Background: NO and BDNF are responsible for numerous functions in the CNS; however, joint actions exerted by these factors have not been studied.
Results: BDNF reversed the block on neural differentiation caused by insufficient NO signaling.
Conclusion: The NO-citrulline cycle and BDNF through up-regulation of p75 expression interact for restoring normal NO signaling and promoting neural differentiation.
Significance: New insights are provided for BDNF and NO-citrulline cycle actions in neurogenesis.
Keywords: Brain-derived Neurotrophic Factor (BDNF), Neural Stem Cell, Neurodifferentiation, Nitric Oxide, Proliferation
Abstract
The diffusible messenger NO plays multiple roles in neuroprotection, neurodegeneration, and brain plasticity. Argininosuccinate synthase (AS) is a ubiquitous enzyme in mammals and the key enzyme of the NO-citrulline cycle, because it provides the substrate l-arginine for subsequent NO synthesis by inducible, endothelial, and neuronal NO synthase (NOS). Here, we provide evidence for the participation of AS and of the NO-citrulline cycle in the progress of differentiation of neural stem cells (NSC) into neurons, astrocytes, and oligodendrocytes. AS expression and activity and neuronal NOS expression, as well as l-arginine and NOx production, increased along neural differentiation, whereas endothelial NOS expression was augmented in conditions of chronic NOS inhibition during differentiation, indicating that this NOS isoform is amenable to modulation by extracellular cues. AS and NOS inhibition caused a delay in the progress of neural differentiation, as suggested by the decreased percentage of terminally differentiated cells. On the other hand, BDNF reversed the delay of neural differentiation of NSC caused by inhibition of NOx production. A likely cause is the lack of NO, which up-regulated p75 neurotrophin receptor expression, a receptor required for BDNF-induced differentiation of NSC. We conclude that the NO-citrulline cycle acts together with BDNF for maintaining the progress of neural differentiation.
Introduction
The gaseous messenger NO has been widely studied because of its significant importance for human cell physiology. Understanding the role of NO in the development of CNS has been a complex task because the analysis of NO accumulation in cell lines does not mimic the large diversity of neural phenotypes in the brain (1). The CNS develops from a specific set of precursor cells that divide in order to form the neural epithelium, migrate to appropriate niches, and differentiate into glia or various neuronal phenotypes. Neural stem cells (NSC)4 can be grown in vitro as neurospheres and still retain their multipotent capacity (2–5). Under controlled experimental conditions, NSC proliferate, migrate, differentiate, and form neural networks, closely reflecting conditions of cortical development. NSC are useful for studying brain development and provide promising tools for cell therapy of neurodevelopmental and neurodegenerative diseases (reviewed in Ref. 5). Here, we have used neurospheres obtained from embryonic rat telencephalon (embryonic day 14) to investigate the roles of NO and enzymes of the NO-citrulline cycle in neural differentiation.
NO is a molecule generated from l-arginine by the action of the enzyme NO synthase (NOS). There are three NOS isoforms: NOS1 or neuronal NOS (nNOS), which is primarily found in the brain, although its expression is also observed in skeletal muscle and other tissues; NOS2 or inducible NOS; and NOS3 or endothelial NOS (eNOS), which was first observed in endothelial cells but is also present in other cell types (reviewed in Ref. 6). l-Arginine is the only substrate of NOS for NO synthesis. This semiessential amino acid can be directly obtained from the breakdown of dietary proteins, but it is also de novo synthesized from l-citrulline (reviewed in Ref. 7).
l-Citrulline is metabolized to l-arginine involving the enzymes argininosuccinate synthase (AS, EC 6.3.4.5) and argininosuccinate lyase (EC 4.3.2.1). AS is responsible for the condensation reaction between l-citrulline and l-aspartate in an ATP-dependent manner in order to form argininosuccinate, AMP, and pyrophosphate; argininosuccinate lyase catalyzes the conversion of argininosuccinate into fumarate and l-arginine, which is then metabolized by NOS into NO and l-citrulline, closing the NO-citrulline cycle (8). AS expression increases during brain development (9), suggesting a possible function for the enzyme in this process. In addition, AS activity has been described as a limiting step for the biosynthesis of NO in numerous tissues (10). Among the many functions of NO (11), this transcellular-signaling molecule regulates proliferation of NSC (12) by acting cytostatically on cell division, which is a prerequisite for cells to enter a program of differentiation (13). It is also known that in spinal cord development, interneurons express NOS during migration to their final destination (14). However, the exact functions of NO and the NO-citrulline cycle enzymes in brain development remain unknown.
We hereby show that AS and nNOS are differentially expressed along differentiation and that l-arginine as well as intracellular and extracellular NO levels, follow up the appearance of neural phenotypes. Moreover, we have observed that eNOS expression was induced by NOS inhibition, indicating that this isoform is amenable to modulation by extracellular cues. Inhibition of NOS and AS enzymatic activities prevented final differentiation, indicating the importance of correct working of the NO-citrulline cycle for the progress of neural differentiation. Nevertheless, no neuroanatomical alterations were detected in adult nNOS or eNOS knock-out mice (15, 16). This discrepancy might be explained by the existence of physiological and biochemical compensation mechanisms. Based on previous observations that BDNF and NO play similar biological activities in the brain (17, 18), and NO inhibits BDNF release (19), we have now investigated the role of BDNF in reverting effects caused by interruption of the NO-citrulline cycle.
Here we show that BDNF reverses the block of neural differentiation caused by insufficient NO signaling. We suggest that BDNF-mediated effects may be mediated by the p75 neurotrophin receptor (p75NTR), whose expression was up-regulated as consequence of inhibition of NO production. Taken together, we present evidence that the NO-citrulline cycle and BDNF interact for promoting neural differentiation.
EXPERIMENTAL PROCEDURES
Isolation, Culture, and Differentiation of NSC
NSC were obtained by dissection of embryonic telencephalons (embryonic day 14) of Wistar rats or telencephalons (embryonic day 13) of C57BL/6 mice representing similar stages of neuronal development. The animals were housed in the animal facility of the Instituto de Química of the Universidade de São Paulo and sacrificed in a CO2 gas chamber using protocols reviewed and approved by the local ethics committee. Telencephalons were dissected under a stereo microscope in aseptic conditions followed by incubation with trypsin for 10 min at 37 °C. Then an equal volume of FBS was added for inactivation of trypsin, and the cells were mechanically dissociated in order to obtain a single cell suspension. Cell viability was evaluated by trypan blue staining (Invitrogen). Cells were plated at a density of 2 × 105 cells/ml in culture medium containing 2% (v/v) B-27 (Life Technologies), 98% (v/v) DMEM/Ham's F-12 medium, 20 ng/ml EGF (Sigma-Aldrich), 20 ng/ml FGF-2 (Sigma-Aldrich), 5 μg/ml heparin (Sigma-Aldrich), and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) and cultured at 37 °C in a water-saturated atmosphere and 5% of CO2. For induction of neural differentiation, neurospheres were plated onto adherent poly-l-lysine- and laminin-precoated cell culture grade dishes and cultured in the absence of EGF and FGF-2. The medium was changed every 2 days. Under these experimental conditions, enrichment of neurons and glias in the culture was confirmed by immunofluorescence staining against β3-tubulin and glial fibrillary acidic protein (GFAP) on day 7 of differentiation (20). On day 14, peak values of expression of proteins characteristic for mature neurons, such as microtubule-associated protein 2 (MAP-2), and for astrocytes, such as GFAP, were observed, whereas expression of Nestin, a marker protein for NSC and neural progenitor cells, was decreased, being in agreement with the conversion of undifferentiated cells into defined neural phenotypes (20). For studying the role of NO-citrulline cycle during differentiation, NSC were also cultured in the presence of substrates of NOS or AS (1 mm l-arginine or 1 mm l-citrulline), or 1 mm l-Nγ-nitroarginine methyl ester (l-NAME), 1 mm α-methyl-dl-aspartic acid (MDLA), or 1 μm 7-nitroindazole (7-Ni), inhibiting all isoforms of NOS, AS, or nNOS, respectively. In other experiments, NSC were cultured in the presence of 1 mm l-NAME and 20 ng/ml BDNF or 1 mm MDLA and 20 ng/ml BDNF, concomitantly, or with 20 ng/ml BDNF alone. The drugs were newly supplied every day along differentiation.
Real Time Polymerase Chain Reaction
Total RNA was extracted from NSC using TRIzol (Invitrogen). All samples were further treated with amplification grade DNase I (Sigma-Aldrich). Reverse transcription for cDNA synthesis was carried out with a thermal cycler using the SuperScript III first strand synthesis system according to the manufacturer's protocol (Invitrogen) in the presence of specific primers listed in Table 1. The transcription rates of selected mRNAs were measured by real time PCR using the ABI Step One Plus instrument (Life Technologies). Real time PCR was performed in 25 μl of buffer reaction containing 1 μl of cDNA, SYBR Green Master Mix (Life Technologies), and 5 pmol of each sequence-specific primers (Table 1). Thermal cycling conditions consisted of a preincubation step for 2 min at 50 °C, then denaturation for 10 min at 95 °C followed by 40 cycles for denaturation for 15 s at 95 °C, and annealing/extension for 1 min at 60 °C. The comparative 2−ΔΔCT method was employed for relative quantification of gene expression as described previously (21) using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression as an internal standard for normalization.
TABLE 1.
