Background: The effect of ADWX-1 on EAE model is unknown.
Results: ADWX-1 selectively inhibits TEM activation through regulating both Kv1.3 activity and expression.
Conclusion: ADWX-1 ameliorates EAE with a cell selectivity mechanism.
Significance: ADWX-1 is a novel potent candidate therapeutic drug for MS.
Keywords: Autoimmune Diseases, Multiple Sclerosis, Peptides, Pharmacology, Toxins
Abstract
The voltage-gated Kv1.3 K+ channel in effector memory T cells serves as a new therapeutic target for multiple sclerosis. In our previous studies, the novel peptide ADWX-1 was designed and synthesized as a specific Kv1.3 blocker. However, it is unclear if and how ADWX-1 alleviates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. In this study, the administration of ADWX-1 significantly ameliorated the rat experimental autoimmune encephalomyelitis model by selectively inhibiting CD4+CCR7− phenotype effector memory T cell activation. In contrast, the Kv1.3-specific peptide had little effect on CD4+CCR7+ cells, thereby limiting side effects. Furthermore, we determined that ADWX-1 is involved in the regulation of NF-κB signaling through upstream protein kinase C-θ (PKCθ) in the IL-2 pathway of CD4+CCR7− cells. The elevated expression of Kv1.3 mRNA and protein in activated CD4+CCR7− cells was reduced by ADWX-1 engagement; however, an apparent alteration in CD4+CCR7+ cells was not observed. Moreover, the selective regulation of the Kv1.3 channel gene expression pattern by ADWX-1 provided a further and sustained inhibition of the CD4+CCR7− phenotype, which depends on the activity of Kv1.3 to modulate its activation signal. In addition, ADWX-1 mediated the activation of differentiated Th17 cells through the CCR7− phenotype. The efficacy of ADWX-1 is supported by multiple functions, which are based on a Kv1.3high CD4+CCR7− T cell selectivity through two different pathways, including the classic channel activity-associated IL-2 pathway and the new Kv1.3 channel gene expression pathway.
Introduction
Multiple sclerosis (MS)4 is a typical autoimmune disease in which inflammatory cells from the peripheral blood invade the central nervous system (CNS) and actively attack axons, myelin sheaths, and oligodendrocytes (1, 2). The neurological disability caused by the demyelination of the axons afflicts millions of young people (3). However, the current MS therapies are expensive, are only partially effective, and are associated with side effects as well as potential toxicity; therefore, there is a need for clinical treatments that can efficiently prevent neuronal injury by attenuating the acute autoimmune exacerbations that occur in the early stages of MS.
Although the pathogenesis of MS is complicated and remains uncertain, among the multiple immune cells that participate in the development of the disease, T lymphocytes play a dominant role. In MS, the infiltrated T lymphocytes that disrupt the blood-brain barrier recognize the specific myelin antigens in the CNS where cells are restimulated. Therapies targeting the recruitment of reactive T cells in the CNS exhibit further immune modulatory effects to prevent inflammatory cytokine secretion and the neurologic deficits caused by demyelination (4, 5).
Recently, the voltage-gated potassium channel Kv1.3 was proposed as a promising new therapeutic target for MS because the numbers of Kv1.3 on autoimmune disease-related CCR7− effector memory T cells (TEM) increase significantly after activation by myelin antigens (6–8). The naive CCR7+ T cells differentiate into auto-reactive CCR7− T cells by repeated autoantigen stimulation. These cells migrate into inflamed tissues and exhibit immediate effector functions, such as inflammatory cytokine secretion (5). Therefore, it is possible that the selective suppression of TEM cells using specific Kv1.3 blockers could efficiently suppress these immune responses and alleviate MS. The toxin peptides (e.g. ShK-F6CA (9), OSK1 (10), margatoxin (11), etc.) derived from naturally venomous animals exhibit greater potency (with picomolar affinity) and selectivity on Kv1.3 blockade than small chemical molecules (e.g. Psora-4 (12), correolide (13), etc.) designed to block Kv1.3 (at nanomolar concentrations) (14). Currently, the newly designed Kv1.3 blocker peptides (e.g. Moka-1 (15), ShK (L5) (5), etc.) were synthesized to target the Kv1.3 channel as a new therapy for MS. However, novel peptides remain required for sufficient specificity to distinguish between Kv1.3 and other related Kv1.x channels (16, 17). Moreover, although Kv1.3 blocker peptides, such as ShK (a 35-AA polypeptide isolated from the sea anemone Stichodactyla helianthus), efficiently attenuate the severity of experimental autoimmune encephalomyelitis (EAE), a model for MS (18, 19), the mechanisms underlying the peptide-mediated inhibition of activation and proliferation of primary TEM cells derived from the EAE model are poorly understood.
In a previous study, we developed the novel Kv1.3 blocker peptide ADWX-1 as a representative drug with a high affinity, high potency, and selectivity toward the Kv1.3 channel (20, 21). This peptide is based on the natural scorpion toxin BmKTX in which three important residues were mutated in a structure-modification strategy to enhance the Kv1.3 channel selectivity (20). ADWX-1 blocked Kv1.3 currents with an IC50 of 1.89 pm and displayed specificity for Kv1.3 over Kv1.1 and Kv1.2 (20). However, to develop new drug treatments for MS in a clinical setting, further functional studies of ADWX-1 in the animal model of MS are required. In this study, we first examined whether ADWX-1, the Kv1.3-targeting peptide, ameliorated the EAE model. Next, we elucidated the effects of ADWX-1 on T cell activation in the EAE model focusing on the selective effect on TEM cells and the TEM cell activation pathways involved.
EXPERIMENTAL PROCEDURES
Peptide Expression and Purification
ADWX-1 peptide was obtained as described previously (20). The molecular mass of the purified ADWX-1 peptide was obtained by MALDI-TOF-MS (Voyager-DESTR, Applied Biosystems). Peptide purity was >95%.
Animals and Cell Culture
Female inbred 8–10-week-old Sprague-Dawley (SD) rats were purchased from the ABSL-III laboratory at Wuhan University and were housed under specific pathogen-free conditions. All animal work was performed in accordance with protocols and guidelines approved by the Institutional Animal Care and Use Committee.
The Jurkat T cells were purchased from the China Center for Type Culture Collection. Ficoll gradients were used to isolate splenocytes and peripheral blood mononuclear cells (PBMCs) from rats. The CD4+ T cells from PBMCs were purified using MACS (Miltenyi Biotech). The separated cells were sorted into CCR7− and CCR7+ subsets using FACS. The primary T cells or total PBMCs were seeded at 105/well with 105 irradiated autologous PBMCs (3,000 rads) in a volume of 200 μl/well in round-bottom 96-well plates. The cells were cultured in 1640 RPMI medium supplemented with 2 mm glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1% homologous rat serum at 37 °C. The T cells derived from the EAE rats were incubated with the Kv1.3 blocker ADWX-1 for 60 min and were stimulated with the myelin-specific antigen myelin basic protein (MBP) (5 μg/ml) for 48–72 h. The T cells derived from control rats or the peripheral blood of healthy donors were stimulated with soluble anti-CD3 antibody (Ab) (1 μg/ml) (eBioscience) or PHA (Sigma).
Rat EAE Induction and Drug Administration
The EAE model was induced using homologous homogenates extracted from the brain and spinal cord of SD rats. Homogenates containing 25 μg of brainstem and spinal cord extracts and 50 μl of PBS were emulsified in 50 μl of complete Freund's adjuvant (Sigma) supplemented with 0.4 mg of Mycobacterium tuberculosis (H37RA). The footpad of each rat was immunized by the subcutaneous injection of this emulsion at a dose of 150 μl/200 g and with an auxiliary injection of 0.2 ml of pertussis toxin. Control rats received PBS plus complete Freund's adjuvant, and the second immunization was administered 7 days after the first injection. The rats were weighed and observed daily. In the prevention trial, the rats were subcutaneously injected once daily with 100 μg/kg ADWX-1 in 1 ml of PBS from days 0 to 4. In the treatment trial, ADWX-1 was administered after the onset of disease for 3 days of continued therapy. The vehicle group received PBS. The clinical scores were recorded daily as follows (19): 0 = no clinical signs; 0.5 = distal limp tail; 1 = limp tail; 2 = mild paraparesis or ataxia; 3 = moderate paraparesis; 4 = complete hind leg paralysis or severe ataxia; 5 = 4+ incontinence; 5.5 = tetraplegia; 6 = death. Special care was required for animals with severe EAE (score of 3 or more).
Ca2+ Measurement
The [Ca2+]i in lymphocytes was calculated as described previously (18), and T cells were attached to an l-lysine-coated glass dish and loaded with 1 μm Fura-2 AM (Dojindo, Japan) for 60 min, and then cells were washed and incubated with 1 or 10 nm ADWX-1 or 0.1% bovine serum albumin (BSA, Sigma) for 50 min. After antigen stimulation with MBP or anti-CD3 Ab in Ca2+-rich medium, the intracellular calcium concentrations were determined by fluorescence intensity. The fluorescence excitation wavelength was set at 480 nm, and emission wavelength was set at 530 nm.
Cell Proliferation
106 PBMCs were seeded in round-bottom 96-well plates (Corning-Costar) and were incubated with 50 or 250 μg/ml homogenate antigen in 200 μl of culture medium supplemented with 1% homologous rat serum. ADWX-1 at various concentrations (0.1, 1, and 10 nm) was added 60 min before antigen stimulation. The cells were cultured for 4 days and were pulsed with [3H]thymidine) (1 μCi per well) 16 h before harvesting. The proliferative response was assessed using a β-scintillation counter (Beckman) to measure the [3H]thymidine incorporation (counts/min value) as follows: background counts in unstimulated (resting) cells were below 600 cpm, and counts in only antigen-activated (maximal) cells were 60,000–80,000 cpm.
In vitro generated CD4+CCR7− TEM cells were suspended in PBS at a concentration of 107 cells/ml and were incubated at 37 °C for 10 min with carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) at a final concentration of 1 μm. The cells were washed, resuspended in medium, and stimulated with antigen. The fluorescence intensity of the CFSE was measured by flow cytometry (FCM) after 4 days (22).
For the signal transduction study, CD4+CCR7− T cells derived from the PBMCs of EAE rats were incubated for 60 min with 1 μm cyclosporin A (CsA, Sigma) or 30 μm rottlerin (Sigma) or 25 μm pyrrolidine dithiocarbamate (Sigma) and were stimulated with MBP for 4 days. ADWX-1, 1 or 10 nm, was added 60 min before antigen stimulation. The proliferative response was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay using a Cell Counting Kit-8 (CCK-8, Dojindo, Japan).
Flow Cytometry
The PBMCs from each group in the in vivo study were directly double-stained directly using PE-Cy5-labeled anti-rat CD4 mAb (Pharmingen) and rabbit anti-CCR7 antibody (Abcam) followed by a secondary PE-conjugated goat anti-rabbit IgG. The PBMCs cultured in vitro were collected after 4 days and were double-stained using PE-Cy5-CD4 and PE-CCR7. The stained cells were analyzed by FCM (Beckman Coulter, Epics Altra II). To determine the Kv1.3 protein expression, the PBMCs cultured in vitro were harvested after 48 h and were triple-stained with PE-Cy5-CD4, PE-CCR7, as well as FITC-conjugated anti-Kv1.3 antibody (Sigma, P4247). The Kv1.3 mean fluorescence intensity (MFI) values were measured using FCM; the PBMCs isolated in vivo were directly stained directly with FITC-Kv1.3 antibody for the FCM assay.
