Background: DAT activity is regulated by protein kinases.
Results: We identify Thr53 as a DAT phosphorylation site in rat striatum by mass spectrometry and a phospho-specific antibody; Thr53 mutation reduced dopamine influx and ablated transporter-mediated efflux.
Conclusion: Phosphorylation of DAT Thr53 is involved in transport activity.
Significance: These results identify Thr53 phosphorylation of DAT in vivo and elucidate associated functional properties.
Keywords: ERK, MAP Kinases (MAPKs), Mass Spectrometry (MS), Protein Kinase C (PKC), SH3 Domains, 1-Methyl-4-phenylpyridinium (MPP+), PP1/2A, cis-trans Isomerization, Phospho-specific Antibody, Proline-directed Phosphorylation
Abstract
In the central nervous system, levels of extraneuronal dopamine are controlled primarily by the action of the dopamine transporter (DAT). Multiple signaling pathways regulate transport activity, substrate efflux, and other DAT functions through currently unknown mechanisms. DAT is phosphorylated by protein kinase C within a serine cluster at the distal end of the cytoplasmic N terminus, whereas recent work in model cells revealed proline-directed phosphorylation of rat DAT at membrane-proximal residue Thr53. In this report, we use mass spectrometry and a newly developed phospho-specific antibody to positively identify DAT phosphorylation at Thr53 in rodent striatal tissue and heterologous expression systems. Basal phosphorylation of Thr53 occurred with a stoichiometry of ∼50% and was strongly increased by phorbol esters and protein phosphatase inhibitors, demonstrating modulation of the site by signaling pathways that impact DAT activity. Mutations of Thr53 to prevent phosphorylation led to reduced dopamine transport Vmax and total apparent loss of amphetamine-stimulated substrate efflux, supporting a major role for this residue in the transport kinetic mechanism.
Introduction
The neurotransmitter dopamine (DA)5 plays a key role in many brain processes, including motor activity, motivation, and reward. Proper dopaminergic function is dependent on the reuptake activity of the dopamine transporter (DAT), which is the primary mechanism responsible for spatial and temporal control of extraneuronal DA (1–3). Dysregulation of transport activity and consequent DA imbalance are hypothesized to contribute to dopaminergic disorders, such as Parkinson disease, depression, attention deficit hyperactivity disorder, and schizophrenia (4–6). DAT is also a target for many drugs of abuse, such as cocaine and amphetamine (AMPH), and for therapeutic agents used to treat DA diseases (7, 8). In particular, AMPH and its congeners induce multiple acute and chronic effects on DAT (9), including reversal of transport direction (10), that lead to substrate efflux and depletion of transmitter stores (11). The mechanism underlying efflux remains to be elucidated, but it is associated with transporter-generated currents (12) that correlate with substrate-releasing capacity (13) and involves N-terminal serine phosphorylation (14).
DAT is subject to extensive acute and chronic regulation that modulates DA neurotransmission in response to momentary physiological demands and to long term disease or drug addiction states (7, 15, 16). Changes in DAT activity and cell surface expression occur in response to the actions of several kinases and phosphatases, including protein kinase C (PKC), extracellular signal-related kinase (ERK), and protein phosphatases 1 and 2A (PP1/2A) (17–20). DAT is phosphorylated in PKC- and phosphatase-dependent manners, but the mechanistic relationships between transporter phosphorylation and regulation remain unclear. Using 32PO4 metabolic labeling, we have found in rat striatal tissue and model cells that ∼90% of rDAT phosphorylation occurs on phosphoserine (Ser(P)) and ∼10% occurs on phosphothreoine (Thr(P)) (17, 21). Phosphorylation occurs at a tonic level that is increased with PKC activators, PP1 inhibitors, and in vitro and in vivo administration of AMPH (22), with the majority of 32P labeling occurring in a serine cluster at the distal end of the N-terminal domain (21–23). We recently showed that a recombinant peptide containing N-terminal residues 1–65 of rDAT (NDAT) was phosphorylated in vitro by the proline-directed kinases ERK1/2, JNK, and p38 MAPK, which require a proline immediately C-terminal to the phosphate acceptor (24–28). We identified the membrane-proximal residue Thr53, which precedes Pro54, as the NDAT ERK phosphorylation site (29) and showed the apparent total loss of Thr(P) from 32PO4 metabolically labeled rDAT carrying a Thr53 → Ala mutation, indicating that Thr53 is a major site or the sole site of Thr(P) in the heterologously expressed protein (29).
In this study, we use mass spectrometry and a phosphospecific antibody as positive function approaches to demonstrate Thr53 phosphorylation of DAT and examine its characteristics without the necessity for 32PO4 labeling or interference from PKC-induced Ser phosphorylation. Our findings verify in vivo phosphorylation of DAT Thr53 in rat and mouse striatum as well as in heterologous model cells and demonstrate its modulation by signaling pathways. DAT mutants containing non-phosphorylatable residues at position 53 possessed reduced DA transport Vmax and in superfusion assays showed complete loss of AMPH-induced substrate efflux, suggesting a crucial role for this residue in transport kinetics. These findings reveal Thr53 phosphorylation as a novel mechanism for regulation of DAT functions and identify the membrane-proximal region of the N terminus as a major locus for regulation of transport kinetics.
EXPERIMENTAL PROCEDURES
Animals and Materials
Protein G- and protein A-Sepharose beads were from GE Healthcare; PMA, OA, recombinant PKCα, and ERK1 were from EMD Calbiochem; goat anti-DAT polyclonal antibody (C-20) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); Colorburst molecular mass standards, alkaline phosphatase-linked anti-mouse and anti-rabbit IgG antibodies, and other fine chemicals were from Sigma-Aldrich; FuGENE 6 transfection reagent and Complete Mini protease inhibitor tablets were from Roche Applied Science; bicinchoninic acid (BCA) protein assay reagent was from Thermo Scientific; [7,8-3H]DA (45 Ci/mmol) was from GE Healthcare; and [125I]RTI82 was synthesized and radioiodinated as previously described (30). The recombinant NDAT was prepared and subjected to in vitro phosphorylation with PKC and ERK1 as described previously (29). Rats were purchased from Charles River Laboratories or the Institute for Animal Genetics, Medical University of Vienna (Himberg), and SV129 mice were obtained from Dr. Eric Murphy (University of North Dakota). All animals were housed and treated in accordance with regulations established by the National Institutes of Health and approved by the University of North Dakota Institutional Animal Care and Use Committee.
Cell Culture and DAT Mutagenesis
Lewis lung carcinoma-porcine kidney (LLC-PK1) cells or LLC-PK1 cells stably expressing WT rDAT (rDAT-LLCPK1) (31) or T53A or T53D rDAT were maintained in α-minimum essential medium supplemented with 5% fetal bovine serum, 2 mm l-glutamine, 200 μg/ml G418, and 100 μg/ml penicillin/streptomycin. tsA201 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS and penicillin/streptomycin. Cells were maintained in a humidified incubation chamber gassed with 5% CO2 at 37 °C. The T53A, T53D, and T53E mutations were made in the rDAT pcDNA 3.0 template using the Stratagene QuikChange® kit with codon substitution verified by sequencing (Alpha Biolabs, Northwoods DNA). For production of pooled stable transformants, transfected cells (FuGENE, Roche Applied Science) were maintained under selection with 800 μg/ml G418 (29). tsA201 cells were transiently transfected with WT rDAT using the ExGen500 reagent (Fermentas) according to the manufacturer's protocol. For experiments with T53E, LLC-PK1 cells were transiently transfected with 0.6 μg of WT or T53E DNA/well using FuGENE and assayed for [3H]DA transport activity after 24 h.
