Background: In heart, the A-kinase anchoring protein (AKAP) Yotiao anchors the KCNQ1 subunit of the slow outward potassium ion current (IKs).
Results: Adenylyl cyclase type 9 (AC9) is associated with the IKs complex and sensitizes PKA phosphorylation of KCNQ1 by isoproterenol.
Conclusion: Yotiao facilitates complex formation between AC9 and IKs in heart.
Significance: Sympathetic regulation of IKs is important for repolarization in heart.
Keywords: Adenylate Cyclase (Adenylyl Cyclase), cAMP, Heart, Potassium Channels, Protein Kinase A (PKA), A-kinase Anchoring Protein (AKAP), KCNQ1, Yotiao
Abstract
The scaffolding protein Yotiao is a member of a large family of protein A-kinase anchoring proteins with important roles in the organization of spatial and temporal signaling. In heart, Yotiao directly associates with the slow outward potassium ion current (IKs) and recruits both PKA and PP1 to regulate IKs phosphorylation and gating. Human mutations that disrupt IKs-Yotiao interaction result in reduced PKA-dependent phosphorylation of the IKs subunit KCNQ1 and inhibition of sympathetic stimulation of IKs, which can give rise to long-QT syndrome. We have previously identified a subset of adenylyl cyclase (AC) isoforms that interact with Yotiao, including AC1–3 and AC9, but surprisingly, this group did not include the major cardiac isoforms AC5 and AC6. We now show that either AC2 or AC9 can associate with KCNQ1 in a complex mediated by Yotiao. In transgenic mouse heart expressing KCNQ1-KCNE1, AC activity was specifically associated with the IKs-Yotiao complex and could be disrupted by addition of the AC9 N terminus. A survey of all AC isoforms by RT-PCR indicated expression of AC4–6 and AC9 in adult mouse cardiac myocytes. Of these, the only Yotiao-interacting isoform was AC9. Furthermore, the endogenous IKs-Yotiao complex from guinea pig also contained AC9. Finally, AC9 association with the KCNQ1-Yotiao complex sensitized PKA phosphorylation of KCNQ1 to β-adrenergic stimulation. Thus, in heart, Yotiao brings together PKA, PP1, PDE4D3, AC9, and the IKs channel to achieve localized temporal regulation of β-adrenergic stimulation.
Introduction
The slow delayed rectifier current (IKs)2 is critical for the late phase repolarization of the cardiac action potential. This outward potassium channel is composed of four KCNQ1 α-subunits assembled with accessory KCNE1 β-subunits (1, 2). In humans, the sympathetic regulation of the cardiac action potential requires PKA-mediated phosphorylation of the KCNQ1 subunit of the IKs channel (3, 4). Phosphorylated KCNQ1 increases IKs current and shortening of the action potential duration that occurs to allow sufficient diastolic intervals in the face of increased heart rate. To facilitate PKA phosphorylation, KCNQ1 must assemble with the A-kinase anchoring protein (AKAP) Yotiao (5). Yotiao is the smallest (250 kDa) splicing variant of AKAP9. Mutations in KCNQ1 or Yotiao that disrupt this complex give rise to variants 1 and 11 of the long-QT syndrome (LQT1 and LQT11), one of the potentially lethal heritable arrhythmia syndromes (6). For example, both the inherited S1570L mutation in Yotiao and the G589D mutation in KCNQ1 that occur in LQT1 and LQT11 patients reduce the interaction between KCNQ1 and Yotiao (5, 7). This eliminates cAMP-induced phosphorylation of the channel and the functional response of the IKs channel to β-adrenergic stimulation.
AKAPs often bring together opposing regulatory molecules such as kinases and phosphatases to set up localized temporal regulation of signal transduction pathways. Yotiao is no exception and recruits PKA and PP1 to the IKs complex to modulate the PKA phosphorylation state of a single serine residue (Ser-27) located in the KCNQ1 N terminus (5, 8, 9). Yotiao itself is also the target of PKA phosphorylation on Ser-43 to further enhance regulation by β-adrenergic receptor stimulation (10, 11). The local levels of cAMP around the channel are tightly regulated by anchoring phosphodiesterase (PDE4D3) to the IKs-Yotiao complex (12). In myocytes, PDE4D3 regulates basal IKs activity but likely also plays a role in the temporal kinetics upon sympathetic activation as well.
We have previously shown that Yotiao can also associate with adenylyl cyclase (AC) isoforms 1, 2, 3, and 9, but surprisingly not with the main cardiac isoforms AC5 and AC6 (13). Thus, it was unclear whether direct anchoring of AC to Yotiao occurred in heart to regulate IKs. We now show that AC9 is part of the IKs complex in heart. Although either AC2 or AC9 can form complexes with Yotiao and KCNQ1 in HEK293 cells, only AC9 is expressed in adult cardiac myocytes. In addition, activity assays and Western blotting demonstrated AC9 association with KCNQ1 in transgenic mouse hearts and endogenous IKs complexes in guinea pig. Finally, expression of Yotiao and AC9 with KCNQ1 enhanced the β-adrenergic stimulation of KCNQ1 phosphorylation. Thus, Yotiao sets up several feedback loops by bringing together cAMP synthesis and degradation along with PKA phosphorylation and PP1 dephosphorylation for tight control of IKs function.
EXPERIMENTAL PROCEDURES
Plasmids
Myc-tagged Yotiao, Myc-tagged fragments of Yotiao, AC isoform expression vectors in pcDNA3, and the fusion of human KCNQ1-human KCNE1 in pcDNA3 were described previously (13–16). GST fusions to AC N termini were created by PCR and included residues 1–61 of bovine AC1, residues 1–43 of rat AC2, residues 1–77 of rat AC3, and residues 1–116 of human AC9 (13, 14). GST-AC2-NT was a generous gift from Dr. Nathan Dascal (Tel Aviv University, Tel Aviv, Israel). The N termini for AC1, AC2, and AC9 were cloned into pGEX-4T (GE Healthcare) using the restriction enzymes BamHI and NotI; AC3-NT was cloned into pGEX-CS using NcoI and EcoRI.
Antibodies
The antibodies used were rabbit anti-PKAR2β (1:1000 dilution; BD Transduction Laboratories), goat anti-KCNQ1 (Santa Cruz Biotechnology), goat anti-AC9 (Santa Cruz Biotechnology), mouse anti-c-Myc (9E10, National Cell Culture Center), and mouse anti-GST (Santa Cruz Biotechnology). Rabbit anti-Yotiao and anti-phospho-Ser-27 KCNQ1 antibodies were generated as described previously (13, 17).
