Background: Difficulty in obtaining sufficient amounts of human hematopoietic stem/multipotent progenitor cells (HSC/MPPs) is a major hurdle that limits their application.
Results: Down-regulation of VentX, a hematopoietic homeobox transcription factor, allows multilineage expansion of hematopoietic cells ex vivo and in vivo in SCID/NODγ2null mice.
Conclusion: VentX is a novel regulator of human HSC/MPP expansion.
Significance: VentX represents a novel target for mechanistic exploration and clinical application of human HSC/MPPs.
Keywords: Cell Growth, Signal Transduction, Stem Cells, Transcription Factors, Tumor Suppressor Gene, VentX
Abstract
Mechanisms that regulate proliferation and expansion of human hematopoietic stem/multipotent progenitor cells (HSC/MPPs) are targets of intensive investigations. Several cell intrinsic factors and signaling pathways have been implicated in the proliferation and differentiation of human HSC/MPPs. Nevertheless, expansion of human HSC/MPPs for clinical application remains a critical challenge. VentX is a human homeobox transcription factor that was recently identified as an anti-proliferation and pro-differentiation factor in human hematopoietic cells. Here, we report that VentX expression is up-regulated during ontogenesis of human hematopoietic cells. Strikingly, suppression of VentX expression led to significant expansion of HSC/MPPs ex vivo and a 20-fold increase in engraftment potential in the NOD/SCID/IL2Rγ2null mouse model. VentX suppression helped preserve the HSC/MPP pools and promote clonogenicity of hematopoietic progenitor cells. Mechanistically, we show that VentX regulates critical cell cycle regulators and Wnt downstream genes previously implicated in HSC/MPP proliferation and expansion.
Introduction
Hematopoietic stem cells (HSCs)3 give rise to multipotent progenitor cells (MPPs) and eventually mature cells of all lineages of blood and immune systems. HSC/MPPs play essential role in tissue homeostasis, regeneration, and host defense. HSC/MPP transplantation has shown great promise in the reconstitution of hematopoietic systems and treatment of malignancies. HSC/MPP-based gene therapy has revealed potentials for correcting hereditary metabolic and autoimmune diseases. The molecular mechanisms underlying HSC/MPP proliferation and differentiation have been explored extensively for effective expansion of human HSC/MPPs, which is required for clinical application of HSC/MPP transplantation-based therapy (1, 2).
Over the past decades, several cell extrinsic signaling pathways and cell intrinsic factors of early embryogenesis have been shown to play important roles in the proliferation and differentiation of HSC/MPPs (3–7). In particular, using mouse models, it has been shown that murine HSCs can be expanded by >100-fold by either enhanced Wnt signaling or overexpression of the homeobox protein HoxB4 (5, 6). In comparison, clinically significant expansion of human HSC/MPPs remained a fundamental challenge. Despite a recent advance in HSC/MPP expansion by cytokine mixtures (8), neither enhanced Wnt signaling nor overexpression of HoxB4 or BMI1 was able to expand human HSC/MPPs by >2∼3-fold (9–11). As such, safe and efficacious expansion of human HSC/MPPs remains a bottle neck that limits the clinical application of HSC/MPP-based therapy.
VentX is a unique human homeobox protein that is expressed restrictedly in human hematopoietic cells in a highly regulated manner. Initially cloned as a human homolog of the vertebrate Vent family of homeobox transcription factors, the function of VentX in cell proliferation and differentiation is being appreciated through reverse genetic modeling of the molecular mechanisms underlying dorsoventral axis formation during early vertebrate embryogenesis (12, 13). As an human homolog of the vertebrate Xom/Vent2 homeobox genes, VentX was found to be a novel lymphoid enhancer factor/T-cell factor-associated antagonist of the canonical Wnt signaling and plays an important role in normal and malignant hematopoiesis (14–16). Our recent investigations showed that, in addition to antagonizing canonical Wnt signaling, VentX is also an activator of the p53/p21 and p16ink4a/Rb pathways and is able to induce senescence phenotypes in tumor cells (17).
Several lines of evidence suggest the potential involvement of VentX in controlling proliferation and differentiation of HSC/MPPs. First, VentX expression is up-regulated during both myeloid and lymphoid ontogenesis and during early hematopoiesis (15, 16, 18). Second, VentX controls signaling pathways and cell cycle regulators that have been previously implicated in the proliferation and differentiation of hematopoietic cells (15–17). Third, VentX has been shown to control differentiation of primary human hematopoietic cells (16, 18).
To further define a potential role of VentX in the proliferation and differentiation of human HSC/MPPs, we examined VentX expression during early hematopoiesis and explored the function of VentX in the expansion of CD34+ HSC/MPPs through gain-of-function and loss-of-function approaches ex vivo and in vivo in the NOD/SCIDγ2null mouse model. We found that VentX inhibition helped preserve the stem cell pool of HSC/MPPs. Strikingly, we observed that knockdown of VentX in bone marrow CD34+ HSC/MPPs resulted in a 20-fold increase in engraftment potential and multilineage development of hematopoietic cells in the NOD/SCIDγ2null mouse model. We found that VentX regulates the expression of several cell cycle regulators and Wnt downstream genes implicated in HSC/MPP proliferation and differentiation. Our data therefore reveal VentX as a novel regulator of HSC/MPP proliferation and differentiation and a potential druggable target for clinical application of HSC/MPP-based therapy.
EXPERIMENTAL PROCEDURES
CD34+ Cell Isolation
Human bone marrow samples were obtained from discarded femoral head tissue after hip replacement surgery at Brigham and Women's Hospital, with Institutional Review Board approval. After Ficoll separation of mononuclear cells, the CD34+ cells were enriched using a magnetically activated cell sorting CD34+ progenitor kit (Miltenyi Biotec). The purity of the bone marrow CD34+ cells was >95% as assessed by flow cytometry. Flow cytometry sorting of different subpopulations of CD34+ cells (common myeloid progenitors, megakaryocyte/erythroid progenitors, and granulocyte/macrophage progenitors) was as described previously (11).