Quantitative RT-PCR
Freshly purified CD4+CCR7− and CD4+CCR7+ T cells from PBMCs were activated in vitro for 48 h, and the cells were resorted into CD4+CCR7− and CD4+CCR7+ populations for quantitative RT-PCR analysis. The RNA was isolated from the cells using TRIzol reagent (Invitrogen), and reversed transcription was performed using the First Strand cDNA synthesis kit (Fermentas), in accordance with the manufacturer's instructions. Total PBMC samples triple-stained for the Kv1.3 MFI test were also sorted into CD4+CCR7− and CD4+CCR7+ populations for quantitative RT-PCR analysis. The expressions of the gene encoding KCNA3 (Kv1.3 channels) were quantified using the SYBR Green PCR master mix kit (Toyobo). The following primer pairs were selected for the amplification of KCNA3 gene: forward, 5′-AGTATATGGTGATCGAAGAGG-3′, and reverse, 5′-AGTGAATATCTTCTTGATGTT-3′. The reactions were performed on an ABI 7500 platform under the following conditions: 95 °C for 5 s, 55 °C for 8 s, and 72 °C for 9 s, preceded by 10 min at 95 °C and followed by 10 min at 95 °C. Melting curves were performed to verify the specificity of the product, and primers for GAPDH were included as an internal reference. The relative gene expression was determined as the ratio of KCNA3 to GAPDH gene expression for each sample.
ELISA
IL-2 levels from the supernatants of the cell cultures were measured using ELISA. Supernatants of cell cultures were collected 16 h after antigen stimulation. Cytokine levels were measured using rat and human IL-2 and IFN-γ Quantikine kits (R&D Systems) according to the manufacturer's instructions. The IL-2, IFN-γ, and TNF-α cytokine production in serum from each group of animal experiments (on day 15) was also assessed by rat Quantikine kits (R&D Systems). Homogenates of rat brain and spinal cord were used to assay the IL-2 levels in tissues by rat IL-2 Quantikine kit (R&D Systems).
T Cell Differentiation
Naive CD4+ T cells from rats, sorted using FACS, were stimulated for 4–5 days with anti-CD3/CD28 beads (Miltenyi Biotec) plus the following recombinant cytokines: TGF-β (5 ng/ml, PeproTech) and IL-6 (10 ng/ml, PeproTech) for polarization of the T cells into the Th17 subset. The cells were incubated with 1 or 10 nm ADWX-1 for 60 min before differentiation. The cells were harvested and double-stained with PE-Cy5-labeled anti-rat CD4 mAb (Pharmingen) and PE-labeled anti-rat IL-17A mAb (eBioscience). The differentiated cells were double-stained with PE-labeled anti-rat IL-17A mAb and rabbit anti-CCR7 antibody (Abcam) followed by a secondary FITC-conjugated goat anti-rabbit IgG. The differentiated cells were restimulated using anti-CD3 Ab alone, and ADWX-1 was added; the expression of CD4+IL-17+ Th17 cells was determined after 3 days. The supernatants from primary differentiated cultures were collected on day 4, and the restimulated cultures were collected after incubation overnight. IL-17 production was assessed using ELISA kit (eBioscience). CD4+ T cells were activated using anti-CD3 Ab, and ADWX-1 was added; the expression of CD4+Foxp3+ Treg cells was determined after 3 days following the intracellular staining of Foxp3 (eBioscience). For the in vivo study, the PBMCs from each group were isolated and stained directly for the FCM assay of the percentage of CD4+IL-17+ and CD4+Foxp3+ cells without incubation. On day 15, IL-17 and TGF-β cytokines in the serum or the homogenates were measured using ELISA.
Western Blotting
In signaling transduction study, CD4+CCR7− T cells from EAE rats were incubated with the indicated compound (rottlerin, pyrrolidine dithiocarbamate) as well as 1 or 10 nm ADWX-1 for 1 h, and then stimulated in vitro by myelin antigen MBP for 4 h. Cells were lysed by RIPA plus 0.1% pepstatin, aprotinin, leupeptin, and 0.1% PMSF for Western blotting analysis. The concentration of proteins was determined by Bradford assay before the lysate was boiled with loading buffer. Proteins were separated on a 10% SDS-polyacrylamide gel and then were transferred to PVDF membranes under 100 V for 80 min (Immobilon-P, Millipore). Membranes were blocked in 5% milk TBST for 1 h. NF-κB activity was determined by phospho-NF-κB p65 (Ser-536) (93H1) rabbit mAb (Cell Signaling) followed by a second antibody of HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology). The membranes were developed using an ECL kit (Pierce) according to the manufacturer's instructions. For the Kv1.3 membrane protein expression study, mouse monoclonal (S23-27) antibody to KCNA3 (Abcam) was used for membrane blot, and monoclonal anti-β-actin antibody (Sigma) was used as control.
Histology
For H&E staining and Luxol fast blue staining, rats were anesthetized with 10% chloraldurate and then perfused transcardially by 0.9% NaCl and pre-fixed with 4% paraformaldehyde in 0.1 m PBS. The lumbar region of the spinal cord and brainstem were collected and fixed in paraformaldehyde solution. The tissues were embedded in paraffin and cut into transverse sections (5 μm) that were stained by hematoxylin and eosin (H&E) to evaluate inflammatory cell distribution and by Luxol fast blue to evaluate demyelination. The number of perivascular inflammatory infiltrates and the degree of demyelination per section were calculated by two blinded investigators as histological scores (23, 24) as follows: grade 1 refers to less than 25% infiltration or demyelination; grade 2 denotes over 25% infiltration or demyelination, and grade 3 denotes widespread infiltration or demyelination. For TEM analysis, tissues were fixed in 3% glutaraldehyde and 1% osmium tetroxide and embedded in Epon 812 to make ultrathin sections that were counterstained by uranyl acetate and lead citrate and then examined in a transmission electron microscope (Hitachi) at 80 kV.
Statistical Analysis
The differences between groups were evaluated using a Student's t test or a one-way analysis of variance followed by a Dunnett's test for multiple comparisons. The one-way analysis of variance was used to compare the clinical score between two groups at every time point and was followed by a Mann-Whitney U test to determine the differences between the two curves in the EAE study. p values less than 0.05 were considered statistically significant. The data were analyzed using the GraphPad Prism software.
RESULTS
ADWX-1 Has a Therapeutic Effect on Rat EAE Model in Vivo and in Vitro
Previously, we designed and synthesized a novel ADWX-1 peptide as a specific Kv1.3 blocker and found that this peptide displayed high potency and selectivity toward Kv1.3 when compared with the related Kv1.1 and Kv1.2 channels (20). To evaluate whether the Kv1.3-specific peptide ADWX-1 is a viable therapeutic agent for the treatment of MS, a rat EAE model was employed to simulate the human MS disease. The rat EAE model is more appropriate than the mouse EAE model, because the Kv1.3 expression pattern in mice differs from humans and is not suitable for evaluating the effects of Kv1.3 blockers (25).
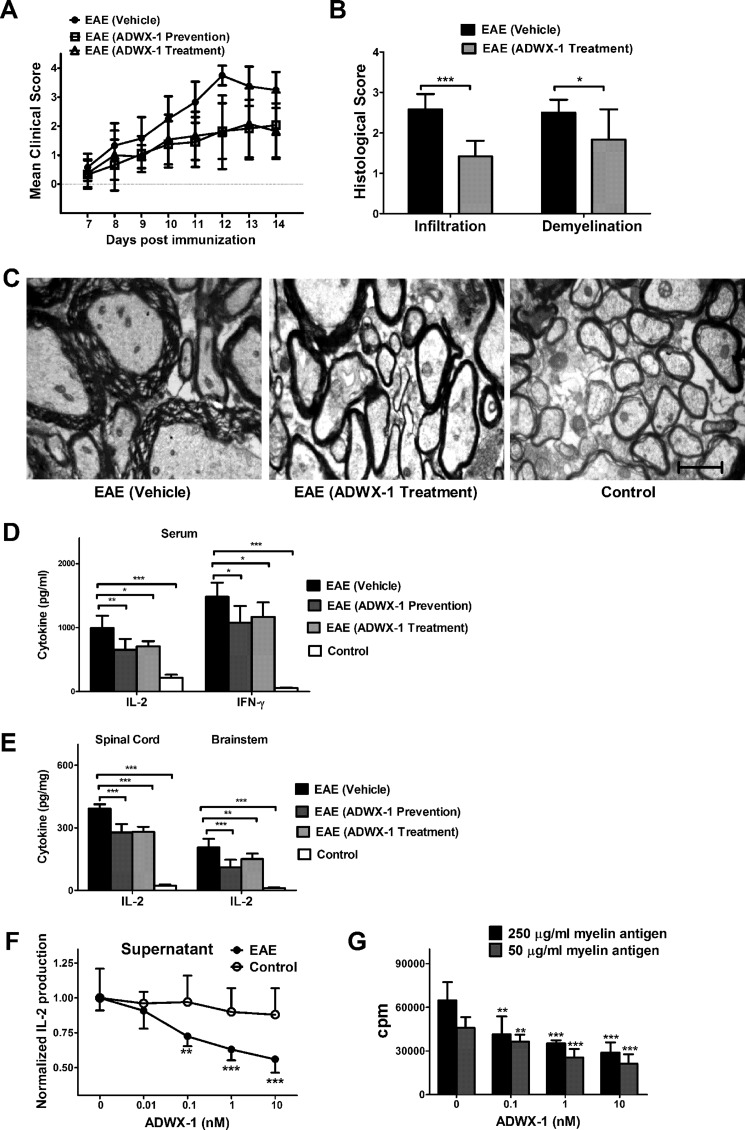
Stable symptoms of acute EAE were induced by immunizing Sprague-Dawley rats. These rats developed an ascending paralysis from day 7 to 14 post-immunization, and the clinical signs peaked at days 11–14 (Fig. 1A). For the prevention trial and the treatment trial, the EAE rats received a subcutaneous injection of 100 μg/kg/day ADWX-1. In the prevention trial, the ADWX-1-pretreated rats exhibited significantly reduced neurological scores compared with vehicle-treated rats on days 10 (p < 0.01), 11 (p < 0.001), 12 (p < 0.001), 13 (p < 0.001), and 14 (p < 0.01, Fig. 1A). In the treatment trial, the ADWX-1-treated rats also showed greatly reduced neurological scores compared with the vehicle-treated rats on days 10 (p < 0.05), 11 (p < 0.001), 12 (p < 0.0001), 13 (p < 0.01), and 14 (p < 0.001, Fig. 1A). Interestingly, the clinical scores of the prevention group were also significantly lower than the vehicle group on days 10–14, exhibiting a delayed onset and delayed peak day for the disease, suggesting that the peptide may modulate T cell autoactivation peripherally. In addition to the neurological score, significant reductions in inflammatory infiltrates (p < 0.001) and demyelination (p < 0.05) in the affected spinal cord, as shown by the histological score, were observed in the ADWX-1-treated EAE rats compared with the vehicle-treated rats (Fig. 1B). The transmission electron microscopy analysis revealed that the ultrastructure of the tissue in ADWX-1-treated rats contained less demyelinated axons compared with the vehicle-treated rats (Fig. 1C).
FIGURE 1.
Therapeutic effect of ADWX-1 on a rat acute EAE model. A, prevention and treatment trial. Rats were immunized with myelin antigens on day 0; the prevention group (from day 0 to day 4) and treatment group (for 3 days after the onset of clinical signs) were subcutaneously injected with ADWX-1 at a dose of 100 μg/kg, and the vehicle group was injected with PBS (n = 12). The clinical score was recorded daily. B, statistical data for H&E staining and Luxol fast blue staining (n = 6) shown as the histological score depicting the inflammatory infiltration and demyelination in the spinal cord of EAE rats treated with ADWX-1 or PBS. C, representative photographs from transmission electron microscopy demonstrating demyelination and axonal loss in the spinal cord of the vehicle group, treatment group, and control group. Scale bar, 1,600 nm. D, Th1 type IL-2 and IFN-γ production in serum from each group (n = 6) was determined by ELISA. E, IL-2 production in CNS tissues from each group (n = 6) was determined by ELISA. **, p < 0.01; ***, p < 0.001 versus EAE (vehicle). F, cell activation in vitro. PBMCs from EAE (black) and control (white) rats were treated with various concentrations of ADWX-1 (0, 0.01, 0.1, 1, and 10 nm) for 1 h before activation with MBP or PHA for 16 h. IL-2 levels in the supernatants were assessed by ELISA. G, cell proliferation in vitro. PBMCs from EAE rats were treated with various concentrations of ADWX-1 (0, 0.1, 1, and 10 nm) and were activated for 96 h with high concentration (250 μg/ml, black) or low concentration (50 μg/ml, gray) of myelin antigen. **, p < 0.01; ***, p < 0.001 versus 0 nm ADWX-1. The data represent the mean ± S.D. from two independent experiments performed in triplicate.