Protein Purification
Gαs-His6 was expressed in Escherichia coli, purified, and activated with [35S]GTPγS (18). GST-tagged proteins were purified on glutathione resin (Amersham Biosciences) and dialyzed into buffer containing 50 mm HEPES (pH 8.0), 100 mm NaCl, 5% glycerol, 2 mm DTT, and 1 mm EDTA as described previously (19).
Cell Culture and Transfections
HEK293 cells and CHO cells were maintained in DMEM or Ham's F-12 medium, respectively, and supplemented with 10% (v/v) fetal bovine serum and 50 μg/ml penicillin and streptomycin. CHO cells stably expressing KCNQ1 were maintained in Ham's F-12 medium containing hygromycin B (500 μg/ml) with 10% FBS (7). For transient expression of proteins, HEK293 cells were seeded at 5 × 106 cells/10-cm culture dish and grown for 1 day prior to transfection with Lipofectamine 2000 (Invitrogen) and analyzed 40–44 h later. Plasmid DNA (total of 10 μg) used to transfect cells in 10-cm dishes included Yotiao (5 μg), AC2 or AC9 (2.5 μg), and pcDNA3-KCNQ1-KCNE1 (2.5 μg). CHO cells were seeded at 4 × 106 cells/10-cm culture dish and grown for 24 h prior to transfection with Lipofectamine 2000 and harvested after 30–36 h.
GST Pulldown Assays
GST pulldown assays were as described previously (13). Briefly, lysates from transfected HEK293 cells (500 μg) were incubated with GST, GST-AC1-NT, GST-AC2-NT, GST-AC3-NT, or GST-AC9-NT (50 μg) for 30 min at 4 °C. Glutathione-agarose (30 μl of resin) was added to each sample for 2 h. Samples were washed three times with wash buffer and analyzed by Western blotting.
Immunoprecipitation-AC (IP-AC) Assay
Assays were performed essentially as described (13, 20, 21). To perform the IP-AC assay, transfected HEK293 or CHO cells were rinsed with PBS and then lysed with buffer (50 mm HEPES (pH 7.4), 1 mm EDTA, 1 mm MgCl2, 150 mm NaCl, 0.5% C12E9, and protease inhibitors) and homogenized using a 23-gauge syringe. For competition experiments, competing proteins were added during homogenization. Cellular debris was removed by centrifugation, and a 30-μl aliquot was saved to measure AC activity in the starting material. Samples were rotated at 4 °C for 1 h with the appropriate antibody, followed by an additional 1 h with 30 μl of protein A-Sepharose. Samples were washed two times with lysis buffer containing 0.05% C12E9, resuspended in buffer lacking NaCl with 0.04% C12E9, and analyzed for AC activity. The indicated stimulators and assay components (22) were added to the immunoprecipitate as described previously, and cAMP was detected by enzyme immunoassay (Assay Designs) or using [γ-32P]ATP.
Preparation of Heart Extracts
Frozen adult transgenic mouse hearts or guinea pig hearts were thawed in PBS with protease inhibitors as described (21). The hearts were rinsed, quartered, and homogenized with a Polytron homogenized in lysis buffer lacking detergent. For competition experiments, GST fusion proteins were added to this homogenization step. After addition of 1.0% Triton X-100, the extracts were further homogenized with a Dounce homogenizer, followed by centrifugation at 14,000 rpm to remove the insoluble fraction. The extracts were used immediately for experiments.
Primary Adult Cardiomyocyte Isolation
Adult mouse cardiomyocytes were isolated as described (23). Briefly, hearts were excised from 2-month-old C57BL mice and placed in ice-cold saline, and the aortas were cannulated. Hearts were perfused with warm perfusion buffer for several minutes, followed by perfusion with digestion buffer containing collagenase. Once digested, tissue was minced and concentrated by centrifugation. After calcium reintroduction, cardiomyocytes were plated and cultured with 2% carbon dioxide for 4–6 h.
AC Isoform Expression Analysis
Total RNA was prepared from adult mouse cardiomyocytes using an RNeasy kit (Qiagen) and quantitated using the NanoDrop® 1000 spectrophotometer. First-strand cDNA was generated from DNase-treated total RNA using Moloney murine leukemia virus reverse transcriptase (New England Biolabs) and oligo(dT) primers. Specific AC cDNAs were amplified by semiquantitative PCR using Taq polymerase (Promega) and forward and reverse PCR primers, previously characterized for mouse AC isoforms (24). Amplified PCR products were run on 1.5% agarose gels from three to four reaction cycles to ensure linearity.
Statistical Analysis
Data were analyzed by analysis of variance with multiple comparisons using the Bonferroni t test or between two groups using Student's t test from an average of at least three experiments, each performed in duplicate or triplicate. Experiments were considered significant for p values <0.05.
RESULTS
There is significant evidence for cAMP- and PKA-dependent regulation of IKs that is dependent on the association of the channel with the AKAP Yotiao, yet the source of cAMP that leads to this regulation is unknown. We have previously identified a subset of AC isoforms that interact with the scaffolding protein Yotiao, including AC1–3 and AC9, but not the predominant cardiac myocyte isoforms AC5 and AC6 (13). Of these, only AC2 and AC9 have previously been reported to be expressed in cardiac myocytes and thus capable of regulating IKs. Therefore, we have focused on AC2 and AC9 as potential sources for cAMP to regulate IKs.