Lentiviral Vector Construct and Transduction of Bone Marrow CD34+ Cells
The lentiviral vector pHAGE-CMV-eGFPW, which expresses a shRNA targeting VentX, was used for the transduction of bone marrow CD34+ cells. The lentiviral vector contains an internal ribosome entry site that allows simultaneous expression of GFP monitoring the transduction rate or for cell sorting. The sequence of VentX shRNA was identical to that of the VentX siRNA described previously (15). A non-effective sequence targeting GFP was used as a control. Lentiviral packaging was carried out in the Dana-Farber/Harvard Cancer Center Vector Core Facility, and viral supernatants were stored at −70 °C in aliquots until used. The CD34+ cells were first cultured in Iscove's modified Dulbecco's medium containing FBS (10%), stem cell factor (100 ng/ml), Flt3 ligand (100 ng/ml), thrombopoietin (100 ng/ml), IL-3 (10 ng/ml), and IL-6 (10 ng/ml) (PeproTech) for 2 days and then transduced with a multiplicity of infection of 5 in the presence of 4 μg/ml Polybrene. GFP-positive cells were sorted using a FACSAria high-speed sorter (BD Biosciences) at 24 h post-transduction at the Dana-Farber Cancer Institute Flow Cytometry Core Facility and used for all ex vivo experiments.
Overexpression of VentX in Bone Marrow CD34+ Cells
Human bone marrow CD34+ cells were transfected with pCS2-GFP or pCS2-GFP-VentX plasmid using a Nucleofector kit for human CD34+ cells (Lonza) as described above. GFP-positive cells were sorted using a FACSAria high-speed sorter at 24 h post-transfection and used for subsequent experiments.
Colony-forming Cell (CFC) and Long-term Culture-initiating Cell (LTCIC) Assays
Assays for in vitro CFCs were performed by plating sorted GFP-positive CD34+ cells (1 × 104/dish) in triplicates in complete methylcellulose medium (MethoCult GF H4434, STEMCELL Technologies Inc.), which contained a mixture of recombinant human cytokines (stem cell factor, IL-3, GM-CSF, and erythropoietin). Colonies were counted after 14 days of incubation at 37 °C and classified according to standard criteria. For the LTCIC assay, transduced GFP-positive cells were sorted on M2-10B4 murine fibroblast cells in limiting dilutions from 30 to 900 cells/well in 96-well plates. Cultures were weekly fed with new medium. After 5 weeks of culture, all cells from the wells were transferred to a 35-mm Petri dish in complete methylcellulose medium as described above. After an additional 2 weeks of culture, wells were scored as positive or negative to yield the LTCIC frequency.
FACS Analysis
Phenotypic analysis of the CD34+ cells and cells of different lineages was performed using flow cytometry after immunolabeling of cells with fluorescent dye-conjugated antibodies. Phycoerythrin (PE)-conjugated anti-human CD34 and CD45 and PE/Cy5-conjugated anti-human CD38 antibodies were obtained from eBioscience. The anti-cyclin D1 primary antibody was purchased from Cell Signaling for intracellular staining. PE-conjugated rabbit anti-mouse IgG antibody was used for anti-cyclin D1 secondary antibody staining. Staining of the cells was performed for 30 min on ice. Mouse bone marrow cells were blocked with anti-Fc antibody for 15 min at 4 °C to avoid nonspecific binding. FACS analyses were performed on a FACScan (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, Inc.).
Transplantation of NSG Mice
8-week-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (commonly known as NOD scid gamma, or NSG) were purchased from The Jackson Laboratory and maintained under specific pathogen-free conditions. Before transplantations, mice were sublethally irradiated with 100 rads. 1 × 106 CD34+ cells were transduced with VentX shRNA or GFP shRNA lentivirus. At 24 h post-transduction, all cells (1 × 106, with ∼20% GFP-positive cells) or positively transduced cells (2 × 104, with all GFP-positive cells) were injected into recipient mice. Human CD45+ cells from the bone marrow of recipient mice were analyzed. For secondary transplantation, the bone marrow of chimeric primary recipient mice was injected into secondary recipient NSG mice without further purification of human cells.
RT-PCR
Total RNA was isolated by the TRIzol method, and the same amount of RNA was used for first-strand cDNA synthesis with a SuperScript first-strand synthesis system (Invitrogen) according to the manufacturer's protocol. The VentX mRNA level was determined as described previously (16). We performed real-time PCR with SYBR Green on a LightCycler® 480 real-time PCR system (Roche Applied Science). The following primer sets were used for amplification: p21, 5′-AAACTTTGGAGTCCCCTCAC-3′ and 5′-AAAGGCTCAACACTGAGACG-3′; p16, 5′-CTTCCCCCACTACCGTAAAT-3′ and 5′-TGCTCACTCCAGAAAACTCC-3′; c-myc, 5′-CAGCTGCTTAGACGCTGGATT-3′ and 5′-GTAGAAATACGGCTGCACCGA-3′; and cyclin D1, 5′-GTTCGTGGCCTCTAAGATG-3′ and 5′-TTGTTCACCAGGAGCAGC-3′.
RESULTS
VentX Expression Is Regulated during Hematopoiesis
VentX is a homeobox transcription factor expressed in all lineages of hematopoietic cells. Our previous studies showed that VentX is a lymphoid enhancer factor/T-cell factor-associated Wnt repressor (15). Given the broad implications of Wnt signaling in hematopoiesis, we further explored the potential involvement of VentX in hematopoiesis by defining the expression pattern of VentX during early hematopoietic development. Through FACS analysis of different subpopulations of CD34+ cells, followed by quantitative PCR analysis, we found that VentX expression was regulated during hematopoiesis (Fig. 1). VentX expression was negligible in primitive CD34+CD38− cells. Consistent with the findings of Rawat et al. (18), VentX expression levels increased significantly in progenitor cells of myeloid lineage (Fig. 1). There was no significant difference in VentX expression among different subgroups of myeloid lineages (Fig. 1).
FIGURE 1.
