Background: The fate of dihydrotestosterone has been characterized only in terms of reduction of the 3-ketone.
Results: Human P450s 19A1 and 3A4 oxidized dihydrotestosterone to several new products, detected in vivo.
Conclusion: The new P450 19A1 and 3A4 pathways introduce an oxidative dimension to the metabolism of dihydrotestosterone.
Significance: Small changes in the steroid A ring can produce major changes in P450 3A4 catalytic selectivity.
Keywords: Cytochrome P450, Mass Spectrometry (MS), Metabolism, NMR, Oxidation-reduction, Steroid, Testosterone, Dihydrotestosterone
Abstract
Dihydrotestosterone is a more potent androgen than testosterone and plays an important role in endocrine function. We demonstrated that, like testosterone, dihydrotestosterone can be oxidized by human cytochrome P450 (P450) 19A1, the steroid aromatase. The products identified include the 19-hydroxy- and 19-oxo derivatives and the resulting Δ1,10-, Δ5,10-, and Δ9,10-dehydro 19-norsteroid products (loss of 19-methyl group). The overall catalytic efficiency of oxidation was ∼10-fold higher than reported for 3α-reduction by 3α-hydroxysteroid dehydrogenase, the major enzyme known to deactivate dihydrotestosterone. These and other studies demonstrate the flexibility of P450 19A1 in removing the 1- and 2-hydrogens from 19-norsteroids, the 2-hydrogen from estrone, and (in this case) the 1-, 5β-, and 9β-hydrogens of dihydrotestosterone. Incubation of dihydrotestosterone with human liver microsomes and NADPH yielded the 18- and 19-hydroxy products plus the Δ1,10-dehydro 19-nor product identified in the P450 19A1 reaction. The 18- and 19-hydroxylation reactions were attributed to P450 3A4, and 18- and 19-hydroxydihydrotestosterone were identified in human plasma and urine samples. The change in the pucker of the A ring caused by reduction of the Δ4,5 bond is remarkable in shifting the course of hydroxylation from the 6β-, 2β-, 1β-, and 15β-methylene carbons (testosterone) to the axial methyl groups (18, 19) in dihydrotestosterone and demonstrates the sensitivity of P450 3A4, even with its large active site, to small changes in substrate structure.
Introduction
P4503 enzymes have long been studied because of their roles in the metabolism of steroids, fat-soluble vitamins, fatty acids, drugs, carcinogens, and other endogenous and xenobiotic chemicals (1–3). The importance of P450s in steroid metabolism is documented by the large number of instances of inherited endocrine diseases now linked to defects in P450 (CYP) genes (4, 5). Roughly one-fourth of the human P450s have important roles in steroid metabolism (6, 7). The effects of small chemical changes in steroids (introduced by P450s and other enzymes) are rather remarkable in terms of the resulting changes in biological activities.
P450 19A1, the steroid aromatase, is a critical enzyme that converts testosterone and androstenedione to estrogens (8). Deficiencies in P450 19A1 present clinical problems, e.g. female virilization (4). However, P450 19A1 is also a drug target in the treatment of estrogen-dependent cancers, e.g. breast and ovary (9). The catalytic mechanism of P450 19A1 involves a three-step reaction, consisting of 19-hydroxylation, oxidation of the 19-hydroxy product to an aldehyde, and the unusual loss of the 19-formyl group as HCO2H accompanying the aromatization of the steroid A ring (10, 11). P450 3A4 is best known as a hepatic/intestinal enzyme and is involved in the metabolism of roughly one-half of drugs (that are metabolized) (12). However, it is also active in the oxidations of many steroids, including cholesterol (13), estradiol (14), progesterone (15), cortisol (16), and testosterone and androstenedione (14, 15). The major reaction observed with testosterone is 6β-hydroxylation (14, 15, 17); hydroxylation at the 2β, 1β, and 15β sites also occurs (17, 18). However, no physiological relevance of any of these hydroxylations has been established. Crystal structures of both P450 19A1 (19) and P450 3A4 (20–23) have been reported, some with bound steroids (19, 21), but the structures have yielded limited rationalization of catalytic selectivity.
Dihydrotestosterone is a more potent androgen than testosterone and is important, particularly in virilization (24). Men with genetic deficiency of steroid 5α-reductase Type 2 show a syndrome termed pseudohermaphroditism (25). The enzymes that reduce testosterone to dihydrotestosterone (5α-steroid reductase, isozymes 1 and 2 (24)) are targets for drugs used to treat prostate hypertrophy, a common problem in older men, as well as some other androgen-dependent conditions (26). The only enzyme reported to be involved in the metabolism of dihydrotestosterone is a 3α-hydroxysteroid dehydrogenase (AKR1C2) that reduces the 3-keto group to an alcohol, which is devoid of androgen activity (27).
We became interested in the possibility of dihydrotestosterone oxidation following a series of mechanistic studies on androgen oxidation by recombinant P450 19A1 (11). Some alternate substrates of P450 19A1 were shown to be oxidized (estrone and 19-nortestosterone), confirming earlier reports. The 2-electron difference in the oxidation states of testosterone and dihydrotestosterone suggested that aromatic (estrogenic) products might not be formed from oxidations of the latter; we identified three unsaturated 19-norsteroids as products, plus a further hydroxylation product of one of these. Human liver microsomes (in the presence of NADPH) also formed oxidation products from dihydrotestosterone, and P450 3A4 was implicated in the production of 18- and 19-hydroxydihydrotestosterone. The latter two hydroxylated products were identified in vivo in human plasma and urine samples.
EXPERIMENTAL PROCEDURES
Enzymes
Human P450 19A1 was heterologously expressed in Escherichia coli and purified as described earlier (11). Recombinant rat NADPH-P450 reductase was also expressed in E. coli and purified as described (28). E. coli membranes expressing human P450 3A4 and human NADPH-P450 reductase were prepared as described previously (29). Microsomes were prepared from human livers as described (30) (samples were originally obtained through the Nashville Regional Organ Procurement Agency, according to Vanderbilt Institutional Review Board procedures).
Plasma and urine samples were obtained from healthy individuals in accord with the Vanderbilt Institutional Review Board policies.
MS and NMR Spectroscopy
HRMS analyses were done with an LTQ-Orbitrap (Thermo) spectrometer in the Vanderbilt facility. Other MS analyses were performed either on Thermo LTQ linear ion-trap or TSQ triple quadrapole instruments. All spectrometers were operated in the positive ESI mode. NMR spectra of reaction products were recorded on a Bruker AV-II-600 instrument, equipped with a cryoprobe, in the Vanderbilt facility or on other Bruker instruments as noted. Spectra were referenced to the residual solvent signals (see above). Two-dimensional spectra (HSQC, HMBC) were acquired using standard Bruker programs.
Chemicals
Steroids were obtained from either Sigma or Steraloids (Wilton, NH). 2,4-DNPH-HCl (Sigma) was recrystallized from C2H5OH before use.
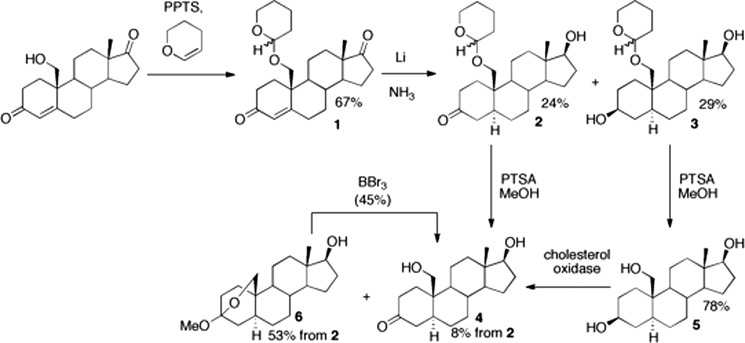
Synthesis of 19-Hydroxydihydrotestosterone (Fig. 1)
FIGURE 1.
Chemical synthesis of 19-hydroxydihydrotestosterone. PPTS, pyridinium p-toluenesulfonate; PTSA, p-toluenesulfonic acid. Yields are shown in the scheme. 19-Hydroxydihydrotestosterone exists in equilibrium with its hemiketal form (31). See “Experimental Procedures” for details.
General
19-Hydroxyandrostenedione was purchased from Waterstone Technologies Ltd. (Carmel, IN). Compounds were monitored by TLC under UV light or staining with CAM reagent (Ce(NH4)2(NO3)6 (0.5%, w/v), ammonium molybdate (12%, w/v), and H2SO4 (20%, v/v) in H2O) for non-UV active compounds (compounds 2, 2a, 3, 4, 5, and 6). Synthetic compounds were characterized by NMR (300 or 400 MHz, CDCl3, or CD3OD solvent). The CDCl3 peak was referenced to δ 7.26 ppm in the 1H NMR spectra and the triplet corresponding to CDCl3 was referenced to 77.16 ppm in the 13C NMR spectra. The methyl peak of CD3OD was referenced to δ 3.49 ppm in the 1H NMR spectra (32).
19-(Tetrahydropyran-2′-yl-oxy)-androst-4-ene-3,17-dione (1 in Fig. 1)
3,4-Dihydro-2H-pyran (0.30 ml, 0.28 g, 2.3 mmol, 8 mol eq) was added to a mixture of 19-hydroxyandrostenedione (95 mg, 0.3 mmol, 1.0 mol eq) and pyridinium p-toluenesulfonate (2.6 mg, 0.1 mmol, 0.3 mol eq) in THF (5 ml). The reaction was stirred at room temperature under an argon atmosphere for 20 h. The reaction was concentrated and purified via preparative TLC (silica gel G, ethyl acetate:hexanes, 1:1, v/v) to furnish a diastereomeric mixture of 19-(tetrahydropyran-2′-yl-oxy)-androst-4-ene-3,17-dione (1, 72 mg, 0.2 mmol, 67%). Rf 0.50 (1:1, ethyl acetate:hexanes, v/v). 1H NMR (400 MHz, CDCl3): δ 5.91 (br s, 1H, H-4), 5.89 (br s, 1H, H-4), 4.63–4.56 (m, 2H), 0.92 (s, 3H, 19-H), 0.91 (s, 3H, 19-H).
19-(Tetrahydropyran-2′-yl-oxy)-5α-androstan-3-one (2)
A modified Birch reduction procedure was based on the work of Oh and Robinson (33). Lithium (0.5 g) was pre-rinsed in hexanes and added to a 3-necked oven-baked round bottom flask equipped with a cold finger, oil bubbler outlet, and stirrer. Ammonia (150 ml) was used to fill the flask (−78 °C). The mixture was stirred for 45 min at −78 °C. A solution of 19-(tetrahydropyran-2′-yl-oxy)-androst-4-ene-3,17-dione (1, 71 mg, 0.18 mmol) in THF (3 ml) was added dropwise. After 10 min, NH4Cl (0.5 g) was added. The reaction was warmed to 23 °C and diluted with ethyl acetate (50 ml) and H2O (20 ml). The organic extract was concentrated in vacuo and purified by flash column chromatography on silica gel (100% hexanes to 50% hexanes/ethyl acetate, v/v) to afford 19-(tetrahydropyran-2′-yl-oxy)-5α-androstan-3,17-diol (3, 21 mg, 53 μmol, 29%) and 17β-hydroxy-19-(tetrahydropyran-2′-yl-oxy)-5α-androstan-3-one (2, 17 mg, 44 μmol, 24%). (Note: if not enough NH4Cl was added to quench the reaction, the 17β-hydroxy group became acetylated upon addition of ethyl acetate to yield 3-oxo-19-(tetrahydropyran-2′-yl-oxy)-5α-androstan-17β-yl acetate 2a, which could be converted back to 2 with stoichiometric potassium carbonate in CH3OH.) Rf of diol 3, 0.22 (1:1, ethyl acetate:hexanes, v/v). 1H NMR of diol 3 (300 MHz, CDCl3): δ 4.63–4.55 (m, 1H), 3.91–3.81 (m, 1H), 2.31–2.19 (m, 1H), 2.10–1.99 (m, 1H), 0.76 (s, 3H), 0.73 (s, 3H). Rf of 3-ketosteroid 2, 0.36 (1:1, ethyl acetate:hexanes, v/v). 1H NMR of 3-ketosteroid 2 (300 MHz, CDCl3): δ 4.64–4.56 (m, 1H), 4.15 (dd, J = 14.4, 10.6 Hz), 0.77 (s, 3H, 19-H), 0.76 (s, 3H, 19-H). Rf of acetate 2a, 0.8 (10:1, CH2Cl2:CH3OH, v/v).
