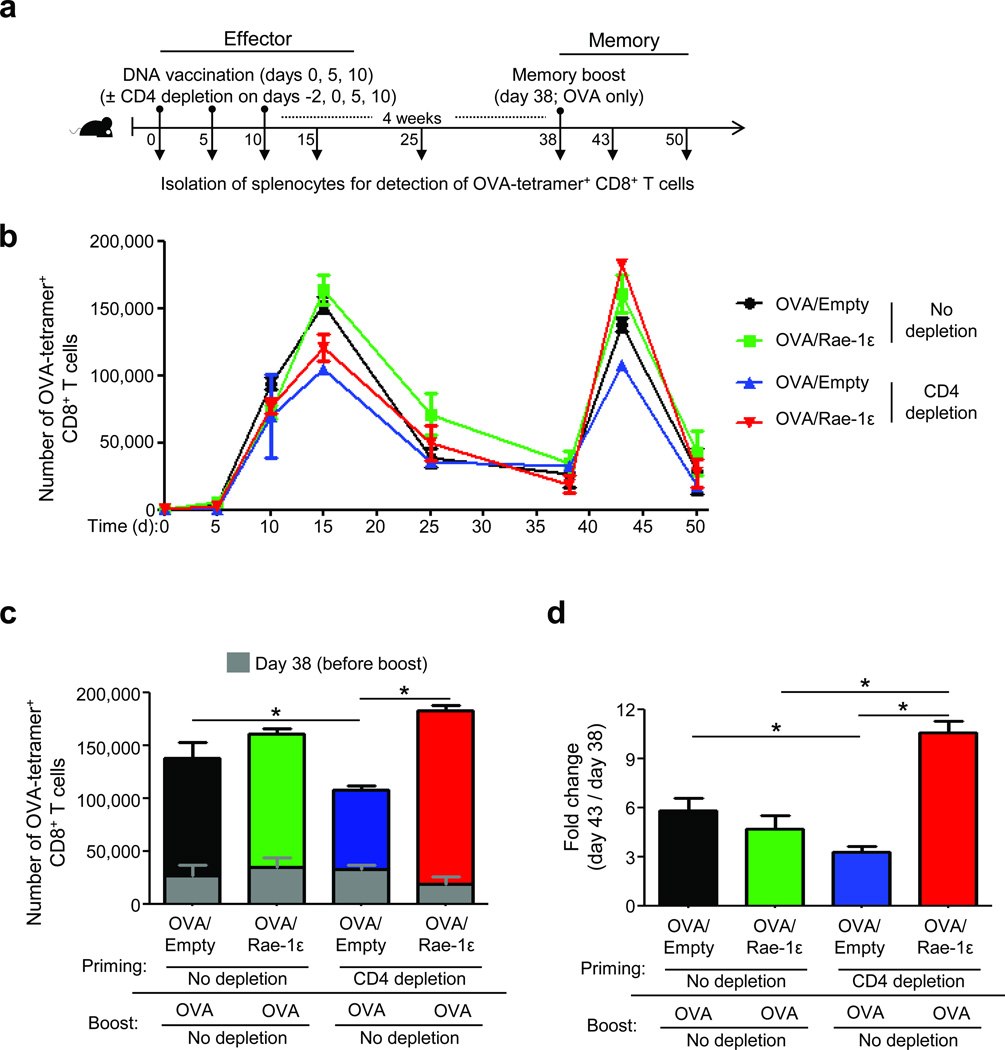
CD8+ T cells that do not receive CD4+ T cell help during priming undergo impaired memory recall expansion6, 20. Because NKG2D engagement on CD8+ T cells contributes to co-stimulation16, 17, we hypothesized that increased NKG2D engagement by Rae-1ε from antigen presenting cells (APCs) during priming would rescue memory recall CD4-unhelped CD8+ T cell expansion. We employed skin gene gun vaccination21 (in vivo biolistic transfection) to co-deliver DNA plasmids encoding chicken ovalbumin (OVA) and NKG2D ligand Rae-1ε (OVA/Rae-1ε), OVA and empty vector (OVA/Empty), or empty vectors (Empty/Empty) to APCs. Using a Rae-1ε-GFP fusion vector22, we verified that skin DNA delivery resulted in elevated expression of Rae-1ε protein on draining lymph node APCs (Supplementary Fig. 1).
Next, we assessed the effects of OVA/Rae-1ε vaccination (the NKG2D co-stimulation regimen) on CD8+ T cell memory recall responses. C57BL/6 mice received gene gun vaccinations three times (days 0, 5, and 10) ± CD4 depletion (days −2, 0, 5, and 10), were rested for 4 weeks during memory formation, and then received one memory boost vaccination (OVA only without Rae-1ε and without CD4 depletion) on day 38 (Fig. 1a). OVA-specific CD8+ T cell numbers were determined by OVA-tetramer staining in the spleen (Fig. 1b and Supplementary Fig. 2) and confirmed in the draining inguinal lymph node (Supplementary Fig. 3). Similar post-contraction levels (day 38, before boost) of OVA-specific CD8+ T cells were observed in all groups (Fig. 1b,c). Remarkably, the NKG2D co-stimulation regimen at priming resulted in complete rescue of CD4-unhelped OVA-specific CD8+ T cells at the memory recall phase (Fig. 1c,d and Supplementary Fig. 3b).
Figure 1.
NKG2D engagement by Rae-1ε rescues CD4-unhelped CD8+ T cell memory recall expansion. (a) Experimental design for vaccination and CD4 depletion. (b) Expansion kinetics of OVA-tetramer+ CD8+ T cells (±SEM) calculated per spleen. (c) Mean number of OVA-tetramer+ CD8+ T cells (+SEM) per spleen on days 38 and 43. (d) Data from (c) shown as a fold change. Data are representative of 3–5 mice analyzed individually per group per experiment from three experiments conducted with similar results. *P < 0.05.