KCNQ1 Forms Complexes with AC2 or AC9 via Interactions with Yotiao
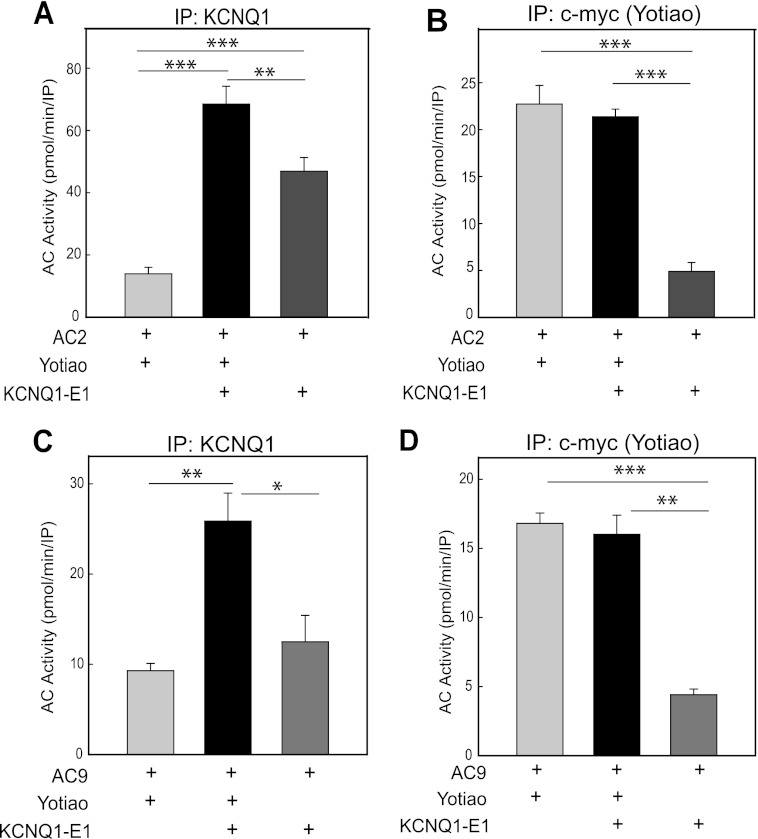
To determine whether KCNQ1-KCNE1 can associate with AC2 or AC9 in a Yotiao-dependent manner, we transfected HEK293 cells with plasmids encoding AC, c-Myc-tagged Yotiao, and/or a fusion of KCNQ1-KCNE1 as indicated (Fig. 1). Protein complexes were immunoprecipitated using antibodies directed against KCNQ1 (Fig. 1, A and C) or the c-Myc tag on Yotiao (Fig. 1, B and D). We then measured the associated AC activity present in the immunoprecipitates (referred to as an IP-AC assay). AC2 and AC9 activities were both associated with KCNQ1-KCNE1 immune complexes, giving rise to ∼5- and 3-fold increases in associated AC activity, respectively, compared with controls lacking KCNQ1-KCNE1 expression (Fig. 1, A and C). The interaction of AC9 with the channel was dependent on the presence of Yotiao, as a significant reduction of AC9 activity was observed in the absence of Yotiao expression. Although Yotiao significantly facilitated AC2-KCNQ1-KCNE1 association, there was significant AC2 (but not AC9) activity associated with KCNQ1-KCNE1 in the absence of Yotiao expression (Fig. 1A). This may be due to weak direct binding between AC2 and KCNQ1-KCNE1 or via interactions with endogenously expressed AKAPs in HEK293 cells, such as AKAP79, which also anchors AC2. KCNQ1-KCNE1 does not compete for AC2 or AC9 association with Yotiao, as no reduction of Yotiao-associated AC activity was observed upon coexpression of KCNQ1-KCNE1 (Fig. 1, B and D).
FIGURE 1.
AC2 and AC9 activities associate with KCNQ1-KCNE1 in a Yotiao-dependent manner. HEK293 cells were transfected with AC2 (A and B) or AC9 (C and D) with or without Myc-tagged Yotiao or KCNQ1-KCNE1 fusion protein. Transfected cell lysates were then subjected to immunoprecipitation (IP) using antibodies against KCNQ1 (A and C) or c-Myc (B and D). The associated AC activity in each immunoprecipitation was measured following stimulation by exogenous addition of 100 nm Gαs plus 50 nm Gβγ for AC2 or 400 nm Gαs for AC9. KCNQ1-KCNE1 did not compete with AC association with Yotiao. Data are represented as the mean ± S.E. of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 using the Bonferroni t test (A and B) or Student's t test (C and D).
Mapping AC2 and AC9 Interaction Domains with Yotiao
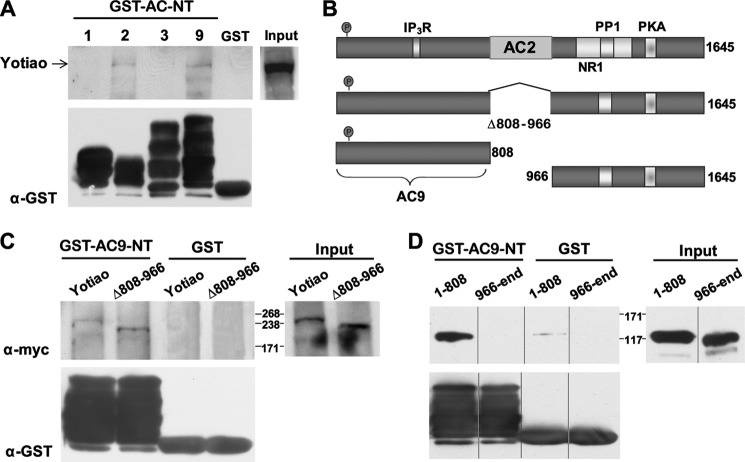
We have previously shown that AC2-NT interacts with residues 808–957 of Yotiao (13), but interaction sites of AC9 and Yotiao have not been mapped. GST-tagged AC N termini from all AC isoforms previously identified as Yotiao-interacting proteins were used to pull down any associated Yotiao (Fig. 2A). AC2-NT and AC9-NT, but not AC1-NT and AC3-NT, bound to Yotiao in GST pulldown assays. The AC9-NT-binding site on Yotiao was determined by similar GST pulldown assays using deletions and truncations of Yotiao (Fig. 2B). Deletion of the AC2-binding site on Yotiao (Δ808–966) had no effect on AC9-NT binding, although this deletion completely abolished AC2 association (21). Rather, the primary interaction site of AC9 was within the first 808 residues of Yotiao, although at least two separate sites of contact within this region were identified when further truncations were tested. AC9-NT also very weakly bound His6-tagged fragment 808–956 as detected by in vitro binding assays (data not shown). This may be due to the repeating nature of Yotiao, which has several stretches within region 1–808 that have weak homology to region 808–956 (25–37% identity). Therefore, we focused on the AC N termini as potential Yotiao-AC9 interaction-disrupting proteins rather than fragments of Yotiao.
FIGURE 2.
AC9-NT binds to amino acids 1–808 of Yotiao. A, GST-tagged AC N termini were screened for interactions with Yotiao by GST pulldown assay using HEK293 cell lysates expressing Myc-tagged Yotiao. Yotiao bound AC2-NT and AC9-NT, but not AC1-NT or AC3-NT (upper). GST protein input for each AC N terminus is shown (lower). B, diagram of Myc-tagged Yotiao fragments. IP3R, inositol 1,4,5-trisphosphate receptor. C and D, Yotiao and/or Yotiao fragments were expressed in HEK293 cells and subjected to GST pulldown assay using GST or GST-AC9-NT. AC9-NT bound Yotiao residues 1–808; deletion of regions 808–966 did not interfere with binding.
