Background: GSK-3α and GSK-3β are crucial kinases that mediate various principal signals.
Results: The compound knock-out mice (Gsk3a−/−;Gsk3b+/−) showed impaired skeletal growth with RelA as the key target.
Conclusion: Their redundant functions through RelA phosphorylation at Thr-254 regulate chondrocyte differentiation.
Significance: This is the first report that shows in vivo evidence of the functional relationship of GSK-3α and GSK-3β and the underlying mechanism.
Keywords: Cartilage, Chondrocytes, Development, Glycogen synthase kinase 3, NF-kappa B (NF-KB)
Abstract
Here we examine the roles of two isoforms of glycogen synthase kinase-3 (GSK-3), GSK-3α and GSK-3β, in skeletal development. Both isoforms were unphosphorylated and active in chondrocyte differentiation stages during SOX9 and type II collagen (COL2A1) expression. Although knock-out of both alleles of Gsk3a (Gsk3a−/−) or a single allele of Gsk3b (Gsk3b+/−) in mice did not significantly affect skeletal development, compound knock-out (Gsk3a−/−;Gsk3b+/−) caused dwarfism with impairment of chondrocyte differentiation. GSK-3α and GSK-3β induced differentiation of cultured chondrocytes with functional redundancy in a cell-autonomous fashion, independently of the Wnt/β-catenin signal. Computational predictions followed by SOX9 and COL2A1 transcriptional assays identified RelA (NF-κB p65) as a key phosphorylation target of GSK-3. Among several phosphorylation residues in RelA, Thr-254 was identified as the critical phosphorylation site for GSK-3 that modulated chondrocyte differentiation. In conclusion, redundant functions of GSK-3α and GSK-3β through phosphorylation of RelA at Thr-254 play a crucial role in early stages of chondrocyte differentiation.
Introduction
In the early stages of skeletal development, mesenchymal progenitor cells are recruited into condensations and differentiate into chondrocytes that produce cartilage-specific matrix proteins such as type II collagen (COL2A1)2 and aggrecan (ACAN). This process is regulated by sex-determining region Y-type high mobility group box protein (SOX9), a master transcriptional activator, in combination with the co-factor SOX6 (1, 2). The chondrocytes then undergo hypertrophy and terminal differentiation, which are characterized by secretion of type X collagen (COL10A1).
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase that phosphorylates numerous substrates including transcription factors, structural proteins, and signaling proteins (3, 4). In mammalian cells, there are two GSK-3 isoforms, GSK-3α and GSK-3β, encoded by independent genes (Gsk3a and Gsk3b, respectively), which share very similar kinase domains but differ substantially outside of this catalytic structure. Unlike most protein kinases, GSK-3 is active under resting conditions and is inactivated upon stimulation through phosphorylation-dependent and phosphorylation-independent mechanisms. When Ser-21 and Ser-9 residues within the N-terminal domain of GSK-3α and GSK-3β, respectively, are phosphorylated by upstream kinases such as PI3K/Akt, cyclic AMP-dependent protein kinase (PKA), and protein kinase C (PKC), the phosphorylated residues block the access of substrates to the GSK-3 catalytic domain, thereby inhibiting substrate phosphorylation by GSK-3. Meanwhile, GSK-3 functions as a negative regulator of the canonical Wnt, signaling independently of N-terminal domain phosphorylation, coupling instead via the scaffolding proteins axin and adenomatous polyposis coli (APC) and causing subsequent phosphorylation and proteolytic inactivation of β-catenin. Because these PI3K/Akt, PKA, PKC, and Wnt/β-catenin signals are known to be crucial for regulation of the chondrocyte differentiation (5–7), their central mediator GSK-3 is likely to play an important role in this process. However, we and others have reported that Gsk3a homozygous knock-out (Gsk3a−/−) mice and Gsk3b heterozygous knock-out (Gsk3b+/−) mice show normal skeletal development (8, 9). Furthermore, although the global Gsk3b homozygous knock-out (Gsk3b−/−) mice are embryonically lethal (10), the cartilage-specific Gsk3b knock-out (Col2a1-Cre;Gsk3bfl/fl) mice exhibit normal skeletal phenotypes (11). More interestingly, the cartilage-specific Gsk3b knock-out mice show up-regulation of GSK-3α protein in the cartilage, implicating redundant or compensatory functions of the two GSK-3 isoforms during chondrocyte differentiation and skeletal development (11). To know the involvement of the GSK-3 signal in skeletal development, the present study initially examined the expression patterns of GSK-3α and GSK-3β during chondrocyte differentiation. Furthermore, we looked at their functions and relationship by creating compound knock-out mice and investigated the underlying molecular mechanism during chondrocyte differentiation.
EXPERIMENTAL PROCEDURES
Mice
All experiments were performed according to protocols approved by the Animal Care and Use Committee of the University of Tokyo. In each experiment, we compared genotypes of littermates that were maintained in a C57BL/6 background. To generate Gsk3a−/−;Gsk3b+/− mice, Gsk3b+/− mice were mated with the homozygous Gsk3a−/− mice to obtain Gsk3a+/−;Gsk3b+/− mice, which were then mated with each other.
Cell Culture
We cultured ATDC5 cells (Riken BRC) in DMEM/F12 (1:1) with 5% FBS in the presence of ITS (insulin, transferrin, and sodium selenite) to induce differentiation. We isolated primary chondrocytes from the ribs of mouse embryos (E18.5). We performed pellet cultures of primary chondrocytes in DMEM/F12 (1:1) with 10% FBS for 1 week and monolayer culture for 1 week in differentiation medium as described previously (12). For toluidine blue staining, cells were fixed with 70% (v/v) ethanol and stained with 0.05% toluidine blue solution (Wako) for 5 min. We assessed cell proliferation using a CCK-8 assay kit (Dojindo) according to the manufacturer's instructions.
Real-time RT-PCR
We extracted total RNA from cells using an RNeasy mini kit (Qiagen). Expression patterns of GSK-3 in human tissues were determined by the Human Total RNA Master Panel II (Clontech). An aliquot (1 mg) was reverse-transcribed with QuantiTect reverse transcription (Qiagen) to make single-stranded cDNA. Real-time RT-PCR was performed using an ABI Prism 7000 sequence detection system (Applied Biosystems) using QuantiTect SYBR Green PCR master mix (Qiagen) according to the manufacturer's instructions. Standard plasmids were synthesized with a TOPO TA cloning kit (Invitrogen). Copy numbers of target gene mRNA in each total RNA were calculated by reference to standard curves and were adjusted to the human or murine standard total RNA (Applied Biosystems) with the human GAPDH or rodent actin. Primer sequence information is available upon request.
Immunoblotting and Immunoprecipitation
Cells were lysed in TNE buffer (10 mm Tris-HCl (pH 7.8), 150 mm NaCl, 1 mm EDTA, 1% Nonidet P-40). For immunoblotting, the cell lysates were fractionated by SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were incubated with primary antibodies to p-GSK-3αS21, p-GSK-3βS9, GSK-3α, GSK-3β, axin, β-catenin, p-IκBS32/36, and IκB (Cell Signaling Technology); APC, p-RelAT254, and RelA (Abcam); actin (Sigma); and IKKα/β (Santa Cruz Biotechnology Inc.). Membranes were incubated with HRP-conjugated antibody (Promega), and immunoreactive proteins were visualized with ECL Plus (Amersham Biosciences). For immunoprecipitation, supernatants precleared with protein G-Sepharose (Invitrogen) were incubated with antibodies to GSK-3α and GSK-3β at 4 °C for 2 h. After centrifugation, immunocomplexes were washed three times with washing buffer (HEPES-NaOH (pH 7.4), 150 mm NaCl, 10 mm EDTA, 0.02% Nonidet P-40), and bound proteins were eluted with SDS sample buffer, subjected to SDS-PAGE, and subjected to immunoblotting as described above.