Considering the importance of Th1-type cytokines, such as IL-2, IFN-γ, and TNF-α during the development of autoimmune disease, various inflammatory cytokines in the serum were analyzed. In the prevention group and the treatment group, IL-2 and IFN-γ productions observed after ADWX-1 engagement were less than that observed in the vehicle-treated rats but higher than that in the control rats (Fig. 1D). Furthermore, the down-regulation of IL-2 in the CNS of the ADWX-1-treated rats further supports that ADWX-1 attenuates cellular infiltration in pathological tissues, including the brainstem and the spinal cord (Fig. 1E).
To confirm the decrease in inflammatory cytokines in the ADWX-1-treated EAE rats, we investigated whether ADWX-1 suppresses T cell activation and proliferation in isolated PBMCs in vitro. Using ELISA, we observed that inflammatory IL-2 secretion in MBP-stimulated PBMCs from EAE rats treated with ADWX-1 (0.01, 0.1, 1, and 10 nm) in vitro was markedly decreased compared with non-ADWX-1-treated cells in a dose-dependent manner (Fig. 1F). However, the T cell response of PHA-stimulated PBMCs from the control rats treated with ADWX-1 (0.01, 0.1, 1, and 10 nm) in vitro was not altered compared with non-ADWX-1-treated cells (Fig. 1F). These results suggest that the suppression of myelin-specific T cell activation in vitro supports the modulation of inflammatory cytokines by ADWX-1 in vivo. Furthermore, ADWX-1 inhibited the EAE rat T cell proliferation triggered by high and low concentrations of myelin antigen in a dose-dependent manner (Fig. 1G).
These in vivo and in vitro data demonstrate that ADWX-1 significantly alleviates EAE. Considering that both the efficacy and safety are crucial issues for long term drug therapies and that the Kv1.3 blocker peptides are toxins derived from naturally venomous animals, we performed 2-week acute toxicity studies using 50- or 100-fold doses of ADWX-1 (5 or 10 mg/kg). These doses failed to induce pathological changes in the behavior or tissues of the rats (data not shown).
ADWX-1 Preferentially Inhibits T Cell Activation and Proliferation in CD4+CCR7− T Cells
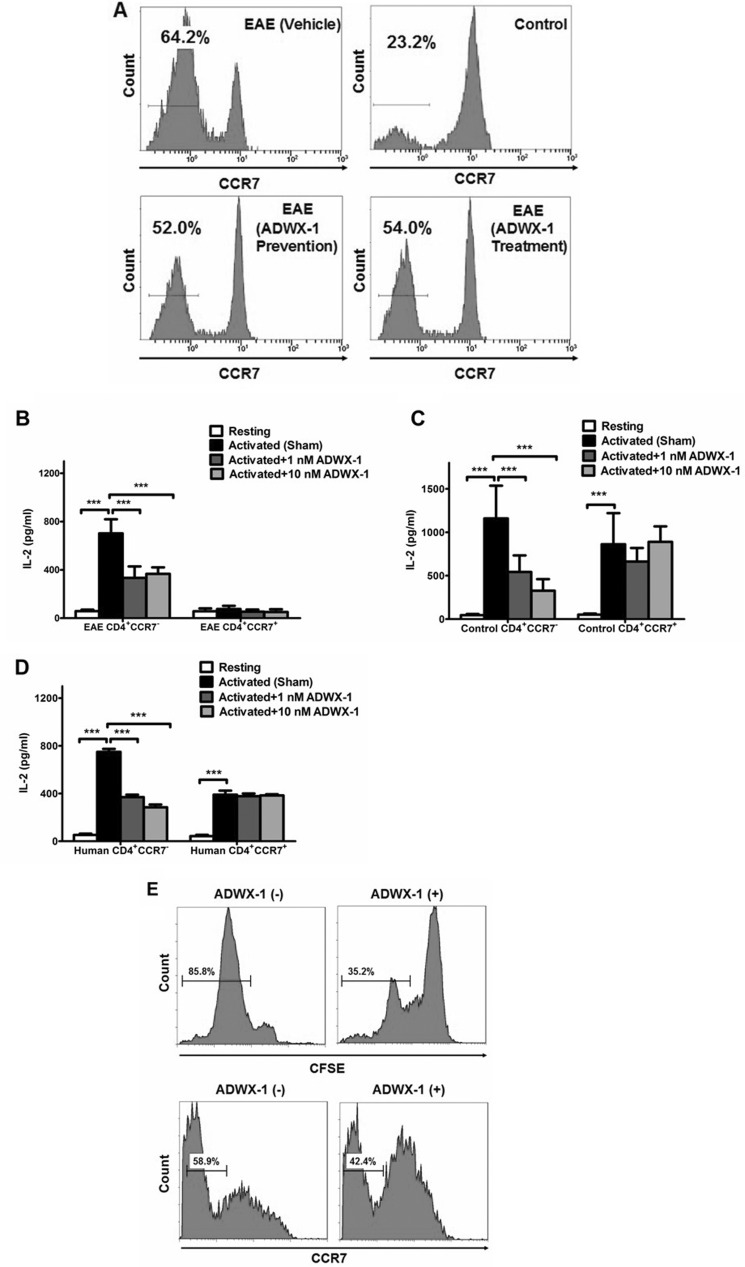
The amelioration of the EAE model by the ADWX-1 peptide prompted us to delineate the possible mechanisms underlying the therapeutic effects of this peptide. We addressed the molecular mechanisms starting with the critical finding that CD4+CCR7− TEM cells in the EAE model were specifically inhibited by ADWX-1 engagement. A 2-fold up-regulation of CD4+CCR7− TEM cells was observed in the EAE model as compared with the control animals (Fig. 2A), possibly because of the in vivo proliferation of the myelin antigen-specific T cells in this population. This hypothesis is consistent with the observation that patients carrying autoimmune diseases generate a high number of effector memory T cells with high expression of Kv1.3 (7, 25). However, the injection of 100 μg/kg/day of ADWX-1 decreased the number of CD4+CCR7− TEM cells by ∼12% in the EAE model in both the prevention and treatment trials (Fig. 2A), indicating that ADWX-1 ameliorates the disease through the physiological modulation of CCR7− TEM proliferation.
FIGURE 2.
Selective regulation of the CD4+CCR7− T cell activation by ADWX-1. A, representative FCM profile depicting CCR7 staining in CD4-gated PBMCs from each group; values in the histogram indicated the percentage of CD4+CCR7− T cells. B, sorted CD4+CCR7− and CD4+CCR7+ T cell populations in PBMCs from EAE rats were treated in vitro with 1 or 10 nm ADWX-1 or PBS (sham) 1 h before activation with MBP for 16 h. Resting control refers to quiescent cells in the base line. IL-2 levels in the supernatants were assessed by ELISA. C, sorted CD4+CCR7− and CD4+CCR7+ T cell populations in PBMCs from control rats were treated as outlined in B; IL-2 secretion in vitro was assessed by ELISA. D, sorted human CD4+CCR7− and CD4+CCR7+ T cells from three healthy donors were treated as outlined in B; IL-2 secretion in vitro was assessed by ELISA. ***, p < 0.001 versus activated (sham). The data represent the mean ± SD from three independent experiments performed in triplicate. E, top panels, CD4+CCR7− T cells sorted from the PBMCs of EAE rats were previously CFSE-labeled and treated with (+) or without (−) 1 nm ADWX-1 and were stimulated with MBP for 96 h in vitro; the numbers in each gate are the percentage of proliferated cells assessed by CFSE staining. Bottom panels, total PBMCs from EAE rats were treated with (+) or without (−) 1 nm ADWX-1 before the stimulation with MBP for 96 h in vitro; the cells were gated on CD4, and numbers in each gate were the percentage of CD4+CCR7− cells. The data are representative of two independent experiments.
To elucidate the mechanism underlying the in vivo suppression of the CCR7− phenotype by the Kv1.3 blocker peptide ADWX-1, we investigated the effects of ADWX-1 on the in vitro immune responses of primary CD4+CCR7+ and CD4+CCR7− T cell subsets purified from PBMCs.
A substantial amount of inflammatory IL-2, a marker of CD4+ T cell activation, was produced in cultured MBP-stimulated CD4+CCR7− T cells from EAE rats compared with resting cells from the same population (p < 0.001), whereas no IL-2 was produced by the CD4+CCR7+ subset (p > 0.05, Fig. 2B). However, the secretion of IL-2 upon activation in vitro was suppressed significantly (∼50%) by the addition of 1 or 10 nm ADWX-1 in CD4+CCR7− but not in CD4+CCR7+ T cells (Fig. 2B), suggesting that Kv1.3-related signaling pathways might be involved in the activation of the CCR7− subset. Similar effects of ADWX-1 were observed in the cells from control rats stimulated with anti-CD3 Ab (Fig. 2C). Similar results in IL-2 production were observed in the splenocytes of rats and in the human T cell line Jurkat T cells (data not shown). To determine whether ADWX-1 is specific to human CD4+CCR7− T cells at a clinical level, we stimulated purified CD4+CCR7− and CD4+CCR7+ T cells from healthy individuals in the presence of ADWX-1. Similar to the rat cells, the ADWX-1 peptide affected the activation of the human CCR7− subset but not the CCR7+ subset within the same CD4+ population (Fig. 2D). In addition to IL-2, an analogous change in IFN-γ production was also observed (data not shown). These results suggest that ADWX-1 plays a selective role in the inhibition of CD4+CCR7− TEM activation in both rats and humans.
It is known that proliferation follows activation. We determined that ADWX-1 selectively inhibited the MBP-triggered proliferation of CFSE-labeled CD4+CCR7− T cells from EAE rats (Fig. 2E), and the number of CCR7− cells in the CD4-gated cell population treated with ADWX-1 dropped after activation (Fig. 2E). These results further confirmed that ADWX-1 is more specific for CD4+CCR7− T cells.