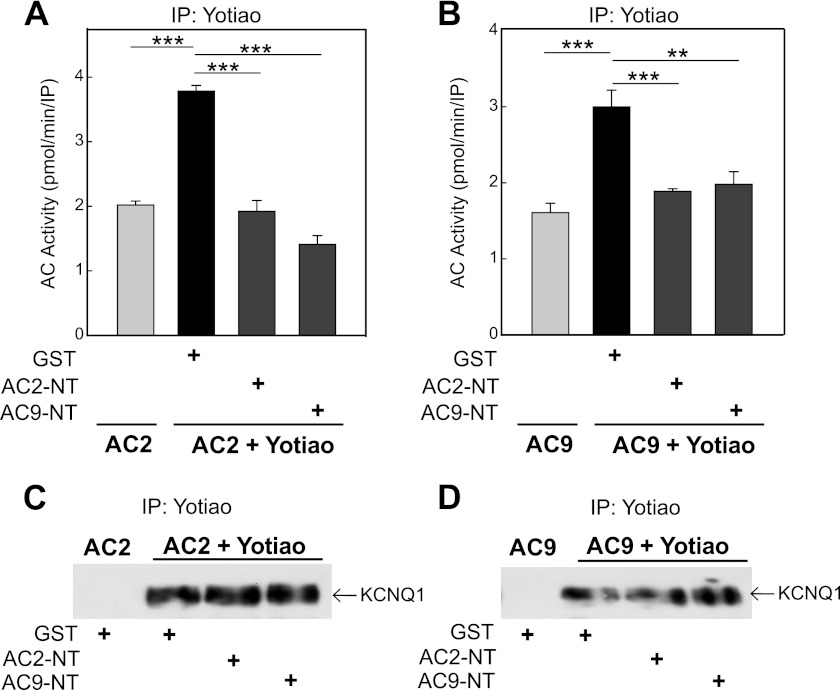
AC2-NT and AC9-NT were both tested for their ability to disrupt Yotiao-AC interactions (Fig. 3, A and B). IP-AC assays for Yotiao-AC2 or Yotiao-AC9 interactions were performed in the presence of GST-tagged AC N termini or GST alone. Surprisingly, AC2-NT and AC9-NT could compete with either isoform for interactions with Yotiao. This may be due to simple steric hindrance or similarities within their respective binding sites; however, neither AC N terminus competed for KCNQ1-Yotiao interactions with AC2 or AC9 (Fig. 3, C and D).
FIGURE 3.
AC9-NT blocks AC9 and AC2 interactions with Yotiao, but not Yotiao binding to KCNQ1. A and B, transfected CHO cell lysates were incubated with GST (5 μm), GST-AC2-NT (5 μm), or GST-AC9-NT (5 μm) prior to immunoprecipitation (IP) with anti-Yotiao antibody. Immunoprecipitates were assayed for associated AC activity stimulation with 100 nm Gαs and 50 nm Gβγ for AC2 (A) or 400 nm Gαs for AC9 (B). Both AC2-NT and AC9-NT were able to inhibit the association of AC2 or AC9 with Yotiao in CHO cells. Data are presented as the mean ± S.E. (n = 3). **, p < 0.01; ***, p < 0.001 using the Bonferroni t test. C and D, CHO cells stably expressing KCNQ1 were transfected with AC2 with or without Yotiao. Cell lysates were incubated with GST (5 μm), GST-AC2-NT (5 μm), or GST-AC9-NT (5 μm) prior to immunoprecipitation with anti-Yotiao antibody and Western blotting for KCNQ1 association.
KCNQ1-KCNE1 Associates with AC9 in Transgenic Mouse Heart
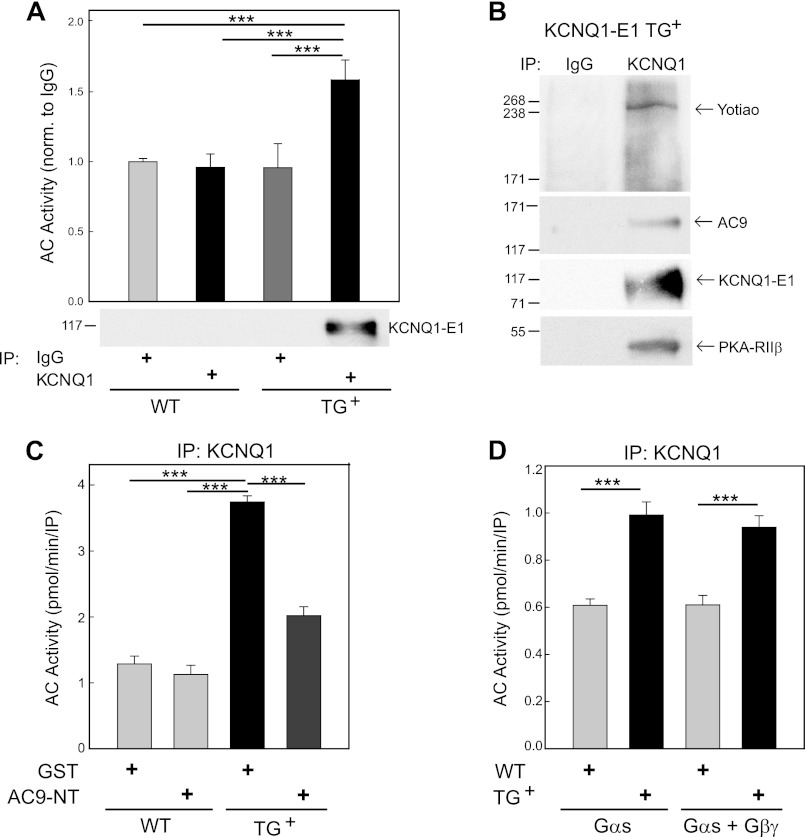
To determine whether AC activity is associated with IKs channels in heart, we used a murine model that we have previously characterized. A fusion of the human KCNQ1-KCNE1 channels was expressed in a targeted manner in the heart (5). These mice expressed functional slow delayed rectifier potassium channel currents (IKs) normally absent in murine cardiac ventricular myocytes. Using an antibody directed against KCNQ1 or goat IgG as a control, we measured the associated Gαs- and forskolin-stimulated AC activity in immunoprecipitates from WT versus KCNQ1-KCNE1 transgenic hearts (Fig. 4A). AC activity was increased by 1.5-fold in KCNQ1 immunoprecipitates from transgenic hearts, the same samples that pulled down the KCNQ1-KCNE1 fusion (Fig. 4A). Yotiao and the regulatory subunits of PKA (RIIβ) were also present in KCNQ1 immunoprecipitates, but not in control IgG (Fig. 4B). In addition, we detected the presence of AC9 (Fig. 4B) but failed to observe any AC2, AC3, or AC5/6 by Western blotting (data not shown). We did not expect to detect AC3 or AC5/6 based upon the low expression of AC3 in adult heart (25) and the lack of interaction of AC5/6 with Yotiao (13). However, AC isoforms are notoriously difficult to detect in native tissues by Western blotting (26).
FIGURE 4.