NKG2D co-stimulation regimen rescues CD4-unhelped CD8+ T cell memory recall cytokine production and cytolytic responses
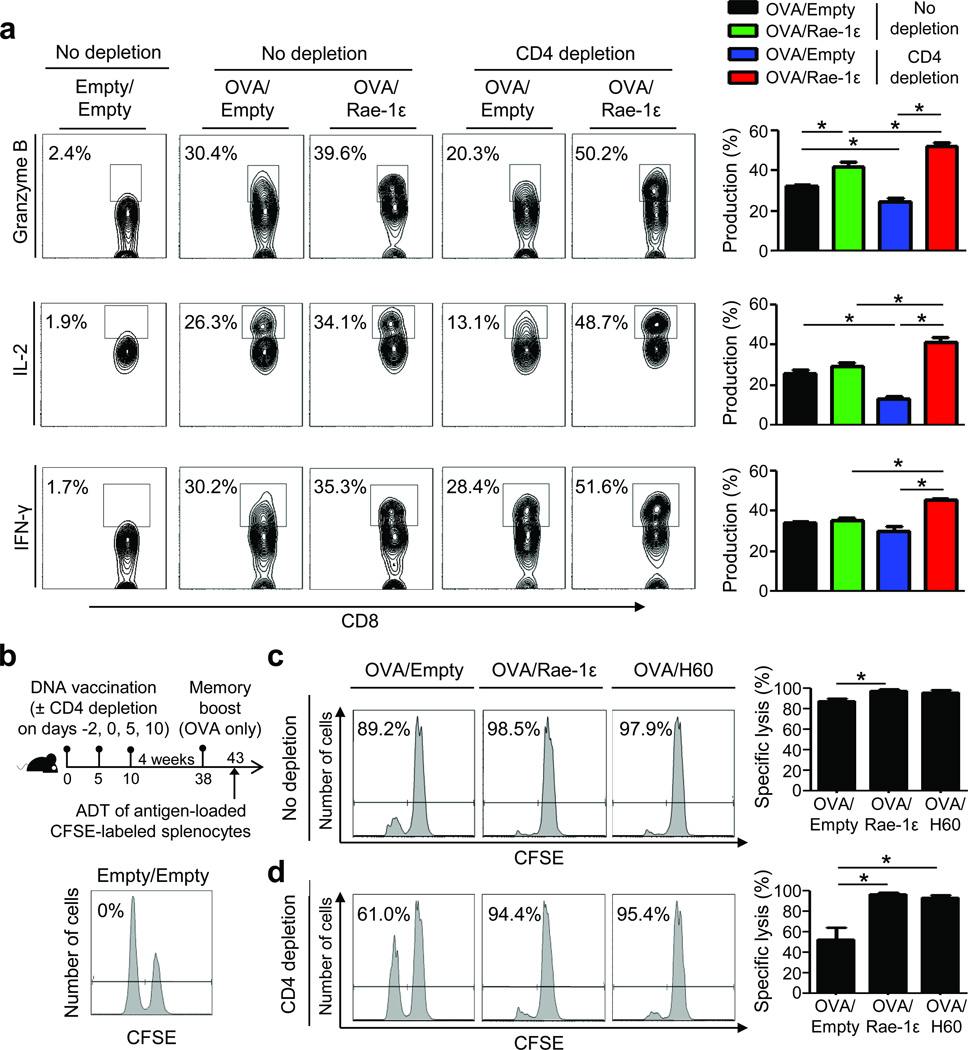
Based on the ability of the NKG2D co-stimulation regimen to augment memory recall expansion, we hypothesized that such engagement during priming may rescue memory cytolytic molecule and cytokine production by CD4-unhelped CD8+ T cells. Notably, upon memory boost with OVA only, CD4-unhelped CD8+ T cells that received the NKG2D co-stimulation regimen during priming displayed complete rescue of granzyme B, IL-2, and IFN-γ production (Fig. 2a). The contribution of the memory boost vaccination (i.e., a single vaccination) on naïve cells on day 38 was negligible (Supplementary Fig. 4).
Figure 2.
NKG2D-mediated rescue of CD4-unhelped CD8+ T cell memory recall responses involves cytokine and lytic molecule production and CTL lysis. (a) Splenic OVA-tetramer+ CD8+ T cell production of granzyme B, IL-2 and IFN-γ on day 43 as described in Fig. 1a. (b) Experimental design and in vivo specific lysis (%) of OVA257–264-loaded (CFSElo) and irrelevant peptide-loaded (hgp10025–33, CFSEhi) targets from a control (Empty/Empty) mouse. (c,d) Specific lysis (%) from mice vaccinated with OVA/Empty, OVA/Rae-1ε, and OVA/H60 as described in (b) without (c) and with (d) CD4 depletion. Data are representative of 3–5 mice analyzed individually per group per experiment from at least three experiments conducted with similar results. *P < 0.05.
To further investigate NKG2D-mediated rescue of CD4-unhelped CD8+ T cell memory recall responses, we examined antigen-specific target lysis ability (Fig. 2b). CD4 depletion at priming significantly weakened CD8+ T cell memory recall CTL lysis (Fig. 2c,d). Importantly, administration of either Rae-1ε (in C57BL/6 mice) or H60 (NKG2D ligand in B6BCF1 mice) during priming resulted in complete rescue of CD4-unhelped CD8+ T cell memory CTL lysis (Fig. 2d). Since CD4+ T cells were present during boost vaccination, we analyzed their potential role in the memory recall responses observed. Addition of CD4 depletion during the memory boost resulted in decreased memory recall CTL lysis in all groups (Supplementary Fig. 5). While the greatest decrease was observed in the group primed without the NKG2D co-stimulation regimen and depleted of CD4+ T cells both during priming and memory boost, rescue was still observed with the NKG2D co-stimulation regimen (Supplementary Fig. 5).
NKG2D on CD8+ T cells is necessary for rescue of CD4-unhelped CD8+ T cell memory recall responses
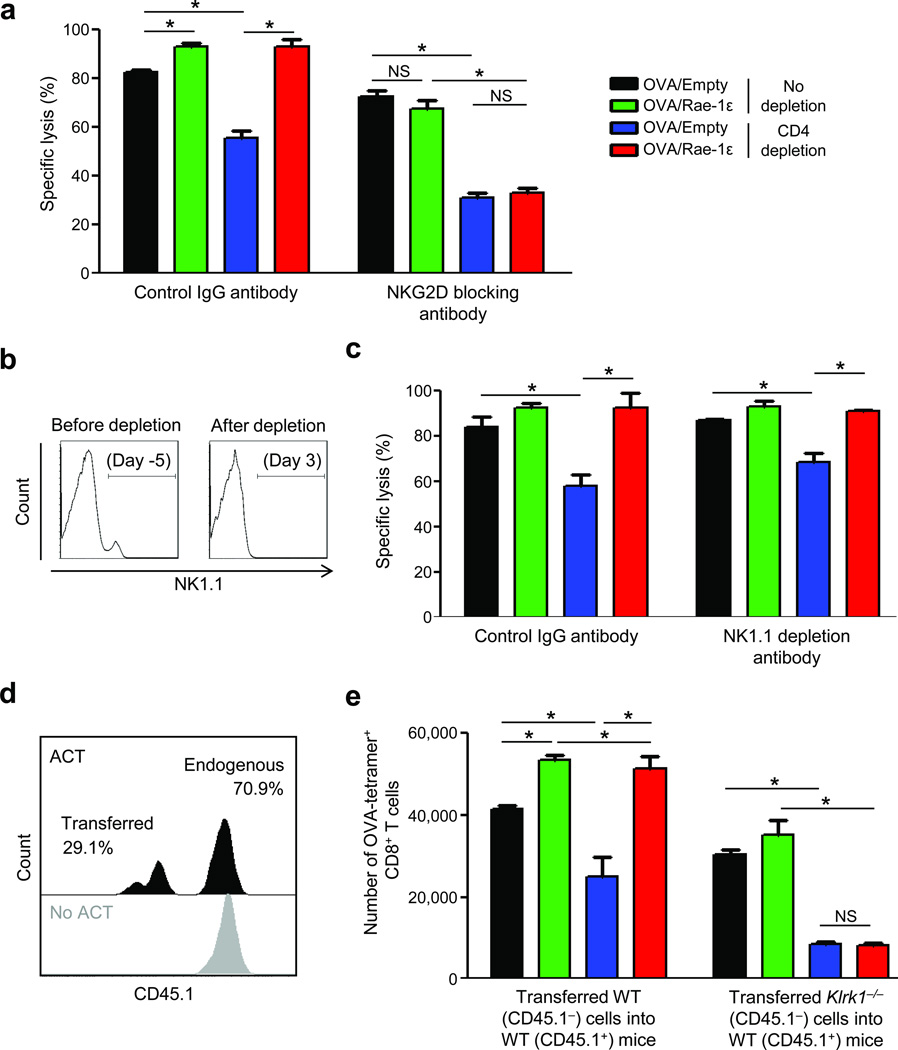
To demonstrate that the observed rescue of memory recall responses is dependent on NKG2D and, specifically, on CD8+ T cell NKG2D engagement, we conducted a series of experiments. First, we blocked NKG2D with antibody during priming and observed decreased memory CTL lysis (Fig. 3a). Vaccination of NKG2D-blocked, CD4-depleted mice with OVA/Rae-1ε did not rescue CD4-unhelped CD8+ T cell lysis (Fig. 3a).