ADWX-1 Inhibits CD4+CCR7− T Cell Activation through a Kv1.3-mediated IL-2 Activation Pathway
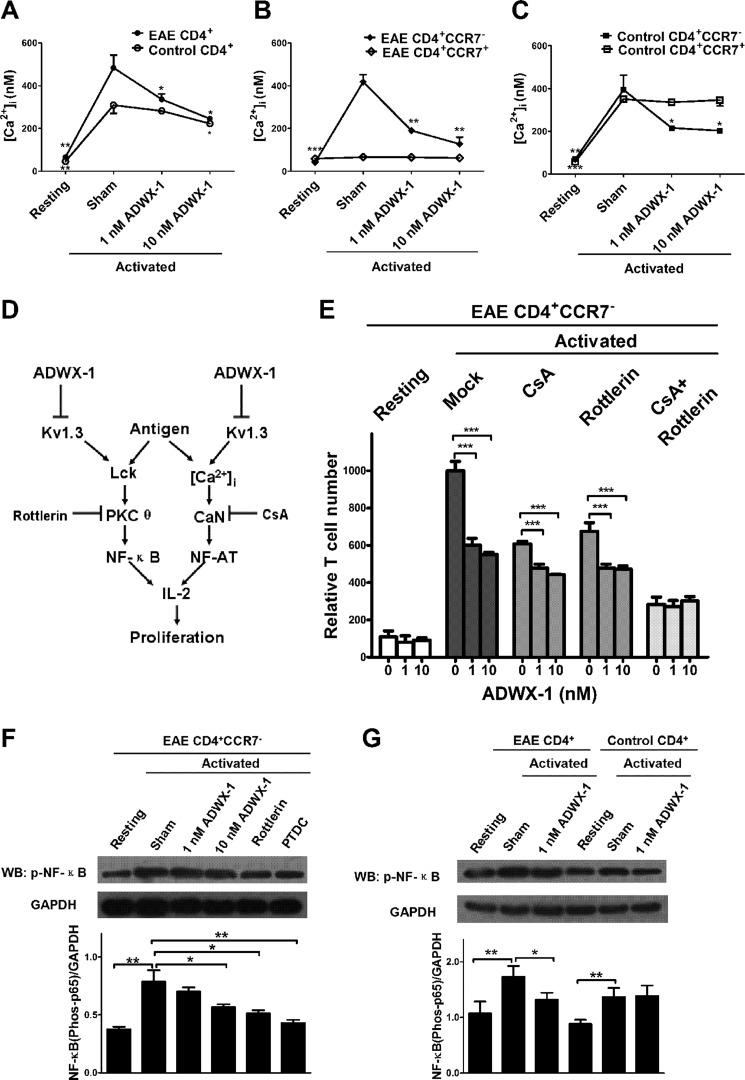
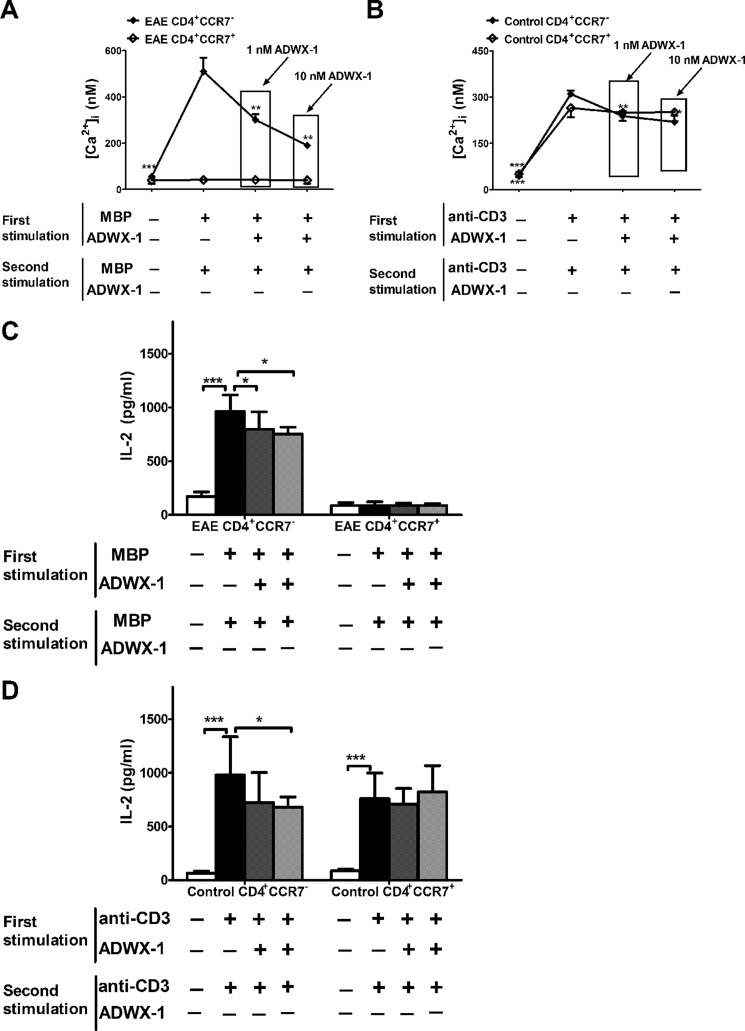
To further elucidate the molecular mechanism underlying the observed selective inhibition of CD4+CCR7− TEM cell activation by ADWX-1, we investigated the signaling activity of the IL-2 pathway, which is critical for the activation of TEM cells. Previously, we determined that ADWX-1 targets the artificially expressed Kv1.3 channels in transfected cells and blocks the channel currents (20). Moreover, the voltage-gated Kv1.3 channels open for potassium efflux after antigen-induced depolarization, which promotes calcium influx through the calcium release-activated calcium channel (26, 27). To determine the effect of ADWX-1 on the initial calcium signaling for downstream IL-2 activation in antigen presentation, [Ca2+]i was measured in accordance with methods described previously (18). It was observed that CD4+ T cells from the EAE and control rats exhibited increased [Ca2+]i following MBP or anti-CD3 Ab stimulation (p < 0.01). However, the increased [Ca2+]i in the activated CD4+ T cells from EAE rats was reduced by the ADWX-1 treatment at concentrations of 1 and 10 nm (p < 0.05, Fig. 3A). In contrast, ADWX-1 was less effective at inhibiting [Ca2+]i in the activated CD4+ T cells from the control rats (Fig. 3A). Furthermore, the ADWX-1 treatment dramatically reduced [Ca2+]i in activated CD4+CCR7− T cells, but not in CD4+CCR7+ T cells, from EAE (Fig. 3B) and control rats (Fig. 3C), likely because the IKCa1 channel (calcium-activated K+ channel) modulates Ca2+ signaling in activated naive/TCM cells in place of the Kv1.3 channel in TEM cells (7, 25).
FIGURE 3.
Specific inhibitory effect of ADWX-1 on the CD4+CCR7− subset IL-2 pathway. A, calcium measurement. The sorted CD4+ T cells from the PBMCs of EAE and control rats were loaded with Fura-2 AM and were preincubated with 1 or 10 nm ADWX-1 for 50 min. The cells were activated for 10 min with MBP or anti-CD3 Ab. [Ca2+]i was calculated as the mean ± S.D. and is the representative of three independent experiments performed in duplicate. B, sorted CD4+CCR7− and CD4+CCR7+ T cells from the PBMCs of EAE rats were treated as outlined in A, and [Ca2+]i was calculated in the two populations. C, sorted CD4+CCR7− and CD4+CCR7+ T cells from the PBMCs of control rats were treated as outlined in A, and [Ca2+]i was calculated in the two populations. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus activated (sham). D, schematic diagram of the ADWX-1-mediated anti-IL-2 activation mechanisms. ADWX-1 preferentially targets the Kv1.3 channels on CD4+CCR7− T cells to prevent the Kv1.3-mediated downstream cascades for IL-2 activation, including the PKCθ pathway (left) and the calcium signaling pathway (right). E, CD4+CCR7− T cells sorted from EAE rats were treated with CsA, rottlerin, or a combination of the two with the indicated concentrations of ADWX-1. After MBP stimulation, the normalized numbers of proliferated cells from Mock- (no inhibitor added), CsA-, rottlerin-, CsA + rottlerin-treated cells were analyzed. ***, p < 0.001 versus 0 nm ADWX-1. F, effects of 1 and 10 nm ADWX-1 on the PKCθ-NF-κB pathway in CD4+CCR7− cells; the activities of NF-κB (phos-p65 Ser-536) stimulated by MBP were detected by Western blotting (WB), and the PKCθ-specific inhibitor rottlerin and the NF-κB inhibitor pyrrolidine dithiocarbamate were used as positive controls. The phosphorylation levels were quantified using Quantity One software, and the results are presented as a ratio to GAPDH. G, effects of 1 nm ADWX-1 on NF-κB activity in CD4+ T cells from EAE (MBP-stimulated) and control (anti-CD3 Ab-stimulated) rats were detected by Western blotting. Proteins were analyzed as outlined in F. *, p < 0.05; **, p < 0.01 versus activated (sham). The data are the representative of three independent experiments performed in triplicate.
To further investigate the IL-2 activation pathway involved in the ADWX-1-induced blockade of the Kv1.3 channel, including the Ca2+ mediated NF-AT pathway and the PKCθ-triggered NF-κB pathway (Fig. 3D), we pretreated purified CD4+CCR7− T cells from EAE rats with pathway-specific inhibitors followed by MBP stimulation in the presence or absence of ADWX-1. Cyclosporin A (CsA), a CaN inhibitor, was used to inhibit the NF-AT cascade to test the effect of ADWX-1 on the NF-κB pathway. Rottlerin, a PKCθ-specific inhibitor, was used to inhibit the NF-κB cascade to test the effect of ADWX-1 on the NF-AT pathway. The Mkb-triggered proliferation of inhibitor-treated cells was reduced in both cases (compared with mock-treated cells, in the absence of ADWX-1, the CsA-treated cells exhibited a reduction of ∼40% and the PKCθ-treated cells exhibited a reduction of ∼30%, Fig. 3E), suggesting that both pathways contribute to the antigen-induced cell activation and proliferation. In the CsA-treated NF-κB pathway, a decrease in proliferation was observed after the ADWX-1 treatment (p < 0.001, Fig. 3E), suggesting that ADWX-1 may be involved in regulating IL-2 activation and cell proliferation through the NF-κB pathway. In the rottlerin-treated NF-AT pathway, a decrease in proliferation was also observed after the ADWX-1 treatment (p < 0.001, Fig. 3E), suggesting that ADWX-1 signals through calcium modulation to trigger an inhibitory signaling cascade of NF-AT, which inhibits IL-2 activation and cell proliferation. The suppression of calcium in CD4+CCR7− T cells by ADWX-1 is shown in Fig. 3B. When both inhibitors were introduced (CsA plus rottlerin), ADWX-1 exerted no apparent effect on proliferation (p > 0.05, Fig. 3E), suggesting that this peptide dose not affect any other IL-2 activation pathway.
Because the calcium signaling-mediated NF-AT pathway has been investigated using other Kv1.3 inhibitors (28), we specifically analyzed the effect of ADWX-1 on the Kv1.3-mediated Lck-PKCθ-NF-κB pathway in CD4+CCR7− cells. Representative Western blots together with the densitometric quantifications of the phosphorylated activation of NF-κB are shown in Fig. 3, F and G. After antigen stimulation, the enhanced level of phosphorylated NF-κB (compared with the quiescent cells, p < 0.05) was suppressed by 1 nm ADWX-1, 10 nm ADWX-1 (p < 0.05), the PKCθ inhibitor rottlerin (p < 0.05), and the NF-κB inhibitor pyrrolidine dithiocarbamate (p < 0.01, Fig. 3F). Furthermore, we examined the activity of NF-κB following the activation of T cells from EAE and control rats. We observed that ADWX-1 preferentially reduces NF-κB activation in MBP-stimulated CD4+ T cells from EAE rats (p < 0.05) but not in anti-CD3 Ab-stimulated cells from control rats (p > 0.05, Fig. 3G). These results suggest that the Kv1.3 channel is activated and recruited in the pathological state and that the Kv1.3 blocker ADWX-1 might specifically inhibit channel activity to modulate both the initial calcium signaling and the signaling events involved in NF-κB activation.