KCNQ1-KCNE1 associates with AC9 activity in transgenic mouse heart. A, heart extracts from WT or KCNQ1-KCNE1 transgenic (TG+) mice were immunoprecipitated (IP) with goat IgG (control) or anti-KCNQ1 antibody. Immunoprecipitates were stimulated with Gαs/forskolin, and associated AC activity was measured and normalized to IgG of WT mouse heart (n = 3). B, immunoprecipitates of KCNQ1 (or control IgG) from WT or KCNQ1-KCNE1 transgenic hearts were subjected to Western blot analysis with anti-Yotiao, anti-KCNQ1, anti-AC9, and anti-RIIβ (PKA regulatory subunit) antibodies. C, WT or KCNQ1-KCNE1 transgenic mouse heart extracts (n = 3) were incubated with GST (5 μm) or GST-AC9-NT (5 μm) before immunoprecipitation with anti-KCNQ1 antibody. Immunoprecipitates were stimulated with 400 nm Gαs (p < 0.05). AC9-NT inhibited KCNQ1-associated AC activity. D, WT and KCNQ1-KCNE1 transgenic heart extracts were immunoprecipitated with anti-KCNQ1 antibody. Immunoprecipitates were stimulated with Gαs versus Gαs/Gβγ, and associated AC activity was measured (n = 3). Gβγ did not activate KCNQ1-associated AC. For A, C, and D, data are presented as the mean ± S.E. (n = 3). ***, p < 0.001 using the Bonferroni t test.
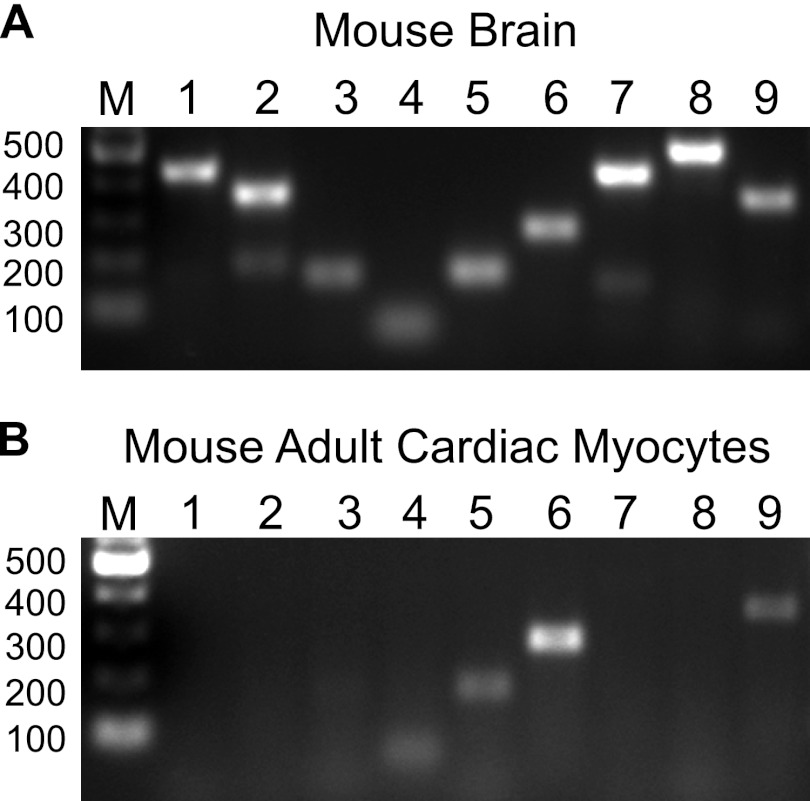
To further confirm that AC activity was specifically associated with an IKs-Yotiao complex, we used AC9-NT as a disrupting agent of Yotiao-AC9 interactions (Fig. 4C). The increase in KCNQ1-associated AC activity was completely blocked by addition of AC9-NT to cardiac extracts prior to immunoprecipitation of KCNQ1-KCNE1 complexes, indicating that this interaction was mediated by the Yotiao scaffolding protein. Because AC9-NT blocks both AC2 and AC9 interactions with Yotiao (Fig. 3), we used Gαs or Gαs + Gβγ to stimulate KCNQ1-associated AC activity to further differentiate between AC isoforms. Gβγ greatly activates AC2, AC4, and AC7 and, to a lesser extent, AC5 and AC6 in the presence of Gαs. However, Gβγ does not regulate the activity of AC9 (21). Addition of Gβγ had no effect on the amount of Gαs-stimulated AC activity detected in immunoprecipitates of KCNQ1 from heart (Fig. 4D), suggesting that no member of the AC2/4/7 or AC5/6 family was present in this complex. Finally, we used semiquantitative PCR to determine the AC isoforms expressed in adult mouse cardiac myocytes. In addition to AC5/6, previous reports have suggested that both AC2 and AC9 are expressed in adult and neonatal cardiac myocytes, respectively (21, 27–29). We detected AC4–6 and AC9 by RT-PCR in adult myocytes (Fig. 5); AC7 was weakly detected upon additional PCR cycles. We did not detect any interaction of Yotiao with AC7 in IP-AC assays (n = 3) (data not shown). We also did not detect AC2 in mouse adult cardiac myocytes, although this isoform has been reported in cardiac fibroblasts (30). Nevertheless, the only Yotiao-interacting isoform detected in adult mouse cardiac myocytes by activity assays or RT-PCR was AC9.
FIGURE 5.
Expression of AC isoforms in adult mouse cardiac myocytes. A and B, AC isoform mRNA expression as determined by semiquantitative PCR from total RNA in mouse brain and adult mouse cardiac myocytes, respectively. Lanes 1–9 indicate PCR amplification of AC1–9. DNA markers (M) are indicated. PCR was performed as described under “Experimental Procedures” using the corresponding primer pairs for all mouse transmembrane AC isoforms (AC1–9). Adult mouse cardiac myocytes expressed AC4–6 and AC9.