Histological and Radiological Analyses
We performed double staining of skeletons of mouse embryos or neonates with a solution containing Alizarin red S and Alcian blue 8GX (Sigma) after fixation in 99.5% ethanol and acetone. Hematoxylin and eosin (H&E) staining was done according to standard protocols after fixation in 4% paraformaldehyde buffered with PBS. For immunohistochemistry, we incubated the sections with the antibodies described above and those to COL2A1 and COL10A1 (LSL Biolafitte) using nonimmune serum as control, diluted 1:250 in blocking reagent. For immunofluorescence, we used a tyramide signal amplification (TSA) fluorescence systems kit (PerkinElmer Life Sciences) as the secondary antibody detection system. For radiological analysis, plain radiographs were taken using a soft x-ray apparatus (Softex CMB-2; Softex).
Construction of Expression Vectors
We prepared expression vectors for luciferase assays in pCMV-HA (Clontech) and siRNA vectors for the murine Gsk3a and Gsk3b gene (NM_001031667: nucleotides 575–599, and NM_019827: nucleotides 1,063–1,087, respectively) in piGENEmU6 vectors (iGENE Therapeutics). We generated retroviral vectors using pMx vectors as described previously (13) and adenovirus vectors by the AdenoX Expression system (Clontech). All vectors were verified by DNA sequencing.
Luciferase Assay
We prepared a reporter construct containing a fragment of the SOX9 gene (from −4,042 to +376 bp relative to the transcription start site), COL2A1 gene (+284 to +3,271 bp connected to the 38-bp basal promoter (minP; Promega)), SOX6 gene (−2,000 to −1 bp), and ACAN gene (−4,000 to −1 bp) by PCR using human genomic DNA as template and cloned these into either pGL3-Basic vector or pGL4.10 (luc2) vector (Promega). The TOPflash system (Upstate Biotech Millipore) was used according to the manufacturer's protocol. We created mutation constructs by PCR, performed luciferase assays with the PicaGene Dual SeaPansy luminescence kit (Toyo Ink Co., Ltd.) using a GloMax 96 Microplate Luminometer (Promega), and showed the data as the ratio of firefly activity to Renilla activity (relative luciferase activity).
Computational Predictions
We used the database and online resource NetworKIN for predicting in vivo kinase-substrate relationships and the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/sites/GDSbrowser; GEO accession GSE7685) for expressions in the proliferative zone of growth plate cartilage.
Statistical Analyses
We performed statistical analyses of experimental data with the unpaired two-tailed Student's t test. p values less than 0.05 were considered significant.
RESULTS
Expressions and Activities of GSK-3α and GSK-3β during Chondrocyte Differentiation
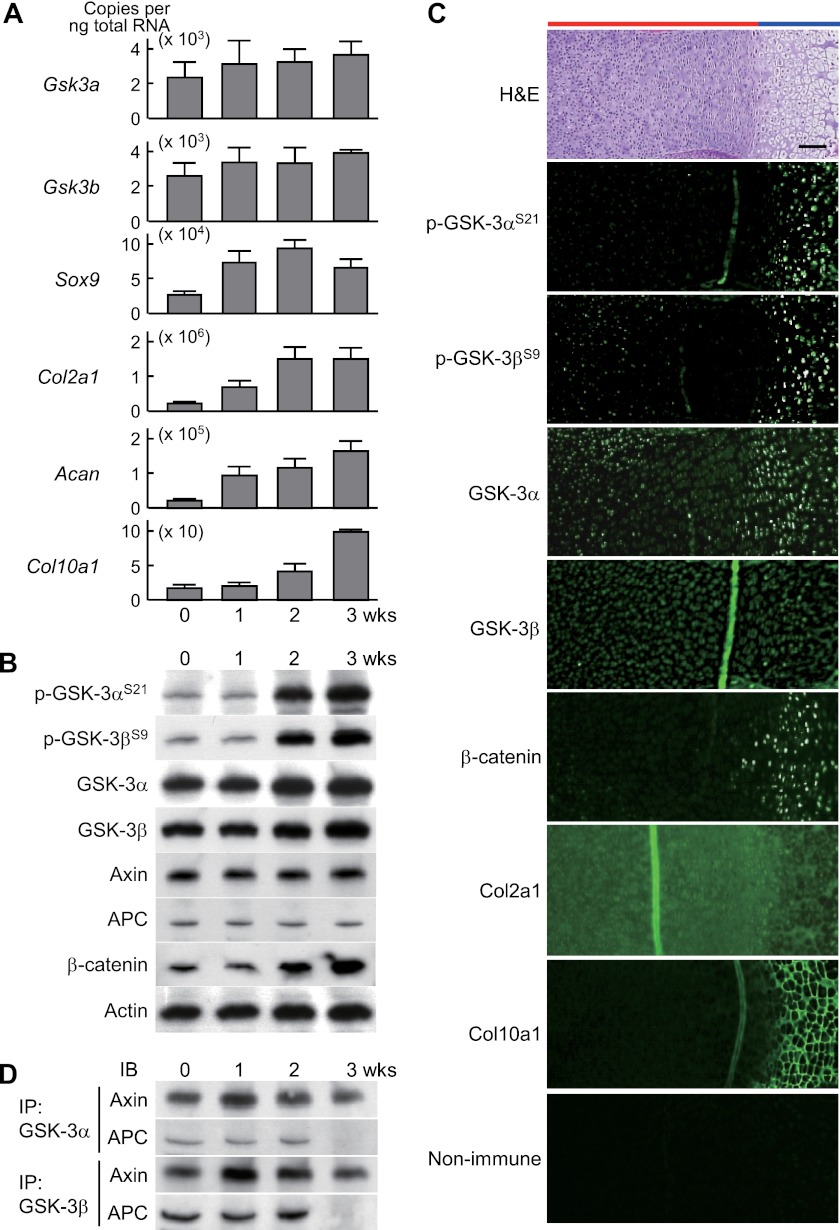
In cultures of murine chondrogenic ATDC5 cells, both Gsk3 isoforms were constitutively expressed in early chondrocyte differentiation stages with Col2a1 and Acan expression, as well as in the later stages with Col10a1 expression (Fig. 1A). Our analysis of the posttranslational regulation of the GSK-3 activity found enhancement of phosphorylation of GSK-3α at Ser-21 and GSK-3β at Ser-9 at 2 weeks of culture and thereafter, indicating that the catalytic activity of both isoforms is suppressed in later stages (Fig. 1B). In the tibial limb cartilage of mouse embryos, the phosphorylated proteins of GSK-3α and GSK-3β were localized in hypertrophic chondrocytes of later stages that express COL10A1, whereas GSK-3α and GSK-3β proteins were widely detected (Fig. 1C). The scaffolding proteins associated with canonical Wnt signaling, axin and APC, were constitutively expressed (Fig. 1B); however, their physical association with GSK-3α and GSK-3β was stronger in the early stages up to 2 weeks (Fig. 1D), during which time levels of β-catenin protein were concordantly suppressed (Fig. 1B). These data indicate that GSK-3 is not regulated at the level of transcription during chondrocyte differentiation, but is inactivated in the later stages, through either phosphorylation-dependent or phosphorylation-independent mechanisms, such that the activity of both GSK-3 isoforms is higher during early chondrocyte differentiation stages.
FIGURE 1.