Primers used for real-time PCR
| Genes | Primers |
|
|---|---|---|
| Forward (5′–3′) | Reverse (5′–3′) | |
| Nestin | TGGAGCGGGAGTTAGAGGCT | ACCTCTAAGCGACACTCCCGA |
| β3-tubulin | AGACCTACTGCATCGACAATGAAG | GCTCATGGTAGCAGACACAAGG |
| GFAP | AAGAGTGGTATCGGTCCAAGTTTG | CAGTTGGCGGCGATAGTCAT |
| MAP-2 | GTTTACATTGTTCAGGACCTCATGG | TCGGTAAGAAAGCCAGTGTGGT |
| eNOS | AAAATGAGCAGAAGGCCA | TTTTGCTGCACTTTTCCTTTC |
| nNOS | CAGCCAAAGCAGAGATGA | ATTGAAGACGCGGTCATT |
| AS | TGCACTCTATGAGGACCGCTATC | CTAGGCACCTCTCTCGCCAGGCCT |
| BDNF | CAACATCGATGCCAGTTGCT | TCCGCAAGCTTCAACTCTCA |
| p75NTR | CGACCAGCAGACCCATACG | GGCTACTGTAGAGGTTGCCATCA |
| GAPDH | TGGCCTCCAAGGAGTAAGAAA | GGCCTCTCTCTTCCTCTCAGTATC |
AS Activity Assay
AS activity was determined based on accumulation of the product pyrophosphate as inorganic phosphate following cleavage by pyrophosphatase. After lysis of NSC by heat shock, 50 μg of total proteins were used for measuring enzymatic activity of AS. Samples were added to the reaction buffer (20 mm Tris-HCl, pH 7.8, 2 mm ATP, 2 mm citrulline, 2 mm aspartate, 6 mm MgCl2, 20 mm KCl, and 0.2 units of pyrophosphatase) to a final volume of 0.2 ml. Reactions were incubated at 37 °C in 96-well microtiter plates and stopped after 30 min by the addition of an equal volume of molybdate buffer (10 mm ascorbic acid, 2.5 mm ammonium molybdate, 2% (v/v) sulfuric acid). Accumulation of phosphate was determined spectrophotometrically at 650 nm, and concentrations were extrapolated from a standard curve of inorganic phosphate (22).
Determination of l-Arginine Concentration
The methodology used to measure total l-arginine levels is described elsewhere (23). Both extra- and intracellular media of NSC were collected for determination of l-arginine concentration by reversed phase high performance liquid chromatography (HP 1100 series HPLC) with amino acid detection analysis following sample separation on a C18 analytical column (250-mm length, 4.6-mm diameter, 5-μm particle size; Merck).
Chemiluminescence Assay for Detection and Quantification of Nitric Oxide Products
NOx (nitrate, nitrite, nitrosothiol, nitrosamines, and iron-nitrosyl complexes) concentration in extracellular and intracellular media of NSC were determined using a chemiluminescence Sievers nitric oxide analyzer (NOA280i; GE Analytical Instruments) according to the procedure optimized by Feelisch et al. (24). Intracellular media were obtained following cell lysis with radioimmune precipitation assay buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 1 mm diethylene triamine pentaacetic acid (DTPA), and 10 mm N-ethylmaleimide) and centrifugation as previously described (25). Extra- and intracellular media of NSC were directly injected into a vessel containing a saturated solution of vanadium (III) chloride in 1 m HCl at 90 °C. Under these conditions, all nitric oxide-derived products (NOx) were reduced and compared with those of standard nitrate solutions (24).
Flow Cytometry Analysis of Neural Marker Proteins and p75NTR Expression
Flow cytometry procedures were in agreement with previously published protocols (5, 20). NSC were detached from the flasks using trypsin, then centrifuged for 5 min at 200 × g, and dissociated to provide a single cell suspension. The cells were fixed for 20 min in ice-cold 1% (v/v) formaldehyde in PBS, washed with PBS supplemented with 2% (v/v) FBS, and incubated for 2 h with primary antibodies specific for neural markers β3-tubulin (Sigma-Aldrich), GFAP (DAKO), and Nestin (Millipore) at 1:500 dilutions in 0.05% (v/v) Triton X-100. For determination of p75NTR expression, NSC were incubated with a primary antibody anti-NGFR p75 C-20 (Santa Cruz) at 1 μg/1 × 106 cells in PBS supplemented with 2% (v/v) FBS. Following a washing step with PBS, cells were incubated with 1:500 Alexa Fluor 488− or 555− conjugated secondary antibodies (Invitrogen) and then analyzed with a flow cytometer (Fc500; Beckman Coulter).
An argon laser line was used for fluorescence excitation (emission wavelengths of FL1, 525 nm, and FL2, 575 nm, were defined by band pass filter). Thirty-thousand events were acquired per sample with fluorescence emission values measured in logarithmic scales. Background fluorescence was determined using unlabeled cells and cells labeled with secondary antibody alone and used to set gating parameters between stained and unstained cell populations. Forward and side light scatter gates were set to exclude cell aggregates and small debris. The data were analyzed using the Flowjo 7.6.4 software (Ashland, OR).
Immunofluorescence Staining Assay
Imunocytochemistry assays were performed according to Ref. 26. Briefly, the cells were blocked for 1 h with 3% (v/v) FBS in PBS, 0.1% (v/v) Triton X-100, followed by a 2-h incubation with primary antibodies against β3-tubulin (Sigma-Aldrich), Nestin (Millipore), and GFAP (DAKO) at 1:500 dilution. NSC were washed with PBS and anti-mouse Alexa 555-conjugated, or anti-rabbit Alexa 488-conjugated secondary antibodies (Invitrogen) at 1:500 dilution were added. After washing with PBS, DAPI solution (Sigma-Aldrich; 0.3 μg/ml) was used as a nuclear stain. Coverslips were mounted, and slides were analyzed under a fluorescence microscope (Axiovert 200, Zeiss, Jena, Germany).
BrdU Incorporation Assay
Cell proliferation was measured following incubation with 0.2 μm 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) for 12 h. The cells were fixed with ice-cold methanol for 10 min, washed with PBS, and incubated for 30 min in 1.5 m HCl. After washing with PBS, they were incubated for 2 h with rat anti-BrdU antibodies (Abcam; 1:200 dilution). Alexa Fluor-488 secondary antibodies (Invitrogen) were used at 1:500 dilution. After another washing step, DAPI solution (Sigma-Aldrich; 0.3 μg/ml) was used as a nuclear stain. Slides were mounted and analyzed under a fluorescence microscope (Axiovert 200, Zeiss). The percentages of BrdU-positive cells were calculated as the ratio of immunolabeled cells over the total number of DAPI-stained cells.
Determination of Protein Expression Levels by Western Blotting
Following lysis of NSC in radioimmune precipitation assay buffer, samples were incubated on ice for a period of 20 mins and centrifuged for 5 min, at 21,000 × g and the supernatant were stored at −80 °C. After quantification of protein concentration, 50 μg of each protein sample was separaed by SDS-PAGE (10%). Proteins were transferred onto nitrocellulose membranes in 0.38 m Tris-HCl, 0.18 m glycine, and 20% (v/v) methanol under constant voltage of 30V for 12 h. The membrane was incubated in 5% (w/v) BSA dissolved in TBS-Tween (20 mm Tris-HCl, pH 7.4, 0.15 m NaCl and 0.05% (v/v) Tween 20). After three washes with TBS-Tween-20, membranes were incubated for 2 h with primary antibodies against eNOS, β3-tubulin, and GFAP. Next, the membrane was incubated with the secondary antibody conjugated to alkaline phosphatase (Promega Corp., Madison, WI) and revealed with alkaline phosphatase solution (5 m NaCl, 1 m Tris-HCl, pH 9.5, 1 m MgCl2 in the presence of 0.02% (w/v) 5-bromo-4-chloro-3-indolyl phosphate and 0.03% (w/v) nitro blue tetrazolium).
Statistical Analysis
Comparisons between experimental data were made by one- or two-way analysis of variance following the Bonferroni post-test using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA). The criteria for statistical significance were set at p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
RESULTS
Expression and Activity of AS during Neural Differentiation
The role of AS as step-limiting enzyme in the synthesis of substrate for NOS (l-arginine) was studied along differentiation of NSC. Real time PCR experiments revealed increasing AS expression during differentiation. Peak values were observed on day 14 when cells were completely differentiated (2.5-fold increase compared to expression on day 0) (Fig. 1A). In agreement with these results, AS enzymatic activity also augmented during differentiation with peak values of 5.62 ± 0.90 μm/min compared to 3.48 ± 0.34 μm/min at the onset of differentiation (day 1) (Fig. 1B). Augmented expression and enzymatic activity of AS was accompanied by an increase in intracellular levels of l-arginine from 217.8 ± 88.9 μm in undifferentiated cells reaching maximal levels of 630.5 ± 104.6 μm on day 14 of differentiation, whereas extracellular l-arginine levels remained constant along the course of differentiation (Fig. 1C).
FIGURE 1.