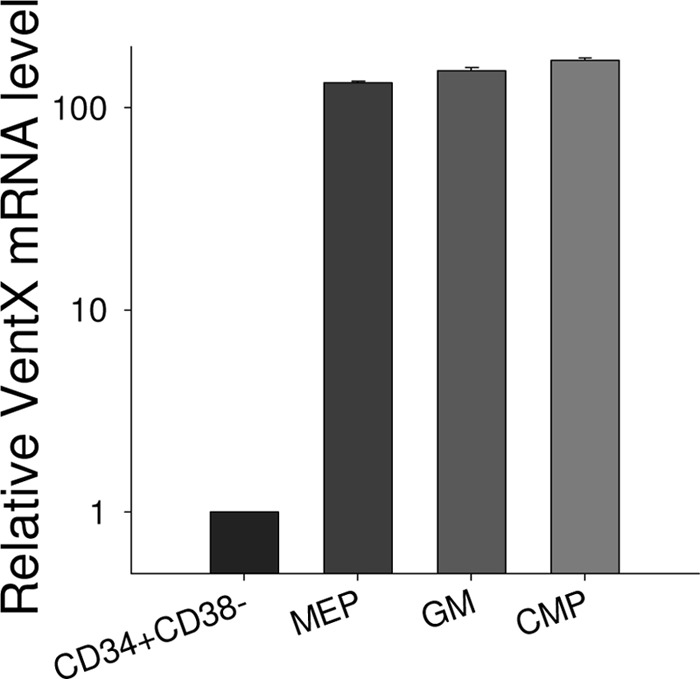
Up-regulation of VentX expression during hematopoiesis. The primitive CD34+CD38− cells and different subpopulations of CD34+ progenitor cells were sorted as described under “Experimental Procedures,” and the mRNA level of VentX was determined by real-time PCR. Results represent one of two independent experiments. MEP, megakaryocyte/erythroid progenitors; GM, granulocyte/macrophage progenitors; CMP, common myeloid progenitors.
VentX Regulates Expansion of Human HSCs ex Vivo
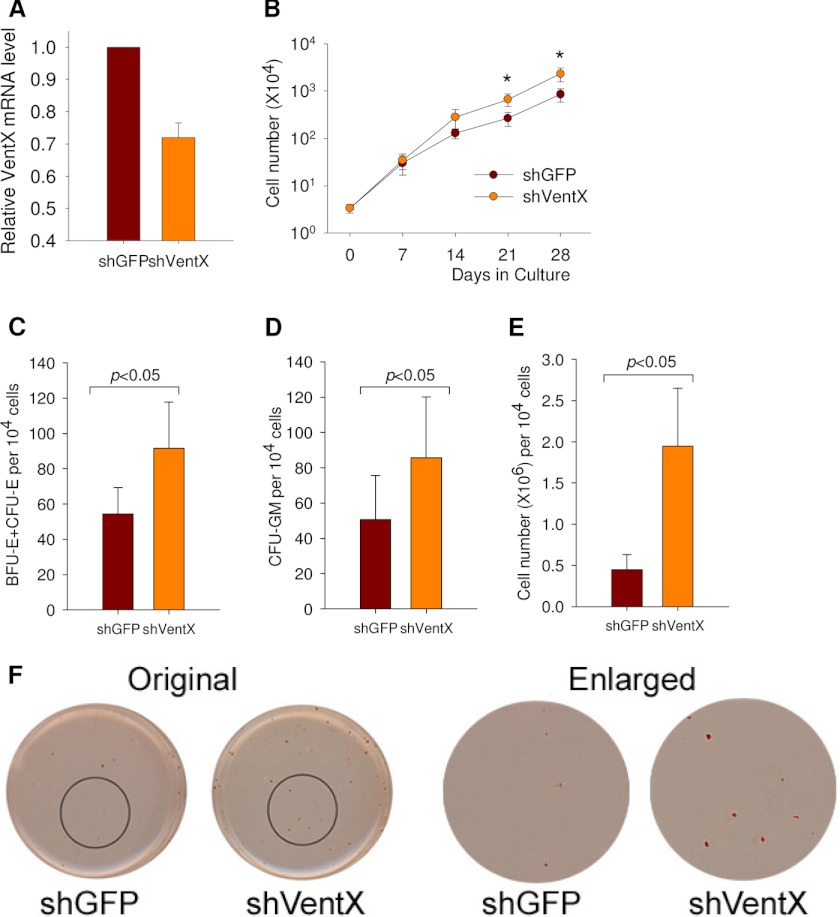
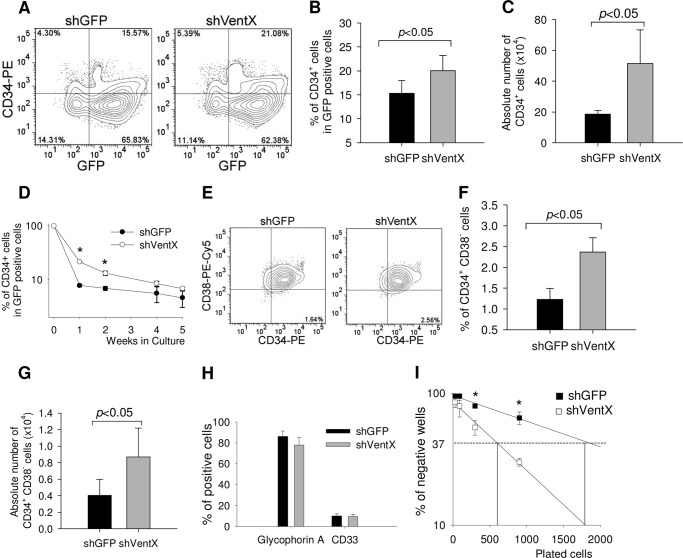
Our previous studies have shown that VentX regulates cell proliferation by controlling key pathways involved in the cell cycle (15–17). The finding that VentX expression is up-regulated during hematopoiesis prompted us to explore the potential role of VentX in the regulation of HSC/MPP expansion. To achieve this goal, VentX expression in human bone marrow CD34+ cells was knocked down with a lentivirus-based shRNA approach. The transduction rates of pHAGE-shGFP (which encodes a non-effective sequence targeting GFP) and pHAGE-shVentX were ∼20%. Using a quantitative PCR approach, the efficiency of VentX knockdown in pHAGE-shVentX-transduced CD34+ cells was determined to be ∼30% (Fig. 2A). Positively transduced cells were plated in cytokine-supplemented stroma-free liquid cultures, and the cell numbers were counted weekly up to 4 weeks. Plotting of weekly cell counts of demi-depopulated cultures showed that knockdown of VentX expression in CD34+ cells led to a 3∼4-fold increase in total cell numbers compared with control cells from three independent experiments (Fig. 2B). To determine the potential effects of VentX on the clonogenicity and lineage development of CD34+ cells, we performed CFC assays. As shown in Fig. 2 (C–F), knockdown of VentX resulted in an ∼2-fold increase in the formation of BFU-E/CFU-E and CFU-GM colonies. The total number of progenitor cells increased by ∼3–4-fold in the CFC assays (Fig. 2E). Knockdown of VentX promoted multilineage expansion of hematopoietic cells, especially erythroid lineage colony formation (Fig. 2F). To determine the effects of VentX knockdown on the HSC pools, we examined the proportion of CD34+ cells in the ex vivo liquid culture and found that knockdown of VentX led to a 30% increase in the percentage of CD34+ cells (Fig. 3, A and B) and an ∼2.5-fold increase in the absolute cell numbers (Fig. 3C). A time course FACS analysis revealed that knockdown of VentX helped preserve the CD34+ cell population for at least 2 weeks (Fig. 3D). In addition, knockdown of VentX also increased the percentage (Fig. 3, E and F) and absolute number (Fig. 3G) of primitive CD34+CD38− cells. FACS staining showed that VentX knockdown did not change the lineage distribution of the differentiated progenitor cells (Fig. 3H). Furthermore, LTCIC frequency increased by ∼3-fold in VentX shRNA-transduced CD34+ cells (Fig. 3I). These results suggest that VentX knockdown may help maintain the CD34+ stem cell pools, thereby promoting CD34+ cell expansion.