Tandem Mass Spectrometry Analysis (LC-MS/MS)
Rat striatal synaptosomes, rDAT-LLCPK1 cells, or tsA201 cells transiently expressing rDAT were solubilized in lysis buffer containing 1% Triton X-100, 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm EDTA, 1 mm sodium orthovanadate, 5 mm sodium fluoride, 5 mm sodium pyrophosphate, and a protease inhibitor mixture (Roche Applied Science) on a tube rotator for 2 h at 4 °C. After centrifugation at 14,000 × g for 30 min at 4 °C, the supernatant was collected and incubated overnight with a goat anti-DAT polyclonal antibody. Immune complexes were collected with protein G beads and washed extensively, and bound proteins were eluted in Laemmli buffer (63 mm Tris-HCl, 10% glycerol, 2% SDS, 3% 2-mercaptoethanol, 100 mm dithiothreitol, 0.0025% bromphenol blue, pH 6.8) at 95 °C for 3 min. Eluted proteins were size-fractionated on SDS-polyacrylamide gels and visualized by Coomassie Brilliant Blue staining, and the indicated band was excised. Gel pieces were destained with 50% acetonitrile in 25 mm ammonium bicarbonate and dried in a speed vacuum concentrator. After reduction and alkylation of cysteine residues, gel pieces were washed and dehydrated. Dried gel pieces were rehydrated with 25 mm ammonium bicarbonate (pH 8.0) containing 10 ng/μl trypsin or chymotrypsin (Promega, Madison, WI) and incubated for 18 h at 37 °C. The digested peptide mixtures were extracted with 50% acetonitrile in 5% formic acid and concentrated in a speed vacuum concentrator for LC-MS/MS. An ion trap mass spectrometer (HCT, BrukerDaltonics, Bremen, Germany) coupled with an Ultimate 3000 nano-HPLC system (Dionex, Sunnyvale, CA) was used for LC-MS/MS data acquisition. A PepMap100 C-18 trap column (300 μm × 5 mm) and PepMap100 C-18 analytic column (75 μm × 150 mm) were used for reverse phase chromatographic separation with a flow rate of 300 nl/min. The two buffers used for the reverse phase chromatography were 0.1% formic acid, water (buffer A) and 0.08% formic acid, acetonitrile (buffer B) with a 125 min gradient (4–30% B for 105 min, 80% B for 5 min, and 4% B for 15 min). Eluted peptides were then directly sprayed into the mass spectrometer to record peptide spectra over the mass range of m/z 350–1500 and MS/MS spectra in information-dependent data acquisition over the mass range of m/z 100–2800. Repeatedly, MS spectra were recorded, followed by three data-dependent collision-induced dissociation MS/MS spectra generated from the four highest intensity precursor ions. The MS/MS spectra were interpreted with the Mascot search engine (Matrix Science, London, UK). Data base searches through Mascot were performed with a mass tolerance of 0.5 Da and an MS/MS tolerance of 0.5 Da; three missing cleavage sites and carbamidomethylation on cysteine, oxidation on methionine, deamidation on asparagine/glutamine, and phosphorylation on serine/threonine were allowed. Each filtered MS/MS spectra exhibiting possible phosphorylation was manually checked and validated (32, 33).
Phospho-specific Antibody Generation
A Threonine 53-phosphospecific polyclonal antibody (Thr(P)53 Ab) against DAT was generated by PhosphoSolutions (Aurora, CO). Briefly, rabbits were immunized with a phosphopeptide based on the DAT N-terminal amino acid sequence: TNSTLINPPQpTPVEAQERTW (Thr(P)53 shown in boldface type). Control peptide consisting of the identical sequence with non-phosphorylated Thr53 was also synthesized. Thr(P)53-specific polyclonal antibody present in immune serum was purified through sequential rounds of chromatography against immobilized phospho- and dephosphopeptide and concentrated to 1 mg/ml. Affinity-purified antibody screened by ELISA showed strong reactivity against the Thr(P)53 peptide antigen and essentially no reactivity to the corresponding dephosphopeptide (not shown).
DAT Immunoblot and Immunoprecipitation
Immunoblotting was performed with mouse monoclonal N-terminal Ab 16 (mAb 16) generated against residues 42–59 to detect total DAT as described previously (34) or with rabbit polyclonal Thr(P)53 Ab generated in this study. Briefly, lysates of rodent striatal membranes or rDAT-LLCPK1 cells were resolved on 4–20% SDS-polyacrylamide gels using ColorBurst (Sigma) molecular mass markers as standards. For regulation studies, rat striatal synaptosomes were prepared and treated with vehicle, phorbol 12-myristate 13-acetate (PMA), or okadaic acid (OA) for 30 min at 30 °C as described previously (35), followed by lysis and electrophoresis. Gels were transferred to PVDF and blocked, followed by incubation with primary antibodies used at 1:1000 dilutions. Where indicated, N-terminal peptides with or without Thr53 phosphorylation were included with the primary antibodies at 50 μg/ml. Immunostaining was detected using anti-mouse or anti-rabbit alkaline phosphatase-conjugated secondary antibodies and chemiluminescent light detection using ImmunStar (Bio-Rad) substrate and a Bio-Rad gel documentation system. For quantification of Thr53 phosphorylation, Thr(P)53 Ab staining was normalized to total DAT levels determined in parallel using mAb 16, and statistical analysis was performed using ANOVA. For immunoprecipitation studies, lysates of unlabeled or [125I]RTI82-labeled rat striatal membranes (36) were immunoprecipitated with polyclonal Ab 16 or Thr(P)53 Ab (3 μg) using procedures previously described (37). Precipitated DATs were resolved on 4–20% SDS-polyacrylamide gels and were transferred for subsequent immunoblotting or were dried and exposed to x-ray film for 3–7 days at −80 °C.
Determination of Thr53 Phosphorylation Stoichiometry
Rat striatal lysates were immunoprecipitated with Thr(P)53 Ab, and bound and unbound fractions were immunoblotted with Thr(P)53 Ab to determine the fraction of Thr53-phosphorylated transporters retained in the pellet. Bound samples were also blotted with mAb 16 to detect total DAT protein in the pellet, and the fraction of input DAT pulled down by Thr(P)53 Ab was determined by comparing the staining intensities of the Thr(P)53 Ab pellet with that of a DAT standard curve generated by titration of input sample and immunoblotted in parallel with mAb 16. The Thr(P)53 stoichiometry estimate was determined by dividing the fraction of input DAT present in the Thr(P)53 Ab pellets by the Thr(P)53 Ab precipitation efficiency.
DA Uptake and Cell Surface Biotinylation Assays
WT or mutant rDAT-LLCPK1 cells were grown in 24-well plates to 70–80% confluence in α-minimum essential medium at 37 °C. Cells were rinsed twice with 0.5 ml of Krebs-Ringer-HEPES (KRH) buffer (25 mm HEPES, 125 mm NaCl, 4.8 mm KCl, 1.2 mm KH2PO4, 1.3 mm CaCl2, 1.2 mm MgSO4, 5.6 mm glucose, pH 7.4) followed by the addition of 0.5 ml of warmed KRH buffer (37 °C) and uptake assay. Uptake was performed in triplicate and initiated by the addition of 10 nm [3H]DA plus 0.3–30 μm unlabeled DA (where indicated). Nonspecific uptake was determined in the presence of 100 μm (−)-cocaine. Uptake was allowed to proceed for 8 min at 37 °C, and cells were rapidly washed three times with ice-cold KRH buffer. Cells were solubilized in 1% Triton X-100, radioactivity contained in lysates was assessed by liquid scintillation counting, and protein content was assessed using BCA colorimetric reagent. For cell surface expression determination, WT or mutant rDAT-LLCPK1 cells were incubated with the membrane-impermeable biotinylating reagent sulfosuccinimidyl-2[biotinamido]ethyl-1,3-dithiopropionate sulfo-NHS-SS-biotin, and biotinylated DATs were purified from cell lysates (25 μg of protein) by chromatography on NeutrAvidin beads, separated by SDS-PAGE, and quantified by immunoblotting (38). For ion dose-response experiments, Na+ and Cl− were replaced across the range of 0–150 mm with N-methyl d-gluconate or sodium acetate, respectively, and uptake was analyzed using 10 nm [3H]DA plus 3 μm DA (39, 40).
Superfusion Experiments
Substrate efflux assays were performed as previously described (41). In brief, culture medium was removed from stably transfected WT or mutant rDAT LLC-PK1 cells (see above; 4 × 105 cells/well grown on coverslips in 96-well plates) and exchanged with KRH buffer. In all superfusion assays, we used [3H]1-methyl-4-phenylpyridinium (MPP+) (85 Ci/mmol; American Radiolabeled Chemicals, St. Louis, MO) as the DAT substrate because it is metabolically inert, cannot diffuse out of the cells, and thereby significantly enhances the signal/noise ratio of the experiment (42). The cells were preincubated with 0.1 μm MPP+ for 20 min at 37 °C in a final volume of 0.1 ml of KRH buffer/well and subsequently transferred into superfusion chambers. Immediately, superfusion was initiated with KRH buffer at 25 °C at a perfusion rate of 0.7 ml/min. After 45 min, a stable efflux of radioactivity was achieved, and the experiment was started with the collection of 2-min fractions. After three fractions, AMPH (3 μm) was added to stimulate the reverse operation of DAT.
