Abstract
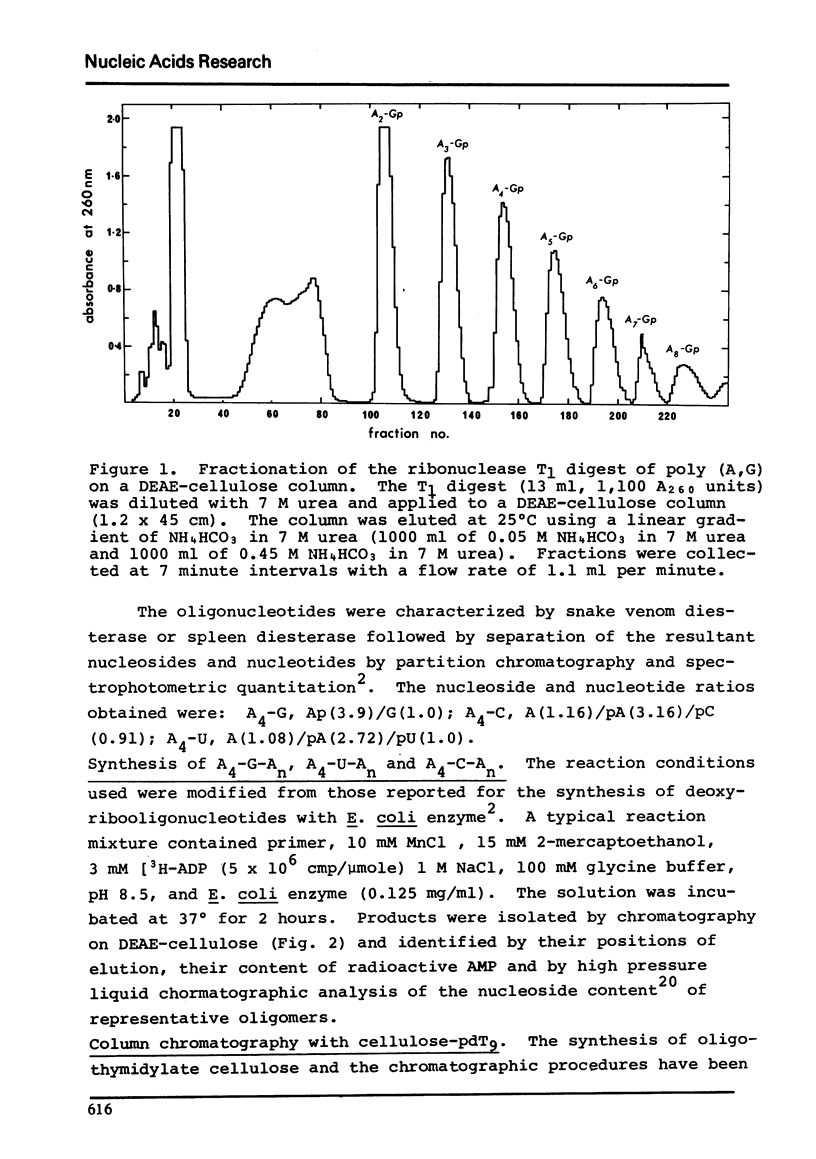
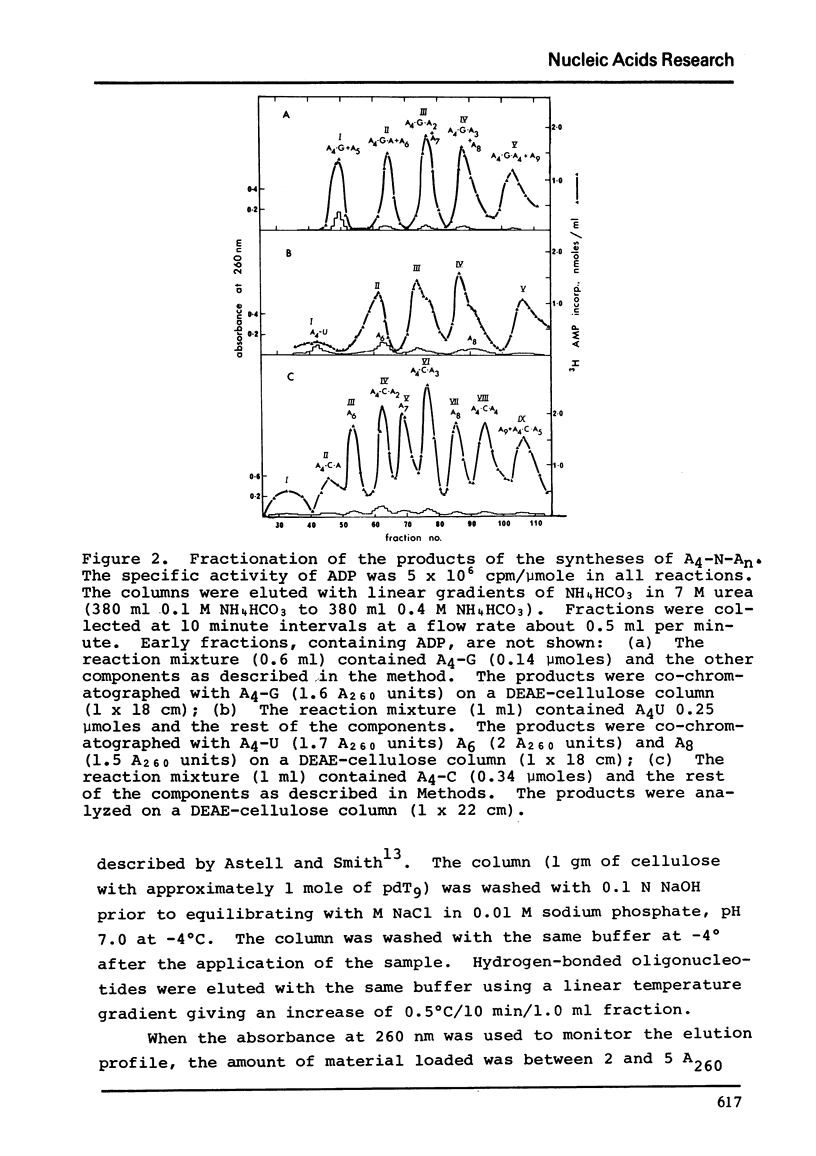
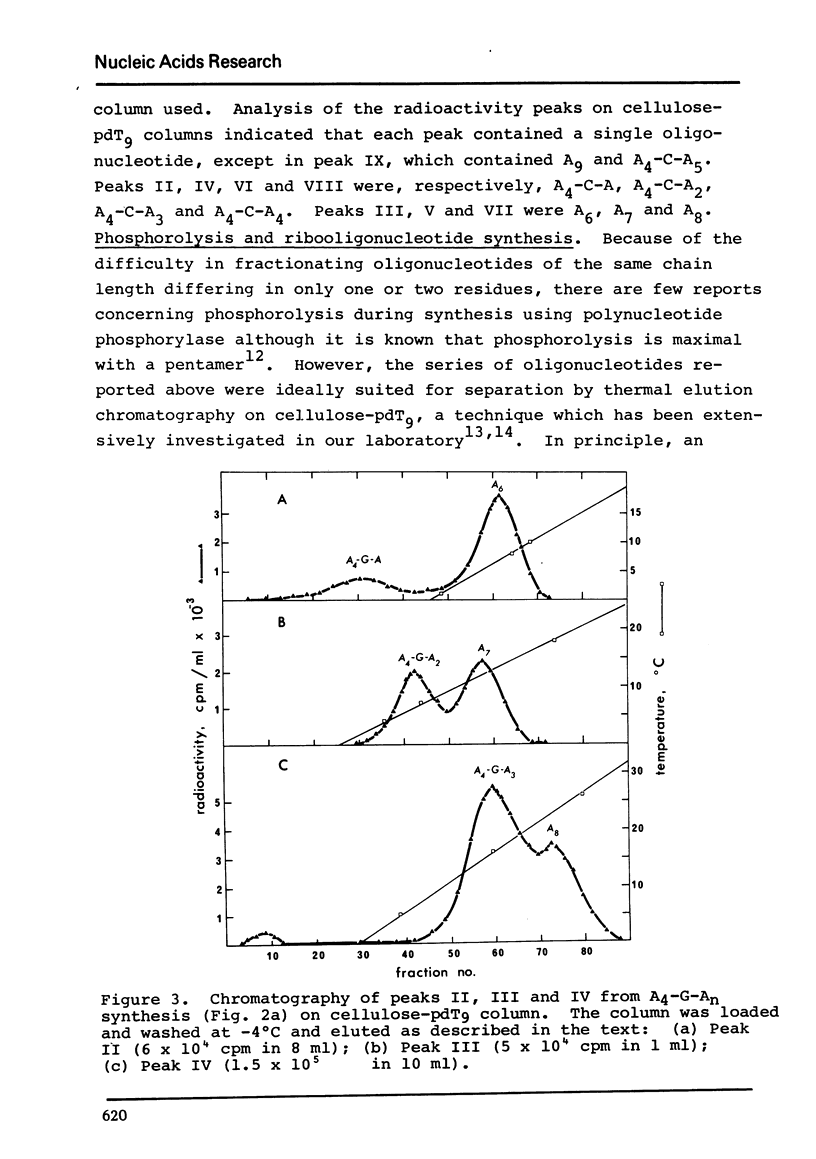
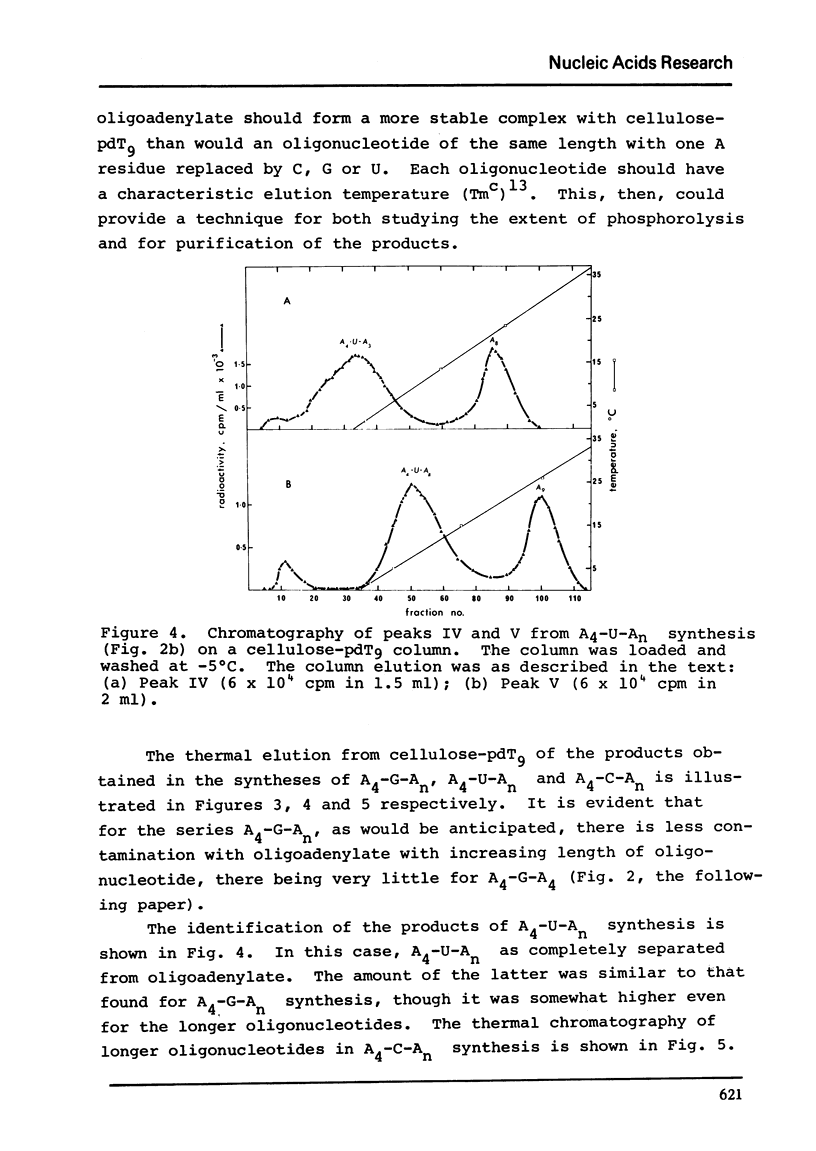
Polynucleotide phosphorylase from Escherichia coli can be used to catalyse the addition of short tracts of deoxyadenylate residues to the 3'-termini of deoxyribooligonucleotides of the type pdAn-dN (where dN = dC, dT or dG) using dADP as donor. Similarly, the enzyme can also be used to catalyse the addition of short tracts of adenylate residues to the 3'-termini of ribooligonucleotides of the type An-N (where N = C, U or G) using ADP as donor. In the ribooligonucleotide series, phosphorolytic cleavage of the primer oligonucleotides is significant and results in the concommitant production of oligoadenylates lacking the N residue. Oligomers of the same length, with and without the residue N, were