19-Hydroxydihydrotestosterone (19-Hydroxy-5α-androstan-3-one) (4), from 2
p-Toluenesulfonic acid monohydrate (123 mg, 646 μmol) was added to a stirring solution of 17β-hydroxy-19-(tetrahydropyran-2′-yl-oxy)-5α-androstan-3-one (2, 65 mg, 167 μmol) in 4 ml of CH3OH:CH2Cl2 (1:1, v/v), and the reaction was stirred for 10 h at 23 °C. The reaction mixture was washed with saturated NaHCO3 solution and purified by flash column chromatography (silica gel G, 100% hexanes to 50% ethyl acetate:hexanes, v/v) to afford a mixture of 19-hydroxydihydrotestosterone 4 (4 mg, 13 μmol, 8%) and 3,19-epoxy-3-methoxy-5α-androstan-17β-ol 6 (30 mg, 89 μmol, 53%). (Note: formation of 6 from 4 increased with reaction time, and the use of HCl instead of p-toluenesulfonic acid monohydrate formed 6 at a faster rate.) Rf of 19-hydroxydihydrotestosterone 4, 0.45 (3:1, ethyl acetate:hexanes, v/v). Rf of 6, 0.76 (3:1, ethyl acetate:hexanes, v/v).
3,19-Epoxy-3-methoxy-5α-androstan-17β-ol (6) from 2a
HCl (12 m, 50 μl) was added to a solution of 2a (80 mg, 190 μmol) in CH3OH (2 ml) and the reaction was stirred for 4 h. The reaction mixture was purified directly by flash column chromatography to afford 3,19-epoxy-3-methoxy-5α-androstan-17β-ol 6 (35 mg, 100 μmol, 53%).
19-Hydroxydihydrotestosterone (4), from 6
A solution of BBr3 (0.1 m solution in CH2Cl2, 500 μl, 50 μmol) was added to a stirring solution of 3,19-epoxy-3-methoxy-5α-androstan-17β-ol (6, 25 mg, 74 μmol) in CH2Cl2 (5 ml) at −78 °C under an argon atmosphere. After stirring for 5 min, the reaction mixture was poured into a flask containing H2O (20 ml). The reaction mixture was extracted with ethyl acetate (3 × 20 ml), and the organic extracts were concentrated in vacuo. The crude material was purified by flash column chromatography (100% hexanes to 50% hexanes in ethyl acetate to 10% CH3OH:CH2Cl2, v/v) to afford 19-hydroxydihydrotestosterone (10 mg, 33 μmol, 45%), which was further purified on a second column to yield material for 1H NMR spectroscopy (1 mg).
19-Hydroxydihydrotestosterone (4), from 3
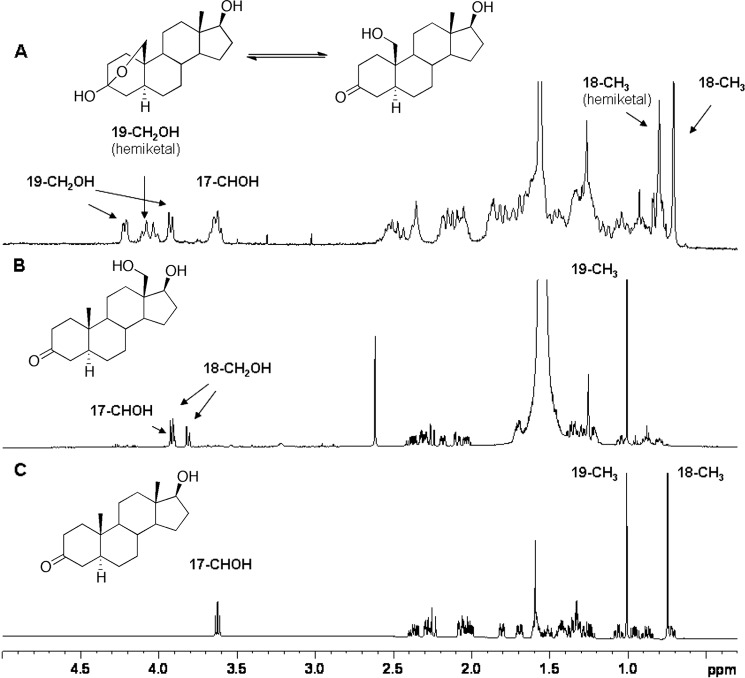
p-Toluenesulfonic acid monohydrate (17 mg, 89 μmol) was added to a solution of tetrahydropyran ether 3 (16 mg, 41 μmol) in 4 ml of CH3OH:CH2Cl2 (1:1, v/v) and the reaction was stirred for 3 h. The reaction mixture was purified directly by flash column chromatography (silica gel G, 100% hexanes to 50% ethyl acetate: hexanes, v/v) to yield the triol 5 (10 mg, 32 μmol, 78%). 1H NMR of triol 5 (300 MHz, CD3OD): δ 4.04 and 3.93 (ABq, 2H, J = 8.7 Hz, 19-H), 3.79–3.66 (m, 2H, 3-H and 17-H), 2.51–2.44 (m, 1H), 2.20–2.09 (m, 1H), 2.03–1.72 (m, 8H), 1.69–1.37 (m, 7H), 1.18–1.05 (m, 3H), 0.96 (s, 3H, 18-H), 0.93–0.82 (m, 1H). The NMR spectrum showed that 4 exists in equilibrium with its 3,19-hemiketal form (Fig. 1), consonant with previous literature (31).
Conversion of Triol 5 to 19-Hydroxydihydrotestosterone (4) using Cholesterol Oxidase
Triol 5 (5 mg), dissolved in CH3OH (50 μl), was incubated with Brevibacterium sp. cholesterol oxidase (Sigma, 25 units) in 50 mm potassium phosphate buffer (pH 7.4) in a total volume of 1 ml (30 °C, shaking at 100 rpm, 3 h). The reaction was terminated by the addition of CH2Cl2 (1 ml) and the organic layer was dried under a stream of N2.
Enzyme Reactions
Dihydrotestosterone-P450 19A1 Titrations
UV-visible spectra were recorded using an Aminco DW2/OLIS spectrophotometer (On-Line Instrument Systems, Bogart, GA). Dihydrotestosterone (final organic solvent concentration ≤3%, v/v) was added to P450 19A1 (2 μm), and duplicate scans were made from 350 to 500 nm and averaged using the manufacturer's software.
Conversion of Estrone to 2-Hydroxyestrone
The reconstituted enzyme system contained 0.4 μm P450 19A1, 0.8 μm NADPH-P450 reductase, and 36 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine (dispersed into vesicles by sonication prior to use, as a 1 mg ml−1 stock solution). Potassium phosphate buffer (100 mm, pH 7.4) and estrone (in CH3OH, final organic solvent content ≤1% (v/v)) were added, and the final incubation volume was 1.0 ml. An NADPH-generating system (30) was added to initiate incubations (in a shaking water bath, 37 °C, 50 rpm shaking, Amerex Instruments Model 903, Lafayette, CA). Reactions were run in duplicate and, after 20 min, quenched with 1.0 ml of CH2Cl2. The layers in the samples were separated by centrifugation (3 × 103 × g, 10 min), and 0.8 ml of each organic layer was removed and dried under N2. HPLC (20-μl injections) was used to separate the compounds (octadecylsilane (C18) column, 6.2 mm × 80 mm, 3 μm, Agilent Technologies, Palo Alto, CA). A Spectra Series UV3000 rapid-scanning UV detector (Thermo Fisher Scientific) (285 nm) was used to quantitate the product 2-hydroxyestrone. The following gradient was used (1.0 ml min−1, solvent A, 90% H2O, 10% CH3OH (v/v); solvent B, 100% CH3OH (v/v)): initial composition was 50% B (v/v) from 0 to 2 min, linear gradient to 100% B from 2 to 4 min, held at 100% B from 4 to 9 min.
Conversion of 19-Norandrostenedione to Estrone
The same incubation conditions were used as for the estrone 2-hydroxylation experiment (see above); 0.20 μm P450 19A1, 0.40 μm NADPH-P450 reductase, and 18 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine were used. Separation was achieved by HPLC (octadecylsilane (C18), 150 mm × 4.6 mm, 5 μm, YMC, Kyoto, Japan), using an isocratic mixture of 40% B (v/v) (1.0 ml min−1, solvent A, 90% H2O, 10% CH3CN (v/v); solvent B, 100% CH3CN (v/v)), with UV detection (A280).
Time Course of the Reaction of Dihydrotestosterone with P450 19A1
The reconstituted enzyme system contained 5 nm P450 19A1, 150 nm NADPH-P450 reductase, and 4.5 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine (dispersed into vesicles by sonication prior to use, as a 1 mg ml−1 stock solution). [1,2,4,5,6,7-3H]Dihydrotestosterone (PerkinElmer Life Sciences) (in CH3OH, ≤1%, v/v) was added (1.0 μm, 0.27 μCi nmol−1), and the sample was incubated at 37 °C, with 50 rpm shaking for 0.5 to 40 min. After quenching reactions with 1.0 ml of CH2Cl2, each sample was mixed with a vortex device and separated by centrifugation (3 × 103 × g, 10 min). The organic layer (0.75 ml) was removed, dried under an N2 stream, and dissolved in CH3OH prior to injection. Radio-HPLC (20-μl injections) was used to separate the products using an octadecylsilane (C18) column (6.2 × 80 mm, 3 μm, Agilent Technologies). The following gradient was used (1 ml min−1; solvent A, 83% H2O, 17% THF (v/v); solvent B, 83% H2O, 17% THF (v/v)): isocratic 20% B from 0 to 11 min, linear gradient to 60% B (v/v) from 11 to 13 min, hold at 60% B (v/v) from 13 to 22 min.
The separations of the P450 19A1 dihydrotestosterone oxidation products were not successful on octadecylsilane HPLC columns without the inclusion of THF. However, THF has undesirable properties in terms of storage and its compatibility with some LC-MS fittings. Accordingly we substituted a phenyl-hexyl column and obtained good resolution with only H2O, CH3CN, and trace HCO2H (see below).
Human Liver Microsomal Incubations with Dihydrotestosterone
Human liver microsomes (mixture of samples from 10 individuals, equal weights combined) (0.5 μm total P450) were incubated with 100 μm dihydrotestosterone (dissolved in CH3OH, ≤1% (v/v)) in 100 mm potassium phosphate buffer (pH 7.4), with an NADPH-generating system (30). After a 20-min incubation at 37 °C, 50 rpm shaking, each reaction was quenched with 1.0 ml of CH2Cl2, mixed with a vortex device, and separated by centrifugation (3 × 103 × g, 10 min). The (lower) organic layer (0.8 ml) was removed and dried under N2. Aliquots were loaded onto a Luna phenyl-hexyl HPLC column (250 × 4.6 mm, 5 μm, Phenomenex) equilibrated with solvent A (95% H2O, 5% CH3CN, 0.1% HCO2H, v/v) and solvent B (5% H2O, 95% CH3CN, 0.1% HCO2H, v/v), using a flow rate of 1.0 ml min−1). The column was maintained at the initial condition of 40% (v/v) solvent B from 0 to 14 min, followed by a linear gradient increasing to 50% (v/v) solvent B over 1 min. This condition was maintained for 10 min and then returned to the initial condition. About 20% of the flow was diverted into a mass spectrometer using a T-shaped sleeve. The mass spectrometer was either a linear ion-trap (LTQ) or an Orbitrap (Thermo), operating in the positive ESI/full-scan mode.