RESULTS
Identification of Phosphorylated Thr53 on DAT by Mass Spectrometry
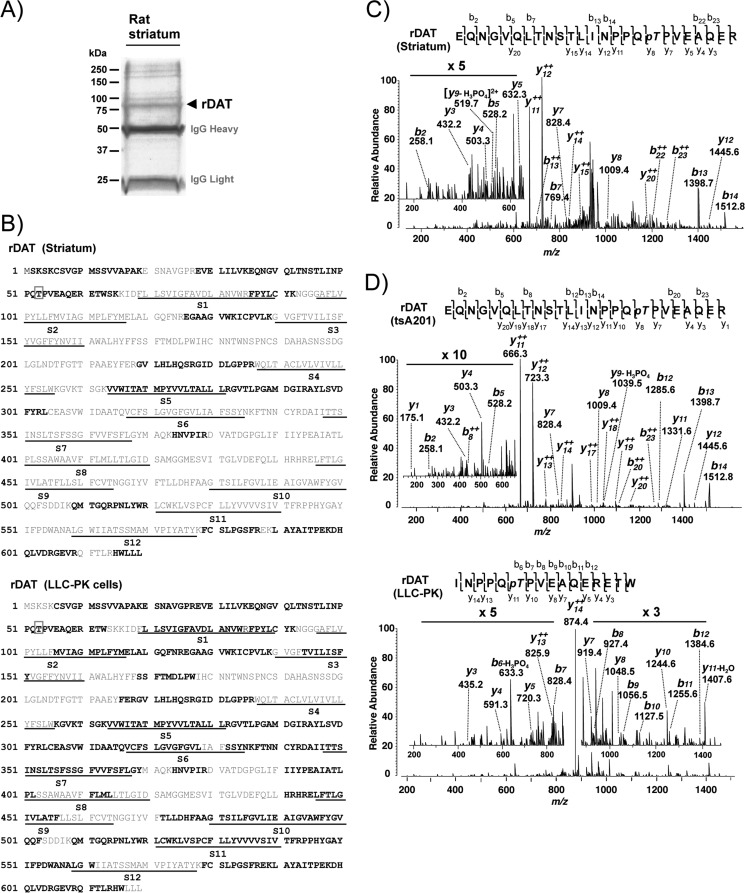
To identify in vivo DAT phosphorylation, we immunopurified DAT from rat striatal lysates and size-fractionated purified proteins by SDS-PAGE (Fig. 1A). The indicated Coomassie Blue-stained band was subjected to trypsin in-gel digestion, and resulting peptides were subjected to liquid chromatography tandem MS (LC-MS/MS). LC-MS/MS identified DAT protein (Swiss-Prot ID: P23977) with 97 matched peptides and 30.2% sequence coverage (Fig. 1B, top). Thr(P) was unambiguously and repeatedly identified at residue 53 in the MS/MS spectra of the tryptic peptide spanning amino acid residues 30–60 (EQNGVQLTNSTLINPPQpTPVEAQER) (Fig. 1C). To confirm phosphorylation of DAT at Thr53 in heterologous cells, we also analyzed rDAT transiently or stably expressed in mammalian cell lines. Using the same MS approach, we found that DAT was phosphorylated at Thr53 in both tsA201 and rDAT-LLCPK1 cell lines (Fig. 1D). The use of chymotrypsin for in-gel digestion substantially increased sequence coverage. MS/MS analysis mapped rDAT protein with individual sequence coverage of 30.7 and 52.7% for tryptic and chymotryptic peptides, respectively (data not shown), summing up to a total sequence coverage of 68.7% of the rDAT protein in rDAT-LLCPK1 cells with Thr(P)53 (Fig. 1B, bottom).
FIGURE 1.
Identification of Thr53 phosphorylation of DAT in rat striatum and heterologous cells by LC-MS/MS. A, Coomassie Blue-stained SDS-PAGE gel with immunopurified rat striatal DAT (arrow). Molecular mass markers are indicated on the left. B, sequence coverage of DAT with identified peptides (boldface type) by MS/MS from rat striatum (top) and rDAT-LLCPK1 cells (bottom). The putative 12 transmembrane segments (S1–S12) of DAT are underlined, and the identified phosphorylation site at Thr53 is indicated by a box. C, the spectrum of triple charged rDAT peptide obtained at m/z 948.45 was fragmented to produce a tandem mass spectrum with y- and b-ion series. The MS/MS spectrum shows phosphorylated Threonine (pT) in the sequence EQNGVQLTNSTLINPPQpTPVEAQER (amino acids 36–60). D, MS/MS spectrum from rDAT transiently expressed in tsA201 cells (top) and stably expressed in LLC-PK1 cells (bottom). The triple charged, tryptic peptide at m/z 948.44 from heterologous tsA201 cells was fragmented to y- and b-ion series that described the sequence EQNGVQLTNSTLINPPQpTPVEAQER with phosphorylation at Thr53. In rDAT-LLCPK1 cells, the spectrum of double charged, chymotryptic peptide at m/z 987.99 presents the unambiguous identification of Thr(P)53 in the sequence INPPQpTPVEAQERETW of rDAT.
Characterization of Thr(P)53 Ab Immunoreactivity
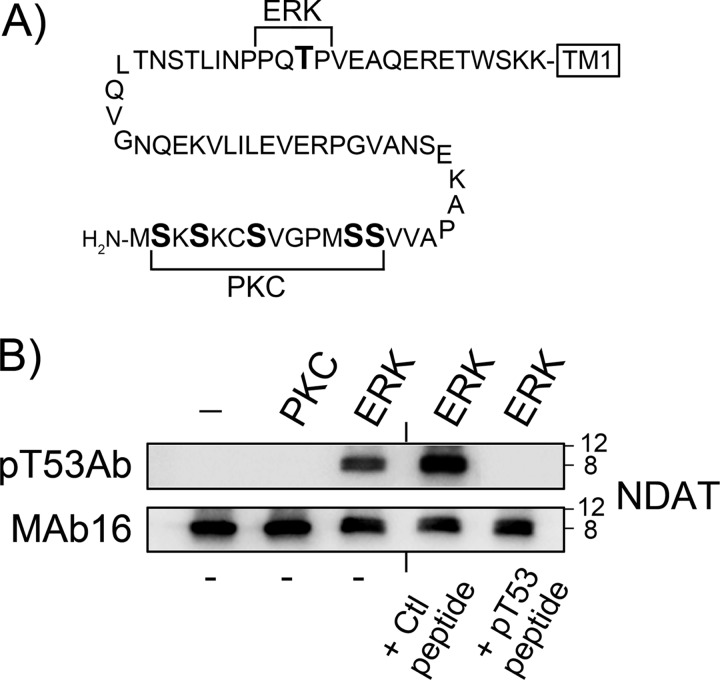
We then assessed the ability of our phospho-specific antibody to detect Thr(P)53 in the DAT sequence. In our previous study (29), we demonstrated that the recombinant N-terminal domain peptide NDAT was phosphorylated in vitro by ERK1/2 at Thr53, whereas more recently we have determined that PKC-catalyzed phosphorylation of NDAT occurs on multiple sites in the distal serine cluster, paralleling PKC-induced metabolic phosphorylation of this domain (21) (Fig. 2A). We used these differentially phosphorylated NDAT samples to test the specificity of the Thr(P)53 Ab in immunoblot assays. Thr(P)53 Ab did not recognize NDAT that was not phosphorylated or was phosphorylated by PKC, but it was strongly reactive against ERK-phosphorylated NDAT (Fig. 2B, top). Staining of ERK-phosphorylated NDAT was lost when Thr(P)53 Ab was preincubated with Thr(P)53 peptide but was not affected by incubation with the corresponding dephosphopeptide (Fig. 2B, top). Staining of blots with mAb 16 verifies equal NDAT protein in all samples (Fig. 2B, bottom) and demonstrates the upward shift of NDAT induced by Thr53 phosphorylation that we previously reported (29). These results demonstrate that Thr(P)53 Ab specifically recognizes DAT N-terminal tail sequence phosphorylated at Thr53 and does not recognize N-terminal sequence that is not phosphorylated or is phosphorylated by PKC on distal serines.
FIGURE 2.
Immunoreactivity of NDAT with Thr(P)53 Ab. A, rat DAT N-terminal amino acid sequence (residues 1–65; NDAT) highlighting the PKC-dependent phosphorylation domain (PKC) and the ERK proline-dependent phosphorylation site (ERK). B, NDAT samples given no kinase treatments or phosphorylated in vitro by PKCα or ERK1 were immunoblotted with Thr(P)53 Ab (pT53Ab) (top) or mAb 16 (bottom). Where indicated, Thr(P)53 Ab was preincubated with phosphorylated (pT53) or non-phosphorylated (Ctl) N-terminal peptide. Molecular mass markers are indicated on the right.