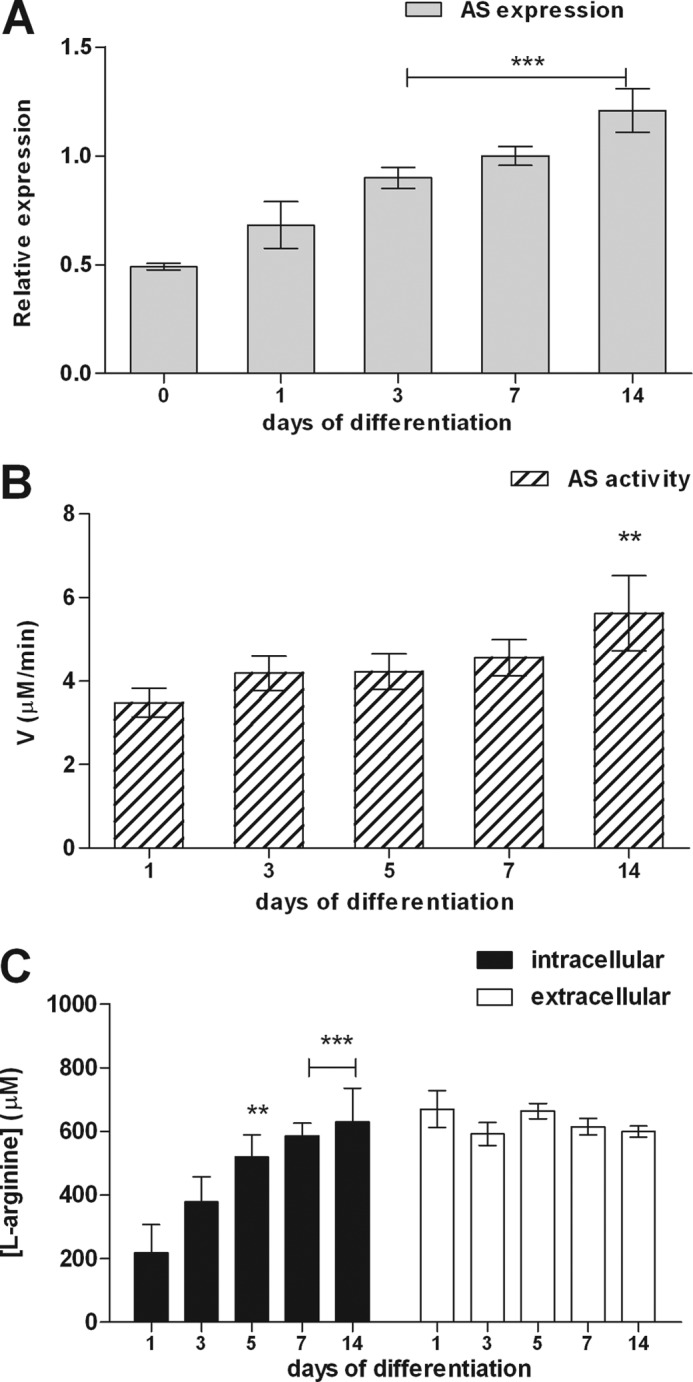
Expression and activity of AS along neural differentiation. A, AS gene expression in neurospheres was determined by real time PCR. Normalization of expression levels was done by comparison with GAPDH RNA transcription levels as internal standard for gene expression. B, determination of AS enzymatic activity of neurospheres along differentiation using a colorimetric assay. C, quantification of intra- and extracellular l-arginine levels during neurosphere differentiation. Cell culture supernatants and intracellular contents were collected and analyzed for l-arginine by HPLC. The experimental data are presented as the mean values ± S.E. **, p < 0.01; ***, p < 0.001, compared with control data collected on day 0 or 1 of differentiation.
Detection of nNOS and eNOS Expression and NO Production during Neural Differentiation
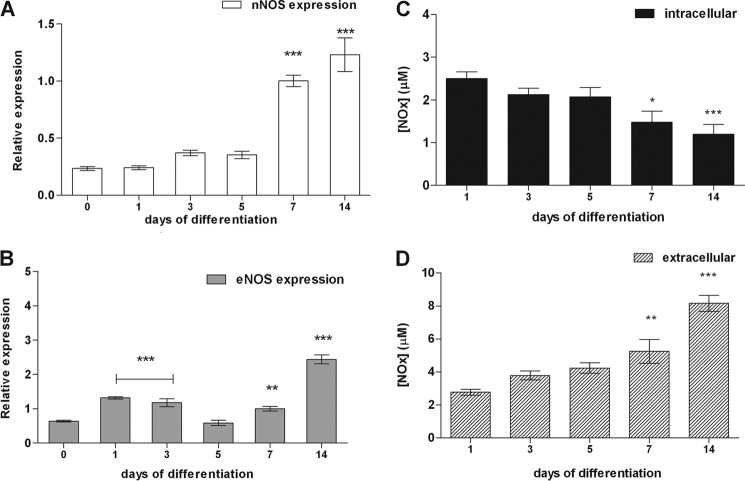
Gene expression of nNOS and eNOS and NO production were detected in undifferentiated NSC and along differentiation into neural phenotypes (Fig. 2). However, inducible NOS could not be detected by Western blot analysis during NSC differentiation (data not shown), in agreement with previous findings (27) excluding inducible NOS as participant in NO production during NSC differentiation. Increased expression of nNOS (Fig. 2A) accompanies rising levels of the neural markers β3-tubulin and GFAP during ongoing differentiation (5, 26). Different from nNOS, the eNOS expression pattern was partially uniform throughout the days of differentiation, with the highest values on day 14 (Fig. 2B).
FIGURE 2.
Gene expression of eNOS and nNOS and NO metabolite (NOx) production along neurosphere differentiation. A and B, nNOS (A) and eNOS (B) gene expression changes in neurospheres cultures from days 0–14 following induction to neural differentiation were analyzed by real time PCR. Normalization of expression levels was done by comparison to GAPDH gene expression. The data are shown as the mean values ± S.E. of three independent experiments. C and D, cells in differentiation were lysed with radioimmune precipitation assay buffer for measuring NOx contents in intracellular (C) and extracellular media (D) by a chemiluminescence assay. The culture medium of neurospheres was changed 1 day before the collection of intra- and extracellular media. The values of concentration of NOx in the basal media used for measurement of nitric oxide were subtracted from the samples collected during NSC differentiation. In addition, we have normalized the production of these metabolites to the protein concentration of the culture. NO concentrations are expressed as mean values ± S.E. of six independent experiments. *, p < 0.01; **, p < 0.01; ***, p < 0.001, compared to control data collected on days 0 or 1 of differentiation.
NO is a gaseous molecule with short half-life, because it is quickly converted into nitrate, nitrite, nitrosothiols, nitrosamines, and nitrosylated compounds (NOx) (24). Intra- and extracellular NOx levels in NSC at different days of differentiation were measured in gaseous phase by a chemiluminescence assay (28). Intracellular NO concentrations decreased from 6.9 ± 0.7 μm on the onset of differentiation to 2.3 ± 0.3 μm on day 14 (Fig. 2C), while extracellular NOx concentration ranged from 1.6 ± 0.4 on day 1 to 3.4 ± 0.2 μm on day 14 of differentiation (Fig. 2D).
Involvement of the NO-Citrulline Cycle in the Progress of Neural Differentiation
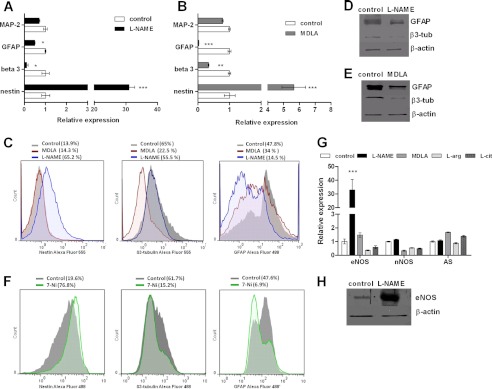
The role of the NO-citrulline cycle in neural differentiation was assessed by treating NSC with l-citrulline or l-arginine, which are AS and NOS substrates, respectively, until day 7 of differentiation. Expression levels of neural markers were compared to those of neurospheres differentiated in the absence of these compounds. l-Arginine promoted NSC differentiation, as shown by 25 and 35% increases in β3-tubulin and GFAP expression, respectively. Chronic treatment of NSC with l-citrulline augmented GFAP expression by 56%, whereas β3-tubulin expression did not change (Fig. 3A). In addition, total NOx production by l-arginine- and l-citrulline-treated NSC was measured on day 7 of differentiation. Increases of 70% in NOx levels were detected in NSC differentiated in the presence of l-citrulline, whereas NO levels of l-arginine-treated differentiated NSC were equal to those of control cells (Fig. 3B). Treatment of cells with inhibitors of AS and NOS enzymes resulted in inhibition of the progress of neural differentiation, measured as alterations in neural marker expression. Both l-NAME- and MDLA-treated NSC along differentiation revealed about 30 (Fig. 4A) and 6 times increased Nestin expression (Fig. 4B), respectively, when compared to cells differentiated in the absence of these drugs. Expression levels of neuronal β3-tubulin and glial GFAP in differentiated cells were drastically reduced by both treatments, whereas expression rates of MAP-2 were not affected. Flow cytometry analysis revealed changes in the percentage of Nestin-, β3-tubulin-, and GFAP-positive cells from the group treated with l-NAME and MDLA when compared to cells differentiated in the absence of these inhibitors. Expression of nestin was detected in 13.9% of untreated neurospheres and in 14.3 and 65.2% of MDLA and l-NAME-treated neurospheres, respectively, on day 7 of differentiation. A slight decrease in β3-tubulin expression (from 65 to 55.5%) and a marked reduction of GFAP-expressing cells (from 47.8 to 14.5%) were observed following chronic treatment with l-NAME along differentiation. The presence of MDLA during differentiation led to a pronounced reduction of β3-tubulin-expressing cells (from 65 to 22.5%), whereas GFAP expression only slightly decreased (from 47.8 to 34%) (Fig. 4C). Western blot analysis of β3-tubulin and GFAP also confirmed the decreased expression of neural markers in cells differentiated in the presence of l-NAME (Fig. 4D) or MDLA (Fig. 4E). Because l-NAME inhibits both NOS isoforms, we have used 7-Ni to identify which isoform is involved in the delay on neural differentiation caused by deficiency in the NO production. The results of flow cytometry analysis showed that nNOS inhibition during differentiation caused changes in the percentage of Nestin-, β3-tubulin-, and GFAP-positive cells, similarly to those observed in the presence of MDLA and l-NAME. The chronic treatment of neurospheres with 7-Ni led to an increase of Nestin-expressing cells (from 19.6 to 76.8%), a decrease in β3-tubulin expression (from 61.7 to 15.2%), and a reduction in GFAP-expressing cells compared to untreated neurospheres (from 47.6 to 6.9%) (Fig. 4F).