P450 19A1 Incubations with Dihydrotestesterone
The LC-MS conditions of the human liver microsomal study were used for the incubations of P450 19A1 with dihydrotestosterone, except that 1.0 μm P450 19A1, 2.0 μm NADPH-P450 reductase, and 90 μm l-α-1,2-dilauroyl-sn-glycero-3-phosphocholine were mixed prior to the addition of dihydrotestosterone.
Analysis of Human Plasma and Urine Samples
Steroid Extraction from Plasma
Pooled plasma samples (600 μl, 200 μl from each of three healthy individuals) were extracted twice with 5 ml of a mixture of ethyl acetate and hexane (4:1, v/v). The organic phases were combined and dried under a stream of N2.
Steroid Extraction from Urine
Urine (20 ml collected from each male volunteer) was diluted with 80 ml of 0.5 m sodium acetate buffer (pH 5.0). β-Glucuronidase type HP-2 (Helix pomatia, Sigma, 105 units) was added and samples were incubated at 55 °C for 24 h. The urinary steroids were recovered either by liquid extraction (using equal volumes of ethyl acetate) or solid phase extraction (Oasis HLB columns, Waters). In the latter case the columns were loaded, washed with H2O, and eluted with CH3OH. The organic phase from liquid extraction was dried using a rotary evaporator, and the eluates from solid phase extraction columns were dried under a stream of N2. The resulting urinary steroids were derivatized with dansyl chloride or 2,4-DNPH (see below) prior to LC-MS analyses.
Dansylation
The dansylation reactions were performed as previously described (34). Briefly, samples were dissolved in 200 μl of CH2Cl2 containing 2 mg of dansyl chloride, 1 mg of 4,4-dimethylaminopyridine, and 20 μl of triethylamine and incubated at 65 °C for 1 h. The samples were dried under an N2 stream and then dissolved in 50 μl of CH3CN for analysis.
Derivatization with 2,4-DNPH
Each sample was dissolved in 100 μl of 2,4-DNPH-saturated CH3OH containing 0.10 m HCl and incubated at room temperature for 16 h. Samples were then directly injected in LC-MS analysis.
Analysis of Dansylated Plasma and Urinary Steroids
Dansylated products were analyzed by LC-MS with an HPLC system connected to a TSQ Quantum mass spectrometer (Thermo) using a Pursuit UPS octadecylsilane (C18) column (2.1 × 100 mm, 3 μm, Varian, Palo Alto, CA). HPLC conditions were as follows. Solvent A contained 95% H2O, 5% CH3CN, and 0.1% HCO2H (v/v), and solvent B contained 5% H2O, 95% CH3CN, and 0.1% HCO2H(v/v). The column was maintained at an initial condition of 78% (v/v) solvent B for 4 min at a flow rate of 300 μl min−1, followed by a linear gradient increasing to 95% (v/v) solvent B over 1 min. This mixture was maintained for 2 min and then returned to the initial condition over 1 min and maintained until the end of a 10-min run. The column temperature was maintained at 25 °C. The injection volume onto the column was 15 μl. MS analyses were performed in a multiple-reaction monitoring mode (dansylated dihydrotestosterone, m/z 524 → 252, collision energy of 35 V; dansylated hydroxydihydrotestosterone, m/z 540 → 252, collision energy of 35 V). The transitions correspond to the release of the dansyl (sulfonic acid) from the derivative. Standard curves were prepared from synthetic material and utilized for quantitation of 19-hydroxydihydrotestosterone in urine.
Quantitation of Urinary Steroids (2,4-DNPH Derivatives)
2,4-DNPH-derivatized products were analyzed by LC-MS using similar LC conditions as in the analysis of dansylated steroids, except that the initial condition was 72% solvent B (v/v). The volume injected onto the column was 10 μl. MS analyses were performed in a multiple-reaction monitoring mode (2,4-DNPH-dihydrotestosterone, m/z 471 → 242, collision energy of 35 V; 2,4-DNPH-19-hydroxydihydrotestosterone, m/z 487 → 268, collision energy of 35 V). The transitions correspond to the loss of a 2,4-dinitroaniline moiety plus H2O (or two molecules of H2O in the case of hydroxydihydrotestosterone). Standard curves were prepared from synthetic material and utilized for quantitation of dihydrotestosterone in urine.
RESULTS
Oxidation of Alternative Substrates by P450 19A1
A P450 19A1 preparation purified from human placental microsomes has been reported to catalyze estrone 2-hydroxylation (35). We also observed this activity with recombinant human P450 19A1, with a kcat of 0.046 ± 0.003 min−1 and Km of 2.7 ± 0.7 μm (Fig. 2A). The catalytic efficiency (kcat/Km) was ∼2 × 104 m−1 min−1, compared with ∼7.5 × 107 m−1 min−1 for the conversion of androstenedione to estrone (11).
FIGURE 2.
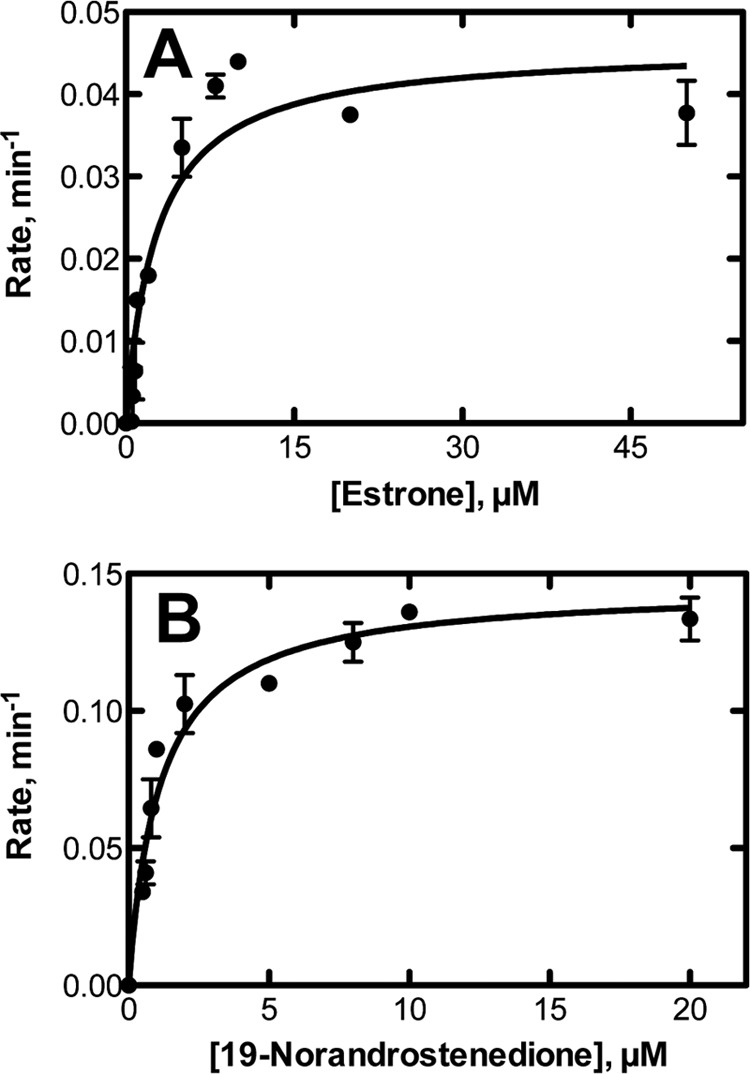
Steady-state kinetics of alternate oxidations catalyzed by P450 19A1. A, 2-hydroxylation of estrone; B, oxidation of 19-norandrostenedione to estrone.
19-Nortestosterone lacks the 19-CH3 group (of testosterone), and Δ1,2-desaturation would yield a product that should rearrange nonenzymatically to estradiol. The oxidation of 19-nortestosterone to estradiol occurred with human recombinant P450 19A1, as reported earlier by Harada (36) with an enzyme purified from placenta, with a kcat of 0.15 (±0.01) min−1 and a Km of 1.1 (± 0.1) μm (Fig. 2B). The catalytic efficiency (kcat/Km) was ∼105 m−1 min−1, or 1/750 of that of conversion of testosterone to estradiol (11).
Binding of Dihydrotestosterone to P450 19A1 and Oxidation
Dihydrotestosterone was found to bind to P450 19A1, as judged by the observed spectral shifts (increase of A392, decrease of A421) indicative of a change in the iron spin-state from low to high spin (11, 37). The apparent Kd was 6.5 (± 0.7) μm (Fig. 3A).
FIGURE 3.
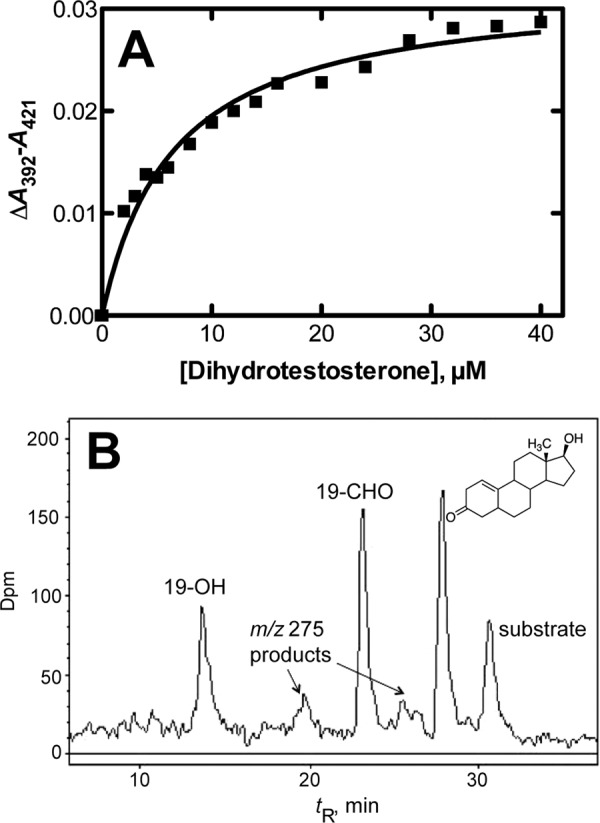
Binding and oxidation of dihydrotestosterone by P450 19A1. A, binding as observed by perturbation of the heme Soret spectra. B, radio-HPLC of oxidation products of dihydrotestosterone formed by P450 19A1. The m/z 275 products, including the Δ1,10 compound whose structure is shown, were identified by MS and NMR (Figs. 4, 5, and 7).