Thr(P)53 Ab Staining of Expressed DAT
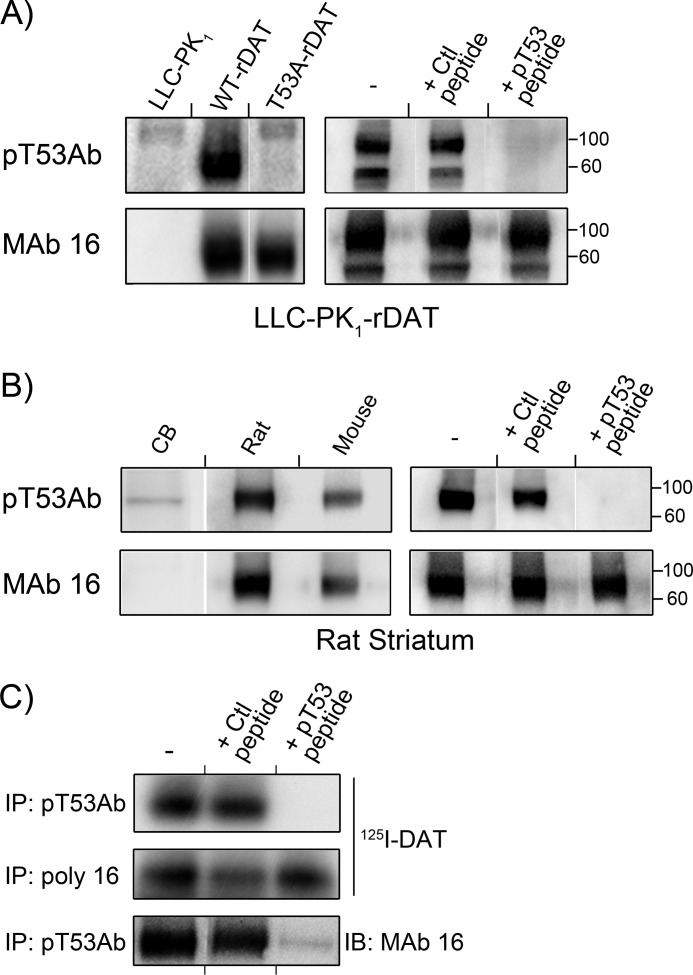
We then assessed the ability of Thr(P)53 Ab to recognize DAT expressed heterologously in LLCPK1 cells (Fig. 3A). Thr(P)53 Ab produced little or no immunostaining of lysates from LLCPK1 cells but showed strong immunoreactivity against a ∼90 kDa band in rDAT-LLCPK1 lysates, indicating specific recognition of rDAT (Fig. 3A, upper panels). Importantly, Thr(P)53 Ab showed no reactivity against T53A rDAT (Fig. 3A, left), and immunostaining of WT DAT was prevented by inclusion of Thr(P)53 peptide but not by the corresponding dephosphopeptide (Fig. 3A, right). Equal levels of WT and T53A rDAT protein and the absence of rDAT in LLCPK1 cells are shown by mAb 16 staining (Fig. 3A, bottom panels). These results demonstrate high specificity of the Thr(P)53 Ab toward phosphorylated Thr53 of rDAT, confirming the mass spectrometry results and supporting our previous loss-of-function evidence for Thr53 phosphorylation in expressed DAT (29).
FIGURE 3.
Immunoreactivity of DAT with Thr(P)53 Ab. Lysates from LLC-PK1 cells, rDAT-LLCPK1 cells, or T53A rDAT-LLCPK1 cells (A) or lysates from rDAT-LLCPK1 cells (B) were immunoblotted with Tyr(P)53 Ab (pT53Ab) (top panels) or mAb 16 (bottom panels). Where indicated, Thr(P)53 Ab was preincubated with phosphorylated (pT53) or non-phosphorylated (Ctl) N-terminal peptide. Molecular mass markers are indicated on the right. C and D, lysates from rat or mouse striatal or rat cerebellar (CB) tissue were immunoblotted with Thr(P)53 Ab (top panels) or mAb 16 (bottom panels). Where indicated, Thr(P)53 Ab was preincubated with phosphorylated (pT53) or non-phosphorylated (Ctl) N-terminal peptide. Molecular mass markers are indicated on the right. E, rat striatal membranes with (top and middle panels) or without (bottom panel) [125I]RTI-82 photoaffinity labeling of DAT were immunoprecipitated (IP) with Thr(P)53 Ab or polyclonal Ab 16 as indicated and resolved by SDS-PAGE. Gels were dried and subjected to autoradiography (top and middle panels) or transferred to PVDF and probed with mAb 16 (bottom panels). Where indicated, Thr(P)53 Ab was preincubated with phosphorylated (pT53) or non-phosphorylated (Ctl) N-terminal peptide.
We also noted in these experiments that Thr(P)53 Ab stained the unglycosylated 60-kDa form of DAT as well as the fully glycosylated 90-kDa form (Fig. 3A, right). The ratio of mature and immature form staining by Thr(P)53 Ab was comparable with that detected by mAb 16, indicating that both forms possess similar levels of Thr53 phosphorylation. We also frequently observed a minor band at >100 kDa in parent cell and T53A rDAT lysates (Fig. 3A, left), indicating the presence of a small degree of cross-reactivity of this Ab with another protein. However the intensity of this staining is negligible in comparison with that of DAT.
Thr(P)53 Ab Staining of Rat and Mouse Striatal DAT
Next we immunoblotted rat and mouse striatal DAT with Thr(P)53 Ab (Fig. 3B). Thr(P)53 Ab was highly reactive against a protein of ∼90 kDa from rat striatal tissue, with only negligible staining detected in cerebellar tissue, which does not express DAT (Fig. 3B, left), strongly supporting the identity of the band as DAT. Staining of the rat striatal band was blocked by inclusion of Thr(P)53 peptide but not by the dephosphopeptide (Fig. 3B, right), demonstrating Ab specificity for phosphorylated Thr53. Levels of DAT protein in each lane are indicated by mAb 16 staining (bottom panels). Mouse DAT, which possesses the Thr53-Pro54 sequence (43), also showed strong immunoreactivity with Thr(P)53 Ab (Fig. 3B, left), whereas human DAT, which possesses the sequence Ser53-Pro54 (44), did not show Thr(P)53 Ab immunostaining (not shown), further supporting the Ab specificity for Thr(P)53. Staining of a minor band in the cerebellar sample in the absence of DAT (Fig. 3B, right) further indicates a slight reactivity of this Ab with a different protein.
Immunoprecipitation of Rat Striatal DAT with Thr(P)53 Ab
We also demonstrated the ability of Thr(P)53 Ab to immunoprecipitate DAT (Fig. 3C). Thr(P)53 Ab readily precipitated rat striatal DATs photoaffinity-labeled with the cocaine analog [125I]RTI-82 (Fig. 3C, top) to levels that were comparable with that precipitated using our standard procedures with polyclonal Ab 16 (Fig. 3C, middle). Non-photolabeled rDATs precipitated by Thr(P)53 Ab could also be detected by immunoblotting with mAb 16 (Fig. 3C, bottom). In both cases, Thr(P)53 Ab-mediated precipitation was blocked with Thr(P)53 peptide but not with the dephosphopeptide (Fig. 3C, top), demonstrating specificity for Thr53 phosphorylation.
Modulation of Thr53 DAT Phosphorylation
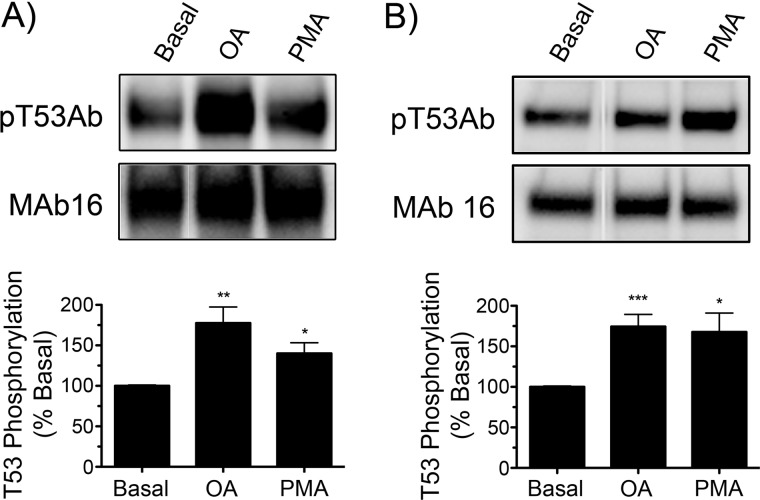
DAT phosphorylation has been studied primarily by metabolic labeling with 32PO4 and has been demonstrated to be modulated by PKC, AMPH, and protein phosphatases (17, 21, 22, 45–47). However, the vast majority of basal and stimulated 32PO4 labeling occurs on distal N-terminal serines (21), making this method unfeasible for characterization of Thr53 phosphorylation responses. To examine the potential for Thr(P)53 Ab to detect regulation of Thr53 phosphorylation, rDAT-LLCPK1 cells and rat striatal tissue were treated with OA to inhibit protein phosphatases or with PMA to activate PKC, followed by blotting with Thr(P)53 Ab or mAb 16. OA and PMA treatments caused Thr53 phosphorylation to increase to 178 ± 20% and 140 ± 13% of basal levels (p < 0.01 and p < 0.05, respectively) in rDAT-LLCPK1 cells (Fig. 4A) and to 174 ± 15% and 168 ± 23% of basal levels (p < 0.001 and p < 0.05, respectively) in rat striatal synaptosomes (Fig. 4B). These results show the ability of Thr(P)53 Ab to detect changes in Thr53 phosphorylation levels and demonstrate the acute modulation of rDAT Thr53 phosphorylation by PKC and phosphatase pathways in both expression systems and native tissue.