Figure 3.
Rescue of CD4-unhelped CD8+ T cell memory recall responses is dependent on NKG2D expression on CD8+ T cells. (a) Memory recall (day 43) specific lysis (% + SEM) of C57BL/6 mice vaccinated as described in Fig. 1a and treated with NKG2D blocking antibody on days 0, 5, and 10. (b,c) NK1.1 expression in the spleen (b) and specific lysis (% + SEM) (c) of C57BL/6 mice depleted of NK1.1 cells on days −5 and −3, adoptively transferred Thy1.1-marked naïve OT-I (OVA257–264-specific) CD8+ T cells (2*106 per mouse) on day −1, and vaccinated on day 0 ± CD4 depletion on days −2 and 0. (d) CD45.1 expression on transferred (CD45.1−) and endogenous (CD45.1+) OVA-tetramer+ CD8+ T cells. ACT = adoptive cell transfer; WT = wild type C57BL/6. (e) Mean number of OVA-tetramer+ CD8+ T cells (+SEM) from (d) per spleen on day 43. Data are representative of 3–5 mice analyzed individually per group per experiment from three experiments conducted with similar results. *P < 0.05; NS: P > 0.05.
Second, since NK and NKT cells express NKG2D, we determined their role in NKG2D-mediated rescue. C57BL/6 mice were depleted of NK1.1+ cells23 and Thy1.1-marked OT-I CD8+ T cells were transferred into these mice. This design allowed induction of detectable OVA-specific responses with a single vaccination, thereby abrogating the need for further NK1.1 depletions, and thus avoiding depletion of activated NK1.1-expressing CD8+ T cells. Here, priming of CD8+ T cells occurred after NK1.1 depletion antibody was cleared (data not shown) and before NK and NKT cells had returned (Fig. 3b). Memory recall OVA-specific CD8+ T cell lysis was not negatively affected by NK1.1 depletion during priming and CD4-unhelped CD8+ T cell lysis was rescued by the NKG2D engagement regimen (Fig. 3c).
Lastly, to demonstrate that NKG2D-mediated rescue is the result of NKG2D-ngagement on CD8+ T cells, we employed vaccination of CD45.1+ C57BL/6 wild type (WT) mice that received adoptively-transferred CD45.1− CD8+ T cells from NKG2D-deficient (Klrk1−/−)24 (Fig. 3d) or CD45.1− WT mice. Compared to transferred WT CD8+ T cells, transferred NKG2D-deficient CD8+ T cells showed decreased memory recall expansion (Fig. 3e). OVA/Rae-1ε vaccination at priming did not rescue memory recall expansion (Fig. 3e) of CD4-unhelped NKG2D-deficient transferred CD8+ T cells but did rescue expansion of CD4-unhelped WT transferred CD8+ T cells (Fig. 3e) and endogenous CD8+ T cells in both hosts (data not shown).
NKG2D-mediated rescue of CD4-unhelped CD8+ T cells is not independent of IL-15
Roles for IL-15 in NKG2D upregulation25 and signaling26, as well as rescue of CD4-unhelped CD8+ T cells27, have been reported. Therefore, we investigated IL-15 presentation (via dendritic cell IL-15Rα expression) and found it to be equal under the four vaccination conditions (Supplementary Fig. 6a).
Memory phase expansion (Supplementary Fig. 6b) and CTL lysis (Supplementary Fig. 6c) of OVA-specific CD8+ T cells were greatly reduced in IL-15-deficient (Il15−/−) mice. The NKG2D co-stimulation regimen was partially able to rescue CD4-unhelped CD8+ T cell responses in the absence of IL-15 to the level observed without CD4 depletion but was unable to rescue to the level observed in WT mice (Supplementary Fig. 6b,c).