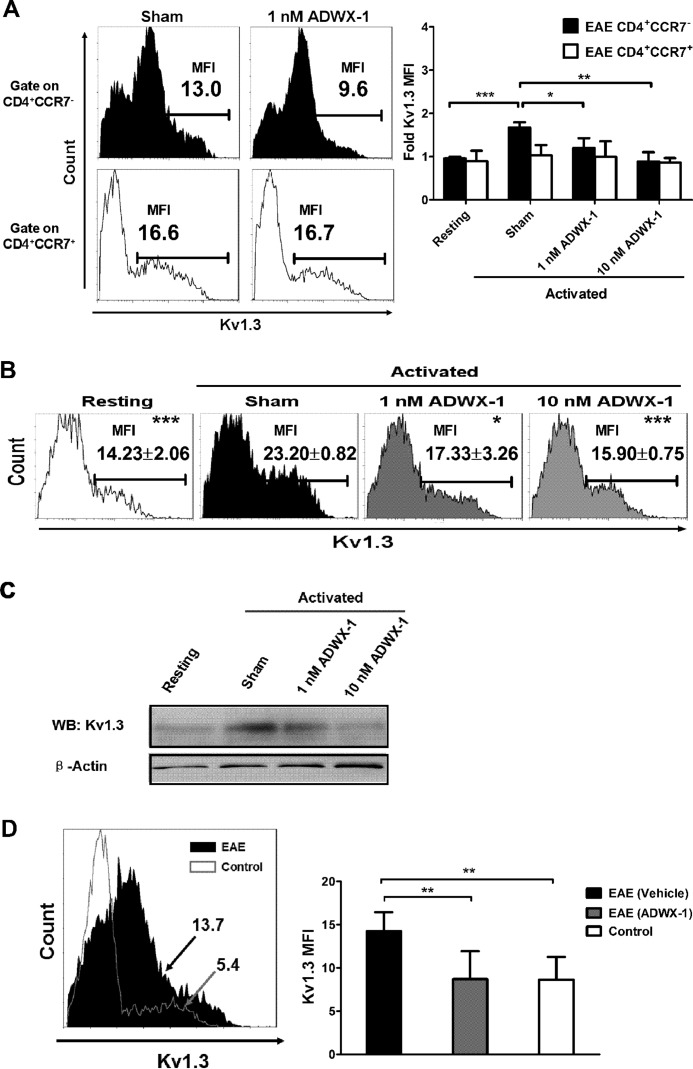
ADWX-1 Preferentially Suppresses Kv1.3 Expression in Activated CD4+CCR7− T Cell Subsets
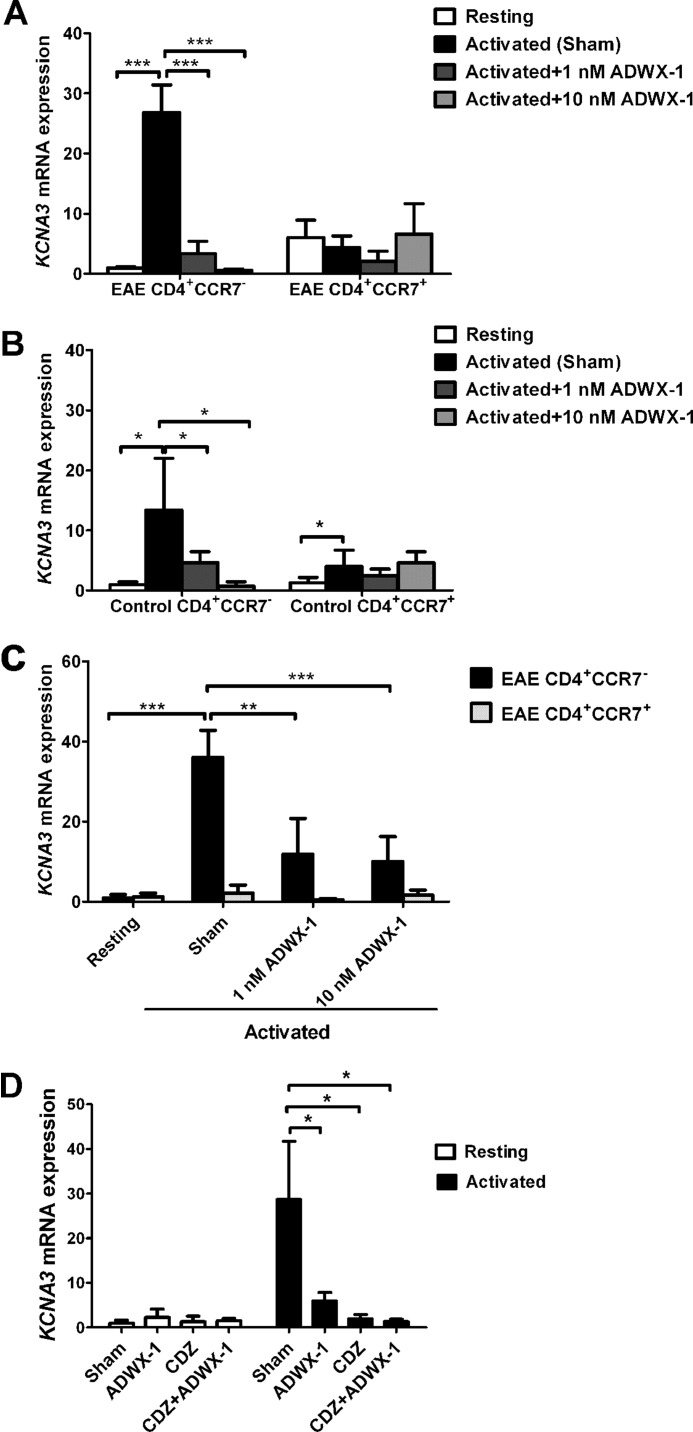
Because the Kv1.3 channel is functionally responsible for the activation of CD4+CCR7− T cells through the NF-AT- and NF-κB-mediated IL-2 pathways, we investigated whether the peptide with a high affinity for the Kv1.3 channel could selectively mediate the expression of Kv1.3 in CD4+CCR7− T cells following activation. Our results show that the mRNA expression of the Kv1.3 gene (KCNA3) in pre-sorted CD4+CCR7− T cells from EAE rats increased dramatically after antigen activation compared with resting cells (p < 0.001, Fig. 4A). However, the increased expression of KCNA3 in the activated CD4+CCR7− T cell subtype was significantly suppressed by ADWX-1 treatment when compared with sham-treated cells (p < 0.001, Fig. 4A). In contrast, no apparent up-regulation of KCNA3 mRNA after antigen stimulation was observed in the pre-sorted CD4+CCR7+ T cells from the EAE rats, and ADWX-1 exhibited no significant effect on the activated T cells. Analogous selective effects of ADWX-1 were observed in cells from control rats stimulated with anti-CD3 Ab (Fig. 4B). Similarly, when the PBMCs from EAE rats were activated for 48 h before sorting into CD4+CCR7− and CD4+CCR7+ populations for quantitative PCR analysis, the MBP stimuli significantly enhanced the KCNA3 mRNA expression only in the CD4+CCR7− T cells (p < 0.001). This increase was reversed significantly by 1 nm ADWX-1 (p < 0.01) and 10 nm ADWX-1 (p < 0.001, Fig. 4C).
FIGURE 4.
Reduced KCNA3 mRNA expression following ADWX-1 engagement in the activated CD4+CCR7− subset. A, EAE PBMCs were previously sorted for CD4+CCR7− and CD4+CCR7+ cells, and the two populations were cultured with MBP for 48 h in the presence (1 or 10 nm) or absence (sham) of ADWX-1. The cells were harvested for the quantitative RT-PCR analysis of KCNA3 (Kv1.3) mRNA levels. Resting CD4+CCR7− T cell gene expression was used as the base line for gene expression, and all gene quantities were normalized to the expression of GAPDH. The relative mRNA expressions are representative of the mean ± S.D. from three independent experiments. B, pre-sorted CD4+CCR7− and CD4+CCR7+ cells from PBMCs of control rats were treated as outlined in A with anti-CD3 Ab stimulation, and KCNA3 mRNA expression was determined by quantitative RT-PCR analysis. C, EAE total PBMCs were cultured with MBP for 48 h in the presence (1 or 10 nm) or absence (sham) of ADWX-1, and the cells were harvested for sorting of CD4+CCR7− (black) and CD4+CCR7+ (white) populations. The KCNA3 mRNA expression in the two populations was determined by quantitative RT-PCR analysis. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 versus activated (sham). D, CD4+CCR7− T cells previously sorted from EAE rats were treated with 1 nm ADWX-1 or 10 μm CDZ or a combination of the two. After MBP activation, the amounts of KCNA3 mRNA in each group were analyzed (black); the effects of the peptide and CDZ on the resting T cells were also determined (white). *, p < 0.05 versus sham group (no ADWX-1 or CDZ added).
Next we investigated the possible mechanisms underlying the modulation of the Kv1.3 expression by ADWX-1 in CD4+CCR7− T cells. Using a bio-information forecast, a series of nuclear transcription factors targeting the cis-acting core motifs of the KCNA3 5′-flanking and 5′-noncoding region nucleotide sequence were identified, including the Ca2+- and calmodulin-dependent nuclear factors nuclear factor of activated T-cells, Smad, cAMP-response element-binding protein, AP1, etc. Thus, we hypothesized that the high expression of the KCNA3 gene following activation is associated with the initial calcium signaling mediated by Kv1.3 channel activity in the CD4+CCR7− T cells. The calmodulin inhibitor calmidazolium (CDZ) was used to investigate the Ca2+ dependence of Kv1.3 expression. As shown in Fig. 4D, CDZ significantly decreased the high expression levels of KCNA3 mRNA in the MBP-activated CD4+CCR7− T cells (p < 0.05), suggesting that a calcium-dependent pathway might be involved in Kv1.3 expression. ADWX-1-treated (1 nm) cells did not exhibit lower KCNA3 mRNA levels than the CDZ-treated cells (Fig. 4D), possibly because CDZ is a more potent inhibitor of Ca2+/calmodulin than ADWX-1. When the CDZ and 1 nm ADWX-1 treatments were combined (CDZ + ADWX-1), the expression of KCNA3 mRNA was slightly lower compared with CDZ treatment alone (Fig. 4D). In addition, ADWX-1 and CDZ had no significant effect on KCNA3 mRNA expression in nonstimulated CD4+CCR7− T cells (Fig. 4D). The results suggest that the selective modulation of calcium, shown in Fig. 3, B and C, is not only responsible for NFAT activation but is also responsible for the selective regulation of Kv1.3 expression in CD4+CCR7− T cells.
To confirm the selective effect of ADWX-1 on Kv1.3 expression at the protein level, we also measured the Kv1.3 expression by staining with a fluorescence-labeled antibody specific for Kv1.3 and gated on the CD4+CCR7− and CD4+CCR7+ populations. The Kv1.3 protein expression was quantified by measuring the MFI of the Kv1.3 channels in the two gated populations (CCR7− and CCR7+) of CD4+ T cells (Fig. 5A). Within the overall PBMC system, the Kv1.3 MFI increased ∼70% in the gated CD4+CCR7− cell population following MBP activation (p < 0.001). ADWX-1 also selectively inhibited the Kv1.3 MFI at 1 nm (p < 0.05) and 10 nm (p < 0.01) in activated CD4+CCR7− cells but not in CD4+CCR7+ cells (Fig. 5A). Furthermore, the Kv1.3 protein expression (MFI) increased ∼64% following activation in the gated CD4+ T cells from the PBMCs of EAE rats, whereas 1 and 10 nm ADWX-1 inhibited this increased expression by ∼67 and 78%, respectively (Fig. 5B). Western blotting analysis was used to examine the membrane protein expression, and the results were consistent with the FCM data confirming the effect of ADWX-1 on Kv1.3 protein expression (Fig. 5C). The real time PCR, coupled with the FCM experiments, demonstrated that 1 and 10 nm doses of ADWX-1 decreased the high levels of Kv1.3 gene (KCNA3) expression following activation of the CD4+CCR7− cells at both the mRNA and protein levels, which parallels the selective inhibitory effect of the peptide on the IL-2 pathway in CD4+CCR7− cells.
FIGURE 5.
Further modulation of Kv1. 3 protein expression by ADWX-1 in the activated CD4+CCR7− subset. A, EAE total PBMCs were treated as outlined in Fig. 4C; the cells were harvested for gating of CD4+CCR7− (black) and CD4+CCR7+ (white) populations. The amounts of Kv1.3 MFI in the two populations were determined by FCM analysis. The representative Kv1.3 MFI in sham- or 1 nm ADWX-1-treated cells in the two populations are shown in the histogram (left panels). Statistical fold changes of the Kv1.3 MFI representing protein expression in the two populations from each group were determined using FCM (right panel). The Kv1.3 MFI in resting T cells was used as the base line for protein expression. B, Kv1.3 MFI measurement in the whole CD4+ population gated from the cells in A; the numbers in the representative histograms are the mean ± S.D. of the Kv1.3 MFI values. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus activated (sham). The data are representative of three independent experiments. C, membrane proteins were isolated from the EAE CD4+ population (as outlined in B) for Western blot (WB) analysis of the Kv1.3 protein level to support the MFI data in B. D, Kv1.3 expression in vivo; the numbers in the histograms (left panel) are representative of the Kv1.3 MFI in the CD4-gated population of PBMCs from EAE (black) and control (white) rats; statistical Kv1.3 MFI in CD4+ populations of PBMCs from each group (n = 6) were assessed (right panel). **, p < 0.01 versus EAE (vehicle).