FIGURE 4.
Regulation of Thr53 phosphorylation by phorbol ester and okadaic acid. A, rDAT-LLCPK1 cells were treated with vehicle, 1 μm OA, or 1 μm PMA for 30 min at 37 °C. B, rat striatal synaptosomes were treated with vehicle, 1 μm OA, or 1 μm PMA for 30 min at 30 °C. Lysates were subjected to SDS-PAGE and immunoblotted with Thr(P)53 Ab (pT53Ab) (top) or mAb 16 (bottom) (representative examples shown). Histograms show quantification of Thr(P)53 staining relative to vehicle controls (means ± S.E.; n = 3) (*, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to basal by ANOVA).
Phosphorylation Stoichiometry
To obtain an estimate of basal phosphorylation stoichiometry, we analyzed lysates of untreated rat striatal tissue. Thr(P)53 Ab was used to specifically extract Thr53-phosphorylated protein in immunoprecipitation procedures. Analysis of the bound and unbound fractions by Thr(P)53 Ab immunoblotting showed that Thr53-phosphorylated DATs were captured with an efficiency of 38 ± 1%. The amount of total DAT in the precipitated sample was estimated to be 20 ± 1% of the input value, as determined by comparison of mAb 16 staining of Thr(P)53 Ab pellets with DAT standard curves generated by dilutions of input striatal lysate and immunoblotted in parallel with mAb 16. Normalizing the fraction of DAT protein in the Thr(P)53 Ab pellet by the Thr(P)53 Ab precipitation efficiency yielded a Thr53 phosphorylation stoichiometry estimate of 53 ± 2% (data not shown; all values means ± S.E. from three independent experiments performed in duplicate).
Kinetic Analysis of Thr53 Mutants
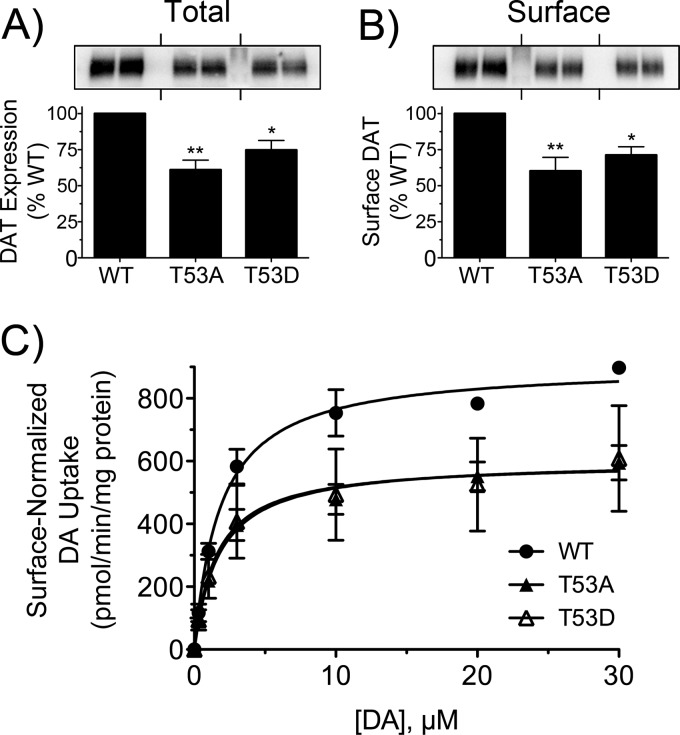
To examine possible functions of Thr53 phosphorylation, we generated T53A non-phosphorylatable and T53D phosphomimetic mutants for stable expression in LLCPK1 cells. mAb 16 immunoblotting showed that T53A and T53D mutants co-migrated with the WT protein at ∼90 kDa on SDS-polyacrylamide gels, indicating full glycosylation and proper biosynthetic processing (48). Total expression levels of the mutants were 61 ± 7% and 75 ± 7% of the WT protein level (Fig. 5A), and plasma membrane levels assessed by cell surface biotinylation were directly proportional to total expression (60 ± 10% and 71 ± 6% of WT surface level) (Fig. 5B), indicating no significant impacts of the mutations on trafficking or surface presentation.
FIGURE 5.
Expression and kinetic analysis of T53A and T53D DATs. rDAT-LLCPK1 cells stably expressing WT, T53A, or T53D DATs were assayed in parallel for total DAT expression levels (A), surface DAT expression levels (B), and DA uptake saturation analysis (C). Blots shown are representative of four independent experiments with samples run in duplicate. Histograms show quantification of DAT levels (**, p < 0.01; *, p < 0.05 relative to controls by ANOVA with Tukey's post hoc test). Transport kinetic parameters were determined by nonlinear regression analysis in three or four independent experiments performed in triplicate. Data are presented as means ± S.E. normalized to cell surface DAT expression levels (**, p < 0.01; *, p < 0.05 relative to controls by ANOVA with Tukey's post hoc test).
Saturation analyses (Fig. 5C) showed that after normalizing DA transport for relative DAT plasma membrane expression, both mutants possessed significantly lower Vmax values than the WT protein (WT, 925 ± 34 pmol/min/mg; T53A, 588 ± 53 pmol/min/mg, p < 0.01; T53D, 664 ± 53 pmol/min/mg, p < 0.05; ANOVA). DA Km values were not different (WT, 2.0 ± 0.2 μm; T53A, 1.8 ± 0.4 μm; T53D, 1.6 ± 0.1 μm; p > 0.05), implicating alteration of transport turnover rate rather than DA recognition as the mechanism for reduced transport in the mutants. To determine if glutamic acid mutation of Thr53 would provide a superior phosphomimetic substitution, we generated T53E DAT for analysis in transiently transfected cells. T53E DAT showed comparable expression relative to the WT protein in mAb 16 immunoblots, but in saturation analyses, it showed DA transport Vmax reductions that were similar in magnitude to that of T53D DAT (not shown), indicating that neither phosphomimetic substitution could rescue the transport reduction seen with the T53A mutation.
To determine if alterations in ion interactions could underlie the reduced Vmax values obtained with Thr53 mutation, we analyzed WT and T53A proteins for Na+ and Cl− concentration dependence. In three independent experiments, we found no significant differences in EC50 of DA uptake for Na+ (WT, 84.4 ± 1.5 mm; T53A, 84.3 ± 4.2 mm; p > 0.05) or for Cl− (WT, 58.9 ± 4.6 mm; T53A, 58.5 ± 1.8 mm; p > 0.05), indicating that loss of Thr53 did not lead to reduced uptake via impacts on the ability of Na+ or Cl− to drive DA translocation.
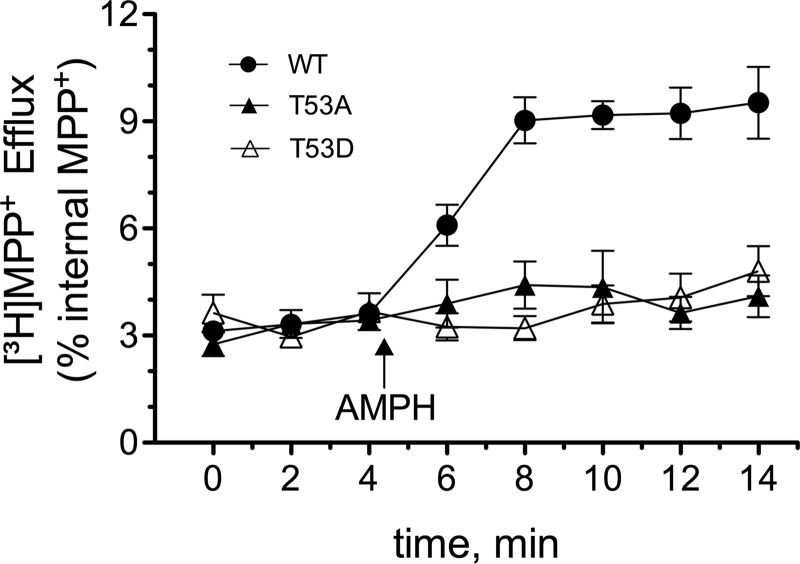
We then used whole-cell superfusion assays to examine the mutants for AMPH-stimulated substrate efflux using [3H]MPP+ as the substrate. MPP+ was robustly transported by the mutants (Vmax WT, 141 ± 7 pmol/min/mg; T53A, 97 ± 14 pmol/min/mg; T53D, 105 ± 12 pmol/min/mg, p > 0.05, n = 3), allowing adequate loading of substrate for efflux analysis. Base-line efflux quantified as a fraction of intracellular substrate did not differ between cell lines (Fig. 6). However, although application of AMPH induced robust MPP+ efflux in the WT rDAT-LLCPK1 cells, it produced no detectable substrate release in the rDAT-T53A and T53D cells (Fig. 6). Lack of AMPH-stimulated efflux activity in the mutants is not due to inability to recognize AMPH because 3 μm AMPH inhibited [3H]DA uptake by 75–85% in all three cell lines (not shown). These results suggest that Thr53 and/or its ability to undergo phosphorylation/dephosphorylation exert a mechanistic role in inward DA transport and constitute a major prerequisite for AMPH-mediated MPP+ efflux.