NKG2D co-stimulation regimen does not rescue CD4-unhelped CD8+ T cell effector responses
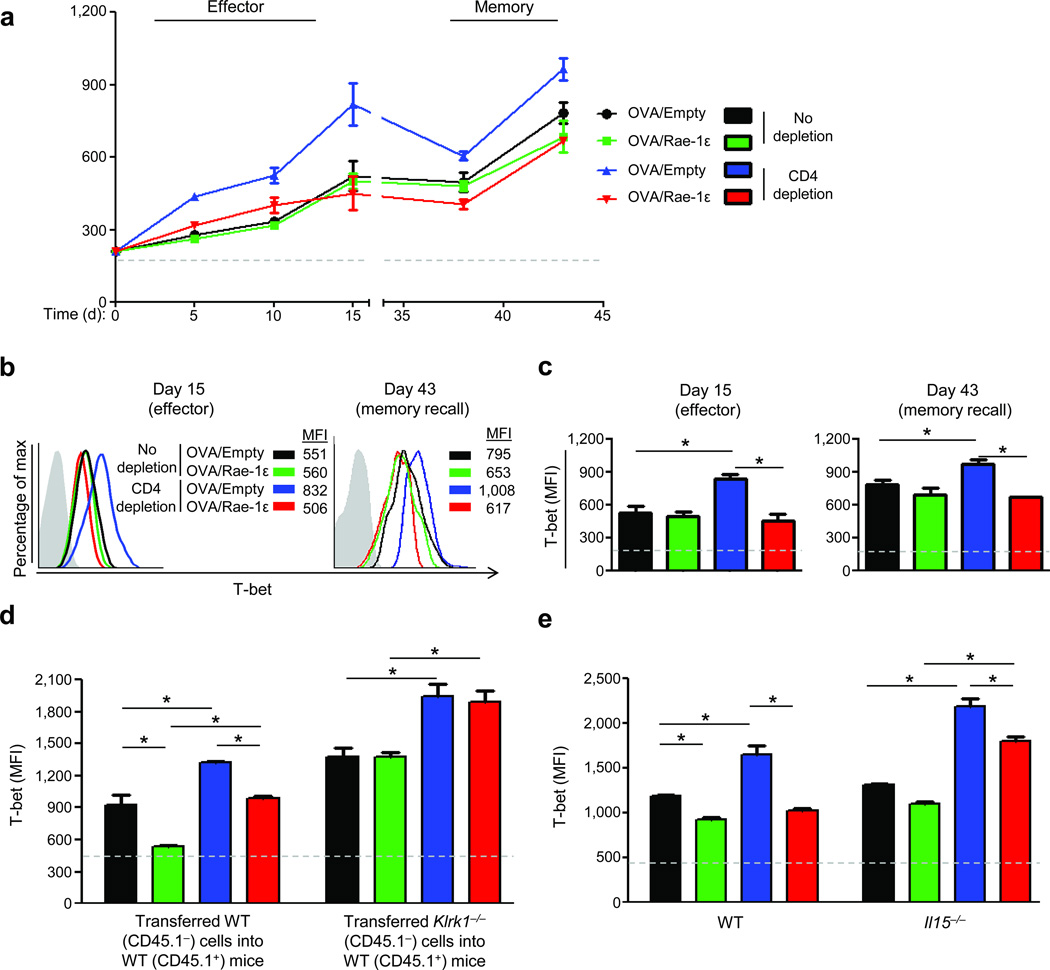
We sought to understand the process by which NKG2D mediates rescue of memory responses by investigating CD8+ T cell responses at the effector phase. A significant decrease in expansion of CD4-unhelped OVA-specific CD8+ T cells was observed at the effector phase (day 15) and, unexpectedly, not augmented by the NKG2D co-stimulation regimen (Fig. 1b and Supplementary Fig. 7).
A significant increase in effector phase granzyme B production (Supplementary Fig. 8a) and lysis (Supplementary Fig. 8b) was observed in non-depleted mice receiving the NKG2D co-stimulation regimen. However, lack of CD4 help resulted in un-rescued minimal target lysis with OVA/Empty, OVA/Rae-1ε, and OVA/H60 vaccination (Supplementary Fig. 8c,d).
NKG2D engagement results in JNK2-mediated suppression of T-bet in CD4-unhelped CD8+ T cells
CD4-unhelped CD8+ T cells express higher levels of T-bet than their CD4-helped counterparts28 and T-bet expression during the effector phase distinguishes short-lived effector cells (SLECs; T-bethi) from memory precursor effector cells (MPECs; T-betlo)28–31. To determine if effector phase T-bet expression correlates with NKG2D-mediated rescue of CD4-unhelped CD8+ T cell memory recall responses, we determined T-bet levels in OVA-specific CD8+ T cells. In the context of CD4 help, OVA-specific CD8+ T cells consistently expressed low T-bet levels throughout the effector and memory phases (Fig. 4a–c). Conversely, CD4-unhelped OVA-specific CD8+ T cells primed without the NKG2D co-stimulation regimen expressed significantly increased levels of T-bet (Fig. 4a–c). Importantly, T-bet levels in CD4-unhelped CD8+ T cells were significantly reduced (45%) with the NKG2D co-stimulation regimen, remained similar to T-bet levels of CD4-helped CD8+ T cells throughout the effector phase, and were significantly lower during the memory phase (Fig. 4a–c). Corroborating these findings, T-bet levels were elevated in NKG2D-deficient versus WT OVA-specific CD8+ T cells transferred into a WT host, especially under CD4-depletion conditions (Fig. 4d), and elevated when compared to endogenous CD8+ T cells (Supplementary Fig. 9a). Likewise, T-bet levels in OVA-specific CD8+ T cells were elevated in IL-15-deficient versus WT hosts, especially under CD4-depletion conditions (Fig. 4e), and only partially repressed by the NKG2D co-stimulation regimen (Fig. 4e and Supplementary Fig. 9b).
Figure 4.
NKG2D engagement reverses the elevated T-bet expression associated with CD4-unhelped CD8+ T cells. (a) T-bet expression MFI (±SEM) in OVA-tetramer+ CD8+ T cells from C57BL/6 mice vaccinated as described in Fig. 1a. (b) T-bet staining on days 15 and 43. MFI = mean fluorescent intensity; gray curve = isotype. (c) T-bet expression from (a) on days 15 and 43. (d) Memory recall (day 43) T-bet expression on OVA-specific CD8+ T cells transferred from CD45.1− NKG2D-deficient (Klrk1−/−) or wild type C57BL/6 (WT) mice into WT CD45.1+ mice. (e) Memory recall (day 43) T-bet expression on OVA-specific CD8+ T cells in IL-15-deficient (Il15−/−) or WT mice vaccinated as described in Fig. 1a. Data are representative of 3–5 mice analyzed individually per group per experiment from three experiments conducted with similar results. Dashed lines represent MFI of background flow cytometric staining for T-bet. *P < 0.05; NS: P > 0.05.