A, mRNA levels of Gsk3a, Gsk3b, and early (Sox9, Col2a1, and Acan) and later (Col10a1) chondrocyte differentiation markers during culture of mouse chondrogenic ATDC5 cells in differentiation medium (ITS) for 3 weeks. Data are expressed as means ± S.D. for three wells/group. B, immunoblotting using antibodies to Ser-21-phosphorylated GSK-3α (p-GSK-3αS21) and Ser-9-phosphorylated GSK-3β (p-GSK-3βS9), GSK-3α, GSK-3β, axin, APC, and β-catenin with actin as the loading control during differentiation of cultured ATDC5 cells as above. C, H&E and immunofluorescence with antibodies to the indicated proteins, as well as the nonimmune control, in the proximal tibias of mouse embryos (E18.5). Scale bar, 100 μm. Red and blue bars indicate layers of proliferative and hypertrophic zones, respectively. D, physical association of GSK-3 with scaffolding proteins axin and APC by immunoprecipitation (IP) and immunoblotting (IB) analyses during differentiation of cultured ATDC5 cells as above.
Roles of GSK-3α and GSK-3β in Skeletal Development
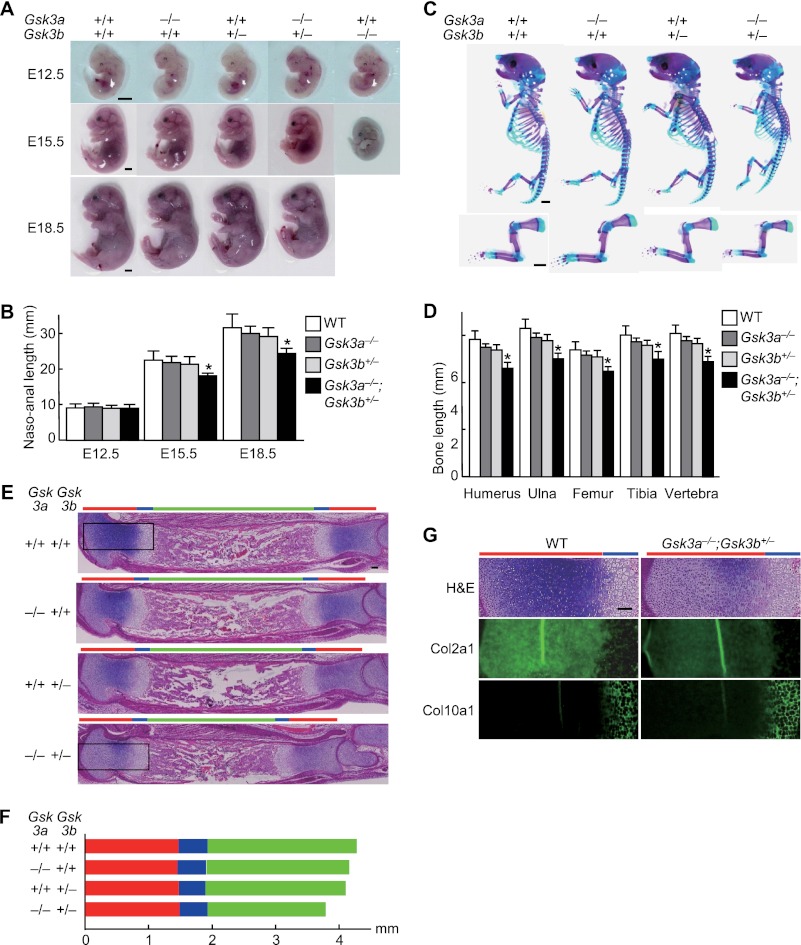
We then examined the physiological role of GSK-3α and GSK-3β in skeletal development using mice in which these genes had been genetically inactivated. Although Gsk3b−/− mice died at mid-gestation, Gsk3a−/− mice and Gsk3b+/− mice developed and grew normally without significant difference from the wild-type littermates (Fig. 2, A–E), as reported previously (8–10). We next generated compound knock-out mice of Gsk3a and heterozygotes of Gsk3b by mating Gsk3a+/− or Gsk3a−/− mice with Gsk3b+/− mice. The Gsk3a+/−;Gsk3b+/− mice showed normal skeletal development (data not shown). Although Gsk3a−/−;Gsk3b+/− mice that harbored only a single GSK-3β allele were viable and generated with the expected Mendelian ratios, these animals displayed dwarfism with significantly shorter long bones and vertebra, as well as lower body weight, than the wild-type littermates both before and after birth (Fig. 2, A–E, and supplemental Fig. 1). In the Gsk3a−/−;Gsk3b+/− limb, the lengths of proliferative and hypertrophic zones were comparable with those of the three other genotypes, suggesting unaffected proliferation or terminal differentiation by the compound knock-out (Fig. 2F). However, metachromasia of the cartilage matrix and expression of COL2A1 was decreased, although that of COL10A1 was little affected (Fig. 2G). These findings demonstrate that the compound knock-out of Gsk3a and Gsk3b, rather than the respective single knock-out, caused impairment of early stages of chondrocyte differentiation.
FIGURE 2.
A, gross appearance of wild-type (Gsk3a+/+;Gsk3b+/+), Gsk3a homozygous knock-out (Gsk3a−/−), Gsk3b heterozygous knock-out (Gsk3b+/−), Gsk3a homozygous and Gsk3b heterozygous knock-out (Gsk3a−/−;Gsk3b+/−), and Gsk3b homozygous knock-out (Gsk3b−/−) littermates (E12. 5, E15.5, and E18.5). All Gsk3b−/− embryos died at mid-gestation. Scale bars, 1 mm. B, total naso-anal length of the four genotypes: wild-type (WT), Gsk3a−/−, Gsk3b+/−, and Gsk3a−/−;Gsk3b+/− littermates (E12.5, E15.5, and E18.5). Data are expressed as means ± S.D. for five mice/group. *, p < 0.05 versus WT. C, double staining with Alizarin red and Alcian blue of the whole skeletons (top) and forelimbs and clavicles (bottom) of the four genotype littermates (E18.5). Scale bars, 1 mm. D, length of long bones (humerus, ulna, femur, and tibia) and vertebra (first to fifth lumbar spines) of the four genotype littermates (E18.5). Data are expressed as means ± S.D. for five mice/group. *, p < 0.05 versus WT. E, H&E staining of whole tibias of the four genotype littermates (E18.5). The boxed areas indicate regions shown in the enlarged images in panel G. Red, blue, and green bars indicate layers of proliferative, hypertrophic zones, and bone area, respectively. Scale bars, 100 μm. F, lengths of proliferative zone (red), hypertrophic zone (blue), and bone area (green) of the four genotype littermates (E18.5). G, H&E staining and immunofluorescence with antibodies to the indicated proteins in the boxed areas above of WT and Gsk3a−/−;Gsk3b+/− littermates (E18.5). Scale bars, 100 μm.