FIGURE 6.
Amphetamine-stimulated efflux of T53A and T53D DATs. LLC-PK1 cells stably expressing WT, T53A, or T53D DATs were preloaded with [3H]MPP+ for 20 min at 37 °C followed by superfusion analysis of efflux as described under “Experimental Procedures.” Upon reaching a stable base line, three 2-min fractions were collected to define basal efflux followed by the addition of 3 μm AMPH (arrow) to stimulate DAT-mediated efflux.
DISCUSSION
In this study, we use mass spectrometry and phospho-specific immunostaining as positive function approaches to identify and characterize phosphorylation of DAT Thr53 in rodent striatum and heterologous cells. Thr53 is present in the membrane-proximal region of the N-terminal tail close to the beginning of transmembrane domain 1 (TM1) in a motif specific for proline-directed kinases, such as ERK1/2, p38 MAPK, and JNK (29). Although we do not yet know which kinase(s) phosphorylate Thr53, our results provide the first evidence that DAT is directly acted on in neurons by this class of enzymes.
The proline-directed kinase with the most well documented effects on DAT is ERK. In cells and striatal tissue, ERK inhibitors induce rapid reductions in DA transport activity (16, 20, 49, 50), whereas activation of D2 and D3 DA receptors induces ERK-dependent up-regulation of DAT (7, 51). These findings indicate that DA transport functionality is positively influenced by tonic ERK signaling and that rapid up-regulation of transport capacity can be achieved by receptor-mediated activation of ERK. Thus, our findings supporting involvement of Thr53 in forward and reverse transport suggest a mechanistic basis for regulation of DAT by ERK. Although we previously found no loss of ERK inhibitor effects on uptake activity of T53A DAT (29), the reduced DA transport Vmax we found in T53A and T53D mutants is consistent with a role for ERK-mediated phosphorylation of Thr53 in maintaining transport activity. If future studies support this idea for ERK or related kinases, our estimate that basal phosphorylation of Thr53 occurs with a stoichiometry of ∼50% suggests a tonic set point readily amenable to either increased or decreased phosphorylation of significant DAT copy numbers to allow robust modulation of transport capacity. Other potential roles for this site could include tyrosine kinase regulation of DAT, which also involves MAPK activity (52), or ERK-associated functions in cocaine and AMPH actions (3, 53). Current efforts are under way in our laboratory to test Thr53 phosphorylation in modulating ERK effects on DAT and to investigate potential regulatory inputs from other proline-directed kinases.
Our results also strongly support a role for Thr53 phosphorylation in the mechanism of AMPH-stimulated efflux, which is considered to be a crucial factor in the reinforcing and neurotoxic properties of AMPH and related drugs (54). The finding that Thr53 exerts a mechanistic role in efflux is striking because the involvement of phosphorylation in reverse transport has previously been attributed to distal N-terminal serines (55). At present, we lack a clear understanding of the relative contributions of these two N-terminal regions in the efflux mechanism and do not know if there is communication between the domains with respect to phosphorylation. However, the importance of the membrane-proximal region of the N terminus in uptake and efflux mechanisms has been shown in several recent mutagenesis studies (6, 56, 57). In particular, the efflux properties of T53A and T53D mutants resemble those found for a SERT construct with the N terminus tethered to the plasma membrane by TAC peptide (56), supporting a crucial role for the precise arrangement and conformational flexibility of the N terminus in the mechanism of transport reversal.
Proline-directed phosphorylation is well established to drive major protein structural rearrangements by inducing cis-isomerization of the peptide backbone around the Ser(P)/Thr(P)–Pro bond (24, 25). This is likely to be the case for DAT as well because ERK phosphorylation of NDAT causes a distinct upward shift in electrophoretic mobility that is not induced by PKC or other AGC kinases (29). Upward shifts in DAT mobility on SDS-polyacrylamide gels induced by phosphorylation conditions have also been noted (16, 45, 58), consistent with altered conformations that could result from Thr53-Pro54 cis-isomerization. Phosphorylation of Thr53 is thus likely to play a major role in directing the conformational state of the membrane-proximal region of the DAT N terminus.
Our findings that T53A and T53D mutants showed reduced Vmax for forward DA transport and complete loss of AMPH-stimulated reverse MPP+ transport strongly support a crucial role for Thr53 in the transport mechanism. TM1 of DAT performs an essential role in substrate transport and psychostimulant drug binding (8), and the proximity of Thr53 to TM1 suggests the potential for its phosphorylation and/or Thr53-Pro54 cis-trans isomerization to impact transport kinetics via effects on TM1 conformation. Alternatively, Thr53 phosphorylation and/or Thr53-Pro54 cis-trans isomerization could potentially impact the ability of nearby intracellular gate residue Arg60 to form a salt bridge with Asp436 (59), which could affect molecular transitions necessary for the transport cycle. At present, we cannot distinguish between Thr53 contributions to uptake and efflux as due to side chain hydrogen bonding, direct phosphorylation, or peptide backbone cis-trans isomerization. However, if an inability to undergo phosphorylation underlies the transport and efflux reductions seen in T53A DAT, the putative phosphomimetic T53D and T53E mutations do not satisfy the requirements necessary to support these functions. This is probably due to the inability of Asp or Glu to fully compensate for phosphoryl group charge interactions or to induce backbone cis-isomerization.
The proline-rich sequence immediately surrounding Thr53 (PPQTP) also constitutes an Src homology 3 (SH3) domain epitope (PXXP) (60) that may serve as a ligand for interactions with SH3 domain proteins. This motif may therefore function to direct DAT oligomer formation or DAT-binding partner interactions (2, 61). Oligomerization is the preferred quaternary state of neurotransmitter transporters (62) and has been shown to play an important role in transporter-mediated efflux (63). DAT oligomerization is also regulated by AMPH (64), suggesting the possibility that AMPH-mediated activation of PKC (65) may regulate DAT monomer-oligomer equilibria through effects on Thr53 phosphorylation. In addition, several proteins that affect DA transport and efflux, including syntaxin 1A and receptor for activated protein kinase C1, interact with the DAT N-terminal domain (66–68), suggesting them as possible intermediaries for Thr53 effects.
We found strongly increased phosphorylation of DAT Thr53 in rat striatal synaptosomes and cells treated with OA, indicating the presence of robust protein phosphatase activity that maintains this residue in the dephosphorylated state (46). The dose of OA used (10 μm) is compatible with inhibition of PP2A and PP1, both of which have been associated with DAT (46, 69). Thr53 phosphorylation is also stimulated by PMA; however, PKC cannot directly phosphorylate proline-directed residues (70) and does not phosphorylate NDAT on Thr53 in vitro (29). Thus, it is likely that stimulation of Thr53 phosphorylation by PMA occurs via PKC cross-talk with pathways for ERK or other proline-dependent kinases (71–76). These findings thus demonstrate the ability of Thr53 phosphorylation to be regulated either directly via proline-directed kinases and phosphatases or indirectly through PKC modulation of downstream pathways, indicating the potential for Thr53 to serve as a locus for integration of DAT regulatory signals. In addition, both OA and PMA induce DAT down-regulation and endocytosis (16), supporting the potential for Thr53 phosphorylation to participate in these processes.
Although the structure of the DAT N terminus is unknown, the sequence consists of two Ser/Thr-rich domains in membrane-distal (amino acids 1–21) and membrane-proximal (amino acids 43–64) regions, separated by a charged/hydrophobic sequence devoid of potential phosphate acceptors (amino acids 22–42) (Fig. 2). We have now demonstrated that DAT is phosphorylated by PKC in the membrane-distal region and by proline-directed kinases in the membrane-proximal region (21, 29). Because PKC and other AGC kinases do not act on Ser/Thr residues that precede proline (27, 70, 77), and none of the distal N-terminal serines are present in proline-directed motifs, our findings provide direct evidence that the proximal and distal phosphorylation domains are acted on by functionally distinct classes of protein kinases. This suggests a separation of N-terminal functional mechanisms that may differentially impact transporter regulation and present potential sites for therapeutic modulation of transport activity in dopaminergic diseases.
Acknowledgments
We thank Drs. Amy Newman and John Lever for supplying [125I]RTI82, Dr. Eric Murphy for supplying SV129 mice, and Drs. Gert Lubec and Wei-Qiang Chen for generous support with mass spectrometry.
This work was supported, in whole or in part, by National Institutes of Health, Grant R01 DA13147 from NIDA (to R. A. V.), ND EPSCoR IIG (to R. A. V. and J. D. F.), P20 RR017699 from the COBRE program of the National Center for Research Resources (to the University of North Dakota), and P20 RR016741 from the IDeA Networks of Biomedical Research Excellence (INBRE) program of the National Center for Research Resources (to the University of North Dakota). This work was also supported by Austrian Research Funds/FWF Grants F3506 and P22893-B1 (to H. H. S.) and P23670-B09 (to J.-W. Y.).