FIGURE 2.
Knockdown of VentX promotes expansion of human CD34+ cells ex vivo. A, real-time PCR showing the knockdown efficiency of VentX shRNA (shVentX) lentivirus in CD34+ cells. shGFP, GFP shRNA. B, VentX knockdown promotes human bone marrow CD34+ cell growth in liquid culture. Human CD34+ cells were transduced with either non-effective GFP shRNA or VentX shRNA lentivirus. Positively transduced cells (2 × 104) were sorted by GFP and grown in stroma-free liquid cultures using a mixture of cytokines as described under “Experimental Procedures.” The cells were counted weekly for 4 weeks, and the mean ± S.D. of three independent experiments is shown. The number of cells at Day 0 represents the number of total transduced cells that were plated. The statistically significant difference (p < 0.05) is marked with asterisks. C–F, knockdown of VentX in CD34+ cells increases the colony formation in semisolid culture. Positively transduced CD34+ cells as described above were plated in triplicate in methylcellulose-based medium and allowed to form colonies for 2 weeks. C and D show the colony numbers of BFU-E/CFU-E and CFU-GM, respectively. E shows total progenitor cells from 2 × 104 CD34+ cells after culturing for 2 weeks. The significant difference between the two groups (three independent experiments for each group) was determined by Student's t test (p < 0.05). F, representative images of CFC assays showing the erythroid colonies in GFP shRNA- or VentX shRNA-transduced CD34+ cells.
FIGURE 3.
Knockdown of VentX in CD34+ cells helps preserve stem cell pools of HSC/MPPs. Human CD34+ cells transduced with either GFP shRNA (shGFP) or VentX shRNA (shVentX) were grown in liquid culture as described in the legend to Fig. 2B. The cells were harvested at the indicated time intervals and stained with PE-conjugated anti-CD34 and PE/Cy5-conjugated anti-CD38 antibodies for FACS analysis. A, representative FACS profile showing the percentage of CD34+ cells from the GFP-positive populations at 2 weeks after plating. B, three independent experiments from A are shown. The significant difference between the two groups was determined by Student's t test (p < 0.05). C, absolute numbers of the CD34+ cells in the two groups at 2 weeks after plating. D, GFP-positive CD34+ cells were harvested at the indicated time points, and the percentage of the remaining CD34+ cells was shown. The statistically significant difference (p < 0.05) is marked with asterisks. E, representative FACS profile showing the percentage of CD34+CD38− cells from GFP-positive populations at 2 weeks after plating. F, three independent experiments from E are shown. The significant difference between the two groups was determined by Student's t test (p < 0.05). G, absolute numbers of CD34+CD38− cells in the two groups at 2 weeks after plating. H, FACS analysis of the erythroid (glycophorin A) and myeloid (CD33) marker staining in the two groups at 2 weeks after plating. I, LTCIC assay of the GFP shRNA- and VentX shRNA-transduced CD34+ cells. The statistically significant difference (p < 0.05) is marked with asterisks.
Ectopic Expression of VentX Blocks CD34+ Cell Proliferation and Expansion
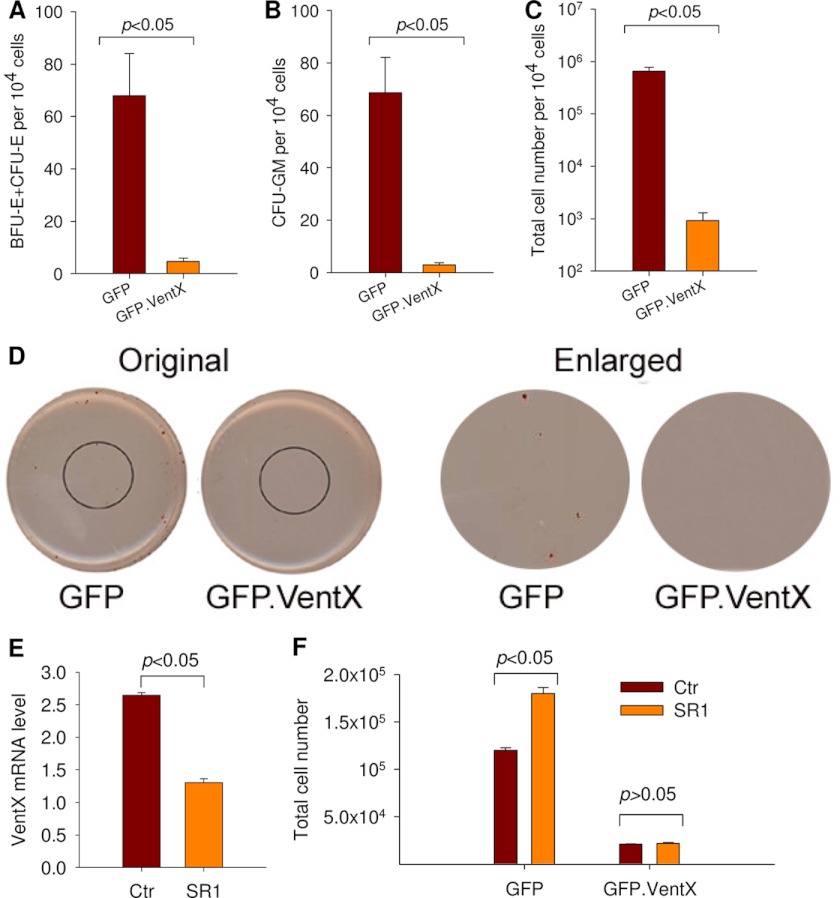
To further determine the effects of VentX on the expansion of human bone marrow HSC/MPPs, we tested whether ectopic expression of VentX would prevent CD34+ cell expansion. As shown in Fig. 4 (A–D), the colony formation of CD34+ cells was largely abrogated by the ectopic expression of VentX, suggesting that VentX is a critical regulator of CD34+ cell proliferation and expansion. A recent study demonstrated that an aryl hydrocarbon receptor antagonist, StemRegenin 1 (SR1), robustly promoted ex vivo expansion of human CD34+ cells (19). To determine whether VentX plays an essential regulatory role in the expansion of human HSC/MPPs, we explored the effects of SR1 treatment on the expression levels of VentX and the effects of VentX on SR1-induced CD34+ expansion. SR1 treatment accelerated the proliferation of CD34+ cells (Fig. 4F, first two bars) and decreased the expression levels of VentX (Fig. 4E). Consistent with the notion that VentX plays an essential regulatory role in CD34+ expansion, we found that ectopic expression of VentX prevented SR1-induced expansion of CD34+ cells (Fig. 4F). Our data indicate that down-regulation of VentX is a prerequisite for proliferation and expansion of CD34+ cells.