Functions of GSK-3α and GSK-3β in Cultured Chondrocytes
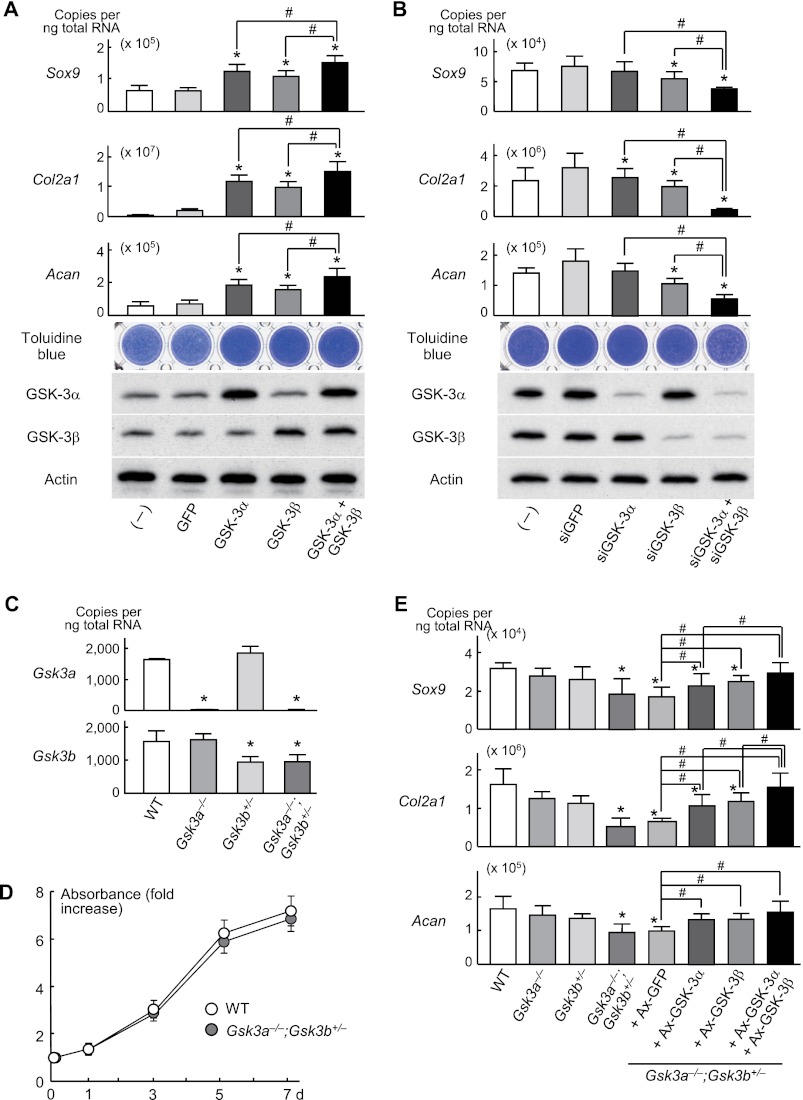
Thus far we had used global knock-out mice rather than tissue-specific knock-out mice. We therefore examined possible cell-autonomous effects of GSK-3α and GSK-3β in chondrocytes by creating stable lines of ATDC5 cells with retroviral overexpression of GSK-3α, GSK-3β, or their combination. Sox9, Col2a1, and Acan, as well as toluidine blue staining, were moderately increased upon single gene overexpression and further enhanced by their combination (Fig. 3A). For loss-of-function analysis, we created stable lines of ATDC5 cells with retroviral overexpression of specific siRNAs for GSK-3α, GSK-3β, or their combination and confirmed inhibition of the differentiation factors noted above by the single knockdown and further by the double knockdown (Fig. 3B). In addition to ATDC5 cells, we looked at cultures of primary chondrocytes derived from wild-type, Gsk3a−/−, Gsk3b+/−, and Gsk3a−/−;Gsk3b+/− mice, which were confirmed to show specific inhibition without significant compensatory up-regulation in cells lacking either Gsk3a or Gsk3b (Fig. 3C). Cell proliferation determined by growth curve analysis was comparable between wild-type and Gsk3a−/−;Gsk3b+/− chondrocytes (Fig. 3D). Contrarily, Sox9, Col2a1, and Acan levels were significantly suppressed in Gsk3a−/−;Gsk3b+/− chondrocytes, but not in Gsk3a−/− or Gsk3b+/− chondrocytes (Fig. 3E). These suppressions by the compound knock-out of Gsk3a and one allele of Gsk3b were significantly restored by overexpression of a single cDNA and further by overexpression of both cDNAs (Fig. 3E), confirming the functional redundancy of these isoforms in chondrocyte differentiation. Taken together, these data are consistent with activity of GSK-3 isoforms during early stages of chondrocytes being important to induce their differentiation in a cell-autonomous fashion.
FIGURE 3.
A, mRNA levels of Sox9, Col2a1, Acan, and toluidine blue staining in stable lines of ATDC5 cells retrovirally transfected with GSK-3α, GSK-3β, a combination of both, or the control GFP and in nontransfected parental cells (−) after 1 week of differentiation induction by ITS treatment. Data are expressed as means ± S.D. for three wells/group. GSK-3α and GSK-3β levels were confirmed by Western blotting, with actin as the loading control. Data are expressed as means ± S.D. for three wells/group. B, analyses of the same read-outs in stable lines of ATDC5 cells retrovirally transfected with siRNA specific for GSK-3α, GSK-3β, a combination of both, or the control siGFP and in nontransfected parental cells (−) after 1 week of the ITS treatment. Data are expressed as means ± S.D. for three wells/group. GSK-3 levels were determined by Western blotting. C, mRNA levels of Gsk3a and Gsk3b in the pellet cultures of primary chondrocytes derived from wild-type (WT), Gsk3a−/−, Gsk3b+/−, and Gsk3a−/−;Gsk3b+/− littermates. Data are expressed as means ± S.D. for three wells/group. D, cell proliferation curves by the CCK-8 assay during 7-day cultures of primary chondrocytes derived from WT and Gsk3a−/−;Gsk3b+/− littermates. There was no significant difference between groups (p > 0.05). Data are expressed as means ± S.D. for three wells/group. E, mRNA levels of Sox9, Col2a1 and Acan in the pellet cultures of primary chondrocytes derived from WT, Gsk3a−/−, Gsk3b+/−, and Gsk3a−/−;Gsk3b+/− littermates. For the rescue experiment, adenoviral (Ax) transfection with GSK-3α, GSK-3β, their combination, or control GFP was performed before pellet formation. All data are expressed as means ± S.D. for three wells/group. *, p < 0.05 versus GFP, siGFP, or WT. #, p < 0.05 as indicated.
Screening of Phosphorylation Target of GSK-3 in Chondrocytes
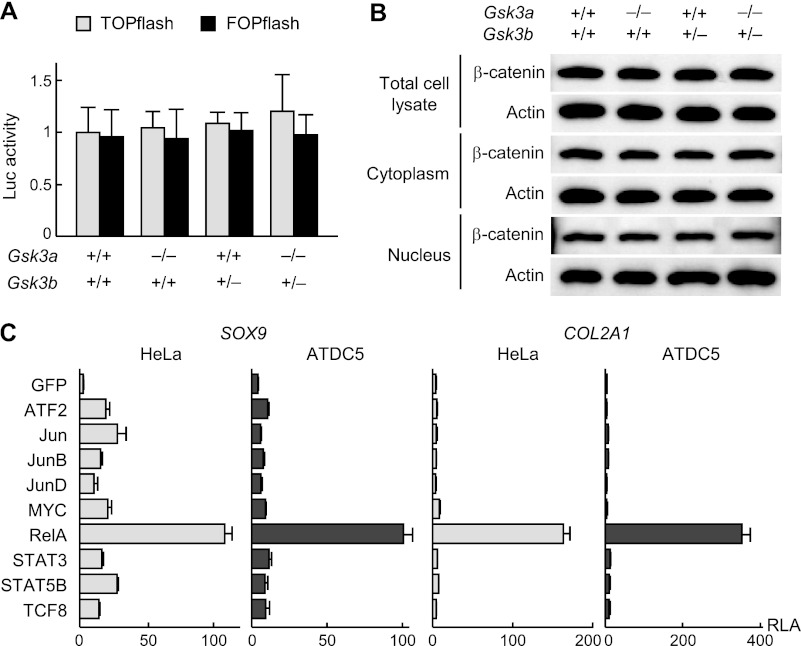
To investigate the molecular networks depending on GSK-3 during chondrocyte differentiation, we sought to identify the relevant transcriptional regulators that were substrates of GSK-3 in chondrocytes. GSK-3 is known to phosphorylate and degrade β-catenin through coupling with the scaffolding proteins axin and APC. We observed binding of GSK-3α and GSK-3β to the scaffolding proteins as well as suppression of β-catenin protein level during the chondrocyte differentiation stages (Fig. 1, B and D). However, none of β-catenin transcriptional activity as determined by the TOPflash system, β-catenin protein levels, or subcellular localization was altered in animals harboring single or compound knockouts of Gsk3a and Gsk3b (Fig. 4, A and B).