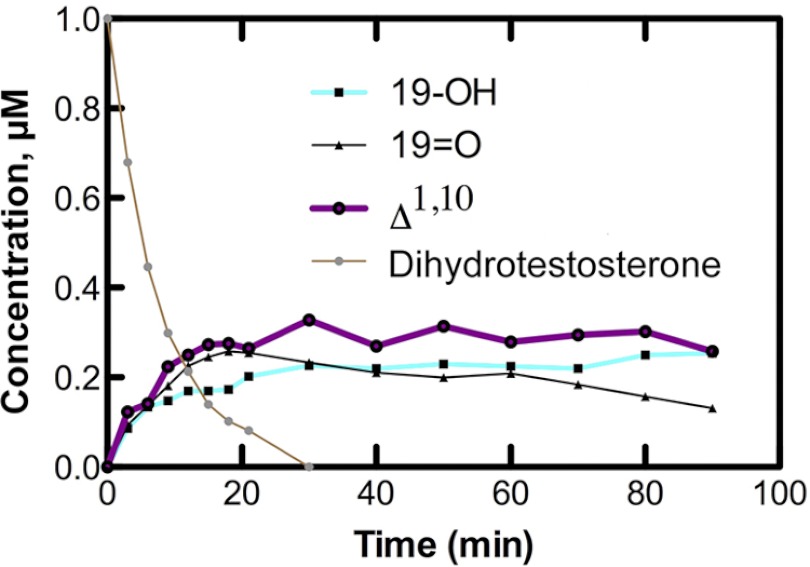
Preliminary incubation experiments with P450 19A1, NADPH-P450 reductase, and NADPH showed the appearance of several products of dihydrotestosterone (Fig. 3B). Subsequent studies (e.g. Fig. 9, see below) showed >95% recovery of the radiolabel from dihydrotestosterone. With a substrate concentration of 1.0 μm and P450 19A1 concentration of 5 nm, dihydrotestosterone disappearance occurred at a rate of ∼7.5 pmol (pmol P450)−1 min−1, suggesting a catalytic efficiency ≥7.5 × 106 m−1 min−1, which is ∼10% of the observed catalytic efficiency of a similar preparation of P450 19A1 with androstenedione as the substrate (11).
FIGURE 9.
Time course of conversion of dihydrotestosterone to products by P450 19A1. The initial concentration of [3H]dihydrotestosterone was 1.0 μm and the P450 19A1 concentration was 0.5 μm (the NADPH-P450 reductase concentration was 1.0 μm).
Identification of Dihydrotestosterone Oxidation Products Formed by P450 19A1
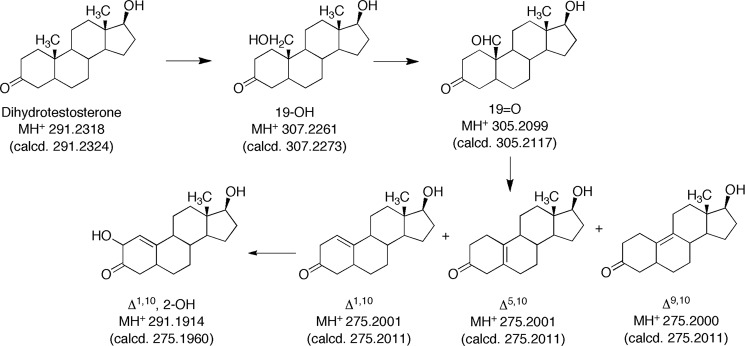
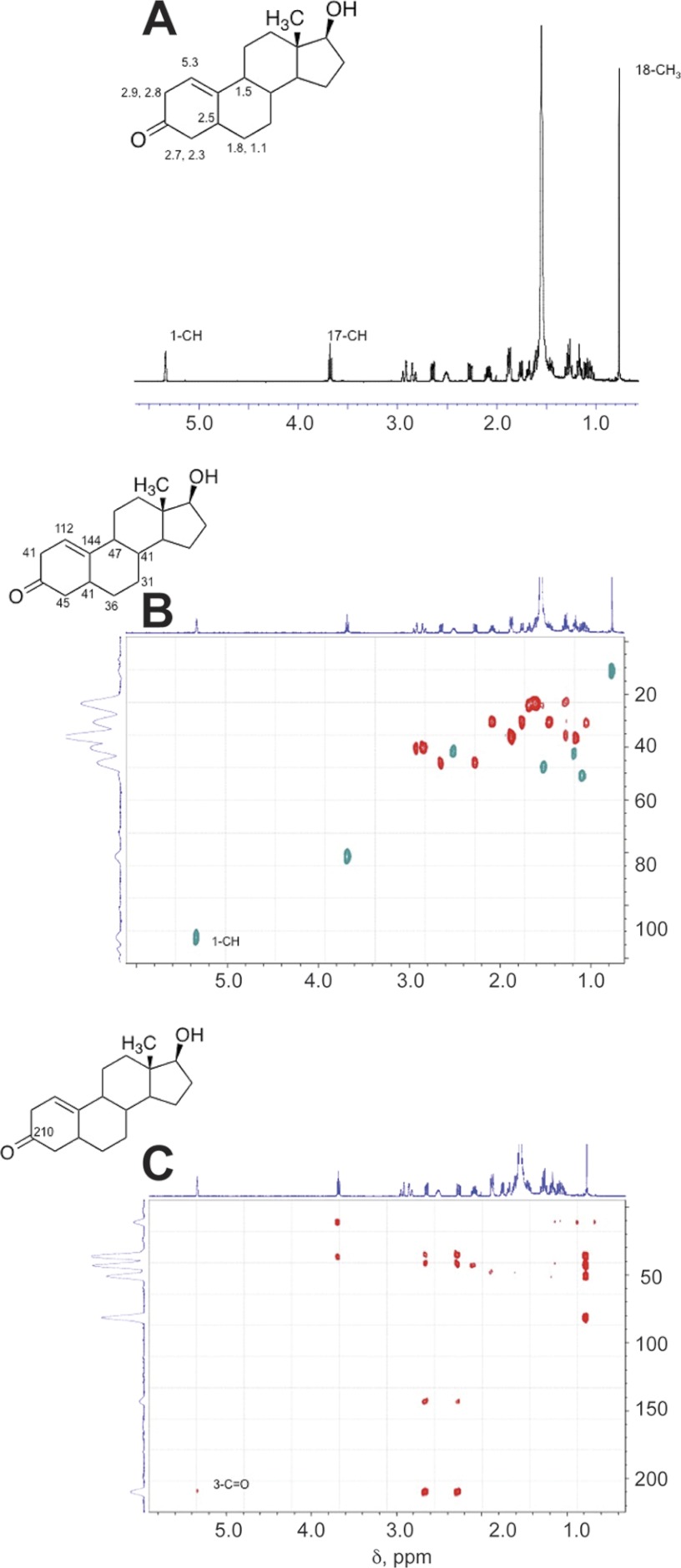
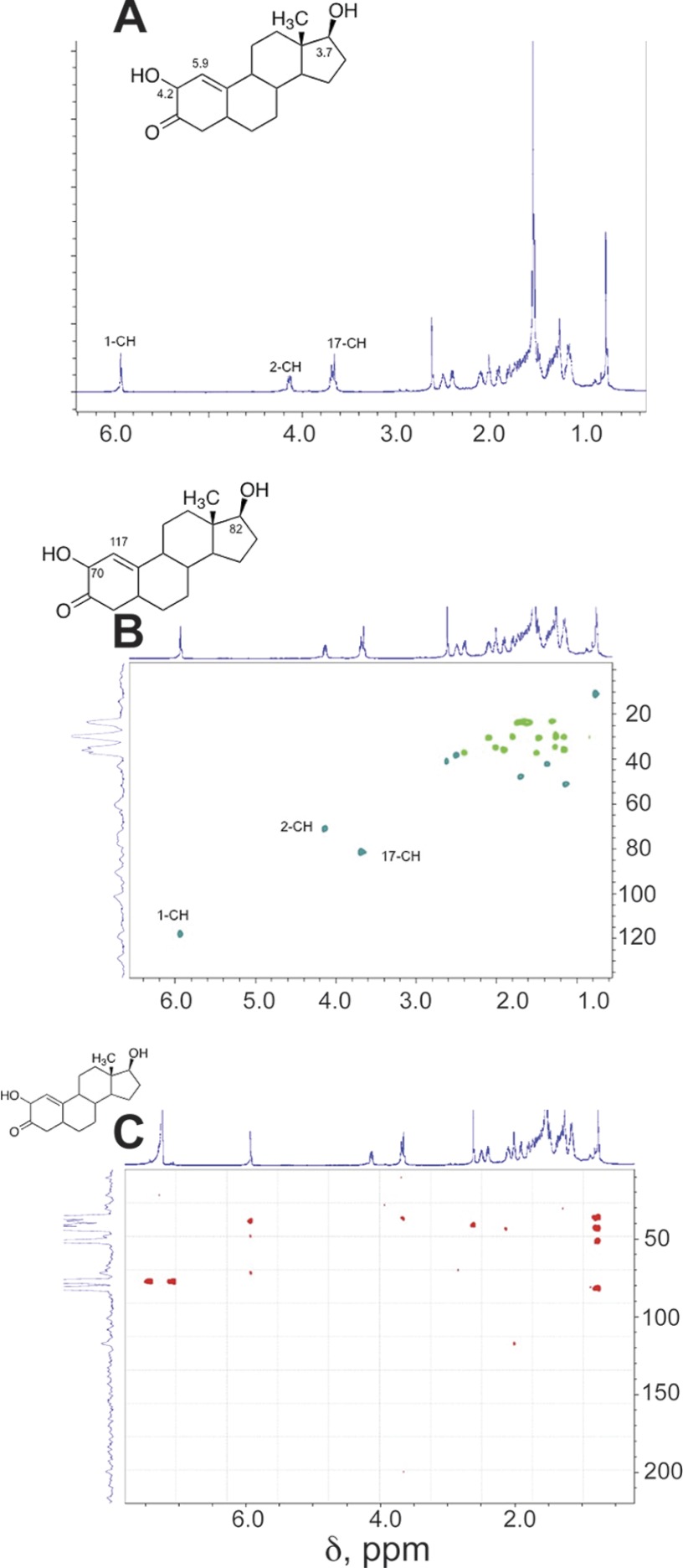
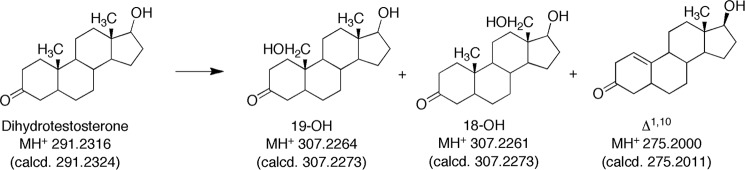
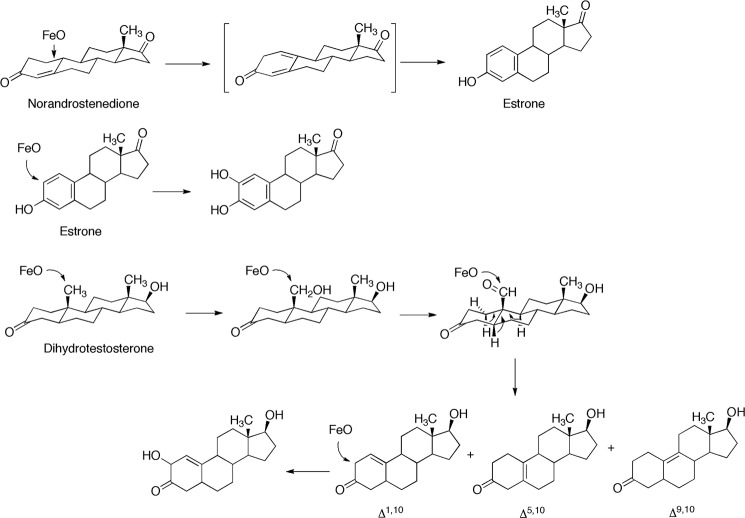
Five products were isolated and identified by MS and NMR. The structures are indicated in Fig. 4 and the NMR spectra are shown in Figs. 5–8.
FIGURE 4.
HRMS of oxidation products of dihydrotestosterone formed by P450 19A1. HRMS parent ions (m/z values) are those determined, with the calculated values (calcd.) are listed below each. All measured values were within 5 ppm of the calculated values.