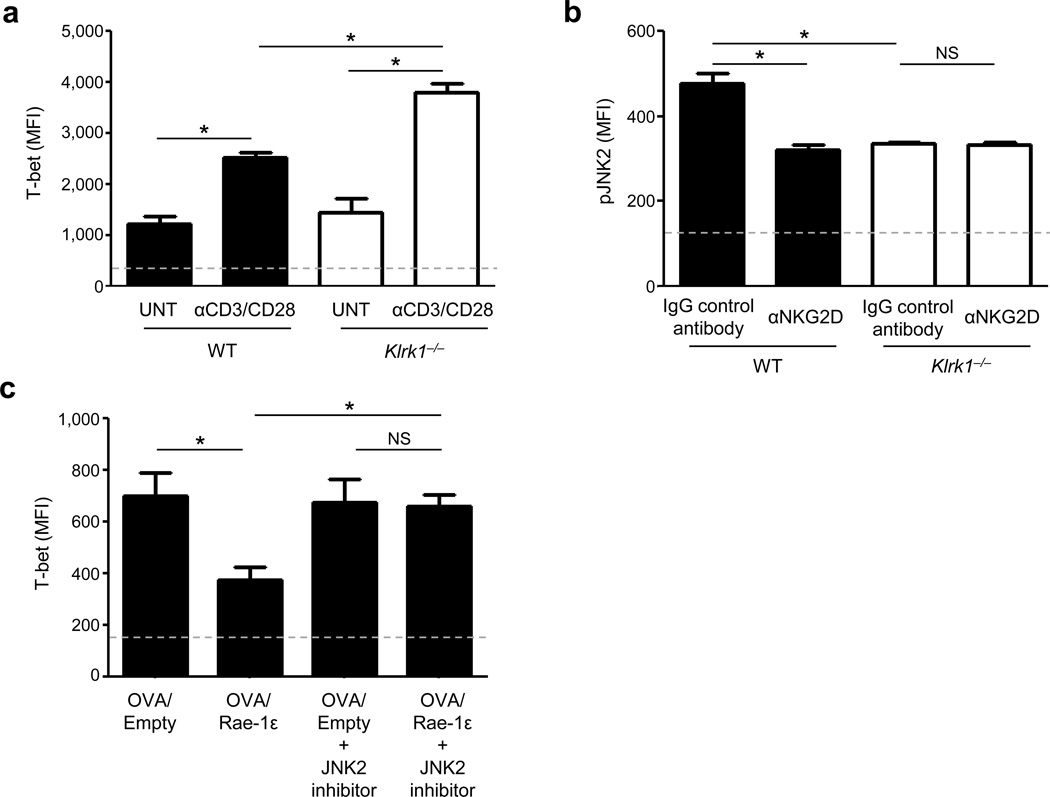
Studies have shown that co-stimulation of CD8+ T cells results in activation of the JNK2 pathway32, and that JNK2 knockout mice have increased levels of T-bet expression33. In our studies, in vitro activated NKG2D-deficient CD8+ T cells expressed elevated T-bet (Fig. 5a) and decreased phosphorylated JNK2 (pJNK2) levels (Fig. 5b) compared to WT counterparts. Additionally, WT CD8+ T cells similarly activated in the presence of NKG2D blocking antibody showed decreased pJNK2 levels (Fig. 5b). Based on these observations and evidence that NKG2D modulates JNK signaling34, 35, we hypothesized that NKG2D signaling represses T-bet via JNK2. JNK2 pathway inhibition of in vitro OT-I CD8+ T cell activation with OVA257–264 peptide-loaded EL4 cells expressing Rae-1ε resulted in a significant increase in T-bet to levels resembling OT-I CD8+ T cells activated without NKG2D co-stimulation (Fig. 5c).
Figure 5.
Suppression of T-bet by NKG2D/Rae-1ε engagement is mediated through JNK2. (a) CD8+ T cell T-bet MFI (+SEM) from NKG2D-deficient (Klrk1−/−) and wild type C57BL/6 (WT) spleens stimulated in vitro with anti-CD3/CD28 antibodies (1 µg ml−1 each) for 72 h or left untreated. (b) pJNK2 MFI (+SEM) from CD8+ T cells stimulated as in (a) ± NKG2D blocking antibody (αNKG2D). (c) T-bet MFI (+SEM) of OT-I CD8+ T cells co-cultured with OVA257–264-loaded EL4 cells transfected with either Rae-1ε or empty vector, and treated with a JNK2 inhibitor (JNK inhibitor IX; 25 nM,). Data are representative of 3–5 mice analyzed individually per group per experiment from at least three experiments conducted with similar results. Dashed lines represent MFI of background flow cytometric staining for the respective markers. *P < 0.05; NS: P > 0.05.
Augmented NKG2D co-stimulation confers protection in CD4-dependent infectious disease models
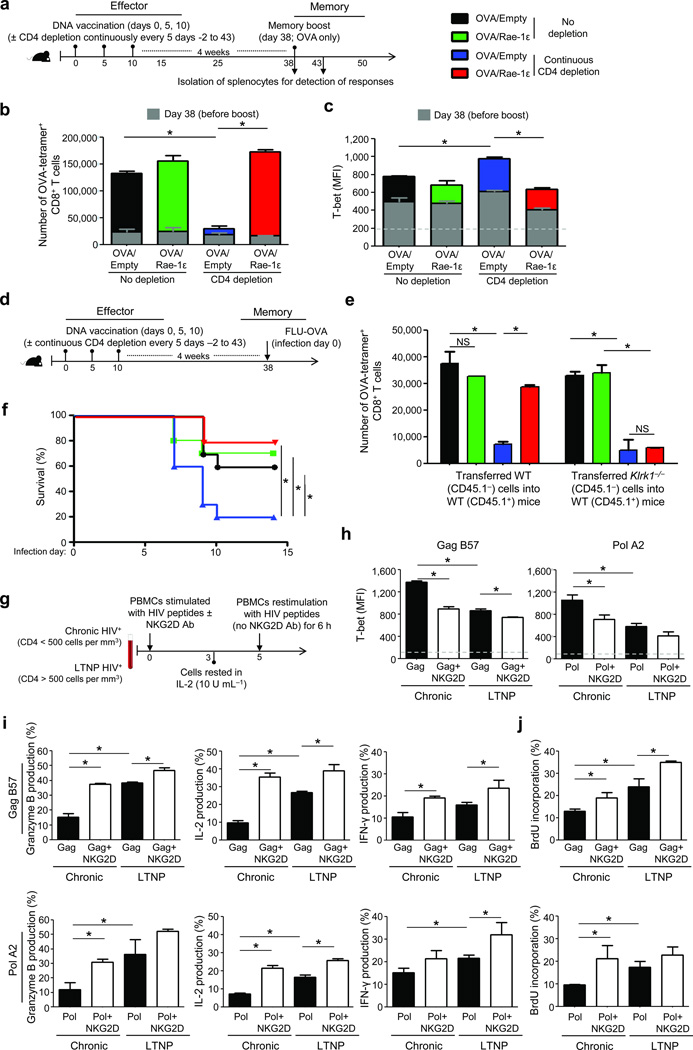
To address the condition in which CD4+ T cells remain continuously low or absent, we characterized the ability of NKG2D to rescue CD8+ T cell responses under continuous CD4 antibody depletion administered every 5 d (days −2 to 43) (Fig. 6a). Here, CD8+ T cell memory expansion was significantly reduced (Fig. 6b), and T-bet further increased (Fig. 6c) compared to CD4 depletion only during priming (Figs. 1 and 4). Further, CD8+ T cells primed with the NKG2D co-stimulation regimen and continuously depleted of CD4+ T cells exhibited rescued memory recall responses on a per cell basis in an ex vivo CTL assay where OVA-tetramer+ CD8+ T cell numbers were equalized from the vaccination conditions (Supplementary Fig. 10).
Figure 6.