FIGURE 4.
A, promoter activity of the β-catenin target T cell-specific factor, as assessed by luciferase (Luc) assay using TOPflash and FOPflash reporter plasmids in primary chondrocytes derived from WT, Gsk3a−/−, Gsk3b+/−, and Gsk3a−/−;Gsk3b+/− littermates. Data are expressed as means ± S.D. for three wells/group. There were no significant differences among the groups (p > 0.05). B, β-catenin protein level as assessed by immunoblotting of total cell lysate, cytoplasmic, and nuclear fractions in primary chondrocytes above. C, luciferase activity after transfection of nine transcription factors selected by the two computational screenings (supplemental Table S1) or the control GFP into HeLa and ATDC5 cells containing either SOX9 gene reporter construct or COL2A1 enhancer-luciferase reporter construct. Data are expressed as means ± S.D. for three wells/group. RLA, relative luciferase activity.
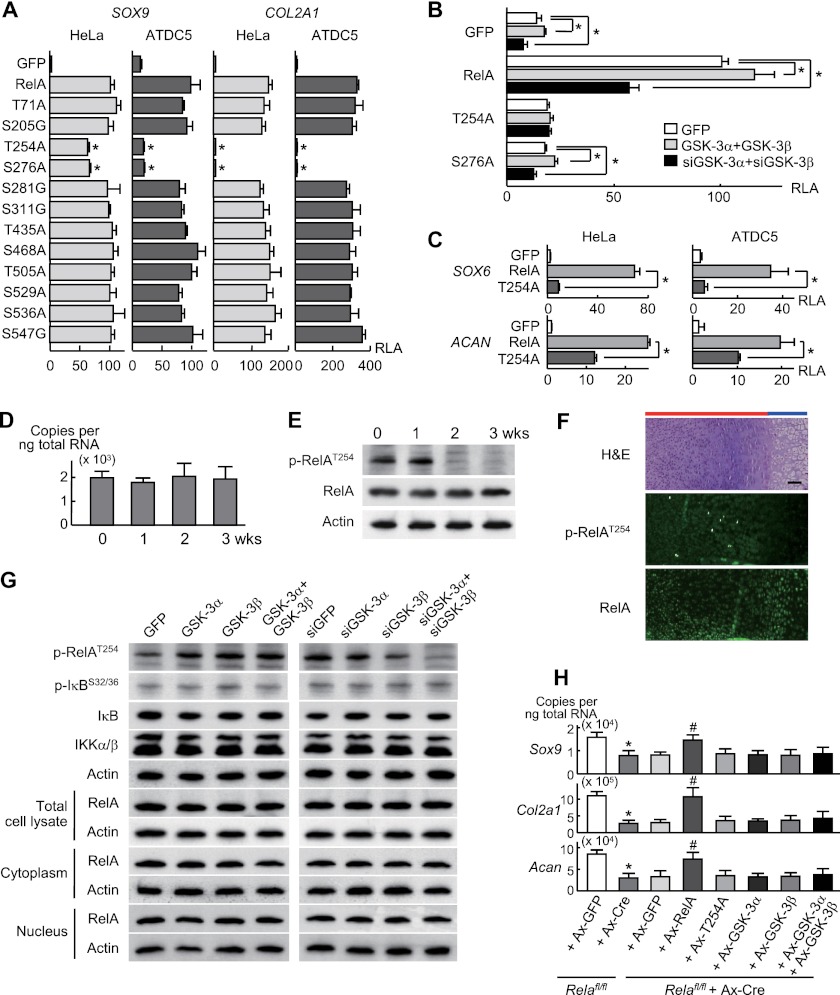
Hence, to identify phosphorylation targets of GSK-3 other than β-catenin, we performed two steps of computational screenings using in silico databases (NetworKIN and GEO database) (supplemental Fig. 2) (14, 15) and predicted nine transcription factors as possible targets (supplemental Table 1). Because GSK-3 was shown to be involved in early stages of chondrocyte differentiation, we compared their effects on transcriptional activities of SOX9 and COL2A1, which are early chondrocyte differentiation markers. Among these, luciferase assays revealed that v-rel reticuloendotheliosis viral oncogene homolog A (RelA or NF-κB p65, encoded by Rela), an essential molecule of the NF-κB signaling pathway, most strongly stimulated the transcriptional activities of both genes in HeLa and ATDC5 cells (Fig. 4C).
Mechanism of RelA Phosphorylation by GSK-3 during Chondrocyte Differentiation
To understand the mechanisms underlying RelA phosphorylation by GSK-3, we created RelA mutants with replacement of putative phosphorylation sites (3, 16) with nonphosphorylatable residues. Among these, replacement of Thr-254 and Ser-276 residues to Ala (T254A and S276A) caused significant suppression of SOX9 and COL2A1 transactivation (Fig. 5A), suggesting that phosphorylation of either site is important for RelA to induce chondrocyte differentiation. Furthermore, the increases and decreases of SOX9 transcriptional activity by overexpression and knockdown of the GSK-3α and GSK-3β combination, respectively, were nullified in the T254A transfected cells, although these effects were unchanged in cells transfected with the S276A mutant (Fig. 5B). In addition to SOX9 and COL2A1, RelA strongly stimulated the transcriptional activity of the SOX6 and ACAN genes in HeLa and ATDC5 cells, both of which were significantly inhibited upon expression of the T254A RelA mutation (Fig. 5C).
FIGURE 5.
A, luciferase activity after transfection with RelA or RelA mutants with replacement of putative phosphorylation sites with nonphosphorylatable residues or control GFP into stable lines of HeLa and ATDC5 cells containing either SOX9 gene reporter construct or COL2A1 enhancer-luciferase reporter construct. Data are expressed as means ± S.D. for three wells/group. *, p < 0.01 versus RelA. RLA, relative luciferase activity. B, luciferase activity after transfection with GFP, RelA, or two RelA mutants (T254A and S276A) in stable lines of ATDC5 cells containing the SOX9 gene reporter construct, which were retrovirally co-transfected with a combination of GSK-3α and GSK-3β, siGSK-3α and siGSK-3β, or GFP. Data are expressed as means ± S.D. for three wells/group. *, p < 0.05 as indicated. C, luciferase activity after transfection with GFP, RelA, or RelA mutant T254A into ATDC5 cells as described above containing the SOX6 (−2,000 to −1 bp) or ACAN (−4,000 to −1 bp) gene reporter construct. Data are expressed as means ± S.D. for three wells/group. *, p < 0.05 as indicated. D, mRNA levels of Rela during differentiation of ATDC5 cells cultured for 3 weeks. Data are expressed as means ± S.D. for three wells/group. E, immunoblotting using antibodies to Thr-254-phosphorylated RelA (p-RelAT254) and RelA in cultured ATDC5 cells above. F, H&E staining and immunofluorescence with antibodies to p-RelAT254 and RelA in the proximal tibias (E18.5). Scale bar, 100 μm. Red and blue bars indicate layers of proliferative and hypertrophic zones, respectively. G, immunoblotting using the antibodies to p-RelAT254, phosphorylated IκB (p-IκB), IκB, and IKKα/β, and RelA (total cell lysate, cytoplasmic and nuclear fractions) in stable lines of ATDC5 cells retrovirally transfected with GFP, GSK-3α, GSK-3β, or their combination (left) and with siRNA specific for GFP, GSK-3α, GSK-3β, or their combination (right) after stimulation with ITS. H, mRNA levels as above after adenoviral transfection with GFP or Cre in primary chondrocytes from Relafl/fl mice. For the rescue experiment, adenoviral transfection with GFP, RelA, T254A, GSK-3α, GSK-3β, and their combination was performed 24 h after Cre transfection in Relafl/fl chondrocytes. *, p < 0.05 versus Relafl/fl+Ax-GFP. #, p < 0.05 versus Relafl/fl+Ax-Cre+Ax-GFP. All data are expressed as means ± S.D. for three wells/group.