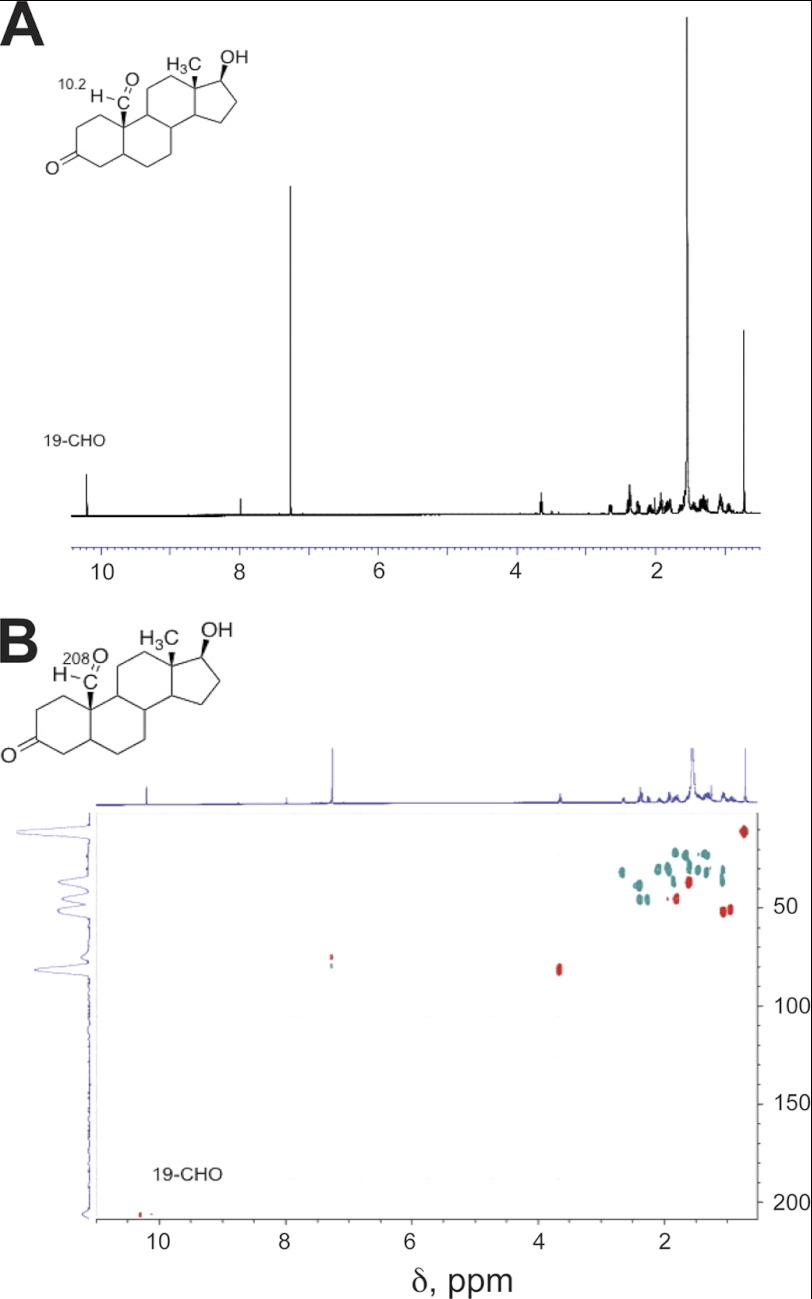
FIGURE 5.
NMR spectra of oxidation products of dihydrotestosterone formed by P450 19A1: dihydrotestosterone 19-aldehyde (see Fig. 3). A, 1H NMR spectrum; B, HSQC spectrum (CDCl3). Key assignments are shown.
FIGURE 6.
NMR spectra of oxidation products of dihydrotestosterone formed by P450 19A1: Δ1,10-dehydro 19-nordihydrotestosterone (see Fig. 3). A, 1H NMR spectrum; B, HSQC spectrum; C, HMBC spectrum (CDCl3). Key assignments are shown.
FIGURE 7.
1H NMR spectra of oxidation products of dihydrotestosterone formed by P450 19A1 (see Fig. 3). A, Δ5,10-dehydro 19-nordihydrotestosterone; B, Δ9,10-dehydro 19-nordihydrotestosterone. The solvent was CDCl3. Key assignments are shown.
FIGURE 8.
NMR spectra of oxidation products of dihydrotestosterone formed by P450 19A1: 2-hydroxy-Δ1,10-dehydro 19-nordihydrotestosterone (see Fig. 3). A, 1H NMR spectrum; B, HSQC spectrum; C, HMBC spectrum (CDCl3). Key assignments are shown.
Initial LC-MS analysis indicated the presence of products with MH+ ions at m/z 307 and 305 and several at m/z 275. Subsequent HRMS yielded molecular formula consistent with the structures indicated in Fig. 4 (all within 5 ppm of calculated). None of the radiolabeled products co-chromatographed with authentic 19-nortestosterone, the Δ4,5-19-norsteroid (Steraloids) (absorbance monitored at 240 nm). We found no evidence for rearrangement of the products following initial isolation, ruling out migration of the olefinic bonds.
19-Hydroxydihydrotestosterone was verified by its 1H NMR spectrum, with a shift of the singlet at δ 1.00 to a doublet (3.92 and 4.21 ppm, data not shown). The peak co-eluted with and had an identical mass spectrum with synthetic 19-hydroxydihydrotestosterone.
The 19-aldehyde product was characterized by HRMS (Fig. 4) and the NMR spectra (Fig. 5). The CHO proton appeared as a singlet at δ 10.2 and, in the HSQC spectrum, was coupled to a carbon peak at 210 ppm, indicative of a carbonyl.
The major peak with MH+ at m/z 275 was assigned the structure Δ1,10-dehydro 19-nordihydrotestosterone (Fig. 4) based on HRMS and the one-dimensional, HSQC, and HMBC NMR spectra (Fig. 6). In particular, the H-1 proton (singlet) appeared at δ 5.3, indicative of a change to an olefin (Fig. 6A). In the HSQC spectrum, that proton is bonded to a carbon at 112 ppm (Fig. 6B). The H-1 proton is linked to the C3 carbonyl at 210 ppm in the HMBC spectrum (Fig. 6C).
Two smaller m/z 275 peaks in the radiochromatogram (Fig. 3B) were characterized, although with less material than the others. Both had HRMS molecular formula of 19-nor products with one degree of unsaturation (Fig. 4). The 1H NMR spectra were used to assign the compounds as the Δ5,10- and Δ9,10-dehydro 19-nor steroids (Fig. 7). Both spectra are devoid of vinyl protons, ruling out several possibilities. In Fig. 7A the H-4 protons are shifted downfield, as would be expected if they were moved into a conjugated system (Δ5,10). In Fig. 7B there is no proton shift characteristic of an olefin, and we assigned this as the Δ9,10-dehydro structure.
2-Hydroxy-Δ5,10-dehydro 19-nordihydrotestosterone (tR ∼ 7 min using the conditions described in Fig. 3B, following prolonged incubation, data not shown for such a chromatogram) had the HRMS formula of a hydroxylated product of one of the dehydro 19-nor products (Fig. 4). The structure was assigned based on the NMR spectra (Fig. 8). Particularly characteristic are the H-1 and H-2 protons (δ 5.9 and 4.2) (Fig. 8A). The H-1 proton is linked to the C-1 carbon (117 ppm) in the HSQC spectrum, and the C-2 carbinol appears at 70 ppm, both linked to the assigned protons (Fig. 8B). The HMBC spectrum (Fig. 8C) shows the coupling to the C-3 carbonyl.
Time Course and Catalytic Efficiencies of Formation of P450 19A1 Dihydrotestosterone Products
P450 19A1 was incubated with a slight excess (2-fold) of dihydrotestosterone, and products were analyzed as a function of time (Fig. 9). The substrate, dihydrotestosterone, was depleted within 30 min. 19-Hydroxydihydrotestosterone increased and did not decrease. The 19-aldehyde (19 = O) product increased and then slowly decreased. The major desaturation product (Δ1,10-dehydro 19-nordihydrotestosterone, Fig. 3B) increased without a noticeable lag.
kcat and Km values were estimated (using a P450 19A1 concentration of 30 nm and a reaction time of 15 min). The exact meaning of these parameters is not clear because of the flux of products (Fig. 9). The following parameters were determined (for the formation of the various products, using dihydrotestosterone as substrate): 19-hydroxydihydrotestosterone, kcat 0.27 min−1, Km 3.8 μm; dihydrotestosterone 19-aldehyde, kcat 0.32 min−1, Km 3.2 μm; Δ1,10-dehydro 19-nordihydrotestosterone, kcat 0.77 min−1, Km 7.6 μm. Thus, the apparent catalytic efficiencies were similar.
Oxidation of Dihydrotestosterone by Human Liver Microsomes
Incubation of [3H]dihydrotestosterone with (a pooled sample of 10) human liver microsomes and NADPH yielded several products (Fig. 10A). The production of the major peaks (except Δ1,10-dehydro 19-nordihydrotestosterone) was strongly inhibited in the presence of 2 μm ketoconazole, a selective inhibitor of P450 3A4 (6, 38). Quinidine (2 μm), sulfaphenazole (2 μm), 4-methylpyrazole (2 μm), nor α-naphthoflavone (2 μm) inhibited product formation (these are, respectively, inhibitors of P450s 2D6, 2C9, 2E1, and 1A2 (6, 38)). Furthermore, E. coli membranes containing recombinant P450 3A4 and NADPH-P450 reductase (29) formed the same major products, except for Δ1,10-dehydro 19-nordihydrotestosterone, in different ratios (Fig. 10B). These results lead to the conclusion that the major catalyst of oxidation is P450 3A4.
FIGURE 10.
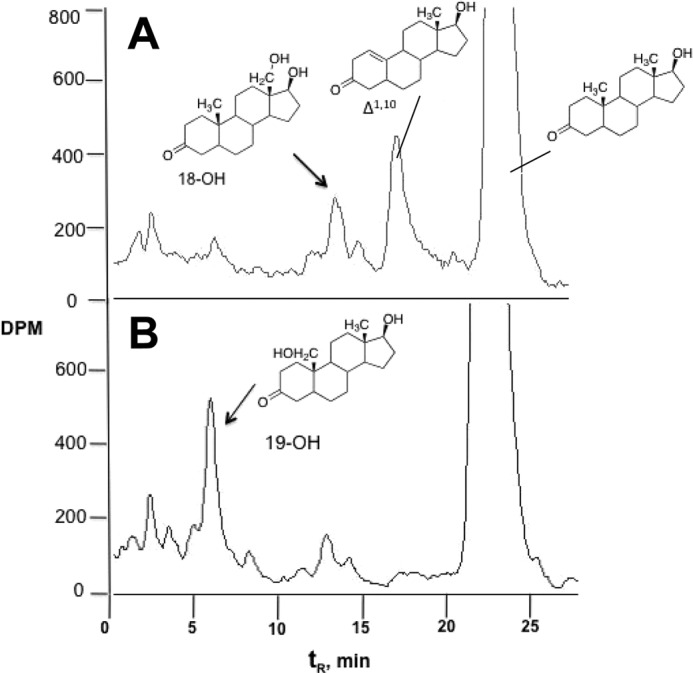
Radio-HPLC traces of [3H]dihydrotestosterone oxidation products. A, human liver microsomes; B, P450 3A4 bicistronic membranes. The reaction time was 60 min in both cases.
Identification of P450 3A4 Oxidation Products
The two major hydroxylated products (Figs. 10B) were isolated from incubations with P450 3A4-containing E. coli membranes and the structures were determined. The Δ1,10-dehydro 19-nordihydrotestosterone product (see above) was also present in liver microsomes (identified by co-chromatography and LC-MS, Figs. 4 and 7) but not the P450 3A4 incubations.