- DA
- dopamine
- DAT
- dopamine transporter
- PMA
- phorbol 12-myristate 13-acetate
- OA
- okadaic acid
- AMPH
- amphetamine
- MPP+
- [3H]1-methyl-4-phenylpyridinium
- NDAT
- recombinant DAT N-terminal tail protein
- rDAT
- rat DAT
- SH3
- Src homology 3
- TM1
- transmembrane domain 1
- Ab
- antibody
- ANOVA
- analysis of variance.
REFERENCES
- 1. Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 [DOI] [PubMed] [Google Scholar]
- 2. Torres G. E. (2006) The dopamine transporter proteome. J. Neurochem. 97, Suppl. 1, 3–10 [DOI] [PubMed] [Google Scholar]
- 3. Lu L., Koya E., Zhai H., Hope B. T., Shaham Y. (2006) Role of ERK in cocaine addiction. Trends Neurosci. 29, 695–703 [DOI] [PubMed] [Google Scholar]
- 4. Miller G. W., Gainetdinov R. R., Levey A. I., Caron M. G. (1999) Dopamine transporters and neuronal injury. Trends Pharmacol. Sci. 20, 424–429 [DOI] [PubMed] [Google Scholar]
- 5. Bannon M. J., Sacchetti P., Granneman J. G. (1995) in Psychopharmacology: the fourth generation of progress (Borroni E., Kupfer D. J., eds) pp. 179–188, Raven Press Ltd., New York [Google Scholar]
- 6. Guptaroy B., Zhang M., Bowton E., Binda F., Shi L., Weinstein H., Galli A., Javitch J. A., Neubig R. R., Gnegy M. (2009) A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward facing conformation. Mol. Pharmacol. 75, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmitt K. C., Reith M. E. (2010) Regulation of the dopamine transporter. Aspects relevant to psychostimulant drugs of abuse. Ann. N.Y. Acad. Sci. 1187, 316–340 [DOI] [PubMed] [Google Scholar]
- 8. Kristensen A. S., Andersen J., Jørgensen T. N., Sørensen L., Eriksen J., Loland C. J., Strømgaard K., Gether U. (2011) SLC6 neurotransmitter transporters. Structure, function, and regulation. Pharmacol. Rev. 63, 585–640 [DOI] [PubMed] [Google Scholar]
- 9. Steinkellner T., Freissmuth M., Sitte H. H., Montgomery T. (2011) The ugly side of amphetamines. Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”), methamphetamine, and d-amphetamine. Biol. Chem. 392, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sitte H. H., Freissmuth M. (2010) The reverse operation of Na(+)/Cl(−)-coupled neurotransmitter transporters. Why amphetamines take two to tango. J. Neurochem. 112, 340–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sulzer D., Sonders M. S., Poulsen N. W., Galli A. (2005) Mechanisms of neurotransmitter release by amphetamines. A review. Prog. Neurobiol. 75, 406–433 [DOI] [PubMed] [Google Scholar]
- 12. Sonders M. S., Zhu S. J., Zahniser N. R., Kavanaugh M. P., Amara S. G. (1997) Multiple ionic conductances of the human dopamine transporter. The actions of dopamine and psychostimulants. J. Neurosci. 17, 960–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sitte H. H., Huck S., Reither H., Boehm S., Singer E. A., Pifl C. (1998) Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J. Neurochem. 71, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 14. Robertson S. D., Matthies H. J., Galli A. (2009) A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol. Neurobiol. 39, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zahniser N. R., Doolen S. (2001) Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters. Drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 92, 21–55 [DOI] [PubMed] [Google Scholar]
- 16. Ramamoorthy S., Shippenberg T. S., Jayanthi L. D. (2011) Regulation of monoamine transporters. Role of transporter phosphorylation. Pharmacol. Ther. 129, 220–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaughan R. A., Huff R. A., Uhl G. R., Kuhar M. J. (1997) Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem. 272, 15541–15546 [DOI] [PubMed] [Google Scholar]
- 18. Garcia B. G., Wei Y., Moron J. A., Lin R. Z., Javitch J. A., Galli A. (2005) Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell surface redistribution. Mol. Pharmacol. 68, 102–109 [DOI] [PubMed] [Google Scholar]
- 19. Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 20. Morón J. A., Zakharova I., Ferrer J. V., Merrill G. A., Hope B., Lafer E. M., Lin Z. C., Wang J. B., Javitch J. A., Galli A., Shippenberg T. S. (2003) Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 23, 8480–8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foster J. D., Pananusorn B., Vaughan R. A. (2002) Dopamine transporters are phosphorylated on N-terminal serines in rat striatum. J. Biol. Chem. 277, 25178–25186 [DOI] [PubMed] [Google Scholar]
- 22. Cervinski M. A., Foster J. D., Vaughan R. A. (2005) Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J. Biol. Chem. 280, 40442–40449 [DOI] [PubMed] [Google Scholar]
- 23. Granas C., Ferrer J., Loland C. J., Javitch J. A., Gether U. (2003) N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J. Biol. Chem. 278, 4990–5000 [DOI] [PubMed] [Google Scholar]
- 24. Lu K. P., Liou Y. C., Zhou X. Z. (2002) Pinning down proline-directed phosphorylation signaling. Trends Cell Biol. 12, 164–172 [DOI] [PubMed] [Google Scholar]
- 25. Lu K. P., Zhou X. Z. (2007) The prolyl isomerase PIN1. A pivotal new twist in phosphorylation signaling and disease. Nat. Rev. Mol. Cell Biol. 8, 904–916 [DOI] [PubMed] [Google Scholar]
- 26. Ando S., Ikuhara T., Kamata T., Sasaki Y., Hisanaga S., Kishimoto T., Ito H., Inagaki M. (1997) Role of the pyrrolidine ring of proline in determining the substrate specificity of cdc2 kinase or cdk5. J. Biochem. 122, 409–414 [DOI] [PubMed] [Google Scholar]
- 27. Gray C. H., Barford D. (2003) Getting in the ring. Proline-directed substrate specificity in the cell cycle proteins Cdc14 and CDK2-cyclinA3. Cell Cycle 2, 500–502 [DOI] [PubMed] [Google Scholar]
- 28. Brown N. R., Noble M. E., Endicott J. A., Johnson L. N. (1999) The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1, 438–443 [DOI] [PubMed] [Google Scholar]
- 29. Gorentla B. K., Moritz A. E., Foster J. D., Vaughan R. A. (2009) Proline-directed phosphorylation of the dopamine transporter N-terminal domain. Biochemistry 48, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lever J. R., Carroll F. I., Patel A., Abraham P., Boja J. W., Lewin A. H., Lew R. (1993) Radiosynthesis of a photoaffinity probe for the cocaine receptor of the dopamine transporter. 3-(p-Chlorophenyl)tropan-2-carboxylic acid m-([125I]-iodo)-p-azidophenethyl ester ([125I]RTI-82). J. Labelled Compd. Radiopharm. 33, 1131–1137 [Google Scholar]
- 31. Gu H., Wall S. C., Rudnick G. (1994) Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem. 269, 7124–7130 [PubMed] [Google Scholar]
- 32. Yang J. W., Vacher H., Park K. S., Clark E., Trimmer J. S. (2007) Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc. Natl. Acad. Sci. U.S.A. 104, 20055–20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng J. F., Patil S. S., Chen W. Q., An W., He J. Q., Höger H., Lubec G. (2009) Hippocampal protein levels related to spatial memory are different in the Barnes maze and in the multiple T-maze. J. Proteome Res. 8, 4479–4486 [DOI] [PubMed] [Google Scholar]
- 34. Gaffaney J. D., Vaughan R. A. (2004) Uptake inhibitors but not substrates induce protease resistance in extracellular loop two of the dopamine transporter. Mol. Pharmacol. 65, 692–701 [DOI] [PubMed] [Google Scholar]
- 35. Foster J. D., Vaughan R. A. (2011) Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J. Biol. Chem. 286, 5175–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaughan R. A., Sakrikar D. S., Parnas M. L., Adkins S., Foster J. D., Duval R. A., Lever J. R., Kulkarni S. S., Hauck-Newman A. (2007) Localization of cocaine analog [125I]RTI 82 irreversible binding to transmembrane domain 6 of the dopamine transporter. J. Biol. Chem. 282, 8915–8925 [DOI] [PubMed] [Google Scholar]
- 37. Vaughan R. A., Kuhar M. J. (1996) Dopamine transporter ligand binding domains. Structural and functional properties revealed by limited proteolysis. J. Biol. Chem. 271, 21672–21680 [DOI] [PubMed] [Google Scholar]
- 38. Foster J. D., Adkins S. D., Lever J. R., Vaughan R. A. (2008) Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 105, 1683–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin Z., Itokawa M., Uhl G. R. (2000) Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J. 14, 715–728 [DOI] [PubMed] [Google Scholar]