Rela mRNA levels were unaltered during differentiation of ATDC5 cells cultured for 3 weeks (Fig. 5D). However, RelA was phosphorylated at Thr-254 during the early stages, which was markedly suppressed at 2 weeks and thereafter (Fig. 5E). In tibial limb cartilage of mouse embryos, Thr-254-phosphorylated RelA was localized in the early stages of differentiation, whereas total RelA protein was more widely detected (Fig. 5F). RelA phosphorylation at Thr-254 during the chondrocyte differentiation stages in vitro and in vivo exhibits a mirror image of phosphorylation of GSK-3α at Ser-21 and GSK-3β at Ser-9 (Fig. 1, B and C). Furthermore, phosphorylated RelA was increased upon overexpression of GSK-3α and GSK-3β and was suppressed by single knockdown and virtually abrogated by double knockdown of Gsk3 in ATDC5 cells (Fig. 5G). Neither RelA protein level nor subcellular localization was affected by Gsk3 overexpression or knockdown, nor was the phosphorylation of IκB or IκB kinase (IKK) levels, which are known to regulate nuclear translocation of free NF-κB complexes (Fig. 5G) (17, 18). Lastly, Cre-mediated deletion of RelA in primary chondrocytes derived from mice homozygous for a floxed Rela allele (Relafl/fl) (19) caused suppression of endogenous mRNA levels of the differentiation markers described above, which were restored upon overexpression of wild type RelA, but not the T254A mutant (Fig. 5H). The transcriptional suppression was not restored by single or combined overexpression of GSK-3α and GSK-3β, demonstrating that RelA acts downstream of and is essential for GSK-3 to induce these markers. These findings clearly demonstrate that the phosphorylation of RelA at Thr-254 is crucial for GSK-3 to induce chondrocyte differentiation.
DISCUSSION
This is the first report of characterization of mice that lack three of the four GSK-3 alleles. It is quite remarkable that these animals develop relatively normally aside from the dwarfism, given the central role of GSK-3 in multiple signaling pathways that are critical for development. The relative fitness of these animals is likely to be a testament to the robustness of signaling systems through feedback mechanisms that allow for resetting of sensitivity to agonists.
GSK-3α and GSK-3β are known to display both overlapping and unique functions depending on the cell and tissue being examined: e.g. they are redundant in regulating the Wnt/β-catenin signal in embryonic stem cells, whereas playing distinct roles in cardiomyocyte differentiation (4, 20–22). The present study is the first to show in vivo evidence of the functional relationship of the two isoforms through analysis of compound knock-out mice that globally express only a single allele of GSK-3β. In chondrocytes, their functions seem to be redundant or compensatory, at least in their early differentiation stages, because the compound knock-out (Gsk3a−/−;Gsk3b+/−) mice showed dwarfism with impairment of chondrocyte differentiation, whereas none of the Gsk3a−/−, Gsk3b+/−, or cartilage-specific Gsk3b−/− mice exhibited significant skeletal abnormalities (Fig. 2) (8–11). Although a previous study detected an increase of GSK-3α protein in cartilage-specific Gsk3b−/− mice (11), the present study failed to show a significant compensatory up-regulation of GSK-3α or GSK-3β level in chondrocytes with deficiency of Gsk3b or Gsk3a, respectively (Fig. 3, B and C), similar to data previously reported in embryonic stem cells (20). This would indicate that the relationship is redundant rather than compensatory in the differentiation stages. Notably, we did not examine Gsk3b−/− tissues, so it is possible that total loss of GSK-3β is required for induction of GSK-3α. Indeed, there seems to be a difference between the isoforms in the strength and extent of the regulation of chondrocyte differentiation. For the chondrocyte differentiation, endogenous GSK-3β seems to be more necessary than GSK-3α because the loss of function of GSK-3β inhibited the differentiation and RelA phosphorylation more potently than that of GSK-3α (Figs. 3B and 5G). Furthermore, contrary to a lack of evidence for a specific role of GSK-3α in chondrocytes, we and others have reported that GSK-3β suppresses later chondrocyte differentiation and osteoblast differentiation by phosphorylating and inactivating β-catenin and Runx2, respectively (9, 23). Hence, GSK-3β may strongly and extensively regulate the skeletal development process, whereas GSK-3α functions as the redundant co-factor mainly at early stages when both isoforms are unphosphorylated and active (Fig. 1B). This functional redundancy may not be important in later stages when both GSK-3 isoforms are phosphorylated because double knock-in mice in which Ser-9 of GSK-3β and Ser-21 of GSK-3α are replaced with nonphosphorylatable residues show normal skeletal development (24).
Although GSK-3 is known to suppress the Wnt/β-catenin signal through coupling with scaffolding proteins to form a β-catenin destruction complex (3, 4), the involvement of phosphorylation of GSK-3 in this pathway remains controversial (4, 9, 25). Our present and previous results show that β-catenin and phosphorylated GSK-3β proteins are co-localized in highly differentiated or hypertrophic chondrocytes (Fig. 1, B and C) (9), and chondrocyte-specific knock-out of β-catenin in mice results in dwarfism with impairment of the differentiation (26). We have also previously shown some interaction between GSK-3β phosphorylation at Ser-9 and binding to axin for the phosphorylation of β-catenin in the chondrocyte differentiation process (9). In the present study, however, the protein level, subcellular localization, and transcriptional activity of β-catenin were unaffected by knock-out of Gsk3a, Gsk3b, or their combined inactivation during chondrocyte differentiation (Fig. 4, A and B). Furthermore, it is reported that the compensatory increase of GSK-3α protein in the cartilage-specific Gsk3b−/− mice is not associated with changes in β-catenin protein levels (11). Taken together, β-catenin might possibly be a phosphorylation target of GSK-3β, but appears independent of the functional redundancy of the two GSK-3 isoforms in the chondrocyte differentiation stages.
Instead of β-catenin, we have identified an NF-κB family member, RelA, as a critical phosphorylation target of GSK-3 for chondrocyte differentiation. Similar to GSK-3, the NF-κB family members, as well as the inhibitory binding protein IκB and the stimulatory kinase IKK, are widely expressed and modulate a number of target genes involved in various cell functions (17, 18). The functional relationship of RelA and GSK-3β is supported by the close resemblance of the phenotypes upon their global knock-out (Rela−/− compared with Gsk3b−/−) as both exhibit massive liver apoptosis causing embryonic lethality (10, 27). The NF-κB family genes are expressed in limb and growth plate cartilage, and NF-κB inactivation by IκB overexpression or knock-out of IKKα in mice causes suppression of skeletal development (28–31). Furthermore, a patient with a heterozygous mutation of the IκBα gene and impaired NF-κB function exhibited short stature (32). Our screening of phosphorylation targets of GSK-3 using two steps of computational screenings, however, did not predict NF-κB family members other than RelA (supplemental Table 1). In fact, RelA has been reported to be a potent regulator of chondrocyte differentiation in vitro (28, 29). Although RelA can act as a homodimer, it generally partners with NF-κB1 (p50) (18). We therefore co-transfected the two in the SOX9 reporter gene transfection assay using HeLa and ATDC5 cells; however, NF-κB1 did not enhance the transactivity by RelA alone (supplemental Fig. 3), suggesting that endogenous NF-κB1 may be sufficient at least in these cells.