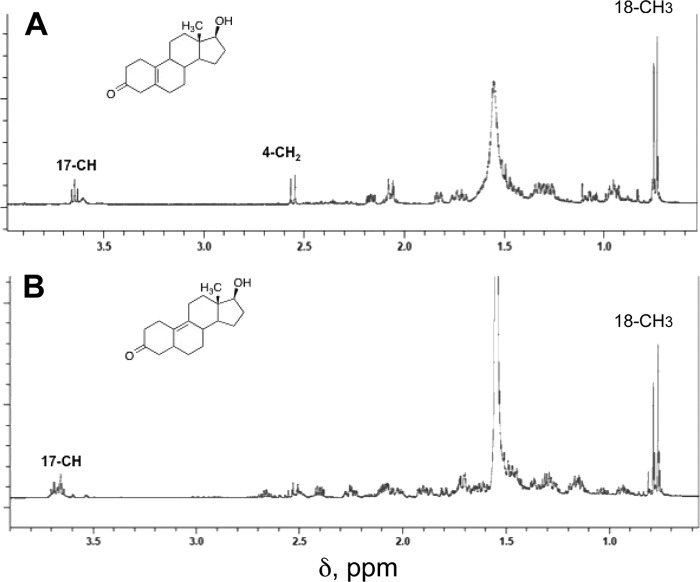
19-Hydroxydihydrotestosterone was identified by co-elution, HRMS, and fragmentation (Figs. 11 and 12) with the product formed by P450 19A1 and the synthetic material (see above). The 1H NMR spectrum was also consistent with the assigned structure (Fig. 13A).
FIGURE 11.
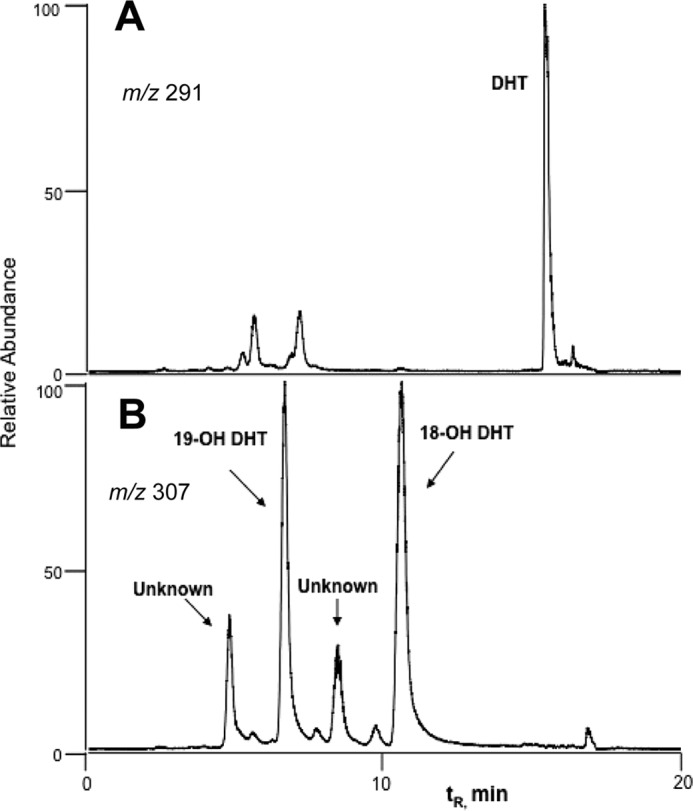
LC-MS of metabolites of dihydrotestosterone formed by P450 3A4. Four hydroxy metabolites (m/z 307) were detected. The two major products were identified as 19-OH dihydrotestosterone (DHT) by co-chromatography and 18-OH DHT by 1H NMR (see Fig. 13), respectively. A, m/z 291; B, m/z 307.
FIGURE 12.
HRMS of oxidation products of dihydrotestosterone formed in human liver microsomes. HRMS parent ions (m/z values) are those determined, with the calculated values (calcd.) listed below each.
FIGURE 13.
One-dimensional 1H NMR spectra of dihydrotestosterone and 18-OH and 19-OH dihydrotestosterone recovered from P450 3A4 incubations. A, 19-hydroxydihydrotestosterone; B, 18-hydroxydihydrotestosterone; C, dihydrotestosterone. The 18-methyl signal of dihydrotestosterone is missing in the 18-OH dihydrotestosterone spectrum and appears (as -CH2OH) at δ 3.82.
18-Hydroxydihydrotestosterone also showed HRMS data consistent with the assigned structure (Fig. 12). The 1H NMR spectrum (Fig. 13B) showed the total loss of the 18-CH3 signal (δ 0.75 ppm) and the appearance of carbinol protons (δ 3.9 ppm) overlapped with the C17 carbinol proton, which had also moved.
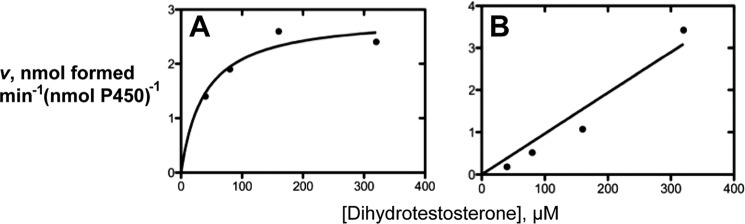
Catalytic Efficiency of Oxidation of Dihydrotestosterone by P450 3A4 and Human Liver Microsomes
Rates of formation of the major oxidation products were measured at varying concentrations of dihydrotestosterone (Fig. 14). With both a recombinant human P450 3A4 system and human liver microsomes, plots of velocity versus substrate concentrations were not very saturable (up to 300 μm dihydrotestosterone). The slopes of these plots provide estimates of catalytic efficiency, i.e. 1.1 × 104 and 7.5 × 103 m−1 min−1 for the formation of 18- and 19-hydroxydihydrotestosterone, respectively, by P450 3A4 and 2400 and 880 m−1 min−1, respectively, for the formation of 18- and 19-hydroxydihydrotestosterone by human liver microsomes (based on total P450). These values are ∼30-fold lower than the catalytic efficiencies of testosterone 6β-hydroxylation reported previously with similar enzyme preparations (3 × 105 m−1 min−1) (17).
FIGURE 14.
Steady-state kinetics of P450 3A4-catalyzed 18- and 19-hydroxylations of dihydrotestosterone. A, 19-hydroxylation by P450 3A4; B, 18-hydroxylation by P450 3A4. The fits shown were done using a hyperbolic fit (A) and linear regression (B) (only slope estimates are considered in the text).
Identification of Dihydrotestosterone Oxidation Products in Vivo
Human plasma and urine samples were analyzed for the presence of the dihydrotestosterone oxidation products. Dansyl (34) and 2,4-DNPH derivatives were analyzed by LC-MS using multiple reaction monitoring mode to achieve appropriate sensitivity.
LC-MS profiles of a sample of urine (from a single individual) and a pooled sample of sera (3 individuals) are shown (Figs. 15 and 16). The identities were established by co-elution with derivatives of the oxidation products identified from the P450 3A4 incubations (18-hydroxy) or synthetic material (19-hydroxy).
FIGURE 15.
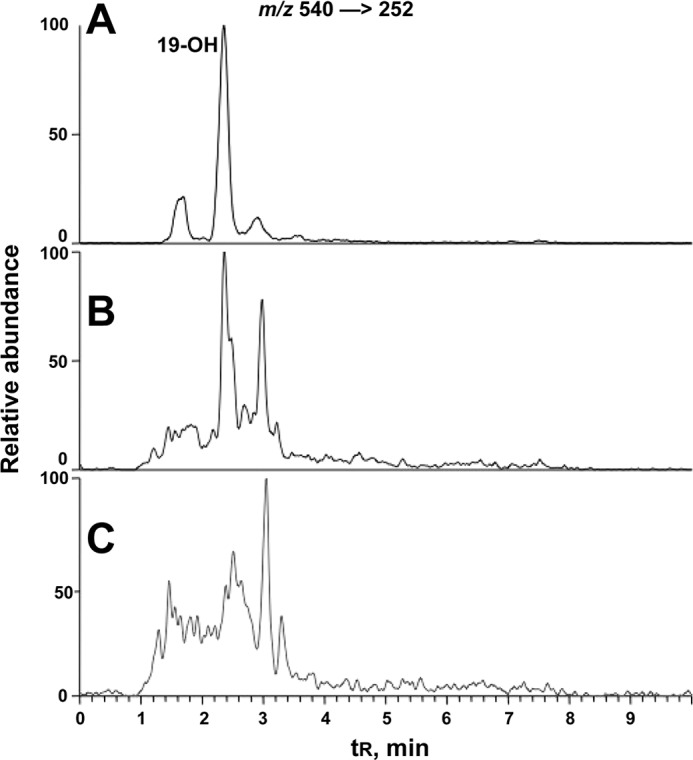
LC-MS of dansylated 19-hydroxydihydrotestosterone in human urine and plasma. The transition m/z 540 [rarrrow] 252 was monitored. A, derivatization of synthetic 19-hydroxydihydrotestosterone led to one major product (identified as a mono-dansyl derivative) and two minor products (unidentified); B, dansylated 19-hydroxydihydrotestosterone in a human urine sample; C, dansylated 19-hydroxydihydrotestosterone in human plasma.
FIGURE 16.
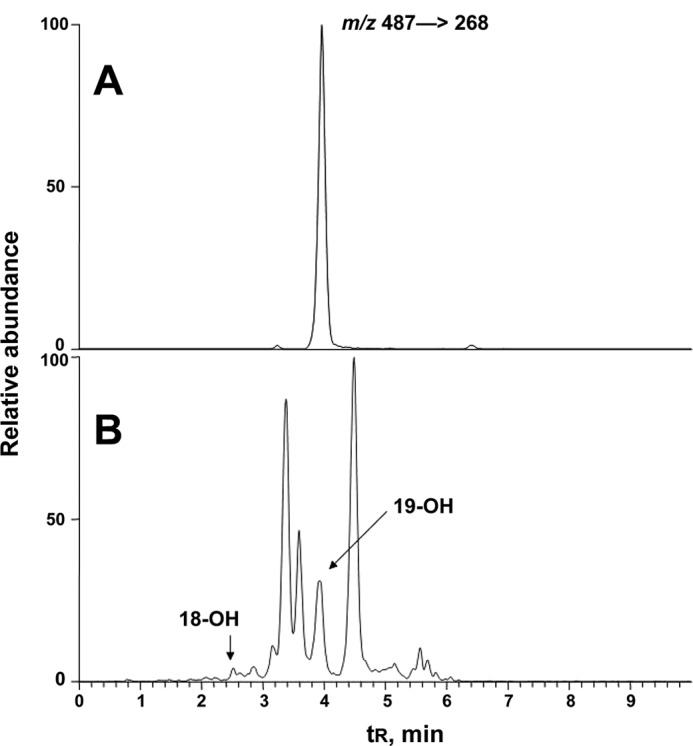
LC-MS of 2,4-DNPH derivatives of 19-hydroxydihydrotestosterone in human urine. The transition m/z 487 → 268 was monitored. A, synthetic 19-hydroxydihydrotestosterone derivatized with 2,4-DNPH; B, a human urine sample extracted and derivatized with 2,4-DNPH.
Concentrations of the oxidation product 19-hydroxydihydrotestosterone could be quantitated, based on the synthetic material and fragmentation of the dansyl group (34), and the values of multiple analyses with several samples of these products were ∼40-fold less than dihydrotestosterone (determined as 2,4-DNPH derivative) in both plasma and urine (Table 1). The range of concentrations was expected to be variable due to variations in individual urinary volumes; the concentrations varied 2-fold. Normalization based on the concentrations of dihydrotestosterone indicated an ∼3-fold variation among the five healthy individuals (Table 1). The concentration of urinary 18-hydroxydihydrotestoterone was much less, but we did not attempt to quantify this in the lack of a standard (other than the material recovered from P450 3A4 incubations, Fig. 13).
TABLE 1.