NKG2D co-stimulation confers protection in CD4-dependent infectious disease models. (a) Experimental design for C57BL/6 mice vaccinated as described in Fig. 1a and continuously CD4 depleted. (b,c) Mean number (c) and T-bet MFI (+SEM) (d) of OVA-tetramer+ CD8+ T cells (+SEM) per spleen on days 38 and 43 from mice in (a). (d) Experimental design of Influenza-PR/8 intranasal infection. (e) Number of OVA-tetramer+ CD8+ T cells from experiment in which WT or NKG2D-deficient (Klrk1−/−) CD8+ T cells were adoptively transferred into WT hosts and treated as described in (d). (f) Survival curve for experiment described in (d) and repeated twice with similar results. (g) Experimental design for stimulation of HIV-positive chronic (Chronic) and long-term non-progressor (LTNP) donor cells with pooled HIV peptides. (h–j) HIV-tetramer+ CD8+ T cell MFI (+SEM) of T-bet (h), mean % producing granzyme B, IL-2, and IFN-γ (+SEM) (i), and mean % incorporating BrdU (+SEM) (j), from at least three donors per group. Dashed lines represent MFI of background flow cytometric staining for the respective markers. *P < 0.05; NS: P > 0.05.
To assess functional responses in an in vivo pathogenic model, we utilized a lethal Influenza-PR/8 infection model, in which the virus expresses OVA and clearance is CD4-dependent (Fig. 6d). Absence of the NKG2D co-stimulation regimen and presence of continuous CD4 depletion in mice resulted in decreased CD8+ T cell memory recall responses (Fig. 6e) and low survival (~20%) (Fig. 6f) after Influenza-PR/8 infection. In contrast, the NKG2D co-stimulation regimen resulted in significantly-augmented CD8+ T cell memory recall responses and survival (Fig. 6e,f). Further, CD8+ T cells from mice lacking NKG2D expression were unable to respond to Influenza-PR/8 challenge despite receiving OVA/Rae-1ε vaccination during priming (Fig. 6e).
To further assess the role of NKG2D co-stimulation in a pathogenic model where chronic CD4 deficiency plays a major role in disease progression, we determined the response of HIV-specific CD8+ T cells from chronic HIV-positive donors, whose lack of CD4+ T cell help leads to CD4-unhelped CD8+ T cells36, and compared these with long-term non-progressor (LTNP) HIV-positive donors whose maintained CD4+ T cell help leads to potent anti-HIV CD8+ T cell responses37 (Supplementary Table 1). To replicate the conditions used in our in vivo mouse studies, donor PBMCs were stimulated with pooled HIV peptides in the presence or absence of exogenous NKG2D stimulating antibody, rested for 2 d, and re-stimulated with pooled HIV peptides (without NKG2D agonist antibody) (Fig. 6g). As in the mouse models, NKG2D co-stimulation of human CD8+ T cells from chronic HIV donors reduced T-bet expression (Fig. 6h) and rescued their recall ability to produce granzyme B, IL-2, and IFN-γ (Fig. 6i) and proliferate (Fig. 6j). Further, NKG2D co-stimulation rescued recall CD8+ T cell responses in chronically-infected HIV-positive donors to resemble the phenotype and functionality associated with HIV-positive long-term non-progressor responses (Fig. 6h–j).
DISCUSSION
We demonstrate that augmented NKG2D engagement on CD8+ T cells rescues CD4-unhelped CD8+ T cell memory recall responses. Contrary to our expectations, the NKG2D co-stimulation regimen did not rescue CD4-unhelped effector responses. This paradoxical finding raises the question as to how memory recall responses can be rescued in the absence of effector responses. We discovered that, through augmentation of phosphorylated JNK2, augmented NKG2D co-stimulation results in CD4-unhelped CD8+ T effector cells with reduced expression of T-bet, a transcription factor which at low levels drives formation of effector cells with increased memory potential28–31. This leads us to propose that effector phase responses and memory potential of CD8+ T cells may be dictated by separate signals, and furthermore, that NKG2D co-stimulation provides CD8+ T cells with memory potential programming for conversion to potent memory CD8+ T cells even in the absence of primary effector responses.
Similar to the impaired effector cytolytic responses of CD4-unhelped CD8+ T cells in our study, work by Janssen et al.5, 38 shows a comparable (i.e., nearly absent) CD4-helpless effector responses on day 14. However, some studies have shown that CD4-unhelped CD8+ T cell effector responses are typically less affected and that memory recall responses are greatly impaired4, 6, 7, 39, 40. This difference may be attributed to the level of inflammation-mediated response during the effector phase. In the studies conducted using infection models, CD4-unhelped CD8+ T cell responses may have arisen from infection-associated inflammation signals. However, skin gene gun delivery causes limited inflammation41 and thus aids in deconstructing effector response mechanisms.
Despite the absence of effector responses from CD4-unhelped CD8+ T cells vaccinated without the NKG2D co-stimulation regimen, memory responses, albeit defective, were observed. These preserved memory responses are attributable to the presence of CD4+ T cell help during memory phase boost vaccination. Recent studies support the notion that CD4-unhelped responses can be rescued in the memory phase5, 42. Importantly, even with continuous CD4 depletion during both the effector and memory phases, the NKG2D co-stimulation regimen still rescued CD8+ T cell memory responses.
Studies have shown that IL-15 can induce NKG2D expression on CD8+ T cells25, and that IL-15 is necessary for NKG2D signaling26. In our study, NKG2D-mediated rescue in CD4-unhelped CD8+ T cells was dependent, albeit partially, on IL-15. These data may provide the mechanism by which IL-15 has been shown to aid in CD4-unhelped memory response rescue27. IL-2, a cytokine augmented in our studies with increased NKG2D engagement, has been reported to compensate for the lack of IL-1543. Future investigation may be warranted in determining whether partial rescue demonstrated in IL-15-deficient mice may be mediated by NKG2D signaling through alternative molecular pathways44 or if other common gamma chain cytokines compensate for the lack of IL-15 in this situation.
Even under deleterious conditions in an in vivo pathogenic model of lethal Influenza-PR/8 infection during continuous immunocompromising CD4 depletion, NKG2D co-stimulation conferred mice with rescued memory recall CD8+ T cells and a high rate of survival. Similar NKG2D-mediated survival was observed in a model of chronic LCMV (clone 13) infection under CD4-deficient conditions (personal observation). These findings raise the possibility of including an NKG2D co-stimulation regimen in human vaccination protocols. Specifically in the context of influenza vaccination, which in a non-adjuvant form does not drive strong CD4 responses45, these data suggest a potential enhancement, via the NKG2D co-stimulation regimen, of the effectiveness of CD8+ T cell responses. Additionally, such an NKG2D co-stimulation regimen may avoid vaccine side effects attributable to robust CD4 activation in the presence of strong adjuvants46.
Finally, we demonstrate that the NKG2D co-stimulation regimen observations equally pertain to a human disease system in which CD4+ T cells are progressively depleted by HIV, resulting in CD8+ T cells that are characteristically CD4-unhelped47. While increased T-bet expression by Nef peptide-stimulated IFN-γ-producing CD8+ T cells from HIV elite controllers (a subset of long-term non-progressors) has been reported48, our study demonstrates that NKG2D co-stimulation reduced T-bet expression of Gag and Pol tetramer-specific CD8+ T cells from chronically-infected HIV donors and rescued their ability to acquire cytolytic potential. Furthermore, such NKG2D co-stimulation rescued CD8+ T cell responses from chronically-infected HIV-positive donors to resemble the phenotype and functionality associated with HIV-positive long-term non-progressor CD8+ T cell responses. Augmented NKG2D co-stimulation may have a role in improving CD8+T cell responses against HIV itself and against AIDS-associated pathogens.