FIGURE 3.
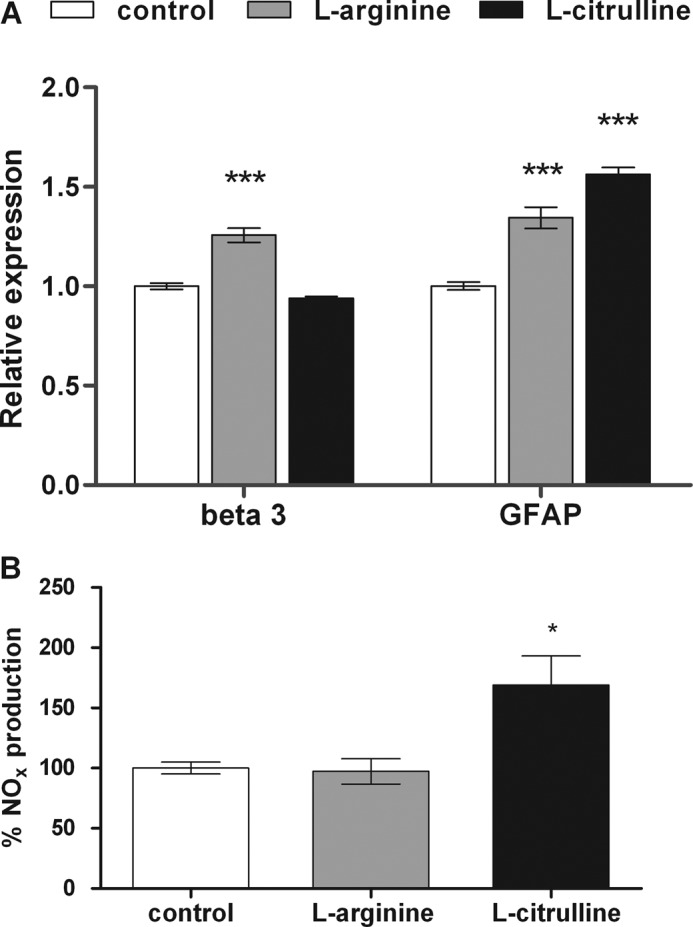
Interference of NO-citrulline cycle intermediates with neurosphere differentiation. A, relative gene expression of specific markers for differentiating neurons (β3-tubulin abbreviated as beta) and glia (GFAP) of l-arginine- or l-citrulline-treated neurospheres on day 7 of differentiation were determined by real time PCR. Neurospheres were maintained in culture until day 7 of differentiation in the presence of 1 mm l-arginine, a natural NOS substrate, or 1 mm l-citrulline, an AS substrate, which were newly supplied every day. The culture medium was changed every 2 days. Nontreated differentiated cells were used as control. B, NO production of neurospheres differentiated in the absence or presence of l-arginine- or l-citrulline on day 7 was measured by a chemiluminescence assay as described under “Experimental Procedures.” NO production of nontreated cells was considered as 100%. The shown data are mean values ± S.E. *, p < 0.05; ***, p < 0.001, compared to control data.
FIGURE 4.
Interference of NO inhibition with neurosphere differentiation. A and B, gene expression levels of specific markers for mature neurons (MAP-2), glia (GFAP), differentiating neurons (β3-tubulin), and progenitor cells (nestin) of l-NAME-treated (A) and MDLA-treated (B) neurospheres on day 7 of differentiation were determined by real time PCR. Neurospheres were maintained in culture until day 7 of differentiation in the absence or presence of 1 mm l-NAME, a nonselective antagonist of NOS, or 1 mm MDLA, an inhibitor of AS, which were newly supplied every day. The medium was changed every 2 days. Cells differentiated in the absence of these compounds were used as control. C, flow cytometry analysis of Nestin, β3-tubulin, and GFAP expression in neurospheres differentiated for 7 days in the absence or presence of 1 mm MDLA or 1 mm l-NAME. Representative histograms compare expression levels of neural markers in differentiated neurospheres (gray) with neurospheres treated with MDLA (red) or l-NAME (blue). D and E, immunoblots of protein extracts from NSC on day 7 of differentiation cultures in the absence or presence of 1 mm l-NAME (D) or 1 mm MDLA (E) were probed for GFAP and β3-tubulin expression levels. F, flow cytometry analysis of Nestin, β3-tubulin, and GFAP expression in murine neurospheres differentiated for 7 days in the presence of 1 μm 7-Ni. Representative histograms compare expression levels of neural markers in untreated neurospheres (gray) and treated with 7-Ni (green). G, regulation of AS, eNOS and nNOS expression in differentiated neurospheres (day 7) in the presence of activators and inhibitors of the NO-citrulline cycle. H, Western blot analysis was performed to confirm increased eNOS expression in l-NAME-treated neurospheres. The data shown are representative for at least two independent experiments. The data are presented as the mean values ± S.E. ***, p < 0.001. β-tub, β-tubulin, l-cit, l-citrulline.
Gene Expression of AS, nNOS, and eNOS in Neurospheres Treated with Substrates and Inhibitors of the NO-Citrulline Cycle
Gene expression of AS, nNOS and eNOS was studied in neurospheres treated with substrates and inhibitors of enzymes of the NO-citrulline cycle. Cells treated during differentiation with the NOS inhibitor l-NAME for a period of 7 days revealed about 30 times elevated eNOS expression compared to untreated cells, whereas nNOS and AS expression did not change under these conditions (Fig. 4, G and H). Exposure of cells to the NOS substrate l-arginine, the AS substrate l-citrulline, or MDLA, a competitive inhibitor of AS, during differentiation, did not affect the expression of any of the analyzed enzymes of the NO-citrulline cycle (Fig. 4G).
Up-regulation of Gene Expression of BDNF in Differentiating Neurospheres and in Neurospheres Treated with Inhibitors of the NO-Citrulline Cycle
Evidence from in vitro studies suggests crucial functions for BDNF in neurogenesis (29). Real time PCR analysis in neurospheres during differentiation revealed that gene expression of BDNF was up-regulated beginning from day 7 of differentiation. Peak values of BDNF expression were reached on day 14 with a 30-fold increase compared to expression levels on day 7 (Fig. 5A). Treatment of cells with the NOS inhibitor l-NAME and the AS antagonist MDLA during differentiation induced BDNF expression by factors of 3- and 20-fold, respectively, whereas chronic exposure of differentiating cells to l-arginine or l-citrulline did not evoke any changes in BDNF expression (Fig. 5B).
FIGURE 5.
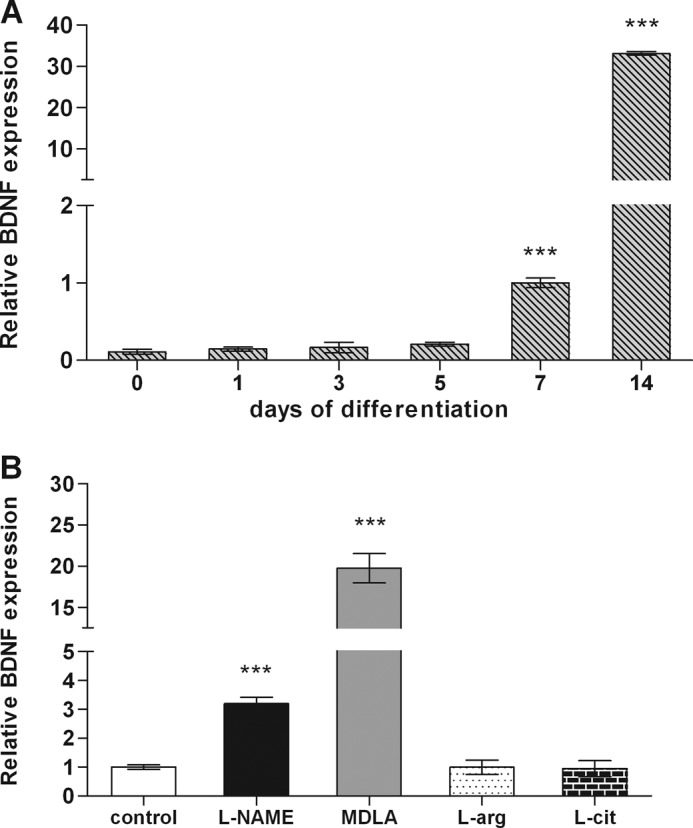
Differential BDNF expression during neurosphere differentiation. For real time PCR experiments, neurospheres were cultured in the absence or presence of 1 mm of l-NAME, 1 mm MDLA, 1 mm l-arginine, or 1 mm l-citrulline and collected on different days of differentiation on day 7 of differentiation. A, BDNF gene expression along neurospheres differentiation. B, BDNF expression changes in neurospheres treated during 7 days of differentiation with inhibitors or substrates of NO-citrulline cycle enzymes. The obtained data are shown as the mean values ± S.E. of three independent experiments. ***, p < 0.001. l-arg, l-arginine; l-cit, l-citrulline.