To further examine the effect of ADWX-1 administration on the Kv1.3 expression in the gated CD4+ T cells from the PBMCs of EAE rats in vivo, we measured the Kv1.3 MFI on freshly isolated T cells from ADWX-1-injected EAE rats. Compared with control rats, elevated Kv1.3 protein expression was observed in CD4+ PBMCs from EAE rats (Fig. 5D). This observation is consistent with the high level of Kv1.3 expression in inflammatory infiltrates in the MS brain (8) because of the breakdown of the blood-brain barrier, and we speculate that the infiltrated cells may be auto-activated repeatedly in the peripheral circulation by myelin antigen injection. However, lower Kv1.3 expression levels were observed in ADWX-1-treated EAE rats when compared with vehicle-treated rats (p < 0.01, Fig. 5D). The effect of ADWX-1 on Kv1.3 expression in vivo suggests that ADWX-1 is responsible for the long term inhibition of activation of the CCR7− subtype, characterized by the enhanced Kv1.3 expression in the periphery.
ADWX-1 Regulates CD4+CCR7− T Cell Restimulation
Considering the in vivo repeated activation of T cells in the EAE model, we tested the efficacy of ADWX-1 on T cell restimulation. Based on the channel expression tests above, we propose that the ability of CD4+CCR7− T cells to reactivate might be impaired by ADWX-1 when the Kv1.3 expression is suppressed by ADWX-1 in the first stimulation. CD4+CCR7− and CD4+CCR7+ T cells purified from EAE and control rats were pretreated with ADWX-1 during the first antigen activation. The cells were washed after 48 h and were allowed to rest for 3 days before rechallenging with antigen in the absence of ADWX-1. The restimulated CD4+CCR7− subtype pretreated with ADWX-1 in the first activation exhibited lower [Ca2+]i compared with the non-ADWX-1-treated cells in both EAE (p < 0.01, Fig. 6A) and control rats (p < 0.01, Fig. 6B). The calcium regulation by ADWX-1 partly contributed to the peptide-mediated signaling of IL-2 activation. Consistently, the restimulation by antigens also resulted in significant IL-2 secretion (p < 0.001) in the CD4+CCR7− T cells from both EAE (Fig. 6C) and control rats (Fig. 6D). However, the IL-2 levels in the supernatant of the sorted CD4+CCR7− T cells from EAE (Fig. 6C) and control rats (Fig. 6D) also decreased (p < 0.05) when the cells were previously activated and treated with ADWX-1 before reactivation. Furthermore, no significant changes were observed in the supernatants from ADWX-1-pretreated CD4+CCR7+ T cells (Fig. 6, C and D). Therefore, the change in the Kv1.3 expression pattern induced by ADWX-1 produced a further and sustained inhibition of the CD4+CCR7− phenotype where Kv1.3 activity was involved in the cell activation signal. The modulation of CD4+CCR7− T cell reactivation in vitro by ADWX-1 also suggests that this peptide may physiologically inhibit the repeated activation of TEM cells in vivo, thus preventing the high expression of Kv1.3 in the EAE model (Fig. 5D) with a parallel down-regulation of the number of CD4+CCR7− T cells (Fig. 2A). The data indicate that the specific Kv1.3 blocker peptide ADWX-1 preferentially inhibits CD4+CCR7− T cells activation, as evidenced by IL-2 production, through regulating both channel activity and channel expression.
FIGURE 6.
Regulation of CD4+CCR7− T restimulation by ADWX-1 pretreatment. A, effect of ADWX-1 on [Ca2+]i after restimulation. Sorted CD4+CCR7− and CD4+CCR7+ T cells from the PBMCs of EAE rats were treated with antigen plus ADWX-1 for 48 h (ADWX-1 pretreated) and were reactivated by antigen alone for the calcium measurement. B, sorted cells from control rats were treated as outlined in A for the calcium measurement. C and D, after restimulation, the supernatants from the EAE group (C) and the control group (D) were collected for IL-2 measurement using ELISA. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 versus reactivated group (first stimulation, antigen +, ADWX-1 −; Second stimulation, antigen +, ADWX-1 −). The data represent the mean ± S.D. from two independent experiments performed in triplicate.
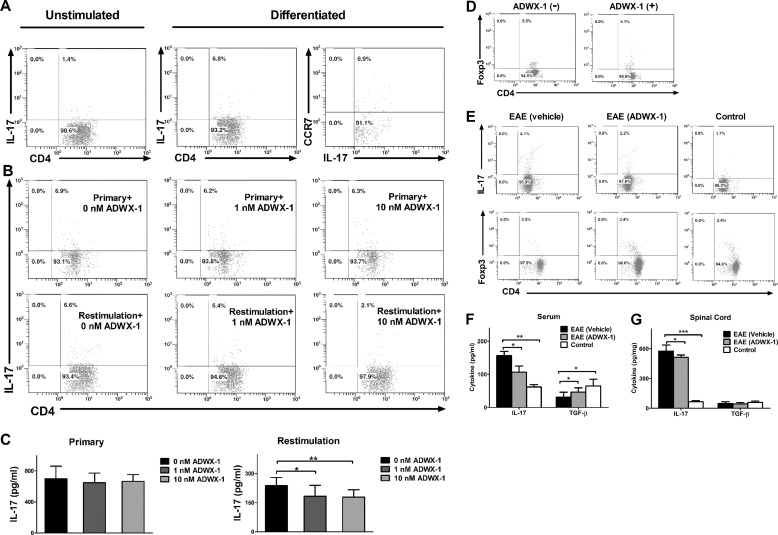
ADWX-1 Suppresses Th17 Activation but Not Differentiation
Considering the effects of ADWX-1 on the activation and proliferation observed in CD4+ T cells and because the polarization process requires antigen stimulation in addition to cytokine induction, we next investigated whether the modulation of CD4+ T cell activation by ADWX-1 regulates CD4+IL-17+ T cell differentiation. In the absence of ADWX-1, ∼7% of the rat naive CD4+ T cells in culture was converted into CD4+IL-17+ cells by IL-6 and TGF-β induction (Fig. 7A), and the differentiated Th17 cells appeared mostly as the CCR7− phenotype (Fig. 7A). The cells differentiated in the presence of ADWX-1 exhibited no obvious differences in the numbers of CD4+IL-17+ cells compared with the cells differentiated in the absence of ADWX-1 (Fig. 7B). However, when the fully differentiated Th17 cells were restimulated with anti-CD3 Ab and were treated with ADWX-1, their expansion was suppressed compared with the untreated cells (Fig. 7B). The ELISA analysis of the primary culture supernatant, as well as the supernatants from effector Th17 cells restimulated with anti-CD3 Ab overnight, revealed a similar decrease in IL-17 production (p < 0.05 or p < 0.01) only in the restimulation culture in the presence of ADWX-1 (Fig. 7C). In addition, ADWX-1 did not exert a significant effect on CD4+Foxp3+ Treg cell proliferation in vitro (Fig. 7D). Our data suggest that ADWX-1 directly regulates the expansion of Th17 cells in vitro but not the differentiation of Th17 cells and that this efficacy is based on the cell selectivity of ADWX-1 on the CD4+CCR7− T cells.
FIGURE 7.
Assessment of ADWX-1 in Th17 differentiation and EAE development. A, dot plots showing the representative frequency of CD4+IL-17+ T cells when naive CD4+ T cells were differentiated into Th17 using TGF-β and IL-6 plus anti-CD3/CD28 beads for 5 days; CD4 and IL-17 double-staining in A and B were gated on CD4. CCR7 phenotype in differentiated Th17 cells is shown in the right panel; CCR7 and IL-17 double-staining were gated on IL-17. B, upper panels, dot plots showing the frequency of CD4+IL-17+ T cells when naive CD4+ T cells were incubated with 1 or 10 nm or without ADWX-1 during cytokine-induced differentiation. Lower panels, dot plots showing the frequency of CD4+IL-17+ T cells when the differentiated T cells were restimulated by anti-CD3 Ab and incubated with 1 to 10 nm or without ADWX-1 for 3 days. C, supernatants from B were analyzed using ELISA. IL-17 production from primary 5-day differentiated or overnight anti-CD3 Ab restimulated cultures were assessed in triplicate. *, p < 0.05; **, p < 0.01 versus 0 nm ADWX-1. The data are the representative of two independent experiments. D, dot plots showing the representative frequency of CD4+Foxp3+ T cells when CD4+ T cells were activated by anti-CD3 Ab and incubated with 10 nm (+) or without ADWX-1 (−) for 3 days. CD4 and Foxp3 double-stained cells were gated on CD4. E, representative profiles exhibited the in vivo expression of CD4+IL-17+ and CD4+Foxp3+ T cells gated on CD4 using FCM. On day 15 post-immunization, the PBMC samples from each group (n = 6) were collected and were stained intracellularly. F and G, IL-17 and TGF-β levels in the serum (F) and the spinal cord (G) from each group (n = 6) were measured using ELISA. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 versus EAE (vehicle).
Furthermore, we used the EAE model to evaluate whether ADWX-1 targets mature Th17 in vivo. The administration of ADWX-1 prevented Th17 cell development in PBMCs (Fig. 7E) and slowed the progress of the disease, which resulted in a less severe decrease in CD4+Foxp3+ Treg cells compared with the model group (Fig. 7E). Consistently, IL-17 production in the serum of the EAE rats was reduced by ADWX-1 (p < 0.05) without further inhibition of TGF-β production (Fig. 7F). Furthermore, IL-17 production in the tissue (spinal cord) of EAE rats decreased after ADWX-1 administration (p < 0.05), without any changes in TGF-β production in the CNS (Fig. 7G). These results demonstrate that ADWX-1 injection reduces Th17 generation in vivo and confers protection against EAE.
DISCUSSION
Using the rat EAE model, we have investigated the therapeutic effect of a novel Kv1.3 blocker peptide, ADWX-1, and the mechanisms underlying the treatment. Our data suggest that ADWX-1, designed from toxin as a specific blocker of the Kv1.3 channel, is a promising drug for the clinical therapy of MS. It has been reported that patients with multiple sclerosis exhibit high numbers of effector memory T cells characterized by a high levels of Kv1.3 expression, mainly because the numbers of Kv1.3 channels on activated CD4+CCR7−CD45RA− TEM are significantly greater (∼1,500) than on resting TEM cells (∼300) after repeated activation (7, 29). The difference in the number of channels following activation suggests that Kv1.3 is a promising drug target for TEM-mediated autoimmune disease and the specific Kv1.3 blocker peptide ShK can suppress the proliferation of TEM cells (5, 7, 30). Based on these data, we proposed that the novel Kv1.3 blocker peptide ADWX-1 exhibits cell selectivity between CCR7− TEM and CCR7+ naive/TCM cells and that the preferential effect of ADWX-1 on the proliferation of TEM cells might be associated with its regulation of the initial signals for cell activation.