FIGURE 4.
Ectopic expression of VentX in CD34+ cells inhibits colony formation. A–C, human bone marrow CD34+ cells were transfected with either the GFP VentX or GFP VentX plasmid as described under “Experimental Procedures.” Positively transfected cells (2 × 104) were sorted and cultured in triplicates in methylcellulose-based medium for CFC enumeration. The number of BFU-E/CFU-E colonies is shown in A, and the number of CFU-GM colonies in B. C, total progenitor cells from 2 × 104 CD34+ cells after culturing for 2 weeks. The significant difference was determined by Student's t test (p < 0.05). D, representative images of the CFC assay showing erythroid colonies in GFP- or GFP-VentX-transfected CD34+ cells. E, human CD34+ cells were treated with 1 μm SR1 for 1 week or mock-treated (control (Ctr)). The treated cells were then harvested for analysis of VentX mRNA expression levels. F, GFP- or GFP-VentX-transfected CD34+ cells (2 × 104) were sorted and treated with 1 μm SR1 for 1 week or mock-treated. The total cell numbers were then counted and plotted.
VentX Regulates HSC/MPP Expansion in Vivo
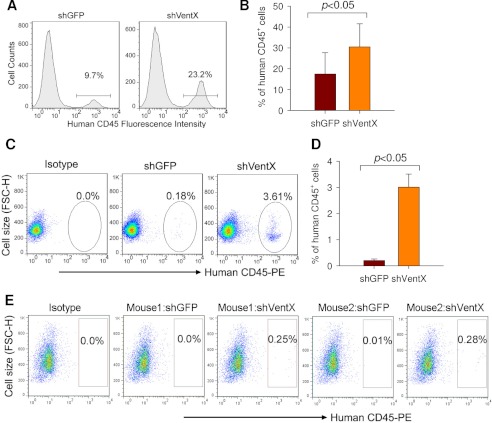
We next sought to determine whether VentX knockdown promotes expansion of HSC/MPPs in vivo using the NOD/SCID/IL2Rγ2null (NSG) mouse model. Human bone marrow CD34+ cells were transduced with lentiviruses expressing either GFP or VentX shRNA. The unsorted transduced CD34+ cells were then transplanted into three pairs of NSG mice. At 8 weeks post-transplantation, engraftment efficiency was determined by FACS analysis of human CD45+ cells from bone marrow of recipient mice. We found that the human cell engraftment rate increased by ∼1.5–2.5-fold in VentX shRNA-transduced CD34+ cells compared with GFP shRNA-transduced CD34+ cells (Fig. 5, A and B). Notably, knockdown of VentX allowed multilineage development of hematopoietic cells (Table 1). Given that the lentiviral transduction rate of the CD34+ cells was only ∼20%, to further explore the effects of VentX knockdown on the expansion of HSC/MPPs, we sorted out the positively transduced CD34+ cells and then transplanted the sorted CD34+ cells into NSG mice. At 13 weeks post-transplantation, the percentage of human CD45+ cells in the bone marrow of recipient mice was determined by FACS analysis. Strikingly, the percentage of human CD45+ cells was consistently increased by 20-fold in VentX shRNA-transduced CD34+ cells compared with GFP shRNA-transduced CD34+ cells (Fig. 5, C and D). Supporting a potential role of VentX knockdown in the expansion of HSC/MPPs, we found that the number of human CD34+ cells increased significantly in bone marrow of mice transplanted with VentX shRNA-transduced CD34+ cells compared with control mice transplanted with GFP shRNA-transduced CD34+ cells (45 ± 7 and 720 ± 13 per 105 total mononuclear cells for GFP shRNA- and VentX shRNA-transduced cells, respectively). To examine the effects of VentX on long-term engraftment of human CD34+ cells, we performed secondary transplantation experiments. Whereas no detectable secondary engraftment was observed in mice transplanted with the control GFP shRNA-transduced CD34+ cells, both of the two mice transplanted with the VentX shRNA-transduced CD34+ cells showed positive human cell engraftment (0.25 and 0.28%, respectively) (Fig. 5E).
FIGURE 5.