AC Association with the KCNQ1-Yotiao Complex Sensitizes PKA Phosphorylation of KCNQ1 to β-Adrenergic Stimulation
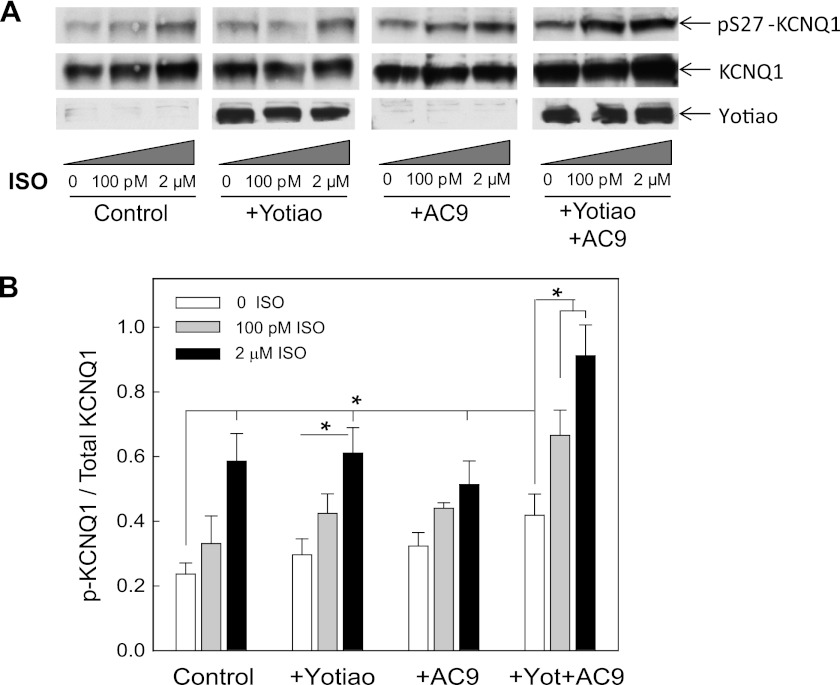
Biochemical experiments show AC9 as a component of the IKs macromolecular complex, but the functional significance of this association was not clear. To determine whether AC9 alters the sensitivity of KCNQ1 phosphorylation to β-agonists, we examined CHO cells stably expressing KCNQ1 with or without expression of Yotiao and AC9. The expression levels of AC9 were maintained to give a 2-fold increase in AC activity in response to Gαs activation in membranes (data not shown). Cells were stimulated with very low levels (100 pm) versus maximal levels (2 μm) of the β-adrenergic agonist isoproterenol and then assayed by Western blotting for PKA phosphorylation at Ser-27 of KCNQ1 versus total KCNQ1 (Fig. 6A). In all cases, stimulation with 2 μm isoproterenol significantly increased PKA phosphorylation of Ser-27 compared with basal phosphorylation of KCNQ1 alone (Fig. 6B). More importantly, expression of both Yotiao and AC9 with KCNQ1 significantly increased sensitivity to PKA phosphorylation under basal conditions and in the presence of 100 pm and 2 μm isoproterenol (n = 5; p < 0.05). Although expression of Yotiao alone anchors PKA in close proximity to the channel, it also couples negative regulation to KCNQ1 in the form of PP1 and PDE4D3 anchoring. Expression of AC9 alone with KCNQ1 increases cAMP levels, but the cAMP is not well coupled to PKA phosphorylation of KCNQ1. It is only in the presence of the entire complex of KCNQ1-Yotiao-AC9 that the negative and positive regulation that are coordinated by Yotiao can be balanced to increase the sensitivity to β-adrenergic stimulation.
FIGURE 6.
Expression of both Yotiao and AC9 increases the sensitivity of KCNQ1 phosphorylation to isoproterenol. A, CHO cells stably expressing KCNQ1 were transfected with pcDNA3 (Control), Yotiao, or AC9 as indicated. Cells were treated with the indicated concentrations of isoproterenol (ISO) for 9 min at 37 °C. Western blotting was performed for KCNQ1 phosphorylated at Ser-27, total KCNQ1, and Yotiao. B, quantification of phospho-KCNQ1 to total KCNQ1 from three to five individual experiments as determined by densitometry using NIH ImageJ software. Data are presented as the mean ± S.E. and were analyzed by analysis of variance of all groups in addition to Student's t test for individual comparisons. *, p < 0.05.
Endogenous IKs-Yotiao Complex from Guinea Pig Also Contains AC9
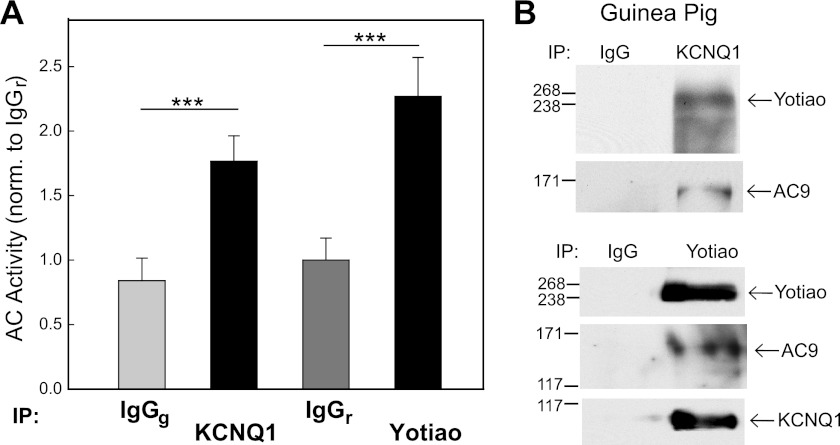
We have shown that a complex of KCNQ1-Yotiao-AC9 can exist in cell culture systems and transgenic mice. Mouse models for IKs are advantageous because they faithfully recapitulate the biology in a carefully controlled system (5, 12). However, because this channel is not normally expressed in mice due to their rapid heart rates, we examined endogenous IKs-Yotiao-AC interactions in guinea pig. IKs and its sympathetic regulation have been extensively examined in guinea pig and provide a better model than mice for many aspects of cardiac disease (31, 32). IP-AC assays were performed using anti-KCNQ1 or anti-Yotiao antibody or control IgG from goat and rabbit, respectively. Immunoprecipitation of KCNQ1 or Yotiao from guinea pig heart gave rise to a 2-fold increase in associated AC activity compared with control IgG (Fig. 7A). Yotiao and KCNQ1 could pull down each other from guinea pig heart extracts as observed by Western blotting of reciprocal immunoprecipitates (Fig. 7B). AC9 was also detected in these complexes, similar to what we observed in mice.
FIGURE 7.
KCNQ1-KCNE1 associates with AC9 activity in guinea pig heart. A, guinea pig heart extracts were immunoprecipitated (IP) with anti-KCNQ1 or anti-Yotiao antibody or control IgG (goat IgG (IgGg), or rabbit IgG (IgGr)). Immunoprecipitates were stimulated with 400 nm Gαs, and associated AC activity was measured. Data are presented as the mean ± S.E. (n = 3). ***, p < 0.001. B, guinea pig heart extracts were subjected to immunoprecipitation with anti-KCNQ1 antibody and goat IgG or with anti-Yotiao antibody and rabbit IgG. Immunoprecipitates were subjected to Western blot analysis using anti-Yotiao, anti-KCNQ1, and anti-AC9 antibodies.