Besides the canonical proteasome-dependent mechanism through IκB phosphorylation by IKK, which causes nuclear translocation, several lines of evidence have suggested that phosphorylation of the NF-κB subunit is required for efficient transcriptional activity (10, 16, 17). In most cells, GSK-3 appears to stimulate the RelA activity via phosphorylation rather than the proteasome-dependent mechanism (10, 33–35). Contrarily, GSK-3 causes inhibition of the NF-κB signal in neuronal and epithelial cells, mainly through suppression of IκB phosphorylation by IKK (36, 37). Hence, GSK-3 may play variable roles through distinct mechanisms in the regulation of NF-κB depending on cell types and conditions. In chondrocytes, the present study showed that GSK-3 caused phosphorylation of RelA without affecting the subcellular localization or the IKK/IκB signal (Fig. 5G). However, the later differentiation stages may be mediated by β-catenin, which is also a substrate of GSK-3 in chondrocytes (9), because β-catenin is reported to interact with RelA to inhibit its transcriptional activity (38). When considering just the mechanisms that act through RelA phosphorylation, the pertinent sites on RelA are different depending on protein kinases and cell types (16). Although phosphorylation of Thr-254 by GSK-3 positively regulates RelA activity in chondrocytes (Fig. 5), phosphorylation of Ser-468 by GSK-3β is reported to negatively regulate basal activity in unstimulated cells (39). These opposing effects may possibly be explained by phosphorylation site-dependent conformational changes of the RelA subunit, which is selective with respect to target genes or epigenetic environments in different cell types and conditions.
Among the transcription factors that were predicted as possible targets of GSK-3 by computational screenings, we have chosen RelA due to the strongest stimulation of the SOX9 and COL2A1 transcriptional activities (Fig. 4C). However, we cannot deny the possibility that other transcription factors might interact with or contribute to the GSK-related chondrocyte differentiation and skeletal development. In fact, c-Myc is reported to participate in proliferation accompanying chondrocyte maturation (40). AP-1 signals also regulate matrix metalloproteinase gene expression in chondrocytes (41, 42).
The mechanism underlying the inhibition of the early differentiation stages in Gsk3a−/−;Gsk3b+/− mice remains unclarified. This might possibly be related to decreased COL2A1 expression (Fig. 2G) because the Col2a1+/− mice exhibit mild dwarfism similar to the Gsk3a−/−;Gsk3b+/− mice (43). In addition to COL2A1, the transcriptional targets of the GSK-3/RelA signal in chondrocyte differentiation include SOX9, SOX6, and ACAN (Figs. 4C and 5, A–C). Among these, SOX9 is confirmed to be transactivated by GSK-3-dependent Thr-254 phosphorylation of RelA (Fig. 5B). In fact, our previous screening of the Sox9 promoter identified RelA as the most potent transcriptional factor through binding to an NF-κB motif (28). Because the present COL2A1 enhancer-luciferase reporter construct contains a stretch of +284 to +3,271 bp including a putative enhancer element in intron 1 (+2,217 to +2,234) to which SOX9 directly binds and activates the transcription (44), the transactivation of COL2A1 by RelA may possibly be mediated by that of SOX9. Our previous study also showed that RelA enhances endogenous expressions of COL2A1 and SOX9, not only in ATDC5 cells, but also in HeLa cells (28). On the other hand, we have previously identified RelA as the most potent transcriptional factor of hypoxia-inducible factor-2α (HIF-2α) that controls later stages of chondrocyte differentiation (45). Hence, RelA may extensively control skeletal development with distinct upstream and downstream signals depending on the stage. In the early stages, however, phosphorylation of RelA at Thr-254 by both GSK-3α and GSK-3β is likely to be essential for chondrocyte differentiation.
Supplementary Material
Acknowledgments
We thank R. Yamaguchi and H. Kawahara for technical assistance.
This study was supported by Grants-in-Aid for Scientific Research 21390416, 22659267, and 22390286 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.

This article contains supplemental Figs. S1–S3 and Table S1.
- COL2A1
- type II collagen α1 chain
- COL10A1
- type X collagen α1 chain
- GSK-3
- glycogen synthase kinase-3
- SOX9
- sex-determining region Y-type high mobility group box protein 9
- SOX6
- sex-determining region Y-type high mobility group box protein 6
- ACAN
- aggrecan
- RelA
- v-rel reticuloendotheliosis viral oncogene homolog A
- NF-κB
- nuclear factor-κB
- APC
- adenomatous polyposis coli
- IKK
- inhibitor of κB kinase
- ITS
- insulin, transferrin, and sodium selenite
- Cre
- cAMP-response element-binding protein
- E
- embryonic day
- p
- phosphorylated.
REFERENCES
- 1. Karsenty G. (2008) Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 [DOI] [PubMed] [Google Scholar]
- 2. Kronenberg H. M. (2003) Developmental regulation of the growth plate. Nature 423, 332–336 [DOI] [PubMed] [Google Scholar]
- 3. Doble B. W., Woodgett J. R. (2003) GSK-3: tricks of the trade for a multitasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel S., Doble B., Woodgett J. R. (2004) Glycogen synthase kinase-3 in insulin and Wnt signaling: a double-edged sword? Biochem. Soc. Trans. 32, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kita K., Kimura T., Nakamura N., Yoshikawa H., Nakano T. (2008) PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells 13, 839–850 [DOI] [PubMed] [Google Scholar]
- 6. Li T. F., Zuscik M. J., Ionescu A. M., Zhang X., Rosier R. N., Schwarz E. M., Drissi H., O'Keefe R. J. (2004) PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp. Cell Res. 300, 159–169 [DOI] [PubMed] [Google Scholar]
- 7. Day T. F., Guo X., Garrett-Beal L., Yang Y. (2005) Wnt/β-Catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739–750 [DOI] [PubMed] [Google Scholar]
- 8. MacAulay K., Doble B. W., Patel S., Hansotia T., Sinclair E. M., Drucker D. J., Nagy A., Woodgett J. R. (2007) Glycogen synthase kinase 3α-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 6, 329–337 [DOI] [PubMed] [Google Scholar]
- 9. Kawasaki Y., Kugimiya F., Chikuda H., Kamekura S., Ikeda T., Kawamura N., Saito T., Shinoda Y., Higashikawa A., Yano F., Ogasawara T., Ogata N., Hoshi K., Hofmann F., Woodgett J. R., Nakamura K., Chung U. I., Kawaguchi H. (2008) Phosphorylation of GSK-3β by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J. Clin. Invest. 118, 2506–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., Woodgett J. R. (2000) Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 406, 86–90 [DOI] [PubMed] [Google Scholar]
- 11. Gillespie J. R., Ulici V., Dupuis H., Higgs A., Dimattia A., Patel S., Woodgett J. R., Beier F. (2011) Deletion of glycogen synthase kinase-3β in cartilage results in up-regulation of glycogen synthase kinase-3α protein expression. Endocrinology 152, 1755–1766 [DOI] [PubMed] [Google Scholar]
- 12. Zhou Z., Xie J., Lee D., Liu Y., Jung J., Zhou L., Xiong S., Mei L., Xiong W. C. (2010) Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev. Cell 19, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitamura T. (1998) New experimental approaches in retrovirus-mediated expression screening. Int. J. Hematol. 67, 351–359 [DOI] [PubMed] [Google Scholar]