Our work here demonstrates that NKG2D co-stimulatory signaling during priming may have therapeutic value, specifically in the development of optimal memory recall responses and reversal of the impotent state of CD4-unhelped CD8+ T cells. These NKG2D-mediated findings are important from the perspective of T cell-based vaccine design and adoptive T cell therapy, where potent effector and memory formation are vital for successful eradication of acute and recurrent disease, especially in situations where CD4+ T cells are depleted as a result of the disease (e.g., HIV/AIDS), exhausted or suppressed as a sequela of the given pathology (e.g., chronic infection, cancer, etc.), or therapeutically depleted to remove immune suppression (e.g., cancer). In such situations, delivery of NKG2D ligand can become a feasible substitute for CD4 help. These findings address limitations of current T cell regimens against intracellular pathogens and cancer by generating better memory recall responses even under severely CD4-compromised conditions.
ONLINE METHODS
Mice, donors, and cells
Six-week-old, specific-pathogen-free, male, C57BL/6 (B6), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), and B6.SJL-PtprcaPepcb/BoyJ (CD45.1+) mice (Jackson Laboratories); C57BL/6NTac-IL15tm1Imx N5 (IL-15-deficient; Il15−/−) and C57BL/6 control mice (Taconic); Thy1.1-marked OT-I and B6BCF1 (C57BL/6×Balb/c F1 hybrid) mice (bred in-house); and NKG2D-deficient (Klrk1−/−) mice were housed under conventional conditions. We conducted experiments in accordance with The University of Chicago Institutional Animal Care and Use Committee (IACUC) and the Loyola University Chicago IACUC guidelines. We obtained peripheral blood from chronic HIV-positive donors (documented HIV+ for at least 5 y without extended antiretroviral therapy, CD4+ T cell count < 500 cells per mm3, and uncontrolled plasma HIV-1 RNA levels) and long-term non-progressor donors (documented HIV+ for at least 5 y without extended antiretroviral therapy, CD4+ T cell count > 500 cells per mm3, and low or undetectable plasma HIV-1 RNA levels). We conducted human cell research in accordance with guidelines on human research and approval of the Institutional Review Board of Rush University Medical Center. We obtained donor informed consent in accordance with the Declaration of Helsinki. All cells were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals), 2mM L-glutamine (Mediatech), and 1% penicillin/streptomycin (Mediatech).
In vitro culture
Mouse EL-4 target cells were loaded (2 h) with OVA257−264 peptide (1 µg ml–−1) and co-cultured for 72 h with CD8+ T cells (isolated by negative selection from OT-I mouse spleens) and 30 U ml−1 IL-2 (R&D Systems) ± JNK Inhibitor IX (N-[3-Cyano-4,5,6,7-tetrahydro-1-benzothien-2-yl]-1-naphthamide; EMD Chemicals; 25 ng ml−1). We primed human HIV-positive donor PBMCs for 3 d with pooled HIV peptides spanning the Gag or Pol regions49 (NIH AIDS Research and Reference Reagent Program) at 2 µg per peptide per ml ± NKG2D stimulation antibody (1D11, 2 µg ml−1), rested them for 2 d in media with minimal IL-2 (10 U ml−1, and restimulated them similarly with HIV peptides (no NKG2D antibody) for 6 h.
DNA vaccination
We performed vaccinations (4 µg plasmid DNA per mouse per vaccination) using a Helios gene gun (Bio-Rad) as previously described21, 50. DNA vaccines (OVA/Rae-1ε, OVA/Empty, OVA/H60, Empty/Empty) were generated using the pCRAN multiple cloning site variant of pcDNA3 (Invitrogen). DNA was produced in large quantities and purified by GeneArt. We generated bullets containing DNA, as previously described21.
Influenza infection
Mice were anesthetized with ketamine and xylazine, weighed, and given Influenza A virus (PR/8; strain A/Puerto Rico/8/1934 H1N1 modified to express OVA) via intranasal administration (80,000 EID50). We euthanized mice on day 3 of infection for spleen recovery, or weighed them throughout the infection and euthanized them when they reached < 70% pre-infection weight.
CTL lysis assays
C57BL/6 splenocyte target cells were loaded (1µg ml−1 of SIINKEKL [OVA257–264] or irrelevant peptide [KVPRNQDWL, hgp10025–33]), CFSE-labeled (0.5 and 8µM, respectively), and adoptively transferred (1:1 ratio, 2×107 cells total) via retroorbital injection. Eighteen hours later spleen cells were analyzed by flow cytometry for CFSE loss and specific lysis was calculated51.
Flow cytometry
We purchased all flow cytometry antibodies from Ebioscience, except mCD3 (BD Biosciences), m/hT-bet (BioLegend), and pJNK2 (Santa Cruz Biotechnology, Inc.). OVA MHC-I Tetramer-PE (SIINFEKL), HIV Gag HLA-A2 Tetramer-PE (KAFSPEVIPMF), and HIV Pol HLA-A2 Tetramer-PE (ILKEPVHGV) were purchased from Beckman Coulter. We performed flow cytometric antibody staining and analysis as previously described49, 52, 53,54. Cell staining data were acquired using a LSR-II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star). We gated on live lymphocytes using LIVE/DEAD staining (R&D Systems), forward scatter area (FSC-A) versus side scatter area (SSC-A), followed by forward scatter width (FSC-W) versus side scatter width (SSC-W), FSC-A versus forward scatter height (FSC-H), and SSC-A versus side scatter height (SSC-H) plots. Cell counts were calculated using PKH2655, 56.
Antibody depletions, blocking, and stimulation
We delivered depletion/blocking monoclonal antibodies (CD4 antibody [GK1.5; BioXcell and The University of Chicago Frank W. Fitch Monoclonal Antibody Facility]; NK1.1 antibody [PK136, BioXcell]; and NKG2D antibody [HMG2D, BioXcell]) via intraperitoneal injection at 500 µg per mouse per depletion or blocking. In vitro NKG2D co-stimulation of human PBMCs was performed using NKG2D/CD314 antibody (1D11, BioLegend).
Adoptive CD8+ T cell transfer
We magnetically isolated cells from mouse spleens via negative selection using the MACS CD8α+ T cell negative isolation kit (Miltenyi Biotec) or a positive isolation kit (naïve [CD44−] CD8+ T cells: R&D Systems, CDllc+ cells: Miltenyi Biotec). Cells were delivered via retro-orbital injections in 100 µl serum-free PBS.