DISCUSSION
It has long been appreciated that the IKs channel requires association of the α-subunit of KCNQ1 with the AKAP Yotiao to respond to sympathetic stimulation, suggesting that local activation of PKA is critically important. This complex also contains PDE4D3 to further regulate local levels of cAMP (12), but the source of cAMP was unknown. We have previously shown that Yotiao anchors a subset of AC isoforms, but the question remained as to whether AC was actually a member of the IKs-Yotiao complex in heart and what isoform of AC was present. In this work, we have shown that both AC9 and AC2 can form a complex with KCNQ1 via their interactions with the AKAP Yotiao. The binding sites for KCNQ1 and AC isoforms are non-overlapping, allowing for assembly of a multiprotein unit. KCNQ1 binding sites on Yotiao have been mapped to amino acid residues 29–56 and the extreme C terminus of Yotiao (7), whereas AC2 binds to residues 808–957 of Yotiao (13). We now show that AC9-NT binds to residues 1–808 of Yotiao, likely with multiple points of contact. Although the major sites on Yotiao for AC2 and AC9 do not appear to overlap, either AC2-NT or AC9-NT can compete with both isoforms for binding to Yotiao. This may be due to the somewhat repetitive nature of Yotiao, whereby several stretches in amino acids 1–808 have similarities to the AC2-binding site consisting of amino acids 808–957. The AC9- and AC2-binding sites on Yotiao are clearly distinct from those on KCNQ1; neither AC9 nor AC2 competes with KCNQ1 for interactions with Yotiao.
Significant AC activity was associated with both KCNQ1 and Yotiao in transgenic mouse heart expressing KCNQ1-KCNE1 and with endogenous KCNQ1 in guinea pig heart. Mouse adult cardiac myocytes expressed AC4–6 and AC9 by RT-PCR. Of the isoforms identified in myocytes, only AC9 can interact with Yotiao (13). By immunoprecipitation of KCNQ1, we confirmed the presence of AC9, Yotiao, and the RIIβ subunit of PKA in this complex by Western blotting. In addition, the association of AC activity with KCNQ1 was inhibited by inclusion of AC9-NT, suggesting that the association of KCNQ1 and AC9 in vivo is mediated by Yotiao.
Increased Sensitivity of AC-AKAP Complexes
The formation of effector-AKAP-PKA complexes increases the sensitivity of the effector to PKA phosphorylation (6, 33). Inclusion of AC in the complex should lead to an even greater sensitivity to cAMP (20). Indeed, the presence of AC9 and Yotiao led to increased phosphorylation of KCNQ1 by PKA in response to low levels of isoproterenol. This increased sensitivity is likely carefully balanced by Yotiao-anchored phosphodiesterase and PP1 to feedback inhibit cAMP production and PKA phosphorylation, respectively. We speculate that anchoring of AC also increases the sensitivity of other cardiac AC-AKAP complexes to cAMP, including AC5-muscle AKAP and AC6-AKAP79 (14, 21, 34).
Role of AC9 in IKs-Yotiao Complexes in Heart
Most of the attention in heart has focused around AC5 and AC6 based upon their deletion or overexpression in heart. Notably, deletion of AC5 protects against stress- and age-induced cardiac myopathy (35–37), consistent with the formation of AC5-muscle AKAP complexes in heart (21). In contrast, deletion of AC6 gives rise to decreased contractile function and calcium handling (38), possibly linked to complexes of AC6 with AKAP79 and L-type Ca2+ channels (34). However, AC5 and AC6 do not interact with Yotiao and were not detected in complexes with KCNQ1 (13). AC1 may play a role in the sinoatrial node to regulate If pacemaker currents, but it is not expressed in adult myocytes (39). Detailed studies of the roles for other AC isoforms have been sorely lacking, due in part to a lack of genetic deletions and/or AC isoform-specific inhibitors.
Physiological roles for AC9 have been largely ignored. AC9 is ubiquitously expressed, and the knock-out of AC9 has been reported to be embryonic lethal (26). The gene is known to be transcriptionally regulated in a number of systems and is the target of the microRNA miR-142–3p to reduce cAMP in a subset of T cells (40). AC9 is also involved in neutrophil chemotaxis but has not been studied in heart (41). However, AC9 has several regulatory properties that make it uniquely qualified as a regulator of IKs, including its ability to sense input from Gs, Gi, Gq, and Ca2+ signaling pathways (reviewed in Refs. 42 and 43). The basal activity of AC9 is inhibited by the Ca2+-dependent phosphatase calcineurin (44, 45), whereas stimulated AC9 is inhibited by novel PKC isoforms and Gi/o proteins (46). Conversely, AC9 can be activated by Gs- and Gq-coupled G protein-coupled receptors; the latter Gq regulation is indirect and dependent on the activation of calmodulin (CaM) kinase II (47, 48). CaM is known to bind to the C terminus of KCNQ1 and to KCNE4. Disruption of CaM interaction with either protein completely suppresses KCNQ1 currents (6, 49, 50). Thus, CaM may already be present in the IKs complex to regulate AC9 activity via CaM kinase II. Not only do Gq-coupled receptors activate AC9, they also regulate IKs in a biphasic manner. Gq-dependent depletion of phosphoinositide 4,5-bisphosphate inhibits IKs, whereas PKC-dependent phosphorylation of KCNE1 activates IKs (51). The feedback sensitivity to calcium-dependent pathways is a hallmark of AC9 regulation through its inhibition by calcineurin and activation by CaM kinase II and may enhance the responsiveness of IKs to both Gs- and Gq-mediated regulation and facilitate cross-talk between these two important cardiac pathways.
Summary
During the normal cardiac cycle, the regular rhythmic pattern of ionic conductance changes is tightly regulated by sympathetic and parasympathetic nervous activities (52). The formation of multiprotein complexes helps to facilitate the temporal changes in IKs phosphorylation states to control the duration of the ventricular action potential. The inclusion of AC9 within a KCNQ1-Yotiao complex sensitizes the system to β-adrenergic stimulation and may serve as a coincidence detector of both Gs- and Gq-mediated regulation of IKs.
Acknowledgments
We thank Dr. Kedryn Baskin for help with isolation of adult cardiac myocytes and Dr. Hongzhen Hu for providing fresh guinea pig heart tissue.
This work was supported, in whole or in part, by National Institutes of Health Grants GM60419 (to C. W. D.) and HL044365 (to R. S. K.). This work was also supported by American Heart Association Grant GRNT2200034 (to C. W. D.).
- IKs
- slow outward potassium ion current
- AKAP
- A-kinase anchoring protein
- AC
- adenylyl cyclase
- NT
- N terminus
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- IP-AC
- immunoprecipitation-AC
- CaM
- calmodulin.