Effects of BDNF on Neurosphere Differentiation
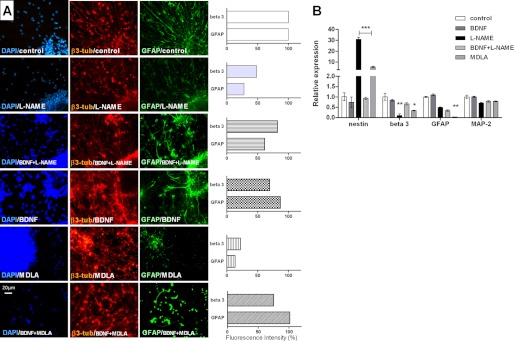
In the present work, BDNF by itself did not affect NSC differentiation; i.e., neurogenesis was not promoted by this factor such as reported in previous studies (30). However, we have verified that BDNF reversed the delay in NSC differentiation caused by inhibition of NO production. Immunofluorescence studies revealed reduced expression of GFAP and β3-tubulin in neurospheres treated along differentiation with MDLA or l-NAME (Fig. 6A). The concomitant treatment of NSC with BDNF and l-NAME or BDNF and MDLA did not affect the progress of neural differentiation when compared to control cells differentiated in the absence of these compounds. In agreement, real time PCR studies confirmed increased nestin and decreased GFAP and β3-tubulin expression in neurospheres differentiated in the presence of l-NAME and MDLA. Expression levels of β3-tubulin in cells differentiated in the presence of BDNF or of both BDNF and l-NAME were identical to those observed in NSC differentiated without any of these compounds (Fig. 6B). MAP-2 expression levels were not affected in any of the mentioned experimental conditions (Fig. 6).
FIGURE 6.
BDNF-mediated reversion of neurosphere differentiation caused by inhibitors of NO-citrulline cycle. A, immunostaining of neurospheres differentiated in the presence of 1 mm l-NAME, 1 mm MDLA, 20 ng/ml BDNF, 20 ng/ml BDNF and 1 mm l-NAME, or 20 ng/ml BDNF and 1 mm MDLA, for β3-tubulin (β-tub) and GFAP expression. Scale bar, 20 μm. The data were analyzed using the NIS Elements software (Nikon) and represented as the ratio of β3-tubulin or GFAP fluorescence intensity over DAPI fluorescence intensity. B, analysis of gene expression of specific markers for mature neurons (MAP-2), glia (GFAP), differentiating neurons (β3-tubulin, beta 3), and progenitor cells (Nestin) on day 7 of neurosphere differentiation by real time PCR. The data are shown as the mean values ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with control experiments obtained with cells differentiated without any of these compounds.
Effects of Endogenous NO and BDNF on Cell Proliferation and Migration
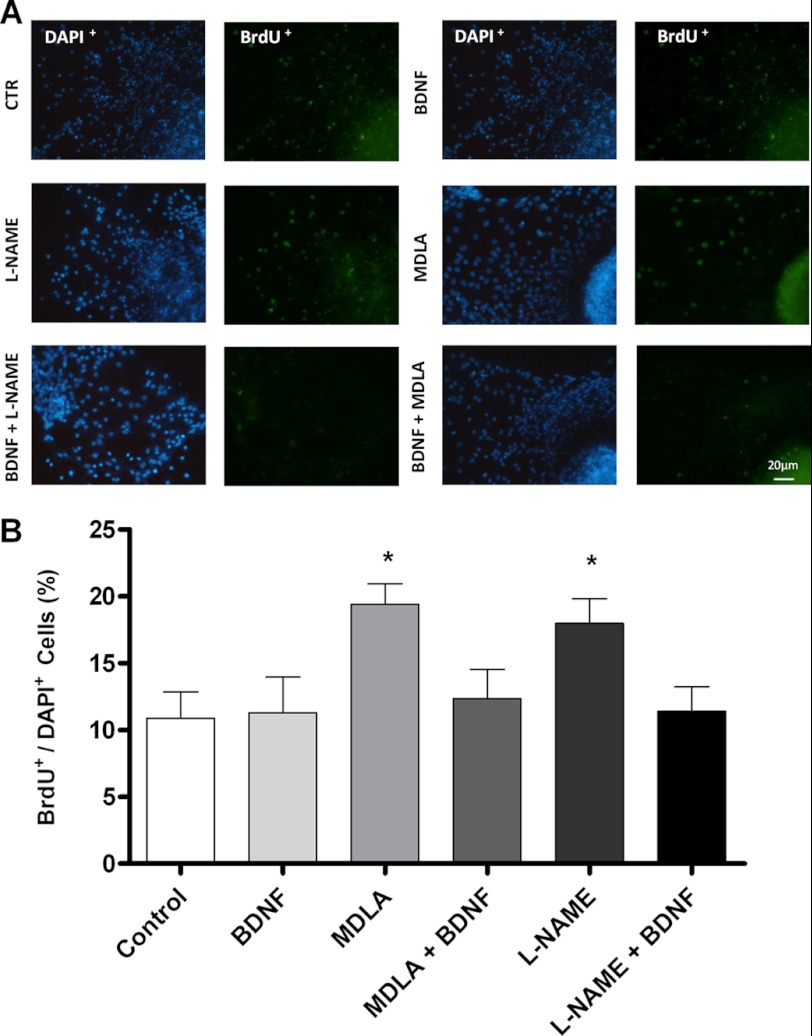
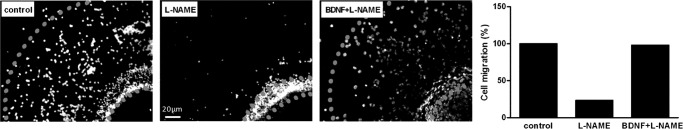
Effects of l-NAME, MDLA, and BDNF on cell proliferation were evaluated. To this end, neurospheres were differentiated for 7 days in the presence of one of those. l-NAME and MDLA induced proliferation rates were about 50% higher than those of untreated control neurospheres, whereas BDNF-treated neurospheres revealed proliferation levels similar to those of untreated cells (Fig. 7A). Co-treatment with BDNF and inhibitors of NO production (l-NAME or MDLA) (31, 32) re-established normal proliferation levels. Neurosphere migration, a prerequisite for neurogenesis, was also inhibited by l-NAME and MDLA, and such as in the proliferation assay, BDNF reversed this effect caused by inhibition of NO production (Fig. 8).
FIGURE 7.
Effects of inhibition of NO production and of BDNF on neural progenitor cell proliferation. A, immunodetection of BrdU incorporation following a 12-h pulse in differentiating neurospheres on day 7 in the presence of 1 mm l-NAME, 1 mm MDLA, 20 ng/ml BDNF, 20 ng/ml BDNF and 1 mm MDLA, or 20 ng/ml BDNF and 1 mm l-NAME. BrdU incorporating nuclei are shown in green. Scale bar, 20 μm. B, quantification of proliferation in different conditions of treatment was performed by determining the ratio of BrdU+ over DAPI+ cells. Six fields were analyzed for each treatment by using the NIS Elements software (Nikon). *, p < 0.05, compared with untreated control cells). CTR, control.
FIGURE 8.
Interference of inhibited NO production with neural stem cell migration. Neurospheres were differentiated for 7 days in the presence or absence of 1 mm l-NAME or 1 mm l-NAME and 20 ng/ml BDNF. The cells were visualized by cell nuclei staining, and the distances of migration were determined by using the NIS Elements software (Nikon).
Involvement of p75NTR in the Effect of BDNF on Differentiating Neurospheres
Experimental evidence indicates that many growth factors including BDNF play an important role in regulating proliferation and differentiation of NSC. However, the effects of BDNF on neurogenesis have not yet been fully elucidated (30, 33). In this context, it is known that p75NTR defines the population of NSC responsive to BDNF (34). Therefore, we have measured the relative expression of p75NTR in untreated and treated NSC on day 7 of differentiation. In fact, treatment with MDLA or l-NAME increased p75NTR gene expression 2- and 3-fold, respectively (Fig. 9A). Moreover, flow cytometry analysis revealed a 31% increase of p75NTR-expressing cells when compared to untreated cells, providing a mechanism for BDNF-driven reversion of inhibition of neural differentiation as consequence of impaired NO production (Fig. 9B).
FIGURE 9.