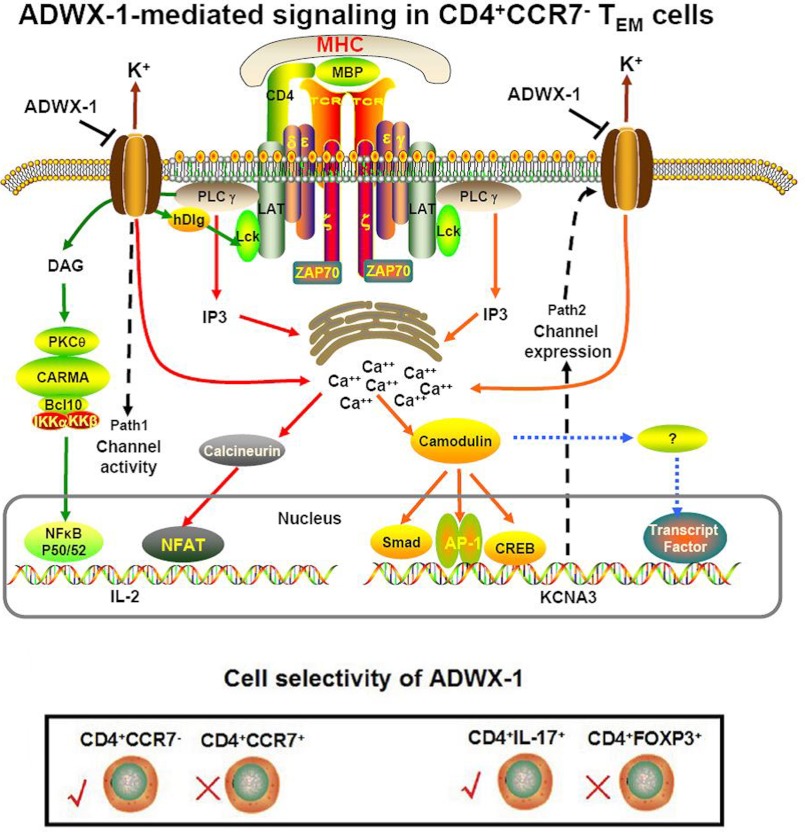
In this study, our results suggest that ADWX-1 preferentially mediates the CD4+CCR7− TEM phenotype through two different inhibitory pathways (Fig. 8). One of these inhibitory pathways is the classic channel activity pattern in which IL-2 is transcriptionally activated by calcium-related nuclear factor NF-AT and the PKCθ-related nuclear factor NF-κB. The other pathway is the channel expression pattern in which the KCNA3 gene expression is transcriptionally activated. Because Kv1.3 channels on CD4+CCR7− TEM cells modulate signals for the binding of nuclear factors to the KCNA3 promoter, blocking the channel may functionally alter the transduction of the KCNA3 promoter signaling and change the expression pattern of Kv1.3 on CD4+CCR7− TEM cells. In return, the expressed channels will influence cell reactivation. Therefore, ADWX-1 exhibits its inhibitory effect on the activation of CD4+ CCR7− phenotype by regulating Kv1.3 from function to gene. Despite the report that hypoxia regulates the expression and activity of Kv1.3 channels in T cell proliferation (31), the Kv1.3 blocker peptide ADWX-1 may directly mediate both the activity and expression of Kv1.3 channels in TEM cell activation.
FIGURE 8.
Putative mechanisms of the effect of the Kv1. 3 blocker ADWX-1 on TEM cells. ADWX-1 preferentially inhibits CD4+CCR7− TEM subtype cells through two inhibitory pathways; one pathway mediates the transcriptional activation of IL-2 via NF-AT and NF-κB, and the other pathway regulates channel expression by affecting the binding of transcriptional factors to the Kv1.3 promoter via the upstream calcium signaling. This cell selectivity mechanism of ADWX-1 on CD4+CCR7−/CCR7+ cells can be expanded to the activation of CD4+IL-17+/CD4+Foxp3+ cells.
First, we determined that the Kv1.3 blocker ADWX-1 specifically inhibited the activation and subsequent proliferation of CD4+CCR7− TEM cells with a limited effect on CD4+CCR7+ naive/TCM cells (Fig. 2, B and C). In the EAE model, CD4+CCR7+ T cells were less sensitive to MBP antigen stimulation, which is consistent with the observation that myelin-reactive T cells from MS patients are predominantly TEM cells (7, 25). However, 1 nm ADWX-1 was more potent at inhibiting the activation of CD4+ T cells from EAE rats than from the normal rats (data not shown). This observation is consistent with the effect of ADWX-1 on the total PBMCs in vitro, as shown in Fig. 1F, possibly because of the dominance of the CD4+CCR7+ naive/TCM phenotype over the CD4+CCR7− TEM cell phenotype in normal rats (Fig. 2A). We also studied primary human T cells because they are physiologically similar to the human leukocyte microenvironment. The inhibitory effect of ADWX-1 on human T cells was similar to the reported effects of ShK on freshly isolated CD4+ T cells, sorted TEM cells, or chronic TEM cells in humans (22).
Considering the question of how ADWX-1 modulates CD4+CCR7− cell activation, we investigated the activation of the IL-2 (Fig. 3D). A non-calcium-dependent but Kv1.3-related IL-2 activation model that involves Lck and Kv1.3 may modulate TCR activation and downstream NF-κB events, and it is reported that Kv1.3, Kvβ2, SAP97, ZIP, and p56lck (Lck) co-cap with CD4 in human CD4+ TEM cells (25, 32). Because the physical association and functional correlation between active Lck and Kv1.3 channel activity have been examined (33), ADWX-1 targeting Kv1.3 might be associated with TCR signaling cascades via Lck in the early stages of T cell activation. A recent report claimed that the Disc large 1 (Dlg1)-tyrosine kinase Lck complex mediates protein kinase AI, which selectively regulates Kv1.3 channels in human T lymphocytes (34), proving the interaction of Lck, Kv1.3, and protein kinase. Because phosphorylation by Lck may be required to induce protein kinase C (PKC) activation (35, 36), and PKCθ as a member of PKC family selectively expresses on T lymphocyte, blocking the Kv1.3 channel may affect the function of Lck and the downstream signaling events for PKCθ activation (37). Furthermore, the downstream Carma1 complex transmits PKCθ signaling to IKKs, which ultimately phosphorylates the IκB in this cascade (38, 39).
It is remarkable that the specific Kv1.3 blocker peptide ADWX-1 regulates Kv1.3 channel expression on activated TEM cells and that the expressed Kv1.3 channels are functionally important for the IL-2 activation of TEM cells. We adopted two different cell culture sorting patterns for the assay of mRNA levels (Fig. 4, A and C). To rule out interference from other cells, we used the sorted cells (CD4+CCR7−/CD4+CCR7+) for ADWX-1 treatment in vitro to investigate the targeting function of ADWX-1 on the two cell subtypes. Meanwhile, the PBMCs from whole blood treated with ADWX-1 in vitro were used to evaluate the effect of the drug on the leukocyte microenvironment because the CCR7+ and CCR7− subsets in CD4+ T lymphocytes are physiologically mixed in vivo. Our results demonstrate that ADWX-1 selectively lowers the enhanced expression of Kv1.3 channels in CD4+CCR7− T cells upon activation at both the mRNA and protein levels. In contrast, the enhancement of Kv1.3 expression was not apparent in the EAE CD4+CCR7+ T cells, and ADWX-1 had no effect on this subtype of T cells because the antigen-specific T cells were mainly CD4+CCR7− TEM Kv1.3high cells in MS/EAE (25). However, in control rats, although the CD4+CCR7+ cells responded to anti-CD3 Ab stimulation, the expression of the Kv1.3 channel increased only slightly without a substantial change in Kv1.3 expression on CD4+CCR7+ T cells after ADWX-1 treatment. It is likely that the activation of CCR7− cells depends on Kv1.3 channels but that CCR7+ cell activation depends on IKCa1 channels, which exhibit functions that are equivalent to Kv1.3 (7, 25). Because the Kv1.3 channel is not mobilized in CCR7+ cells, the level of Kv1.3 expression was not as high as in CCR7− cells and the Kv1.3 blocker ADWX-1 did not have a functional effect.
The effect of ADWX-1 on channel expression is similar to that reported for diclofenac, a nonsteroidal anti-inflammatory drug that inhibits Kv1.3 expression in activated T lymphocytes (40). However, diclofenac is not a specific Kv1.3 channel blocker, and the mechanism involved in its inhibition of Kv1.3 expression is unknown. In this study, we elucidated the tentative mechanism of the ADWX-1 peptide on channel expression based on the Kv1.3 promoter and Ca2+ signal transduction (Fig. 4D). Interestingly, the high expression of Kv1.3 in CD4+CCR7− T cells was dependent on calcium and calmodulin, and calcium signaling is in turn mediated by the Kv1.3 channel in this cell subtype (Fig. 3B); thus, there is a cross-talk between channel activity and channel expression in CD4+CCR7− T cells. Furthermore, the lower Kv1.3 expression impairs the Kv1.3-dependent CD4+CCR7− cell reactivation, which is Kv1.3-dependent; thus, pretreatment with ADWX-1 can also specifically lower IL-2 production in CD4+CCR7− cells when they are restimulated (Fig. 6, C and D). However, the selective inhibition of channel expression differs from expression elimination by siRNA or other channel gene knockdown methods in that quiescent T cell channel expression remains unchanged after ADWX-1 treatment (Fig. 4D). Therefore, when compared with other identified immunological genes associated with the risk of developing MS (41), the changes in expression of KCNA3 suggest that it is a gene that specifically regulates TEM cells activation (41).
In addition to the classic Th1 type CD4+ T cells, the latest studies show that Th17 and regulatory T cells (Treg) play vital roles in autoimmune diseases and inflammation. As a new lymphocyte subtype found in the EAE model, Th17 promotes inflammatory development, which is contrary to the anti-inflammatory role of Treg cells, and the receptors on the Th17 subsets contribute to their differentiation (42–44). To understand whether ADWX-1 affects the inflammatory Th17 cells, we shifted the focus of our research from cell proliferation to cell differentiation. The results showed that ADWX-1 did not suppress the Th17 cell polarization and IL-17 secretion in vitro, possibly because the cells were differentiated from naive CD4+, which is a CCR7+ phenotype, and that ADWX-1 was not effective in this type of cell. However, the peptide affected the restimulation of fully differentiated Th17 cells due to the CCR7− phenotype in these polarized CD4+IL-17+ T cells. In contrast, there was no apparent change in CCR7+-based Treg cells (45) after ADWX-1 treatment (Fig. 7D). Indeed, the CCR7+CD45RA+ naive T cells and the CCR7+CD45RA− TCM cells with the entry code of CCR7 were retained in the lymph node, whereas the CCR7−CD45RA− TEM cells without CCR7 infiltrated the inflamed tissue, resulting in effects such as cytokine secretion (5). The large proportion of CCR7− cells in the Th17 population accounts for the recruitment of Th17 to the lesion tissues in autoimmune diseases. The IL-17 levels were lowered in the tissue in particular following ADWX-1 treatment. Although Treg cells were down-regulated in the EAE model, ADWX-1 inhibited the exacerbation of the disease by suppressing Th17 cell development but not inhibiting the Treg cells further. These results demonstrate that ADWX-1 balances Th17 and Treg cells in vivo and ameliorates the MS/EAE disease. Similarly, studies of other peptides used for the treatment of MS report the involvement of Treg cells (46); however, the mechanisms for targeting Th17/Treg cells may differ for these peptides. The role of ADWX-1 in differentiation involves neither the replacement of cytokines, which trigger differentiation, nor synergy with cytokines to promote differentiation but rather the suppression of differentiated Th17 cells without affecting the Treg cells.
The selective effects of ADWX-1 on CCR7− T cell activation rather than on CCR7+ T cells restore the balance of CCR7−/CCR7+ T cells, which might differ from the way Kv1.3 mediates the reversion of T effectors to central memory lymphocytes via cell cycle signaling (47). As a representative Kv1.3 blocker peptide, the mechanisms of ADWX-1 can be expanded to other Kv1.3-targeting toxin peptides or even to other chemical Kv1.3 blockers. The cell selectivity of ADWX-1 provides further support for the therapeutic potential of Kv1.3-specific channel blockers in TEM-mediated autoimmune disease without side effects and broad immunosuppression (5, 48). At the same time, disease-associated autoreactive T cells in rheumatoid arthritis or type-1 diabetes mellitus patients mainly consist of CD4+CCR7−CD45RA− effector memory T cells (TEM cells) with elevated Kv1.3 potassium channel expression, and ADWX-1 may ameliorate these diseases in rat models (25). Furthermore, it is reasonable to suggest ADWX-1 for broader immunotherapies in other effector memory T-related autoimmune diseases, such as psoriasis, chronic graft versus host disease, inflammatory bowel disease, and systemic lupus erythematosus.
In conclusion, the Kv1.3 blocker peptide ADWX-1 inhibited the excessive activation and proliferation of CD4+CCR7− effector T cells by suppressing the channel activity-mediated IL-2 signaling and the expression of the Kv1.3 channel. Although MS is a multifocal demyelinating disease with multiple inducements, the multiple functions of ADWX-1, especially its dual inhibitory roles in both channel activity and channel expression in specific CD4+CCR7−TEM cells, make it a promising candidate drug for MS therapy.
Acknowledgment
We thank the Center for Medical Research of Wuhan University for providing the flow cytometry sample test and transmission electron microscopy image capture.