VentX knockdown promotes expansion of human CD34+ cells in vivo in NSG mice. A and B, human bone marrow CD34+ cells were transduced with GFP shRNA (shGFP) or VentX shRNA (shVentX) lentivirus. The total unsorted transduced cells (1 × 106, with transduction efficiency at ∼20%) were injected into sublethally irradiated NSG mice. The human CD45+ chimerism levels were determined from the bone marrow of recipient mice at 8 weeks post-transplantation. A, representative FACS profile showing the percentage of human CD45+ cells. B, mean ± S.D. of three independent experiments from A. The significant difference was determined by Student's t test (p < 0.05). C and D, human bone marrow CD34+ cells were transduced with either GFP shRNA or VentX shRNA lentivirus. Positively transduced cells (2 × 104) were sorted and then injected into sublethally irradiated NSG mice. The human CD45+ chimerism levels were determined from the bone marrow of recipient mice at 13 weeks post-transplantation. C, representative FACS profile showing the percentage of human CD45+ cells. D, mean ± S.D. of four independent experiments from C. The significant difference was determined by Student's t test (p < 0.05). E, bone marrow cells from the primary recipients were used to perform secondary bone marrow transplants, and the chimerism levels were analyzed after an additional 6 weeks. Results from two pairs of mice are shown. FSC-H, forward scatter height.
TABLE 1.
Knockdown of VentX allows multilineage differentiation of human CD34+ cells in vivo
GPA, glycophorin A.
| Human CD45 cells |
||||||
|---|---|---|---|---|---|---|
| CD45 | CD3 | CD19 | CD14 | CD33 | GPA | |
| % | ||||||
| Mouse1 | ||||||
| GFP shRNA | 100.0 | 20.2 | 60.6 | 37.5 | 32.7 | 21.2 |
| VentX shRNA | 100.0 | 18.5 | 59.2 | 30.3 | 35.5 | 19.9 |
| Mouse2 | ||||||
| GFP shRNA | 100.0 | 7.5 | 47.6 | 27.6 | 47.0 | 7.2 |
| VentX shRNA | 100.0 | 8.5 | 50.5 | 20.8 | 38.0 | 6.9 |
| Mouse3 | ||||||
| GFP shRNA | 100.0 | 6.1 | 63.6 | 35.4 | 23.2 | 12.1 |
| VentX shRNA | 100.0 | 5.0 | 75.7 | 24.3 | 14.0 | 8.6 |
VentX Targets Cell Cycle Regulators in CD34+ Cells
Our recent studies have shown that VentX is a regulator of the Wnt signaling pathway and several components of the cell cycle machinery, such as cyclin D1, p21, and p16ink4a (15, 16). To explore the mechanisms underlying VentX-regulated HSC/MPP expansion, we performed a targeted screen to determine the potential effects of VentX on these critical cell cycle regulators in CD34+ cells. As shown in Fig. 6A, quantitative PCR demonstrated that overexpression of VentX increased the expression of p21 and p16ink4a but down-regulated the expression of cyclin D1 and c-myc in primary CD34+ cells. The results were further confirmed by a loss-of-function approach in which knockdown of VentX by siRNA decreased the expression of p21 but increased the expression of cyclin D1 and c-myc (Fig. 6B). Cyclin D1 has been implicated in CD34+ cell proliferation (20–22). The finding that the cyclin D1 mRNA level was pronouncedly regulated by VentX prompted us to further examine whether the protein levels of cyclin D1 are also subject to VentX regulation. As shown by FACS analysis of the intracellular staining of cyclin D1, VentX regulated the expression of cyclin D1 at the protein level (Fig. 6, C and D).
FIGURE 6.
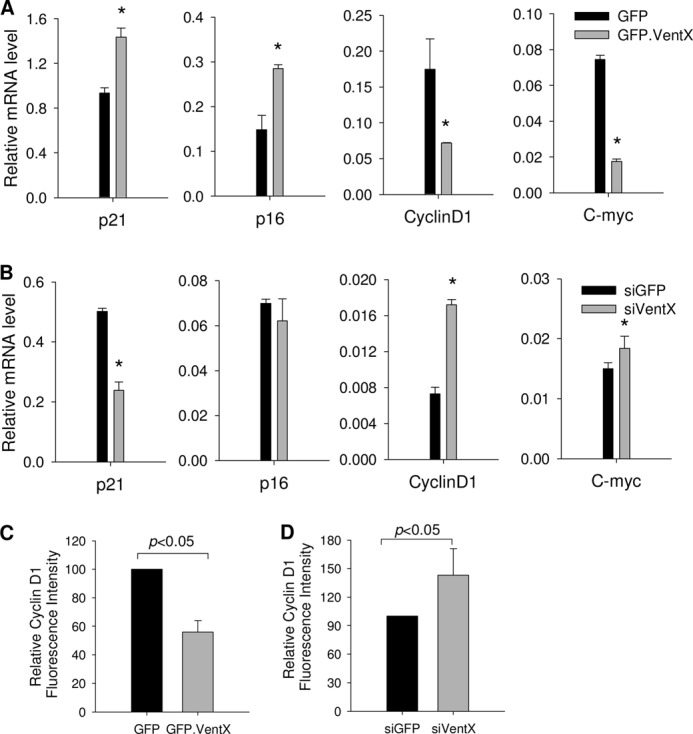
VentX targets critical cell cycle regulators in CD34+ cells. A, human bone marrow CD34+ cells were transfected with either GFP or GFP-VentX as described under “Experimental Procedures.” GFP-positive cells were sorted at 2 days post-transfection and then used for quantitative analysis of mRNA levels of the indicated genes. *, p < 0.05. B, CD34+ cells were transfected with either GFP siRNA (siGFP) or VentX siRNA (siVentX), and cells were harvested 3 days post-transfection. Quantitative PCR analysis was carried out to determine the mRNA levels of the indicated genes. *, p < 0.05. Results show the mean ± S.D. of triplicates from one of two independent experiments. C, CD34+ cells were transfected with either GFP or GFP-VentX, and the intracellular staining of cyclin D1 and FACS analysis were performed at 2 days post-transfection. GFP-positive cells were gated for analysis of mean fluorescence intensity. D, CD34+ cells were cotransfected with either GFP siRNA (non-effective) or VentX siRNA and with plasmid encoding GFP. The intracellular staining of cyclin D1 and FACS analysis were performed at 3 days post-transfection. GFP-positive cells were gated for analysis. Results show the mean ± S.D. of three independent experiments.