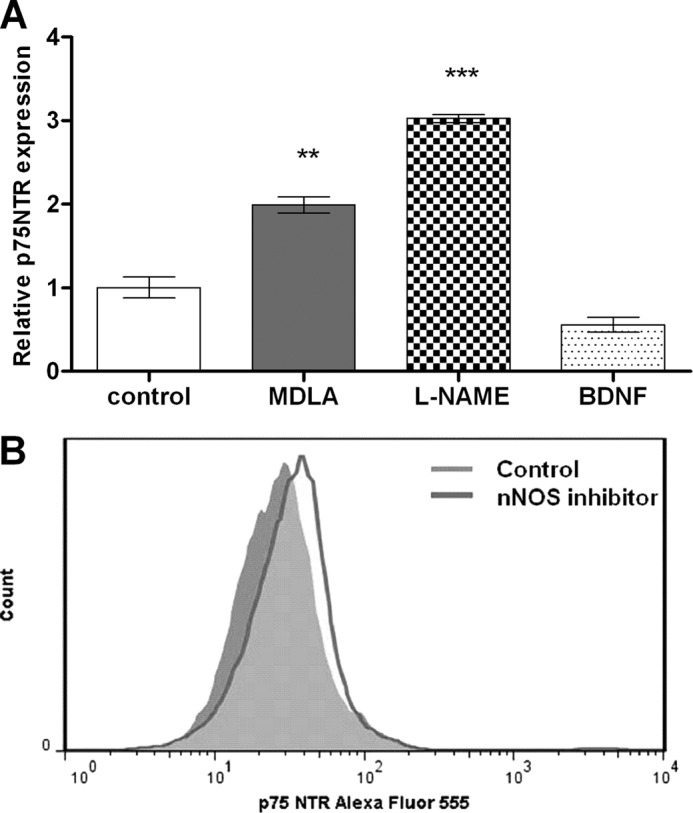
Modulation of p75 neurotrophin receptor expression in neurospheres. The cells were culture in the presence of 1 mm MDLA, 1 mm l-NAME, 20 ng/ml BDNF, or 1 μm 7-Ni. A, neurospheres were collected on day 7 of differentiation for RNA extraction, and p75NTR expression was analyzed by real time PCR. B, flow cytometry analysis of p75NTR expression in murine neurospheres differentiated for 7 days in the absence or presence of the nNOS inhibitor. The data are shown as mean values ± S.E. of three independent experiments. **, p < 0.01; ***, p < 0.001, compared with untreated control cells.
DISCUSSION
Evidence collected in many studies indicates crucial effects of NO signaling in promotion of neural differentiation (reviewed in Ref. 35); however, excessive levels of NO can be deleterious to the organism. In the NO-citrulline cycle, NOS is the enzyme that synthesizes NO from l-arginine, whereas AS is a step-limiting enzyme in the supply of this substrate for NOS. Therefore, AS has been considered as such important as NOS in the process of NO formation, because the regulation of l-arginine supply may be of pivotal importance for a delicate balance of NO benefiting physiological actions and avoiding the induction of pathological events. In the present work, we have addressed the role of the NO-citrulline cycle in regulating neurogenesis and gliogenesis.
We show here that nNOS expression progressively increased throughout NSC differentiation, in agreement with previous studies describing the pattern of nNOS gene expression in developing rat cerebral cortex (36). We also observed that the enzymatic activity of AS, as well as its expression, increased during differentiation, augmenting the concentration of intracellular l-arginine, concomitantly with the onset of in vitro differentiation of NSC into neural phenotypes. These data are in line with the work of Husson et al. (9) showing differential gene expression of this enzyme in the brain of adult and fetal rats and suggesting the physiological significance of AS during brain development.
Expression of eNOS did not uniformly increase during differentiation, such as observed for AS and nNOS. Peak expression of eNOS was reached on day 14 together with maximal immunostaining for MAP-2 and GFAP. Expression of eNOS was induced by chronic inhibition of NOS, indicating that this isoform is subject to modulation by extracellular signals. High concentrations of nNOS are present in the CNS; thus, other mechanisms may exist for compensation of interruption of NO production including up-regulation of eNOS activity (37). Expression of eNOS is relatively uniform throughout the brain development of ewe fetuses, whereas peak expression is observed during pregnancy. It is known that both isoforms of NOS are expressed in the developing and mature brain; however, eNOS expression does not present a differential pattern of expression such as observed for rising nNOS expression accompanying the increasing complexity of neural development (38).
Several studies have attempted to identify factors that regulate NSC proliferation and subsequently lineage specifications. We hypothesized that NO production is a determinant for the progress of NSC differentiation and neural phenotype determination. NO can be directly and rapidly synthesized in response to growth factor stimulation and other extracellular signals and then rearrange gene expression. We observed that inhibition of NOS and AS activity prevented the progress of differentiation.
Moreover, our results revealed that extracellular production of NOx increased, whereas intracellular NOx levels diminished during neural maturation. The synthesized NO diffuses into neighboring cells without the need for packaging, vesicle secretion, or membrane receptors and can interact with a variety of intracellular proteins. It is a gaseous molecule with an extremely short half-life being rapidly converted into other products such as nitrite or nitrate or incorporated into proteins by nitrosylation (24). Opposing changes of quantities of NO metabolites in intra- and extracellular environments throughout differentiation suggest differences in expression patterns of proteins that are nitrosylated along neural differentiation. NO can exert its effects on neuronal function through modification of sulfhydryl groups such as S-nitrosylation (39, 40). For example, the impaired dendrite outgrowth of nNOS−/− mice was explained by the absence of nitrosylation of the collapsin response mediator protein (41, 42). Target proteins for S-nitrosylation as studied in brain lysates, include metabolic enzymes, ion channels such as NMDA-glutamate receptors and structural proteins such as neurofilament heavy chain (NF-H) and β3-tubulin (43).
We have also investigated which is the most important NOS isoform for neural differentiation, using a selective inhibitor of nNOS. The obtained results indicate that nNOS inhibition alone was sufficient to block the progress of neural differentiation, suggesting key functions for nNOS in NO production during CNS formation.
Specific blockade of nNOS during neural differentiation resulted in increased expression of the NSC and progenitor antigen Nestin and decreased expression of neuron-specific β3-tubulin and glia-specific GFAP. MAP-2 expression levels were not affected in any of the mentioned experimental conditions, suggesting that NO signaling is essential for the initial progress of neural fate determination and less important for final neuronal maturation. An underlying mechanism for the observed effects during the onset of differentiation might be the failure of transition into cytostasis in the presence of l-NAME or MDLA. Inhibition of AS in cultured cells blocked production of l-arginine and consequently NO formation (44). NOS inhibition maintained NSC in a proliferative state, thereby suppressing neuronal differentiation; furthermore, l-NAME administration into lateral ventricle of adult mice significantly increased the number of proliferating cells (45). Thus, besides being important for triggering neural differentiation, NO plays an important signaling role in proliferation. As further evidence for NO as an essential factor in neural development, the addition of the substrates of NOS and AS, l-arginine and l-citrulline, respectively, to the culture medium during differentiation resulted in an increase of the number of cells expressing neural marker proteins, despite an increase in NOx production was only observed in cultures that had received l-citrulline, a phenomenon that can be explained by the “l-arginine paradox” (46). The underlying mechanism implies the existence of separate intracellular pools of l-arginine directed to different pathways. This l-arginine is synthesized in the NO-citrulline cycle by l-citrulline recycling (44). The enhancement of NOx production by exogenous l-citrulline can therefore be attributed to the capacity of NSC to efficiently regenerate l-arginine from l-citrulline. Besides providing a source for NO production, l-arginine is a basic amino acid that has versatile metabolic roles, being involved in the generation of a wide range of biologically active intermediates such as NO, polyamines, creatine, and l-amino acids (47). As an alternative to NO production, l-arginine can be deviated to polyamines synthesis (reviewed in Ref. 7).
Agmatine derived from l-arginine decarboxylation (48) is a central neuromodulator with high affinity for α2-adrenoceptors and imidazoline-binding sites, in addition to blocking calcium influx, particularly by inhibiting receptors of the NMDA class (49). Moreover, it has been shown that agmatine increases neurogenesis by recruiting NSC in the hippocampus of adult mice because of blockade of NMDA receptors (50). Furthermore, treatment with drugs with marked affinity to imidazoline-binding sites led to increased levels of GFAP immunostaining together with increased density of imidazoline-binding sites in rat brain (51). Thus, l-arginine, even without altering NO concentration, increased GFAP and β3-tubulin expression, possibly because of induction of polyamine synthesis, such as agmatine. At the same time l-citrulline only affected GFAP expression. Having in mind that NO-induced effects depend on the dose and local of production of this gaseous messenger (7, 42), the incapability of l-citrulline in promoting neurogenesis can be explained by the lack of NO production in most of the cells of the heterogeneous population of differentiating NSC. Consequently, NO is only produced in a subset of cells expressing l-citrulline transporters (51), by yet unknown mechanisms supporting gliogenesis but not neurogenesis. Further studies will be necessary to validate such a hypothesis. Cheng et al. (45) have demonstrated that regulation of proliferation and/or differentiation fate occurred under the control of BDNF, acting in a positive feedback loop with NO for the correct neuronal phenotype choice. Moreover, there are very similar biological activities of NO and this other diffusible factor, BDNF (17, 18), implying potential interaction and association between these neurotransmitter. It is known that endogenous NO regulates BDNF production, supporting the hypothesis that BDNF and NO influence each other and may function as trans-synaptic signaling molecules in the brain (52). We have observed that BDNF expression increased along with neural differentiation of NSC and neuronal maturation, similarly to the pattern of nNOS expression.