This work was supported by the National Basic Research Program of China Grant 2010CB529803, the National Natural Sciences Foundation of China Grants 81171127, 31000344, and 81171577, the National Science and Technology Major Projects of New Drugs Grants 2012ZX09103301-028, the Natural Science Foundation of Hubei Province of China Grant 2010CDA045, Fundamental Research Funds for the Central Universities, and National High Technology Research and Development Program of China (2012AA020304).
- MS
- multiple sclerosis
- EAE
- experimental autoimmune encephalomyelitis
- TEM
- effector memory T cells
- PBMC
- peripheral blood mononuclear cells
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- MBP
- myelin basic protein
- MFI
- mean fluorescent intensity
- CDZ
- calmidazolium
- PE
- phycoerythrin
- FCM
- flow cytometry
- CsA
- cyclosporin A.
REFERENCES
- 1. Noseworthy J. H., Lucchinetti C., Rodriguez M., Weinshenker B. G. (2000) Multiple sclerosis. N. Engl. J. Med. 343, 938–952 [DOI] [PubMed] [Google Scholar]
- 2. Merrill J. E. (2009) In vitro and in vivo pharmacological models to assess demyelination and remyelination. Neuropsychopharmacology 34, 55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nylander A., Hafler D. A. (2012) Multiple sclerosis. J. Clin. Invest. 122, 1180–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goverman J. (2009) Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 9, 393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beeton C., Pennington M. W., Wulff H., Singh S., Nugent D., Crossley G., Khaytin I., Calabresi P. A., Chen C. Y., Gutman G. A., Chandy K. G. (2005) Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 67, 1369–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rangaraju S., Chi V., Pennington M. W., Chandy K. G. (2009) Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin. Ther. Targets 13, 909–924 [DOI] [PubMed] [Google Scholar]
- 7. Wulff H., Calabresi P. A., Allie R., Yun S., Pennington M., Beeton C., Chandy K. G. (2003) The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J. Clin. Invest. 111, 1703–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rus H., Pardo C. A., Hu L., Darrah E., Cudrici C., Niculescu T., Niculescu F., Mullen K. M., Allie R., Guo L., Wulff H., Beeton C., Judge S. I., Kerr D. A., Knaus H. G., Chandy K. G., Calabresi P. A. (2005) The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc. Natl. Acad. Sci. U.S.A. 102, 11094–11099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beeton C., Wulff H., Singh S., Botsko S., Crossley G., Gutman G. A., Cahalan M. D., Pennington M., Chandy K. G. (2003) A novel fluorescent toxin to detect and investigate Kv1.3 channel up-regulation in chronically activated T lymphocytes. J. Biol. Chem. 278, 9928–9937 [DOI] [PubMed] [Google Scholar]
- 10. Mouhat S., Visan V., Ananthakrishnan S., Wulff H., Andreotti N., Grissmer S., Darbon H., De Waard M., Sabatier J. M. (2005) K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem. J. 385, 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Calvo M., Leonard R. J., Novick J., Stevens S. P., Schmalhofer W., Kaczorowski G. J., Garcia M. L. (1993) Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J. Biol. Chem. 268, 18866–18874 [PubMed] [Google Scholar]
- 12. Vennekamp J., Wulff H., Beeton C., Calabresi P. A., Grissmer S., Hänsel W., Chandy K. G. (2004) Kv1.3-blocking 5-phenylalkoxypsoralens. A new class of immunomodulators. Mol. Pharmacol. 65, 1364–1374 [DOI] [PubMed] [Google Scholar]
- 13. Felix J. P., Bugianesi R. M., Schmalhofer W. A., Borris R., Goetz M. A., Hensens O. D., Bao J. M., Kayser F., Parsons W. H., Rupprecht K., Garcia M. L., Kaczorowski G. J., Slaughter R. S. (1999) Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry 38, 4922–4930 [DOI] [PubMed] [Google Scholar]
- 14. Chandy K. G., Wulff H., Beeton C., Pennington M., Gutman G. A., Cahalan M. D. (2004) K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 25, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takacs Z., Toups M., Kollewe A., Johnson E., Cuello L. G., Driessens G., Biancalana M., Koide A., Ponte C. G., Perozo E., Gajewski T. F., Suarez-Kurtz G., Koide S., Goldstein S. A. (2009) A designer ligand specific for Kv1.3 channels from a scorpion neurotoxin-based library. Proc. Natl. Acad. Sci. U.S.A. 106, 22211–22216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han S., Hu Y., Zhang R., Yi H., Wei J., Wu Y., Cao Z., Li W., He X. (2011) ImKTx88, a novel selective Kv1.3 channel blocker derived from the scorpion Isometrus maculates. Toxicon 57, 348–355 [DOI] [PubMed] [Google Scholar]
- 17. Chen Z. Y., Hu Y. T., Yang W. S., He Y. W., Feng J., Wang B., Zhao R. M., Ding J. P., Cao Z. J., Li W. X., Wu Y. L. (2012) Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family. J. Biol. Chem. 287, 13813–13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beeton C., Barbaria J., Giraud P., Devaux J., Benoliel A. M., Gola M., Sabatier J. M., Bernard D., Crest M., Béraud E. (2001) Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. J. Immunol. 166, 936–944 [DOI] [PubMed] [Google Scholar]
- 19. Beeton C., Wulff H., Barbaria J., Clot-Faybesse O., Pennington M., Bernard D., Cahalan M. D., Chandy K. G., Béraud E. (2001) Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 98, 13942–13947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S., Yi H., Yin S. J., Chen Z. Y., Liu H., Cao Z. J., Wu Y. L., Li W. X. (2008) Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoimmune disease. J. Biol. Chem. 283, 19058–19065 [DOI] [PubMed] [Google Scholar]
- 21. Yin S. J., Jiang L., Yi H., Han S., Yang D. W., Liu M. L., Liu H., Cao Z. J., Wu Y. L., Li W. X. (2008) Different residues in channel turret determining the selectivity of ADWX-1 inhibitor peptide between Kv1.1 and Kv1.3 channels. J. Proteome Res. 7, 4890–4897 [DOI] [PubMed] [Google Scholar]
- 22. Hu L., Pennington M., Jiang Q., Whartenby K. A., Calabresi P. A. (2007) Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J. Immunol. 179, 4563–4570 [DOI] [PubMed] [Google Scholar]
- 23. Merrill J. E., Hanak S., Pu S. F., Liang J., Dang C., Iglesias-Bregna D., Harvey B., Zhu B., McMonagle-Strucko K. (2009) Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J. Neurol. 256, 89–103 [DOI] [PubMed] [Google Scholar]
- 24. Paintlia A. S., Paintlia M. K., Singh I., Singh A. K. (2008) Combined medication of lovastatin with rolipram suppresses severity of experimental autoimmune encephalomyelitis. Exp. Neurol. 214, 168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beeton C., Wulff H., Standifer N. E., Azam P., Mullen K. M., Pennington M. W., Kolski-Andreaco A., Wei E., Grino A., Counts D. R., Wang P. H., LeeHealey C. J., S Andrews B., Sankaranarayanan A., Homerick D., Roeck W. W., Tehranzadeh J., Stanhope K. L., Zimin P., Havel P. J., Griffey S., Knaus H. G., Nepom G. T., Gutman G. A., Calabresi P. A., Chandy K. G. (2006) Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. U.S.A. 103, 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feske S., Giltnane J., Dolmetsch R., Staudt L. M., Rao A. (2001) Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2, 316–324 [DOI] [PubMed] [Google Scholar]
- 27. Feske S. (2007) Calcium signaling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 28. Ren Y. R., Pan F., Parvez S., Fleig A., Chong C. R., Xu J., Dang Y., Zhang J., Jiang H., Penner R., Liu J. O. (2008) Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PloS one 3, e4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beeton C., Chandy K. G. (2005) Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist 11, 550–562 [DOI] [PubMed] [Google Scholar]
- 30. Wulff H., Castle N. A., Pardo L. A. (2009) Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 8, 982–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conforti L., Petrovic M., Mohammad D., Lee S., Ma Q., Barone S., Filipovich A. H. (2003) Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes. A possible role in T cell proliferation. J. Immunol. 170, 695–702 [DOI] [PubMed] [Google Scholar]
- 32. Cahalan M. D., Chandy K. G. (2009) The functional network of ion channels in T lymphocytes. Immunol. Rev. 231, 59–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szigligeti P., Neumeier L., Duke E., Chougnet C., Takimoto K., Lee S. M., Filipovich A. H., Conforti L. (2006) Signaling during hypoxia in human T lymphocytes. Critical role of the Src protein tyrosine kinase p56Lck in the O2 sensitivity of Kv1.3 channels. J. Physiol. 573, 357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuras Z., Kucher V., Gordon S. M., Neumeier L., Chimote A. A., Filipovich A. H., Conforti L. (2012) Modulation of K(V)1.3 channels by protein kinase AI in T lymphocytes is mediated by the disc large l-tyrosine kinase Lck complex. Am. J. Physiol. Cell Physiol. 302, 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayashi K., Altman A. (2007) Protein kinase Cθ (PKCθ). A key player in T cell life and death. Pharmacol. Res. 55, 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melowic H. R., Stahelin R. V., Blatner N. R., Tian W., Hayashi K., Altman A., Cho W. (2007) Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cθ. J. Biol. Chem. 282, 21467–21476 [DOI] [PubMed] [Google Scholar]
- 37. Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) T cell activation. Annu. Rev. Immunol. 27, 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity 23, 575–585 [DOI] [PubMed] [Google Scholar]
- 39. Rueda D., Thome M. (2005) Phosphorylation of CARMA1. The link(er) to NF-κB activation. Immunity 23, 551–553 [DOI] [PubMed] [Google Scholar]
- 40. Villalonga N., David M., Bielańka J., González T., Parra D., Soler C., Comes N., Valenzuela C., Felipe A. (2010) Immunomodulatory effects of diclofenac in leukocytes through the targeting of Kv1.3 voltage-dependent potassium channels. Biochem. Pharmacol. 80, 858–866 [DOI] [PubMed] [Google Scholar]
- 41. Fugger L., Friese M. A., Bell J. I. (2009) From genes to function. The next challenge to understanding multiple sclerosis. Nat. Rev. Immunol. 9, 408–417 [DOI] [PubMed] [Google Scholar]
- 42. Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., Weiner H. L. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 [DOI] [PubMed] [Google Scholar]
- 43. Gulen M. F., Kang Z., Bulek K., Youzhong W., Kim T. W., Chen Y., Altuntas C. Z., Sass Bak-Jensen K., McGeachy M. J., Do J. S., Xiao H., Delgoffe G. M., Min B., Powell J. D., Tuohy V. K., Cua D. J., Li X. (2010) The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity 32, 54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farez M. F., Quintana F. J., Gandhi R., Izquierdo G., Lucas M., Weiner H. L. (2009) Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol. 10, 958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider M. A., Meingassner J. G., Lipp M., Moore H. D., Rot A. (2007) CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 204, 735–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shapira E., Brodsky B., Proscura E., Nyska A., Erlanger-Rosengarten A., Wormser U. (2010) Amelioration of experimental autoimmune encephalitis by novel peptides. Involvement of T regulatory cells. J. Autoimmun 35, 98–106 [DOI] [PubMed] [Google Scholar]
- 47. Hu L., Gocke A. R., Knapp E., Rosenzweig J. M., Grishkan I. V., Baxi E. G., Zhang H., Margolick J. B., Whartenby K. A., Calabresi P. A. (2012) Functional blockade of the voltage-gated potassium channel Kv1.3 mediates reversion of T effector to central memory lymphocytes through SMAD3/p21cip1 signaling. J. Biol. Chem. 287, 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matheu M. P., Beeton C., Garcia A., Chi V., Rangaraju S., Safrina O., Monaghan K., Uemura M. I., Li D., Pal S., de la Maza L. M., Monuki E., Flügel A., Pennington M. W., Parker I., Chandy K. G., Cahalan M. D. (2008) Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29, 602–614 [DOI] [PMC free article] [PubMed] [Google Scholar]