- 40. Henry L. K., Iwamoto H., Field J. R., Kaufmann K., Dawson E. S., Jacobs M. T., Adams C., Felts B., Zdravkovic I., Armstrong V., Combs S., Solis E., Rudnick G., Noskov S. Y., DeFelice L. J., Meiler J., Blakely R. D. (2011) A conserved asparagine residue in transmembrane segment 1 (TM1) of serotonin transporter dictates chloride-coupled neurotransmitter transport. J. Biol. Chem. 286, 30823–30836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scholze P., Nørregaard L., Singer E. A., Freissmuth M., Gether U., Sitte H. H. (2002) The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. 277, 21505–21513 [DOI] [PubMed] [Google Scholar]
- 42. Scholze P., Sitte H. H., Singer E. A. (2001) Substantial loss of substrate by diffusion during uptake in HEK-293 cells expressing neurotransmitter transporters. Neurosci. Lett. 309, 173–176 [DOI] [PubMed] [Google Scholar]
- 43. Wu X., Gu H. H. (1999) Molecular cloning of the mouse dopamine transporter and pharmacological comparison with the human homologue. Gene 233, 163–170 [DOI] [PubMed] [Google Scholar]
- 44. Giros B., el Mestikawy S., Bertrand L., Caron M. G. (1991) Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 295, 149–154 [DOI] [PubMed] [Google Scholar]
- 45. Vaughan R. A. (2004) Phosphorylation and regulation of psychostimulant-sensitive neurotransmitter transporters. J. Pharmacol. Exp. Ther. 310, 1–7 [DOI] [PubMed] [Google Scholar]
- 46. Foster J. D., Pananusorn B., Cervinski M. A., Holden H. E., Vaughan R. A. (2003) Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Brain Res. Mol. Brain Res. 110, 100–108 [DOI] [PubMed] [Google Scholar]
- 47. Foster J. D., Cervinski M. A., Gorentla B. K., Vaughan R. A. (2006) Regulation of the dopamine transporter by phosphorylation. Handb. Exp. Pharmacol., 197–214 [DOI] [PubMed] [Google Scholar]
- 48. Li L. B., Chen N., Ramamoorthy S., Chi L., Cui X. N., Wang L. C., Reith M. E. (2004) The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 279, 21012–21020 [DOI] [PubMed] [Google Scholar]
- 49. Carvelli L., Morón J. A., Kahlig K. M., Ferrer J. V., Sen N., Lechleiter J. D., Leeb-Lundberg L. M., Merrill G., Lafer E. M., Ballou L. M., Shippenberg T. S., Javitch J. A., Lin R. Z., Galli A. (2002) PI 3-kinase regulation of dopamine uptake. J. Neurochem. 81, 859–869 [DOI] [PubMed] [Google Scholar]
- 50. Zapata A., Kivell B., Han Y., Javitch J. A., Bolan E. A., Kuraguntla D., Jaligam V., Oz M., Jayanthi L. D., Samuvel D. J., Ramamoorthy S., Shippenberg T. S. (2007) Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J. Biol. Chem. 282, 35842–35854 [DOI] [PubMed] [Google Scholar]
- 51. Lee F. J., Pei L., Moszczynska A., Vukusic B., Fletcher P. J., Liu F. (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoover B. R., Everett C. V., Sorkin A., Zahniser N. R. (2007) Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J. Neurochem. 101, 1258–1271 [DOI] [PubMed] [Google Scholar]
- 53. Shi X., McGinty J. F. (2006) Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience 138, 1289–1298 [DOI] [PubMed] [Google Scholar]
- 54. Sulzer D. (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khoshbouei H., Sen N., Guptaroy B., Johnson L., Lund D., Gnegy M. E., Galli A., Javitch J. A. (2004) N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2, E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sucic S., Dallinger S., Zdrazil B., Weissensteiner R., Jørgensen T. N., Holy M., Kudlacek O., Seidel S., Cha J. H., Gether U., Newman A. H., Ecker G. F., Freissmuth M., Sitte H. H. (2010) The N terminus of monoamine transporters is a lever required for the action of amphetamines. J. Biol. Chem. 285, 10924–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guptaroy B., Fraser R., Desai A., Zhang M., Gnegy M. E. (2011) Site-directed mutations near transmembrane domain 1 alter conformation and function of norepinephrine and dopamine transporters. Mol. Pharmacol. 79, 520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huff R. A., Vaughan R. A., Kuhar M. J., Uhl G. R. (1997) Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J. Neurochem. 68, 225–232 [DOI] [PubMed] [Google Scholar]
- 59. Kniazeff J., Shi L., Loland C. J., Javitch J. A., Weinstein H., Gether U. (2008) An intracellular interaction network regulates conformational transitions in the dopamine transporter. J. Biol. Chem. 283, 17691–17701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mayer B. J. (2001) SH3 domains. Complexity in moderation. J. Cell Sci. 114, 1253–1263 [DOI] [PubMed] [Google Scholar]
- 61. Egaña L. A., Cuevas R. A., Baust T. B., Parra L. A., Leak R. K., Hochendoner S., Peña K., Quiroz M., Hong W. C., Dorostkar M. M., Janz R., Sitte H. H., Torres G. E. (2009) Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci. 29, 4592–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sitte H. H., Farhan H., Javitch J. A. (2004) Sodium-dependent neurotransmitter transporters. Oligomerization as a determinant of transporter function and trafficking. Mol. Interv. 4, 38–47 [DOI] [PubMed] [Google Scholar]
- 63. Seidel S., Singer E. A., Just H., Farhan H., Scholze P., Kudlacek O., Holy M., Koppatz K., Krivanek P., Freissmuth M., Sitte H. H. (2005) Amphetamines take two to tango. An oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol. Pharmacol. 67, 140–151 [DOI] [PubMed] [Google Scholar]
- 64. Chen N., Reith M. E. (2008) Substrates dissociate dopamine transporter oligomers. J. Neurochem. 105, 910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giambalvo C. T. (2003) Differential effects of amphetamine transport versus dopamine reverse transport on particulate PKC activity in striatal synaptoneurosomes. Synapse 49, 125–133 [DOI] [PubMed] [Google Scholar]
- 66. Lee K. H., Kim M. Y., Kim D. H., Lee Y. S. (2004) Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem. Res. 29, 1405–1409 [DOI] [PubMed] [Google Scholar]
- 67. Carvelli L., Blakely R. D., DeFelice L. J. (2008) Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc. Natl. Acad. Sci. U.S.A. 105, 14192–14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Binda F., Dipace C., Bowton E., Robertson S. D., Lute B. J., Fog J. U., Zhang M., Sen N., Colbran R. J., Gnegy M. E., Gether U., Javitch J. A., Erreger K., Galli A. (2008) Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol. Pharmacol. 74, 1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bauman A. L., Apparsundaram S., Ramamoorthy S., Wadzinski B. E., Vaughan R. A., Blakely R. D. (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 20, 7571–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sossin W. S. (2007) Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem. 14, 236–246 [DOI] [PubMed] [Google Scholar]
- 71. Brändlin I., Hübner S., Eiseler T., Martinez-Moya M., Horschinek A., Hausser A., Link G., Rupp S., Storz P., Pfizenmaier K., Johannes F. J. (2002) Protein kinase C (PKC)η-mediated PKC μ activation modulates ERK and JNK signal pathways. J. Biol. Chem. 277, 6490–6496 [DOI] [PubMed] [Google Scholar]
- 72. Mauro A., Ciccarelli C., De Cesaris P., Scoglio A., Bouché M., Molinaro M., Aquino A., Zani B. M. (2002) PKCα-mediated ERK, JNK, and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J. Cell Sci. 115, 3587–3599 [DOI] [PubMed] [Google Scholar]
- 73. Clark J. A., Black A. R., Leontieva O. V., Frey M. R., Pysz M. A., Kunneva L., Woloszynska-Read A., Roy D., Black J. D. (2004) Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J. Biol. Chem. 279, 9233–9247 [DOI] [PubMed] [Google Scholar]
- 74. Wen-Sheng W. (2006) Protein kinase C alpha trigger Ras and Raf-independent MEK/ERK activation for TPA-induced growth inhibition of human hepatoma cell HepG2. Cancer Lett. 239, 27–35 [DOI] [PubMed] [Google Scholar]
- 75. Guerrero C., Lecuona E., Pesce L., Ridge K. M., Sznajder J. I. (2001) Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 281, L79–L85 [DOI] [PubMed] [Google Scholar]
- 76. Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways. Regulation and physiological functions. Endocr. Rev. 22, 153–183 [DOI] [PubMed] [Google Scholar]
- 77. Gold M. G., Barford D., Komander D. (2006) Lining the pockets of kinases and phosphatases. Curr. Opin. Struct. Biol. 16, 693–701 [DOI] [PubMed] [Google Scholar]