- 14. Linding R., Jensen L. J., Ostheimer G. J., van Vugt M. A., Jørgensen C., Miron I. M., Diella F., Colwill K., Taylor L., Elder K., Metalnikov P., Nguyen V., Pasculescu A., Jin J., Park J. G., Samson L. D., Woodgett J. R., Russell R. B., Bork P., Yaffe M. B., Pawson T. (2007) Systematic discovery of in vivo phosphorylation networks. Cell 129, 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James C. G., Stanton L. A., Agoston H., Ulici V., Underhill T. M., Beier F. (2010) Genome-wide analyses of gene expression during mouse endochondral ossification. PLoS One 5, e8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang B., Yang X. D., Lamb A., Chen L. F. (2010) Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell. Signal. 22, 1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen L. F., Greene W. C. (2004) Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 5, 392–401 [DOI] [PubMed] [Google Scholar]
- 18. Hayden M. S., Ghosh S. (2008) Shared principles in NF-κB signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 19. Algül H., Treiber M., Lesina M., Nakhai H., Saur D., Geisler F., Pfeifer A., Paxian S., Schmid R. M. (2007) Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J. Clin. Invest. 117, 1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doble B. W., Patel S., Wood G. A., Kockeritz L. K., Woodgett J. R. (2007) Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 12, 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuda T., Zhai P., Maejima Y., Hong C., Gao S., Tian B., Goto K., Takagi H., Tamamori-Adachi M., Kitajima S., Sadoshima J. (2008) Distinct roles of GSK-3α and GSK-3β phosphorylation in the heart under pressure overload. Proc. Natl. Acad. Sci. U.S.A. 105, 20900–20905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho J., Rameshwar P., Sadoshima J. (2009) Distinct roles of glycogen synthase kinase (GSK)-3α and GSK-3β in mediating cardiomyocyte differentiation in murine bone marrow-derived mesenchymal stem cells. J. Biol. Chem. 284, 36647–36658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kugimiya F., Kawaguchi H., Ohba S., Kawamura N., Hirata M., Chikuda H., Azuma Y., Woodgett J. R., Nakamura K., Chung U. I. (2007) GSK-3β controls osteogenesis through regulating Runx2 activity. PLoS One 2, e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McManus E. J., Sakamoto K., Armit L. J., Ronaldson L., Shpiro N., Marquez R., Alessi D. R. (2005) Role that phosphorylation of GSK-3 plays in insulin and Wnt signaling defined by knock-in analysis. EMBO J. 24, 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baryawno N., Sveinbjörnsson B., Eksborg S., Chen C. S., Kogner P., Johnsen J. I. (2010) Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/β-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 70, 266–276 [DOI] [PubMed] [Google Scholar]
- 26. Akiyama H., Lyons J. P., Mori-Akiyama Y., Yang X., Zhang R., Zhang Z., Deng J. M., Taketo M. M., Nakamura T., Behringer R. R., McCrea P. D., de Crombrugghe B. (2004) Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18, 1072–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beg A. A., Sha W. C., Bronson R. T., Ghosh S., Baltimore D. (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κ B. Nature 376, 167–170 [DOI] [PubMed] [Google Scholar]
- 28. Ushita M., Saito T., Ikeda T., Yano F., Higashikawa A., Ogata N., Chung U., Nakamura K., Kawaguchi H. (2009) Transcriptional induction of SOX9 by NF-κB family member RelA in chondrogenic cells. Osteoarthritis Cartilage 17, 1065–1075 [DOI] [PubMed] [Google Scholar]
- 29. Wu S., Flint J. K., Rezvani G., De Luca F. (2007) Nuclear factor-κB p65 facilitates longitudinal bone growth by inducing growth plate chondrocyte proliferation and differentiation and by preventing apoptosis. J. Biol. Chem. 282, 33698–33706 [DOI] [PubMed] [Google Scholar]
- 30. Kanegae Y., Tavares A. T., Izpisúa Belmonte J. C., Verma I. M. (1998) Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature 392, 611–614 [DOI] [PubMed] [Google Scholar]
- 31. Li Q., Lu Q., Hwang J. Y., Büscher D., Lee K. F., Izpisua-Belmonte J. C., Verma I. M. (1999) IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 13, 1322–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janssen R., van Wengen A., Hoeve M. A., ten Dam M., van der Burg M., van Dongen J., van de Vosse E., van Tol M., Bredius R., Ottenhoff T. H., Weemaes C., van Dissel J. T., Lankester A. (2004) The same IκBα mutation in two related individuals leads to completely different clinical syndromes. J. Exp. Med. 200, 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinbrecher K. A., Wilson W., 3rd, Cogswell P. C., Baldwin A. S. (2005) Glycogen synthase kinase 3β functions to specify gene-specific, NF-κB-dependent transcription. Mol. Cell Biol. 25, 8444–8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ougolkov A. V., Fernandez-Zapico M. E., Savoy D. N., Urrutia R. A., Billadeau D. D. (2005) Glycogen synthase kinase-3β participates in nuclear factor κB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 65, 2076–2081 [DOI] [PubMed] [Google Scholar]
- 35. Gong R., Rifai A., Ge Y., Chen S., Dworkin L. D. (2008) Hepatocyte growth factor suppresses proinflammatory NF-κB activation through GSK-3β inactivation in renal tubular epithelial cells. J. Biol. Chem. 283, 7401–7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graham J. R., Tullai J. W., Cooper G. M. (2010) GSK-3 represses growth factor-inducible genes by inhibiting NF-κB in quiescent cells. J. Biol. Chem. 285, 4472–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bachelder R. E., Yoon S. O., Franci C., de Herreros A. G., Mercurio A. M. (2005) Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J. Cell Biol. 168, 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deng J., Miller S. A., Wang H. Y., Xia W., Wen Y., Zhou B. P., Li Y., Lin S. Y., Hung M. C. (2002) β-Catenin interacts with and inhibits NF-κB in human colon and breast cancer. Cancer Cell 2, 323–334 [DOI] [PubMed] [Google Scholar]
- 39. Buss H., Dörrie A., Schmitz M. L., Frank R., Livingstone M., Resch K., Kracht M. (2004) Phosphorylation of serine 468 by GSK-3β negatively regulates basal p65 NF-κB activity. J. Biol. Chem. 279, 49571–49574 [DOI] [PubMed] [Google Scholar]
- 40. Iwamoto M., Yagami K., Lu Valle P., Olsen B. R., Petropoulos C. J., Ewert D. L., Pacifici M. (1993) Expression and role of c-myc in chondrocytes undergoing endochondral ossification. J. Biol. Chem. 268, 9645–9652 [PubMed] [Google Scholar]
- 41. Ray A., Bal B. S., Ray B. K. (2005) Transcriptional induction of matrix metalloproteinase-9 in the chondrocyte and synoviocyte cells is regulated via a novel mechanism: evidence for functional cooperation between serum amyloid A-activating factor-1 and AP-1. J. Immunol. 175, 4039–4048 [DOI] [PubMed] [Google Scholar]
- 42. Liacini A., Sylvester J., Li W. Q., Zafarullah M. (2002) Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1), and nuclear factor κB (NF-κB) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 21, 251–262 [DOI] [PubMed] [Google Scholar]
- 43. Li S. W., Prockop D. J., Helminen H., Fässler R., Lapveteläinen T., Kiraly K., Peltarri A., Arokoski J., Lui H., Arita M. (1995) Transgenic mice with targeted inactivation of the Col2 α1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 9, 2821–2830 [DOI] [PubMed] [Google Scholar]
- 44. Lefebvre V., Huang W., Harley V. R., Goodfellow P. N., de Crombrugghe B. (1997) SOX9 is a potent activator of the chondrocyte-specific enhancer of the Proα1(II) collagen gene. Mol. Cell Biol. 17, 2336–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saito T., Fukai A., Mabuchi A., Ikeda T., Yano F., Ohba S., Nishida N., Akune T., Yoshimura N., Nakagawa T., Nakamura K., Tokunaga K., Chung U. I., Kawaguchi H. (2010) Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.