readily separated by thermal elution from cellulose-pdT9 columns. This latter procedure therefore provides a simple method for purification of the oligoadenylates containing an internal base substitution and it also provides a convenient assay for oligonucleotide phosphorolysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Doel M. T., Jahnke P. A., Smith M. Further studies on the properties of oligonucleotide cellulose columns. Biochemistry. 1973 Dec 4;12(25):5068–5074. doi: 10.1021/bi00749a007. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Smith M. Synthesis and properties of oligonucleotide--cellulose columns. Biochemistry. 1972 Oct 24;11(22):4114–4120. doi: 10.1021/bi00772a014. [DOI] [PubMed] [Google Scholar]
- Hsieh W. T. Polymerization of deoxyribonucleoside diphosphates with an enzyme from an Escherichia coli mutant lacking deoxyribonucleic acid polymerase activity. J Biol Chem. 1971 Mar 25;246(6):1780–1784. [PubMed] [Google Scholar]
- Klee C. B. The proteolytic conversion of polynucleotide phosphorylase to a primer-dependent form. J Biol Chem. 1969 May 25;244(10):2558–2566. [PubMed] [Google Scholar]
- Leder P., Singer M. F., Brimacombe R. L. Synthesis of trinucleoside diphosphates with polynucleotide phosphorylase. Biochemistry. 1965 Aug;4(8):1561–1567. doi: 10.1021/bi00884a015. [DOI] [PubMed] [Google Scholar]
- Mackey J. K., Gilham P. T. New approach to the synthesis of polyribonucleotides of defined sequence. Nature. 1971 Oct 22;233(5321):551–553. doi: 10.1038/233551a0. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Singer M. F. Polynucleotide phosphorylase of Micrococcus luteus. Studies on the polymerization reaction catalyzed by primer-dependent and primer-independent enzymes. J Biol Chem. 1970 May 10;245(9):2414–2422. [PubMed] [Google Scholar]
- Pike L. M., Rottman F. The determination of 2'-O-methylnucleosides in RNA. Anal Biochem. 1974 Oct;61(2):367–378. doi: 10.1016/0003-2697(74)90404-7. [DOI] [PubMed] [Google Scholar]
- SINGER M. F. Phosphorolysis of oligoribonucleotides by polynucleotide phosphorylase. J Biol Chem. 1958 May;232(1):211–228. [PubMed] [Google Scholar]
- TOMLINSON R. V., TENER G. M. THE EFFECT OF UREA, FORMAMIDE, AND GLYCOLS ON THE SECONDARY BINDING FORCES IN THE ION-EXCHANGE CHROMATOGRAPHY OF POLYNUCLEOTIDES OF DEAE-CELLULOSE. Biochemistry. 1963 Jul-Aug;2:697–702. doi: 10.1021/bi00904a013. [DOI] [PubMed] [Google Scholar]
- Thach R. E., Sundararajan T. A., Dewey K. F., Brown J. C., Doty P. Translation of synthetic messenger RNA. Cold Spring Harb Symp Quant Biol. 1966;31:85–97. doi: 10.1101/sqb.1966.031.01.015. [DOI] [PubMed] [Google Scholar]