Because BDNF signaling is associated with neurogenesis, we have investigated the possible interference with BDNF production in conditions of chronic exposure of NSC to l-NAME and MDLA along differentiation. Corroborating such hypothesis, we hereby show that the blocked endogenous NO production in culture as consequence of NOS or AS inhibition led to augmented BDNF gene expression. As further support for our experimental data, cerebroventricular administration of a NOS inhibitor increased BDNF content in the neocortex (52).
As an underlying mechanism, NSC-induced BDNF expression may be an attempt to compensate missing signaling caused by the lack of NO. However, neither induction of endogenous BDNF production nor induction of eNOS expression as a consequence of missing NO availability were enough to restore normal signaling in vitro. We hypothesized that endogenous paracrine effects of this neuron-derived factor might not be sufficient for restoring the normal progress of differentiation, because just few neurons, being able to secrete this factor, had been originated from differentiating NSC (53). As expected, the addition of 20 ng/ml BDNF to the culture medium reversed the block on neural differentiation caused by insufficient NO signaling. In addition to inducing neural differentiation, NO plays a role as negative regulator of precursor proliferation (35). As further evidence of re-establishing missing NO signaling by BDNF treatment, inhibition of proliferation caused by l-NAME and MDLA was re-established in the presence of the neurotrophic factor. On the other hand, treatment of differentiating neurospheres with BDNF in the absence of any inhibitor of NO production did not induce any changes in the progress of NSC differentiation and cell fate determination when compared with control differentiation assays performed without any of these compounds.
BDNF exerts its signaling by acting through two receptors: the high affinity trkB and the low affinity tyrosine receptor p75NTR, which does not have any enzymatic activity. The p75NTR is required for BDNF-induced blockade of proliferation and induction of differentiation of NSC, even in NSC populations that did not express trkB, suggesting that BDNF induced neurogenesis via p75NTR activation alone (34, 53, 54). NSC cultures obtained from embryonic rat telencephalon are not responsive to BDNF, indicating a low expression of p75NTR. However, our data show that the lack of NOx production following treatment with l-NAME, MDLA, or 7-Ni, induced p75NTR expression; subsequently NSC became BDNF-responsive. These data are in line with previous studies that relate inhibition of NO expression to the up-regulation of p75NTR expression (55, 56).
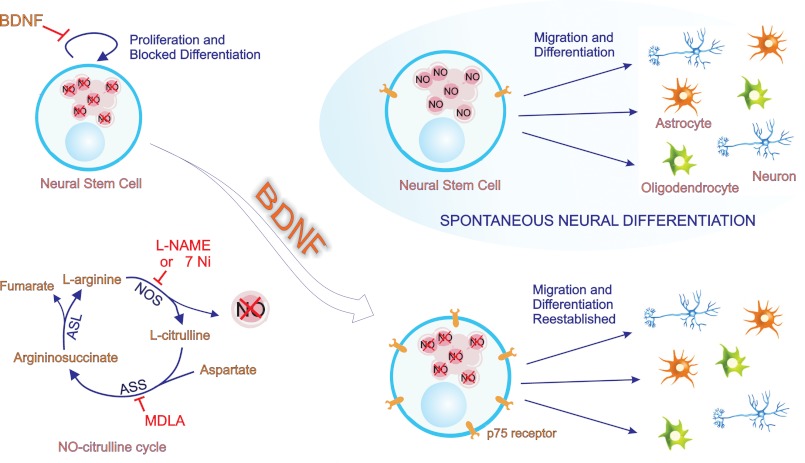
In summary, we have shown for the first time that AS and NO are directly involved in the progress of NSC differentiation by using an in vitro system, which reflects differentiation conditions occurring in the developing cortex (5). Moreover, our results indicate that the cross-talk between BDNF- and NOx-mediated signaling represents a mechanism by which NSC regulate their maintenance as precursor cells and subsequent neural differentiation (Fig. 10). Thus, this work provides new insights for understanding the involvement of the NO-citrulline cycle in regulation of NSC proliferation and differentiation, underlying the complex process of brain development. Further studies may reveal the roles of NO and BDNF in the maintenance of neurogenesis throughout postnatal life.
FIGURE 10.
BDNF restores neural differentiation inhibited by lack of nitric oxide. NO signaling is essential for the spontaneous progress of neural fate determination. After plating, neural stem cells spontaneously differentiate into neurons, astrocytes, and oligodendrocytes. However, when formation of endogenous NO is interrupted by inhibitors of the NO-citrulline cycle (l-NAME, 7-Ni, and MDLA inhibiting all isoforms of NOS, AS, and nNOS, respectively), the progress of neural differentiation is blocked. In these conditions, NSC remain in a proliferative state; they do not migrate, nor do they differentiate into neural cells. BDNF does not affect neural differentiation in the absence of the above-cited inhibitors, but it re-establishes migration and differentiation of NSC when the NO-citrulline cycle is blocked. The addition of BDNF to NSC cultures treated with l-NAME, 7-Ni, or MDLA decreases proliferation and promotes migration and neural differentiation. These BDNF-induced effects are suggested to result from an increase in p75NTR expression induced by lack of NO. The p75NTR is required for BDNF-induced differentiation of neural stem cells, pointing at a novel mechanism for joint actions between the NO-citrulline cycle and BDNF for the maintenance of normal neurodevelopment processes.
This work was supported by Brazilian Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 06/61285-9 and by funds from the Conselho Nacional de Desenvolvimento Científico e Tecnológico of Brazil (to H. U.).
- NSC
- neural stem cell(s)
- AS
- argininosuccinate synthase
- GFAP
- glial fibrillary acidic protein
- MAP-2
- microtubule-associated protein 2
- NOS
- nitric-oxide synthase
- eNOS
- endothelial NOS
- nNOS
- neuronal NOS
- L-NAME
- l-Nγ-nitroarginine methyl ester
- MDLA
- α-methyl-dl-aspartic acid
- 7-Ni
- 7-nitroindazole
- p75NTR
- p75 neurotrophin receptor.
REFERENCES
- 1. Moroz L. L., Gillette R., Sweedler J. V. (1999) Single-cell analyses of nitrergic neurons in simple nervous systems. J. Exp. Biol. 202, 333–341 [DOI] [PubMed] [Google Scholar]
- 2. Reynolds B. A., Weiss S. (1996) Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175, 1–13 [DOI] [PubMed] [Google Scholar]
- 3. McKay R. (1997) Stem cells in the central nervous system. Science 276, 66–71 [DOI] [PubMed] [Google Scholar]
- 4. Gage F. H. (2000) Mammalian neural stem cells. Science 287, 1433–1438 [DOI] [PubMed] [Google Scholar]
- 5. Trujillo C. A., Schwindt T. T., Martins A. H., Alves J. M., Mello L. E., Ulrich H. (2009) Novel perspectives of neural stem cell differentiation. From neurotransmitters to therapeutics. Cytometry A 75, 38–53 [DOI] [PubMed] [Google Scholar]
- 6. Madhusoodanan K. S., Murad F. (2007) NO-cGMP signaling and regenerative medicine involving stem cells. Neurochem. Res. 32, 681–694 [DOI] [PubMed] [Google Scholar]
- 7. Lameu C., de Camargo A. C., Faria M. (2009) l-Arginine signalling potential in the brain. The peripheral gets central. Recent Pat. CNS Drug Discov. 4, 137–142 [DOI] [PubMed] [Google Scholar]
- 8. Flam B. R., Eichler D. C., Solomonson L. P. (2007) Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 17, 115–121 [DOI] [PubMed] [Google Scholar]
- 9. Husson A., Brasse-Lagnel C., Fairand A., Renouf S., Lavoinne A. (2003) Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 270, 1887–1899 [DOI] [PubMed] [Google Scholar]
- 10. Xie L., Gross S. S. (1997) Argininosuccinate synthetase overexpression in vascular smooth muscle cells potentiates immunostimulant-induced NO production. J. Biol. Chem. 272, 16624–16630 [DOI] [PubMed] [Google Scholar]
- 11. Ignarro L. J. (ed) (2000) Nitric Oxide: Biology and Pathobiology, Academic Press, San Diego [Google Scholar]
- 12. Matarredona E. R., Murillo-Carretero M., Moreno-López B., Estrada C. (2004) Nitric oxide synthesis inhibition increases proliferation of neural precursors isolated from the postnatal mouse subventricular zone. Brain Res. 995, 274–284 [DOI] [PubMed] [Google Scholar]
- 13. Peunova N., Enikolopov G. (1995) Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature 375, 68–73 [DOI] [PubMed] [Google Scholar]
- 14. Foster J. A., Phelps P. E. (2000) Neurons expressing NADPH-diaphorase in the developing human spinal cord. J. Comp. Neurol. 427, 417–427 [DOI] [PubMed] [Google Scholar]
- 15. Huang P. L., Dawson T. M., Bredt D. S., Snyder S. H., Fishman M. C. (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75, 1273–1286 [DOI] [PubMed] [Google Scholar]
- 16. Huang P. L., Huang Z., Mashimo H., Bloch K. D., Moskowitz M. A., Bevan J. A., Fishman M. C. (1995) Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 [DOI] [PubMed] [Google Scholar]
- 17. Lo D. C. (1995) Neurotrophic factors and synaptic plasticity. Neuron 15, 979–981 [DOI] [PubMed] [Google Scholar]
- 18. Stoop R., Poo M. M. (1996) Synaptic modulation by neurotrophic factors. Prog. Brain Res. 109, 359–364 [DOI] [PubMed] [Google Scholar]