EL4 target cell preparation
We generated Rae-1ε and H60 constructs using the pcDNA3 vector (Invitrogen) and transfected them using lipofectamine into EL4 cells not expressing Rae-1ε, as described previously50. Control EL4 cells were transfected with empty pCRAN.
Statistical analyses
We used the logrank test for comparison of survival curves. For the remainder of statistical analyses we used Student’s t test (two-tailed). A P value of < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
For their kind gifts, we thank: A. Houghton (pCRAN vector and OVA DNA; Memorial Sloan-Kettering Cancer Center, NY); V. Kumar and L. Chlewicki (non-Rae-1ε-expressing EL-4 cells; The University of Chicago, IL); L. Lanier (Rae-1ε, Rae-1ε-GFP, and H60 genes; UCSF, CA); A. Tenorio (HIV-positive donor identification; Rush University Medical Center, IL); W. Yokoyama (NKG2D-deficient mouse spleens; Washington University School of Medicine, MO), and D. Raulet (NKG2D-decifient mice, University of California, Berkeley, CA). We thank B. Jabri (The University of Chicago, IL), A. Bendelac (The University of Chicago, IL), M. Nishimura (Loyola University Chicago, IL), and M.J. Turk (Dartmouth Medical School, NH) for constructive discussions and manuscript edits. We are grateful to the flow cytometry facilities at The University of Chicago and at Loyola University Chicago for their invaluable support and to the Frank W. Fitch Monoclonal Antibody Facility of The University of Chicago Cancer Center (funded by NCI Cancer Center Support Grant #5P30CA014599-35) for CD4 depletion antibody production. HMG2D antibody was used with permission from H. Yagita (Juntendo University School of Medicine, Tokyo, Japan). This work was supported in part by the American Cancer Society (ACSLIB112496-RSG) to J.A.G.; American Cancer Society-Illinois Division (Young Investigator Award Grant #07-20) to J.A.G.; Croatian Ministry of Science, Education, and Sports (062-0621261-1271), as well as the Croatian-Israeli Grant to B.P.; the US National Institutes of Health R21CA127037 to J.A.G., PO1AI082971 to L.A., K22AI077714 to P.G.T., and T32 Immunology Training Grant, The University of Chicago, 5T32AI007090 to A.Z., F.J.K., and J.A.O.; and the Cancer Research Foundation (Young Investigator Award) to J.A.G.
Footnotes
The authors have no financial conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
A.Z., F.J.K., and J.A.G. designed the study and wrote the manuscript; J.A.G. supervised the project. G.E.L. generated the DNA constructs and performed WT CTL experiments. A.Z. performed the cytokine, kinetics, T-bet/JNK2, HIV, and remainder of CTL experiments. J.M.S. designed the cytokine staining and JNK2 experiments. T.V.M., V.V., J.W.W., and A.Z. performed the influenza experiments. F.J.K., A.T.L., J.A.O., M.C.J., E.C.B., A.Z., and J.A.G. performed DNA vaccinations and flow cytometric experiments. L.A. collaborated on the HIV experiment; A.L.M., P.G.T., and A.I.S. collaborated on the influenza experiment. B.P. and B.Z. developed the NKG2D-deficient mice and collaborated on associated experiments. All authors edited the manuscript.
REFERENCES
- 1.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 4.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 5.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 6.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 11.Raulet DH, Held W. Natural killer cell receptors: the offs and ons of NK cell recognition. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 12.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 16.Groh V, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson AM, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Van Belle TL, von Herrath MG. The role of the activating receptor NKG2D in autoimmunity. Mol Immunol. 2009;47:8–11. doi: 10.1016/j.molimm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Kaech SM, Ahmed R. Immunology. CD8 T cells remember with a little help. Science. 2003;300:263–265. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 21.Guevara-Patino JA. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J Clin Invest. 2006;116:1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodoen M, et al. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafirova B, et al. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31:270–282. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 26.Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. 2007;8:1345–1352. doi: 10.1038/ni1524. [DOI] [PubMed] [Google Scholar]
- 27.Oh S, et al. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaech SM. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 32.Blonska M, et al. The CARMA1-Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity. 2007;26:55–66. doi: 10.1016/j.immuni.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao J, et al. JNK2 negatively regulates CD8+ T cell effector function and anti-tumor immune response. Eur J Immunol. 2007;37:818–829. doi: 10.1002/eji.200636726. [DOI] [PubMed] [Google Scholar]
- 34.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Tang F, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J Exp Med. 2009;206:707–719. doi: 10.1084/jem.20071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuerten S, et al. The TRAIL of helpless CD8+ T cells in HIV infection. AIDS Res Hum Retroviruses. 2008;24:1175–1183. doi: 10.1089/aid.2008.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 38.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 39.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 41.Matthews K, Rhind SM, Gossner AG, Dalziel RG, Hopkins J. The effect of gene gun-delivered pGM-CSF on the immunopathology of the vaccinated skin. Scand J Immunol. 2007;65:298–307. doi: 10.1111/j.1365-3083.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 42.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 43.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Upshaw JL, et al. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 45.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt CS, Morrow WJ, Sheikh NA. Smart adjuvants. Expert Rev Vaccines. 2007;6:391–400. doi: 10.1586/14760584.6.3.391. [DOI] [PubMed] [Google Scholar]
- 47.Kuerten S, et al. Dissociated production of perforin, granzyme B, and IFN-gamma by HIV-specific CD8(+) cells in HIV infection. AIDS Res Hum Retroviruses. 2008;24:62–71. doi: 10.1089/aid.2007.0125. [DOI] [PubMed] [Google Scholar]
- 48.Hersperger AR, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zloza A, et al. Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that coexpresses CD4 on its surface. Blood. 2009;114:3841–3853. doi: 10.1182/blood-2009-02-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zloza A, et al. Engagement of NK receptor NKG2D, but not 2B4, results in self-reactive CD8+ T cells and autoimmune vitiligo. Autoimmunity. 2011;44:599–606. doi: 10.3109/08916934.2011.593599. [DOI] [PubMed] [Google Scholar]
- 51.Byers AM, Kemball CC, Moser JM, Lukacher AE. Cutting edge: rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol. 2003;171:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Bellavance EC, et al. Development of tumor-infiltrating CD8+ T cell memory precursor effector cells and antimelanoma memory responses are the result of vaccination and TGF-beta blockade during the perioperative period of tumor resection. J Immunol. 2011;186:3309–3316. doi: 10.4049/jimmunol.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zloza A, et al. CD8 co-receptor promotes susceptibility of CD8+ T cells to transforming growth factor-beta (TGF-beta)-mediated suppression. Cancer Immunol Immunother. 2011;60:291–297. doi: 10.1007/s00262-010-0962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 55.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JK, O'Gorman MR. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Site Investigators and The NIAID DAIDS New Technologies Evaluation Group. Clin Diagn Lab Immunol. 2000;7:336–343. doi: 10.1128/cdli.7.3.336-343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. Adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. J Immunol. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.