DISCUSSION
Molecular mechanisms controlling HSC/MPP proliferation and differentiation are targets of extensive investigations for their role in the management of diseases involving HSCs, such as primary and secondary bone marrow failure syndrome, refractory anemia, and myelodysplastic syndromes. Difficulties in obtaining sufficient numbers of HSC/MPPs also present a major hurdle for potential clinical applications of HSC/MPP transplantation. Multiple cell intrinsic factors and signaling pathways have been implicated in HSC/MPP expansion; nevertheless, the functions and mechanisms of these pathways in HSC/MPP expansion remain as topics of active debate (23–25). For example, although Wnt signaling was shown to be able to expand mouse HSCs, it seems that the levels of Wnt signaling play an important role in HSC/MPP expansion (26). In addition to the complex signaling events and their interpretations, there is growing appreciation of the species-specific property of HSC/MPPs (27). Evidently, although it is relatively easy to expand mouse HSCs, it remained a fundamental challenge to expand human HSC/MPPs by targeting cell intrinsic factors.
In this study, we have shown that VentX, a recently appreciated hematopoietic homeobox transcription factor, is a novel regulator of HSC/MPP expansion. We showed that suppression of VentX expression led to significant expansion of CD34+ cells ex vivo and a >20-fold increase in engraftment potential in the NOD/SCID/IL2Rγ2null mouse model (Fig. 5). We showed that knockdown of VentX helped preserve the stem cell pools (Fig. 3) of HSC/MPPs, promoted clonogenicity, and allowed multilineage development of hematopoietic cells. The essential role of VentX in controlling expansion of CD34+ cells was further illustrated by the finding that VentX expression was down-regulated upon application of SR1, a recently identified small molecule that promotes expansion of CD34+ cells, and by the finding that ectopic expression of VentX blocked SR1-induced CD34+ cell expansion.
VentX represents the first cell intrinsic factor whose down-regulation leads to significant expansion of human bone marrow CD34+ cells. In comparison with other known cell intrinsic factors, such as HoxB4 and BMI1 (9–11), VentX knockdown allowed more significant expansion of CD34+ cells. In our current experimental system, a 30% knockdown of VentX led to a 20-fold increase in engraftment potential in NOD/SCID/IL2Rγ2null mice (Fig. 5). Theoretically, the efficacy of HSC/MPP expansion by VentX down-regulation could still be improved through more efficient knockdown of VentX. It should be noted, however, that we focused on the effects of VentX knockdown on the expansion of CD34+ cells from human bone marrow. With the increasing application of cord blood in HSC transplantation and the notion that even modest HSC expansion would significantly increase the potential application of cord blood in adult HSC transplantation (28), the potential effects of VentX knockdown on the expansion of human cord blood CD34+ cells would be of great interest for future exploration. The description of cell surface markers of bona fide human HSCs is constantly improving (29). In this study, we focused on CD34+ HSC/MPPs. The clinical importance of these cells was reflected by the direct correlation with time to neutrophil recovery, an indicator of post-transplant mortality (27). The finding of VentX as the first cell intrinsic factor whose down-regulation promotes CD34+ cell expansion provides a novel therapeutic target for diseases involving HSC/MPPs and HSC/MPP-based clinical therapy.
We began to explore the mechanisms underlying VentX regulation of CD34+ cell proliferation and differentiation. VentX expression is relatively low in early HSC/MPPs but increases dramatically during the proliferative differentiation of CD34+ cells. Consistent with its role as a pro-differentiation and anti-proliferation hematopoietic transcription factor, we found that knockdown of VentX promoted proliferation of CD34+ cells (Fig. 2) and deferred their differentiation into lineage-specific progenitor cells (Fig. 3). As the stemness of HSC/MPPs decreases gradually during the proliferative differentiation of HSCs, our data suggest that VentX knockdown promotes the expansion of human HSC/MPPs by deferring differentiation of human HSC/MPPs. Although VentX knockdown promoted proliferation of CD34+ cells, no obvious alteration of the cell cycle profile was observed by knockdown of VentX (data not shown).
At the molecular level, we found that VentX regulates the expression of genes downstream of canonical Wnt signaling, such as cyclin D1 and c-myc, and other critical cell cycle regulators involved in HSC/MPP proliferation and differentiation, such as p21 (30–32). Interestingly, we found that although overexpression of VentX increases the expression of p16, knockdown of VentX does not alter p16 expression significantly, suggesting that other factors are involved in controlling p16 expression in HSC/MPPs (Fig. 6, A and B). As such, the exact position of VentX in the molecular hierarchy of controlling HSC/MPP proliferation and differentiation remains to be further defined.
VentX was identified as an inhibitor of the oncogenic Wnt and as an activator of the p53/p21 and p16/pRb tumor suppressor pathways. Therefore, caution needs to be exercised for potential application of VentX knockdown in the expansion of human HSC/MPPs.
Acknowledgments
We thank Drs. D. Cohen and R. Blumberg for critical reagents and use of their facilities. We thank Yinke Yang and Weixiong Ke for technical support.
Footnotes
- HSC
- hematopoietic stem cell
- MPP
- multipotent progenitor cell
- CFC
- colony-forming cell
- LTCIC
- long-term culture-initiating cell
- PE
- phycoerythrin
- BFU-E
- burst-forming unit-erythroid
- CFU-E
- colony-forming unit-erythroid
- CFU-GM
- colony-forming unit-granulocyte/macrophage
- SR1
- StemRegenin 1.