Concentrations of steroids in human urine samples
| Dihydrotestosterone | 19-Hydroxy-dihydrotestosterone | 19-Hydroxy-dihydrotestosterone/dihydroestosterone | |
|---|---|---|---|
| ng/ml | % | ||
| Subject 1 | 2.2 | 0.13 | 5.9 |
| Subject 2 | 11.2 | 0.23 | 2.0 |
| Subject 3 | 11.2 | 0.20 | 1.8 |
| Subject 4 | 7.6 | 0.22 | 2.8 |
| Subject 5 | 10.9 | 0.29 | 2.6 |
DISCUSSION
Recombinant human P450 19A1 was demonstrated to use dihydrotestosterone as a substrate, with a 3- to 4-step reaction pathway similar to that demonstrated for testosterone and androstenedione (Fig. 4). An interesting feature is the abstraction of any of several hydrogens, 1, 5, and 9, to yield the resulting dehydro 19-norsteroids. Dihydrotestosterone was also oxidized by human liver microsomes to yield one of the dehydro 19-norsteroid products (Δ1,10) observed with P450 19A1. The major products obtained with liver microsomes were 18- and 19-hydroxy dihydrotestosterone, involving the action of P450 3A4. These latter two compounds were also found in vivo.
Estrone 2-hydroxylation and the conversion of 19-norandrostenedione to estrone had previously been reported as reactions of horse (39, 40) and human P450 19A1 preparations (35, 36, 41). We confirmed these reactions with our purified recombinant P450 19A1 (Fig. 2). The apparent catalytic efficiencies were ∼3,000- and 750-fold lower than for the conversion of androstenedione or testosterone to the respective estrogens (11).
Characterization of two dihydrotestosterone oxidation products formed by P450 19A1, the 19-alcohol and 19-aldehyde (Fig. 4), was straightforward in that the mass spectra were expected from the known pathways for testosterone and androstenedione (3, 8), and the NMR data (19-aldehyde, Fig. 5) are supportive. Three desaturated products were characterized (Fig. 4). The Δ1,10-dehydro product was the major one, and the HRMS and two-dimensional NMR data are quite clear regarding its identity. The Δ5,10-dehydro product, obtained in lower yield and not amenable to two-dimensional analysis, was identified by the change in the C-4 protons in the 1H NMR spectrum (Fig. 5), as well as the absence of any vinyl protons (cf. Δ1,10, Fig. 5). Assigning the Δ9,10 structure was more difficult; this product had the same molecular ion/formula (Fig. 4) and was devoid of vinyl protons (Fig. 7B). A Δ8,9-dehydro structure is conceivable (in the absence of more NMR data) but is considered less likely in any mechanistic context.
A time course with a 2-fold molar excess of dihydrotestosterone relative to P450 19A1 showed disappearance of the substrate and immediate formation of the 19-hydroxy, 19-aldehyde, and Δ1,10-dehydro products (Fig. 9). Levels of the 19-hydroxy and Δ1,10-dehydro products did not decrease with time but the 19-aldehyde did. Apparent kcat and Km values were estimated for the conversion of dihydrotestosterone to each of the observed products. However, these values can be misleading for a multistep reaction. A more useful parameter was obtained based on disappearance of dihydrotestosterone (1 μm initial concentration) in the presence of a limiting amount of P450 19A1 (5 nm), yielding a rate of ∼7.5 min−1. A lower limit of 1.2 × 105 m−1 s−1 (i.e. 7.5 × 106 m−1 min−1) can be estimated, which is ∼10-fold greater than the kcat/Km of 104 m−1 s−1 reported for reduction of dihydrotestosterone by 3α-steroid dehydrogenase AKR1C2 (27).
Incubation of dihydrotestosterone with P450 19A1 yielded several additional minor products with molecular ions (m/z 275 for MH+) corresponding to the elemental formula of the dehydro products (Δ1,10-, Δ5,10-, Δ9,10-), observed in LC-MS, but the amounts were too small for further identification (control experiments showed lack of isomerization of the isolated products under these conditions). Also, the Δ1,10-dehydro product was shown to be subsequently hydroxylated at C-2, but the stereochemistry was not established (Figs. 4 and 8).
Incubation of dihydrotestosterone with human liver microsomes yielded the 18- and 19-hydroxy products and the Δ1,10-dehydro product (Fig. 10). The characterization of the two hydroxylated products was straightforward with NMR and, with 19-hydroxydihydrotestosterone, comparison with the synthetic and other biosynthetic material (Figs. 11 and 13). The formation of the 18- and 19-hydroxy products was catalyzed by P450 3A4, as shown by inhibition studies and work with the recombinant enzyme (Fig. 10B). At least two other putative hydroxylated products were formed from dihydrotestosterone by P450 3A4 (Fig. 11B). Formation of the Δ1,10-dehydro product, which presumably proceeds from the 19-hydroxy product, was not attributed to a specific P450. Although we did not address this issue further, our tentative view is that the Δ1,10-dehydro 19-nordihydrotestosterone product (Fig. 10A) may result from P450 19A1. The literature indicates some ambiguity about the expression of P450 19A1, with some studies reporting the absence of P450 19A1 in adult human liver (42). However, in other work the aromatization of androstenedione (43) and the presence of P450 19A1 mRNA (44, 45) have been reported in (noncancerous) adult human liver. However, the role of another, unidentified P450 in the liver cannot be ruled out at this time. The presence of 19-hydroxydihydrotestosterone and the Δ1,10-dehydro 19-norsteroid product are consistent with the involvement of P450 19A1 (Fig. 9). 19-Hydroxydihydrotestosterone was clearly shown to be formed by P450 3A4, however (Figs. 10B and 11B).
Initial in vivo experiments were done with human plasma, and 18- and 19-hydroxydihydrotestosterone were identified in a pooled sample (Fig. 15C). These two hydroxylated steroids were also identified in individual urine samples (Figs. 15B and 16B, Table 1). The extent of variation (normalized for dihydrotestosterone) was 3-fold (Table 1). At this time we cannot define the origin of the 19-hydroxydihydrotestosterone in the urine or plasma. P450 3A4 was much less efficient than P450 19A1 in forming 19-hydroxydihydrotesotosterone (∼103-fold) but the amount of P450 3A4 in human liver (46) is probably much larger than the total amount of P450 19A1 in the body. Two other issues are (i) the tendency of P450 19A1 to further oxidize 19-hydroxydihydrotestosterone (Fig. 9) and (ii) the differential locations of P450 19A1 and P450 3A4 in tissues and the excretion of 19-hydroxydihydrotestosterone from these into plasma and urine. Although the concentration of 19-hydroxydihydrotestosterone in urine was much lower than that of dihydrotestosterone, we do not know if this reflects the ratios in any tissues. To expand on this latter point, P450 19A1 is preferentially expressed in female steroidogenic tissues (e.g. ovaries, placenta) and adipose tissue, and expression in tissues of most relevance to dihydrotestosterone action (e.g. prostate) is very low (44). We do not know the levels of expression of P450 19A1 protein relative to the 3α-reductase AKR1C2, the other enzyme that acts on dihydrotestosterone. Finally (regarding relevance to physiology), the biological activity of the products we have identified here is unknown. We might presume that any of the oxidations would decrease the androgenic activity of dihydrotestosterone. 19-Hydroxylation of testosterone appears to decrease its androgenic activity in rat models, although the ready conversion to estradiol in vivo is a complicating factor (in some models 19-hydroxytestosterone has been used as an estrogen precursor) (47, 48).
The catalytic mechanisms of the oxidations are considered here for P450s 19A1 and 3A4 (Figs. 17 and 18). P450 19A1 can catalyze oxidations at carbons 1, 2, and 19 of these steroids. The oxidation of the 19-formyl group could proceed via an iron peroxy complex (FeOO−) (49, 50) or the “Compound I” FeO3+ species invoked for other P450 reactions (10, 51). Regardless of the oxidizing species, the formation of the characterized dehydro products requires the removal of protons at the 1, 5β, or 9β sites. We presume that the 1β-hydrogen is removed, in the absence of direct evidence, in that H-1β is known to be removed in the usual P450 19A1 aromatase reaction with 3-keto-Δ4,5-dehydro steroids (52). It is not clear if the iron-oxygen complex assists in the proton removal, as shown for P450 N-dealkylation reactions (53). Alternatively base catalysis by amino acid residues might be involved in catalysis (19), or even water-bonded networks to amino acids. The ability of P450 19A1 to remove a variety of β-hydrogens is of interest (Fig. 17).
FIGURE 17.
Some oxidations of steroids catalyzed by P450 19A1. Site of oxidation by P450 FeO complexes are shown. The losses of individual protons of dihydrotestosterone 19-aldehyde are shown with arrows.
FIGURE 18.
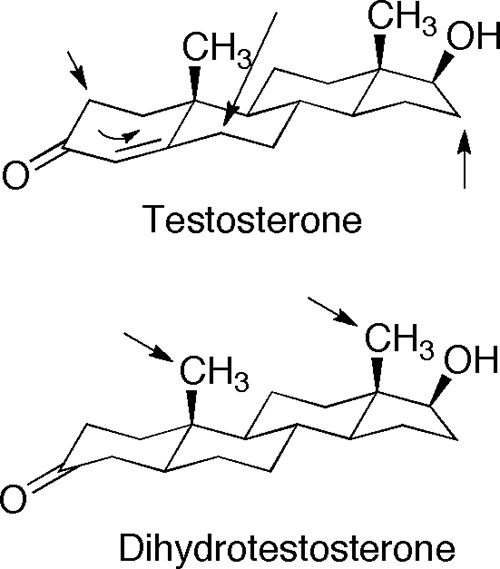
Oxidations of testosterone and dihydrotestosterone catalyzed by P450 3A4. Sites of hydroxylation are shown with arrows. All testosterone hydroxylations occur at the β face (18).
The catalytic selectivity of P450 3A4 in dihydrotestosterone oxidation (18-, 19-hydroxylation) was unexpected, in that the well characterized 6β, 1β, 2β, and 15β hydroxylations of testosterone (14, 18) were not observed (Fig. 18). The only difference between the structures of testosterone and dihydrotestosterone is in the electronics and the pucker of the A ring (Fig. 18). Reduction of the Δ4,5 bond remarkably shifts the sites of oxidation to the attached axial (β) methyl groups (18 and 19), which are chemically more difficult to oxidize than methylenes due to C-H bond strength (54, 55). This specificity was unexpected and argues for caution in relying on predictions of P450 reaction sites.
In conclusion, we report several oxidations of the important androgen dihydrotestosterone. Several interesting reactions occur with the steroid aromatase, P450 19A1. One of these products (Δ1,10-dehydro 19-nordihydrotestosterone, Fig. 4) was also formed in liver microsomes. Two major liver microsomal products, the 18- and 19-alcohols, were also detectable in vivo, in plasma and urine. Because of the variability of P450 3A4 activity among humans (56, 57), variation in the rates of degradation may occur (Table 1) and influence the homeostasis of dihydrotestosterone.
Acknowledgments
We thank M. V. Martin and L. M. Folkmann for preparing NADPH-P450 reductase, D. Stec for assistance with some of the NMR spectroscopy, M. W. Calcutt for assistance with MS, and K. Trisler for assistance in preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R37 CA090426, R01 GM069970, T32 ES007028, and P30 ES000267.
- P450
- cytochrome P450
- 2,4-DNPH
- 2,4-dinitrophenylhydrazine
- ESI
- electrospray ionization
- HMBC
- heteronuclear multiple bond correlated (spectroscopy)
- HRMS
- high-resolution mass spectrometry
- HSQC
- heteronuclear single quantum correlation (spectroscopy)
- THF
- tetrahydrofuran
- dansyl
- 5-dimethylaminonaphthalene-1-sulfonyl
- dihydrotestosterone
- refers to testosterone reduced at the Δ4,5 double bond.