- 19. Hsieh H. Y., Robertson C. L., Vermehren-Schmaedick A., Balkowiec A. (2010) Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J. Neurosci. Res. 88, 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martins A. H., Alves J. M., Trujillo C. A., Schwindt T. T., Barnabé G. F., Motta F. L., Guimaraes A. O., Casarini D. E., Mello L. E., Pesquero J. B., Ulrich H. (2008) Kinin-B2 receptor expression and activity during differentiation of embryonic rat neurospheres. Cytometry A 73, 361–368 [DOI] [PubMed] [Google Scholar]
- 21. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 22. Hao G., Xie L., Gross S. S. (2004) Argininosuccinate synthetase is reversibly inactivated by S-nitrosylation in vitro and in vivo. J. Biol. Chem. 279, 36192–36200 [DOI] [PubMed] [Google Scholar]
- 23. Guerreiro J. R., Lameu C., Oliveira E. F., Klitzke C. F., Melo R. L., Linares E., Augusto O., Fox J. W., Lebrun I., Serrano S. M., Camargo A. C. (2009) Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide. Role in arginine and nitric oxide production. J. Biol. Chem. 284, 20022–20033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feelisch M., Rassaf T., Mnaimneh S., Singh N., Bryan N. S., Jourd'Heuil D., Kelm M. (2002) Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids. Implications for the fate of NO in vivo. FASEB J. 16, 1775–1785 [DOI] [PubMed] [Google Scholar]
- 25. Lameu C., Pontieri V., Guerreiro J. R., Oliveira E. F., da Silva C. A., Giglio J. M., Melo R. L., Campos R. R., de Camargo A. C., Ulrich H. (2010) Brain nitric oxide production by a proline-rich decapeptide from Bothrops jararaca venom improves baroreflex sensitivity of spontaneously hypertensive rats. Hypertens Res. 33, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 26. Schwindt T. T., Trujillo C. A., Negraes P. D., Lameu C., Ulrich H. (2011) Directed differentiation of neural progenitors into neurons is accompanied by altered expression of P2X purinergic receptors. J. Mol. Neurosci. 44, 141–146 [DOI] [PubMed] [Google Scholar]
- 27. Kim S. J., Lim M. S., Kang S. K., Lee Y. S., Kang K. S. (2008) Impaired functions of neural stem cells by abnormal nitric oxide-mediated signaling in an in vitro model of Niemann-Pick type C disease. Cell Res. 18, 686–694 [DOI] [PubMed] [Google Scholar]
- 28. Tarpey M. M., Fridovich I. (2001) Methods of detection of vascular reactive species. Nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 89, 224–236 [DOI] [PubMed] [Google Scholar]
- 29. Li T., Jiang L., Zhang X., Chen H. (2009) In-vitro effects of brain-derived neurotrophic factor on neural progenitor/stem cells from rat hippocampus. Neuroreport 20, 295–300 [DOI] [PubMed] [Google Scholar]
- 30. Larsson E., Mandel R. J., Klein R. L., Muzyczka N., Lindvall O., Kokaia Z. (2002) Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp. Neurol. 177, 1–8 [DOI] [PubMed] [Google Scholar]
- 31. Kumar A., Patel S., Gupta Y. K., Singh M. P. (2006) Involvement of endogenous nitric oxide in myeloperoxidase mediated benzo(a)pyrene induced polymorphonuclear leukocytes injury. Mol. Cell Biochem. 286, 43–51 [DOI] [PubMed] [Google Scholar]
- 32. Shen L. J., Beloussow K., Shen W. C. (2005) Accessibility of endothelial and inducible nitric oxide synthase to the intracellular citrulline-arginine regeneration pathway. Biochem. Pharmacol. 69, 97–104 [DOI] [PubMed] [Google Scholar]
- 33. Lee J., Duan W., Long J. M., Ingram D. K., Mattson M. P. (2000) Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 15, 99–108 [DOI] [PubMed] [Google Scholar]
- 34. Young K. M., Merson T. D., Sotthibundhu A., Coulson E. J., Bartlett P. F. (2007) p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J. Neurosci. 27, 5146–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Contestabile A., Ciani E. (2004) Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem. Int. 45, 903–914 [DOI] [PubMed] [Google Scholar]
- 36. Bredt D. S., Snyder S. H. (1994) Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron 13, 301–313 [DOI] [PubMed] [Google Scholar]
- 37. Ye S., Nosrati S., Campese V. M. (1997) Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J. Clin. Invest. 99, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Northington F. J., Koehler R. C., Traystman R. J., Martin L. J. (1996) Nitric oxide synthase 1 and nitric oxide synthase 3 protein expression is regionally and temporally regulated in fetal brain. Brain Res. Dev. Brain Res. 95, 1–14 [DOI] [PubMed] [Google Scholar]
- 39. Ahern G. P., Klyachko V. A., Jackson M. B. (2002) cGMP and S-nitrosylation. Two routes for modulation of neuronal excitability by NO. Trends Neurosci. 25, 510–517 [DOI] [PubMed] [Google Scholar]
- 40. Riccio A., Alvania R. S., Lonze B. E., Ramanan N., Kim T., Huang Y., Dawson T. M., Snyder S. H., Ginty D. D. (2006) A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell 21, 283–294 [DOI] [PubMed] [Google Scholar]
- 41. Inglis F. M., Furia F., Zuckerman K. E., Strittmatter S. M., Kalb R. G. (1998) The role of nitric oxide and NMDA receptors in the development of motor neuron dendrites. J. Neurosci. 18, 10493–10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quinn C. C., Gray G. E., Hockfield S. (1999) A family of proteins implicated in axon guidance and outgrowth. J. Neurobiol. 41, 158–164 [PubMed] [Google Scholar]
- 43. Jaffrey S. R., Erdjument-Bromage H., Ferris C. D., Tempst P., Snyder S. H. (2001) Protein S-nitrosylation. A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 3, 193–197 [DOI] [PubMed] [Google Scholar]
- 44. Flam B. R., Hartmann P. J., Harrell-Booth M., Solomonson L. P., Eichler D. C. (2001) Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide 5, 187–197 [DOI] [PubMed] [Google Scholar]
- 45. Cheng A., Wang S., Cai J., Rao M. S., Mattson M. P. (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev. Biol. 258, 319–333 [DOI] [PubMed] [Google Scholar]
- 46. Erez A., Nagamani S. C., Shchelochkov O. A., Premkumar M. H., Campeau P. M., Chen Y., Garg H. K., Li L., Mian A., Bertin T. K., Black J. O., Zeng H., Tang Y., Reddy A. K., Summar M., O'Brien W. E., Harrison D. G., Mitch W. E., Marini J. C., Aschner J. L., Bryan N. S., Lee B. (2011) Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat. Med. 17, 1619–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu G., Morris S. M., Jr. (1998) Arginine metabolism. Nitric oxide and beyond. Biochem. J. 336, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Regunathan S., Reis D. J. (2000) Characterization of arginine decarboxylase in rat brain and liver. Distinction from ornithine decarboxylase. J. Neurochem. 74, 2201–2208 [DOI] [PubMed] [Google Scholar]
- 49. Reis D. J., Regunathan S. (1999) Agmatine. An endogenous ligand at imidazoline receptors is a novel neurotransmitter. Ann. N.Y. Acad. Sci. 881, 65–80 [DOI] [PubMed] [Google Scholar]
- 50. Li Y. F., Chen H. X., Liu Y., Zhang Y. Z., Liu Y. Q., Li J. (2006) Agmatine increases proliferation of cultured hippocampal progenitor cells and hippocampal neurogenesis in chronically stressed mice. Acta Pharmacol. Sin. 27, 1395–1400 [DOI] [PubMed] [Google Scholar]
- 51. Olmos G., Alemany R., Escriba P. V., García-Sevilla J. A. (1994) The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br. J. Pharmacol. 111, 997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong H., Yamada K., Han D., Nabeshima T., Enikolopov G., Carnahan J., Nawa H. (1999) Mutual regulation between the intercellular messengers nitric oxide and brain-derived neurotrophic factor in rodent neocortical neurons. Eur. J. Neurosci. 11, 1567–1576 [DOI] [PubMed] [Google Scholar]
- 53. Pruginin-Bluger M., Shelton D. L., Kalcheim C. (1997) A paracrine effect for neuron-derived BDNF in development of dorsal root ganglia. Stimulation of Schwann cell myelin protein expression by glial cells. Mech. Dev. 61, 99–111 [DOI] [PubMed] [Google Scholar]
- 54. Hosomi S., Yamashita T., Aoki M., Tohyama M. (2003) The p75 receptor is required for BDNF-induced differentiation of neural precursor cells. Biochem. Biophys. Res. Commun. 301, 1011–1015 [DOI] [PubMed] [Google Scholar]
- 55. Wu W. (1996) Potential roles of gene expression change in adult rat spinal motoneurons following axonal injury. A comparison among c-jun, off-affinity nerve growth factor receptor (LNGFR), and nitric oxide synthase (NOS). Exp. Neurol. 141, 190–200 [DOI] [PubMed] [Google Scholar]
- 56. Wu W., Han K., Li L., Schinco F. P. (1994) Implantation of PNS graft inhibits the induction of neuronal nitric oxide synthase and enhances the survival of spinal motoneurons following root avulsion. Exp. Neurol. 129, 335–339 [DOI] [PubMed] [Google Scholar]