REFERENCES
- 1. Sorrentino B. P. (2004) Clinical strategies for expansion of hematopoietic stem cells. Nat. Rev. Immunol. 4, 878–888 [DOI] [PubMed] [Google Scholar]
- 2. Mimeault M., Hauke R., Batra S. K. (2007) Stem cells: a revolution in therapeutics–recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin. Pharmacol. Ther. 82, 252–264 [DOI] [PubMed] [Google Scholar]
- 3. Bhardwaj G., Murdoch B., Wu D., Baker D. P., Williams K. P., Chadwick K., Ling L. E., Karanu F. N., Bhatia M. (2001) Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2, 172–180 [DOI] [PubMed] [Google Scholar]
- 4. Antonchuk J., Sauvageau G., Humphries R. K. (2001) HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp. Hematol. 29, 1125–1134 [DOI] [PubMed] [Google Scholar]
- 5. Reya T., Duncan A. W., Ailles L., Domen J., Scherer D. C., Willert K., Hintz L., Nusse R., Weissman I. L. (2003) A role for Wnt signaling in self-renewal of hematopoietic stem cells. Nature 423, 409–414 [DOI] [PubMed] [Google Scholar]
- 6. Antonchuk J., Sauvageau G., Humphries R. K. (2002) HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109, 39–45 [DOI] [PubMed] [Google Scholar]
- 7. Bhatia M., Bonnet D., Wu D., Murdoch B., Wrana J., Gallacher L., Dick J. E. (1999) Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J. Exp. Med. 189, 1139–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C. C., Kaba M., Iizuka S., Huynh H., Lodish H. F. (2008) Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 111, 3415–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amsellem S., Pflumio F., Bardinet D., Izac B., Charneau P., Romeo P. H., Dubart-Kupperschmitt A., Fichelson S. (2003) Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat. Med. 9, 1423–1427 [DOI] [PubMed] [Google Scholar]
- 10. Krosl J., Austin P., Beslu N., Kroon E., Humphries R. K., Sauvageau G. (2003) In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat. Med. 9, 1428–1432 [DOI] [PubMed] [Google Scholar]
- 11. Rizo A., Dontje B., Vellenga E., de Haan G., Schuringa J. J. (2008) Long-term maintenance of human hematopoietic stem/progenitor cells by expression of BMI1. Blood 111, 2621–2630 [DOI] [PubMed] [Google Scholar]
- 12. Gao H., Wu B., Giese R., Zhu Z. (2007) Xom interacts with and stimulates transcriptional activity of LEF1/TCFs: implications for ventral cell fate determination during vertebrate embryogenesis. Cell Res. 17, 345–356 [DOI] [PubMed] [Google Scholar]
- 13. Yang Y. (2007) Xom as a novel partner of LEF/TCFs during dorsal-ventral patterning of the Xenopus embryo. Cell Res. 17, 307–308 [DOI] [PubMed] [Google Scholar]
- 14. Moretti P. A., Davidson A. J., Baker E., Lilley B., Zon L. I., D'Andrea R. J. (2001) Molecular cloning of a human Vent-like homeobox gene. Genomics 76, 21–29 [DOI] [PubMed] [Google Scholar]
- 15. Gao H., Le Y., Wu X., Silberstein L. E., Giese R. W., Zhu Z. (2010) VentX, a novel lymphoid-enhancing factor/T-cell factor-associated transcription repressor, is a putative tumor suppressor. Cancer Res. 70, 202–211 [DOI] [PubMed] [Google Scholar]
- 16. Wu X., Gao H., Ke W., Giese R. W., Zhu Z. (2011) The homeobox transcription factor VentX controls human macrophage terminal differentiation and proinflammatory activation. J. Clin. Invest. 121, 2599–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X., Gao H., Ke W., Hager M., Xiao S., Freeman M. R., Zhu Z. (2011) VentX transactivates p53 and p16ink4a to regulate cellular senescence. J. Biol. Chem. 286, 12693–12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rawat V. P., Arseni N., Ahmed F., Mulaw M. A., Thoene S., Heilmeier B., Sadlon T., D'Andrea R. J., Hiddemann W., Bohlander S. K., Buske C., Feuring-Buske M. (2010) The vent-like homeobox gene VENTX promotes human myeloid differentiation and is highly expressed in acute myeloid leukemia. Proc. Natl. Acad. Sci. U.S.A. 107, 16946–16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boitano A. E., Wang J., Romeo R., Bouchez L. C., Parker A. E., Sutton S. E., Walker J. R., Flaveny C. A., Perdew G. H., Denison M. S., Schultz P. G., Cooke M. P. (2010) Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Istvanffy R., Kröger M., Eckl C., Gitzelmann S., Vilne B., Bock F., Graf S., Schiemann M., Keller U. B., Peschel C., Oostendorp R. A. (2011) Stromal pleiotrophin regulates repopulation behavior of hematopoietic stem cells. Blood 118, 2712–2722 [DOI] [PubMed] [Google Scholar]
- 21. Ko K. H., Holmes T., Palladinetti P., Song E., Nordon R., O'Brien T. A., Dolnikov A. (2011) GSK-3β inhibition promotes engraftment of ex vivo expanded hematopoietic stem cells and modulates gene expression. Stem Cells 29, 108–118 [DOI] [PubMed] [Google Scholar]
- 22. Zou P., Yoshihara H., Hosokawa K., Tai I., Shinmyozu K., Tsukahara F., Maru Y., Nakayama K., Nakayama K. I., Suda T. (2011) p57Kip2 and p27Kip1 cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 9, 247–261 [DOI] [PubMed] [Google Scholar]
- 23. Cobas M., Wilson A., Ernst B., Mancini S. J., MacDonald H. R., Kemler R., Radtke F. (2004) β-Catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirstetter P., Anderson K., Porse B. T., Jacobsen S. E., Nerlov C. (2006) Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 7, 1048–1056 [DOI] [PubMed] [Google Scholar]
- 25. Koch U., Wilson A., Cobas M., Kemler R., Macdonald H. R., Radtke F. (2008) Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood 111, 160–164 [DOI] [PubMed] [Google Scholar]
- 26. Luis N. M., Morey L., Mejetta S., Pascual G., Janich P., Kuebler B., Cozutto L., Roma G., Nascimento E., Frye M., Di Croce L., Benitah S. A. (2011) Regulation of human epidermal stem cell proliferation and senescence requires Polycomb-dependent and -independent functions of Cbx4. Cell Stem Cell 9, 233–246 [DOI] [PubMed] [Google Scholar]
- 27. Doulatov S., Notta F., Laurenti E., Dick J. E. (2012) Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 [DOI] [PubMed] [Google Scholar]
- 28. Brunstein C. G., Wagner J. E. (2006) Umbilical cord blood transplantation and banking. Annu. Rev. Med. 57, 403–417 [DOI] [PubMed] [Google Scholar]
- 29. Notta F., Doulatov S., Laurenti E., Poeppl A., Jurisica I., Dick J. E. (2011) Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333, 218–221 [DOI] [PubMed] [Google Scholar]
- 30. Liu Y., Elf S. E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J. M., Deblasio A., Menendez S., Antipin J., Reva B., Koff A., Nimer S. D. (2009) p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleming H. E., Janzen V., Lo Celso C., Guo J., Leahy K. M., Kronenberg H. M., Scadden D. T. (2008) Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baena E., Ortiz M., Martínez-A C., de Alborán I. M. (2007) c-Myc is essential for hematopoietic stem cell differentiation and regulates Lin−Sca-1+c-Kit− cell generation through p21. Exp. Hematol. 35, 1333–1343 [DOI] [PubMed] [Google Scholar]