REFERENCES
- 1. Conney A. H. (1967) Pharmacological implications of microsomal enzyme induction. Pharmacol. Rev. 19, 317–366 [PubMed] [Google Scholar]
- 2. Miller J. A. (1970) Carcinogenesis by chemicals. An overview. G. H. A. Clowes Memorial Lecture. Cancer Res. 30, 559–576 [PubMed] [Google Scholar]
- 3. Ortiz de Montellano P. R. (ed) (2005) Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed., Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 4. Nebert D. W., Russell D. W. (2002) Clinical importance of the cytochromes P450. Lancet 360, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 5. Zhao B., Lei L., Kagawa N., Sundaramoorthy M., Banerjee S., Nagy L. D., Guengerich F. P., Waterman M. R. (2012) A three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with binary substrate occupancy reveals locations of disease-associated variants. J. Biol. Chem. 287, 10613–10622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guengerich F. P. (2005) in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R., ed) 3rd Ed., pp. 377–530, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 7. Guengerich F. P., Cheng Q. (2011) Orphans in the human cytochrome P450 family. Approaches to discovering function and relevance to pharmacology. Pharmacol. Rev. 63, 684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryan K. J. (1958) Conversion of androstenedione to estrone by placental microsomes. Biochim. Biophys. Acta 27, 658–659 [DOI] [PubMed] [Google Scholar]
- 9. Brodie A. M. (1993) Aromatase, its inhibitors and their use in breast cancer treatment. Pharmacol. Ther. 60, 501–515 [DOI] [PubMed] [Google Scholar]
- 10. Ortiz de Montellano P. R., De Voss J. J. (2005) in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R., ed) 3rd Ed., pp. 183–245, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 11. Sohl C. D., Guengerich F. P. (2010) Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J. Biol. Chem. 285, 17734–17743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams J. A., Hyland R., Jones B. C., Smith D. A., Hurst S., Goosen T. C., Peterkin V., Koup J. R., Ball S. E. (2004) Drug-drug interactions for UDP-glucuronosyltransferase substrates. A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 32, 1201–1208 [DOI] [PubMed] [Google Scholar]
- 13. Bodin K., Andersson U., Rystedt E., Ellis E., Norlin M., Pikuleva I., Eggertsen G., Björkhem I., Diczfalusy U. (2002) Metabolism of 4β-hydroxycholesterol in humans. J. Biol. Chem. 277, 31534–31540 [DOI] [PubMed] [Google Scholar]
- 14. Guengerich F. P., Martin M. V., Beaune P. H., Kremers P., Wolff T., Waxman D. J. (1986) Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J. Biol. Chem. 261, 5051–5060 [PubMed] [Google Scholar]
- 15. Waxman D. J., Attisano C., Guengerich F. P., Lapenson D. P. (1988) Cytochrome P-450 steroid hormone metabolism catalyzed by human liver microsomes. Arch. Biochem. Biophys. 263, 424–436 [DOI] [PubMed] [Google Scholar]
- 16. Ged C., Rouillon J. M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. (1989) The increase in urinary excretion of 6β-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br. J. Clin. Pharmacol. 28, 373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krauser J. A., Guengerich F. P. (2005) Cytochrome P450 3A4-catalyzed testosterone 6β-hydroxylation. Stereochemistry, kinetic deuterium isotope effects, and rate-limiting steps. J. Biol. Chem. 280, 19496–19506 [DOI] [PubMed] [Google Scholar]
- 18. Krauser J. A., Voehler M., Tseng L. H., Schefer A. B., Godejohann M., Guengerich F. P. (2004) 1β-Hydroxylation of testosterone by human cytochrome P450 3A4. Eur. J. Biochem. 271, 3962–3969 [DOI] [PubMed] [Google Scholar]
- 19. Ghosh D., Griswold J., Erman M., Pangborn W. (2009) Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457, 219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yano J. K., Wester M. R., Schoch G. A., Griffin K. J., Stout C. D., Johnson E. F. (2004) The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-Å resolution. J. Biol. Chem. 279, 38091–38094 [DOI] [PubMed] [Google Scholar]
- 21. Williams P. A., Cosme J., Vinkovic D. M., Ward A., Angove H. C., Day P. J., Vonrhein C., Tickle I. J., Jhoti H. (2004) Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 305, 683–686 [DOI] [PubMed] [Google Scholar]
- 22. Ekroos M., Sjögren T. (2006) Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl. Acad. Sci. U.S.A. 103, 13682–13687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sevrioukova I. F., Poulos T. L. (2012) Structural and mechanistic insights into the interaction of cytochrome P450 3A4 with bromoergocryptine, a type I ligand. J. Biol. Chem. 287, 3510–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Russell D. W., Wilson J. D. (1994) Steroid 5α-reductase. Two genes/two enzymes. Annu. Rev. Biochem. 63, 25–61 [DOI] [PubMed] [Google Scholar]
- 25. Andersson S., Berman D. M., Jenkins E. P., Russell D. W. (1991) Deletion of steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature 354, 159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frye S. V. (2006) Discovery and clinical development of dutasteride, a potent dual 5α-reductase inhibitor. Curr. Top. Med. Chem. 6, 405–421 [DOI] [PubMed] [Google Scholar]
- 27. Jin Y., Penning T. M. (2006) Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase. Implications for the elimination of androgens. Biochemistry 45, 13054–13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanna I. H., Teiber J. F., Kokones K. L., Hollenberg P. F. (1998) Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch. Biochem. Biophys. 350, 324–332 [DOI] [PubMed] [Google Scholar]
- 29. Parikh A., Gillam E. M., Guengerich F. P. (1997) Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat. Biotechnol. 15, 784–788 [DOI] [PubMed] [Google Scholar]
- 30. Guengerich F. P., Bartleson C. J. (2007) in Principles and Methods of Toxicology (Hayes A. W., ed) 5th Ed., pp. 1981–2048, CRC Press, Boca Raton, FL [Google Scholar]
- 31. Knox L. H., Blossey E., Carpio H., Cervantes L., Crabbé P., Velarde E., Edwards J. A. (1965) Steroids. CCLXXVIII. Reductions of 19-substituted androst-4-en-3-ones and related compunds. J. Org. Chem. 30, 2198–2205 [Google Scholar]
- 32. Gottlieb H. E., Kotlyar V., Nudelman A. (1997) NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 62, 7512–7515 [DOI] [PubMed] [Google Scholar]
- 33. Oh S. S., Robinson C. H. (1994) Synthesis of and chemical model reaction studies with 3-deoxyandrogens. Evidence supporting a 2,3-enolization hypothesis in human placental aromatase catalyasis. J. Chem. Soc. Perkin Trans. 1, 2237–2243 [Google Scholar]
- 34. Tang Z., Guengerich F. P. (2010) Dansylation of unactivated alcohols for improved mass spectral sensitivity and application to analysis of cytochrome P450 oxidation products in tissue extracts. Anal. Chem. 82, 7706–7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osawa Y., Higashiyama T., Shimizu Y., Yarborough C. (1993) Multiple functions of aromatase and the active site structure. Aromatase is the placental estrogen 2-hydroxylase. J. Steroid Biochem. Mol. Biol. 44, 469–480 [DOI] [PubMed] [Google Scholar]
- 36. Harada N. (1988) Novel properties of human placental aromatase as cytochrome P-450. Purification and characterization of a unique form of aromatase. J. Biochem. 103, 106–113 [DOI] [PubMed] [Google Scholar]
- 37. Schenkman J. B., Remmer H., Estabrook R. W. (1967) Spectral studies of drug interaction with hepatic microsomal cytochrome P-450. Mol. Pharmacol. 3, 113–123 [PubMed] [Google Scholar]
- 38. Correia M. A. (2005) in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R., ed) 3rd Ed., pp. 247–322, Kluwer Academic/Plenum Press, New York [Google Scholar]
- 39. Almadhidi J., Moslemi S., Drosdowsky M. A., Séralini G. E. (1996) Equine cytochrome P450 aromatase exhibits an estrogen 2-hydroxylase activity in vitro. J. Steroid Biochem. Mol. Biol. 59, 55–61 [DOI] [PubMed] [Google Scholar]
- 40. Gaillard J. L., Silberzahn P. (1987) Aromatization of 19-norandrogens by equine testicular microsomes. J. Biol. Chem. 262, 5717–5722 [PubMed] [Google Scholar]
- 41. Okubo K., Jinbo M., Toma Y., Shimizu Y., Yanaihara T. (1996) Aromatase and estrogen 2-hydroxylase activities of human placental microsomes in pregnancy-induced hypertension. Endocr. J. 43, 363–368 [DOI] [PubMed] [Google Scholar]
- 42. Carruba G. (2009) Aromatase in nontumoral and malignant human liver tissues and cells. Ann. N.Y. Acad. Sci. 1155, 187–193 [DOI] [PubMed] [Google Scholar]
- 43. Smuk M., Schwers J. (1977) Aromatization of androstenedione by human adult liver in vitro. J. Clin. Endocrinol. Metab. 45, 1009–1012 [DOI] [PubMed] [Google Scholar]
- 44. Harada N., Utsumi T., Takagi Y. (1993) Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 90, 11312–11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harada N., Ota H., Yoshimura N., Katsuyama T., Takagi Y. (1998) Localized aberrant expression of cytochrome P450 aromatase in primary and metastatic malignant tumors of human liver. J. Clin. Endocrinol. Metab. 83, 697–702 [DOI] [PubMed] [Google Scholar]
- 46. Guengerich F. P. (1990) Mechanism-based inactivation of human liver cytochrome P-450 IIIA4 by gestodene. Chem. Res. Toxicol. 3, 363–371 [DOI] [PubMed] [Google Scholar]
- 47. Hamburger-Bar R., Rigter H. (1977) Peripheral and central androgenic stimulation of sexual behavior of castrated male rats. Acta Endocrinol. 84, 813–828 [DOI] [PubMed] [Google Scholar]
- 48. Leschber G., Nishino Y., Neumann F. (1989) Influence of an aromatase inhibitor (4-acetoxy-4-androstene-3,17-dione) on experimentally induced impairment of spermatogenesis in immature rats. Andrologia 21, 529–534 [PubMed] [Google Scholar]
- 49. Akhtar M., Calder M. R., Corina D. L., Wright J. N. (1982) Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem. J. 201, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cole P. A., Robinson C. H. (1988) A peroxide model reaction for placental aromatase. J. Am. Chem. Soc. 110, 1284–1285 [Google Scholar]
- 51. Hackett J. C., Brueggemeier R. W., Hadad C. M. (2005) The final catalytic step of cytochrome P450 aromatase. A density functional theory study. J. Am. Chem. Soc. 127, 5224–5237 [DOI] [PubMed] [Google Scholar]
- 52. Townsley J. D., Brodie H. J. (1968) Studies on the mechanism of estrogen biosynthesis. 3. The stereochemistry of aromatization of C19 and C18 steroids. Biochemistry 7, 33–40 [DOI] [PubMed] [Google Scholar]
- 53. Okazaki O., Guengerich F. P. (1993) Evidence for specific base catalysis in N-dealkylation reactions catalyzed by cytochrome P450 and chloroperoxidase. Differences in rates of deprotonation of aminium radicals as an explanation for high kinetic hydrogen isotope effects observed with peroxidases. J. Biol. Chem. 268, 1546–1552 [PubMed] [Google Scholar]
- 54. Kerr J. A. (1966) Bond dissociation energies by kinetic methods. Chem. Rev. 66, 465–500 [Google Scholar]
- 55. Carey F. A., Sundberg R. J. (1990) Advanced Organic Chemistry, 3rd Ed., Part A, p. 12, Plenum Press, New York [Google Scholar]
- 56. Guengerich F. P. (1999) Human cytochrome P-450 3A4. Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 39, 1–17 [DOI] [PubMed] [Google Scholar]
- 57. Yang X., Zhang B., Molony C., Chudin E., Hao K., Zhu J., Gaedigk A., Suver C., Zhong H., Leeder J. S., Guengerich F. P., Strom S. C., Schuetz E., Rushmore T. H., Ulrich R. G., Slatter J. G., Schadt E. E., Kasarskis A., Lum P. Y. (2010) Genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res. 20, 1020–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]