REFERENCES
- 1. Sanguinetti M. C., Curran M. E., Zou A., Shen J., Spector P. S., Atkinson D. L., Keating M. T. (1996) Co-assembly of KVLQT1 and MinK (IsK) proteins to form cardiac IKs potassium channel. Nature 384, 80–83 [DOI] [PubMed] [Google Scholar]
- 2. Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996) KVLQT1 and IsK (MinK) proteins associate to form the IKs cardiac potassium current. Nature 384, 78–80 [DOI] [PubMed] [Google Scholar]
- 3. Kass R. S., Wiegers S. E. (1982) The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac Purkinje fibers. J. Physiol. 322, 541–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh K. B., Kass R. S. (1988) Regulation of a heart potassium channel by protein kinases A and C. Science 242, 67–69 [DOI] [PubMed] [Google Scholar]
- 5. Marx S. O., Kurokawa J., Reiken S., Motoike H., D'Armiento J., Marks A. R., Kass R. S. (2002) Requirement of a macromolecular signaling complex for β-adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295, 496–499 [DOI] [PubMed] [Google Scholar]
- 6. Ciampa E. J., Welch R. C., Vanoye C. G., George A. L., Jr. (2011) KCNE4 juxtamembrane region is required for interaction with calmodulin and for functional suppression of KCNQ1. J. Biol. Chem. 286, 4141–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L., Marquardt M. L., Tester D. J., Sampson K. J., Ackerman M. J., Kass R. S. (2007) Mutation of an A-kinase anchoring protein causes long-QT syndrome. Proc. Natl. Acad. Sci. U.S.A. 104, 20990–20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin J. W., Wyszynski M., Madhavan R., Sealock R., Kim J. U., Sheng M. (1998) Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J. Neurosci. 18, 2017–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westphal R. S., Tavalin S. J., Lin J. W., Alto N. M., Fraser I. D., Langeberg L. K., Sheng M., Scott J. D. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285, 93–96 [DOI] [PubMed] [Google Scholar]
- 10. Kurokawa J., Motoike H. K., Rao J., Kass R. S. (2004) Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 101, 16374–16378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L., Kurokawa J., Kass R. S. (2005) Phosphorylation of the A-kinase anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J. Biol. Chem. 280, 31347–31352 [DOI] [PubMed] [Google Scholar]
- 12. Terrenoire C., Houslay M. D., Baillie G. S., Kass R. S. (2009) The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J. Biol. Chem. 284, 9140–9146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piggott L. A., Bauman A. L., Scott J. D., Dessauer C. W. (2008) The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc. Natl. Acad. Sci. U.S.A. 105, 13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Efendiev R., Samelson B. K., Nguyen B. T., Phatarpekar P. V., Baameur F., Scott J. D., Dessauer C. W. (2010) AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and AC6 to α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors. J. Biol. Chem. 285, 14450–14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen-Goodspeed M., Lukan A. N., Dessauer C. W. (2005) Modeling of Gαs and Gαi regulation of human type V and VI adenylyl cyclases. J. Biol. Chem. 280, 1808–1816 [DOI] [PubMed] [Google Scholar]
- 16. Wang W., Xia J., Kass R. S. (1998) MinK-KVLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J. Biol. Chem. 273, 34069–34074 [DOI] [PubMed] [Google Scholar]
- 17. Kurokawa J., Chen L., Kass R. S. (2003) Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc. Natl. Acad. Sci. U.S.A. 100, 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dessauer C. W., Tesmer J. J., Sprang S. R., Gilman A. G. (1998) Identification of a Gαi-binding site on type V adenylyl cyclase. J. Biol. Chem. 273, 25831–25839 [DOI] [PubMed] [Google Scholar]
- 19. Salim S., Sinnarajah S., Kehrl J. H., Dessauer C. W. (2003) Identification of RGS2 and type V adenylyl cyclase interaction sites. J. Biol. Chem. 278, 15842–15849 [DOI] [PubMed] [Google Scholar]
- 20. Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., Hoshi N., Langeberg L. K., Cooper D. M., Dessauer C. W., Scott J. D. (2006) Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapiloff M. S., Piggott L. A., Sadana R., Li J., Heredia L. A., Henson E., Efendiev R., Dessauer C. W. (2009) An adenylyl cyclase-mAKAPβ signaling complex regulates cAMP levels in cardiac myocytes. J. Biol. Chem. 284, 23540–23546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dessauer C. W. (2002) Kinetic analysis of the action of P-site analogs. Methods Enzymol. 345, 112–126 [DOI] [PubMed] [Google Scholar]
- 23. O'Connell T. D., Rodrigo M. C., Simpson P. C. (2007) Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 357, 271–296 [DOI] [PubMed] [Google Scholar]
- 24. Landa L. R., Jr., Harbeck M., Kaihara K., Chepurny O., Kitiphongspattana K., Graf O., Nikolaev V. O., Lohse M. J., Holz G. G., Roe M. W. (2005) Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 β-cell line. J. Biol. Chem. 280, 31294–31302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanoune J., Defer N. (2001) Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 41, 145–174 [DOI] [PubMed] [Google Scholar]
- 26. Antoni F. A., Wiegand U. K., Black J., Simpson J. (2006) Cellular localization of adenylyl cyclase: a post-genome perspective. Neurochem. Res. 31, 287–295 [DOI] [PubMed] [Google Scholar]
- 27. Ping P., Anzai T., Gao M., Hammond H. K. (1997) Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am. J. Physiol. 273, H707–H717 [DOI] [PubMed] [Google Scholar]
- 28. Baragli A., Grieco M. L., Trieu P., Villeneuve L. R., Hébert T. E. (2008) Heterodimers of adenylyl cyclases 2 and 5 show enhanced functional responses in the presence of Gαs. Cell. Signal. 20, 480–492 [DOI] [PubMed] [Google Scholar]
- 29. Cui H., Green R. D. (2001) Cell-specific properties of type V and type IX adenylyl cyclase isozymes in 293T cells and embryonic chick ventricular myocytes. Biochem. Biophys. Res. Commun. 283, 107–112 [DOI] [PubMed] [Google Scholar]
- 30. Ostrom R. S., Naugle J. E., Hase M., Gregorian C., Swaney J. S., Insel P. A., Brunton L. L., Meszaros J. G. (2003) Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 278, 24461–24468 [DOI] [PubMed] [Google Scholar]
- 31. Kurokawa J., Abriel H., Kass R. S. (2001) Molecular basis of the delayed rectifier current IKs in Heart. J. Mol. Cell. Cardiol. 33, 873–882 [DOI] [PubMed] [Google Scholar]
- 32. Hasenfuss G. (1998) Animal models of human cardiovascular disease, heart failure, and hypertrophy. Cardiovasc. Res. 39, 60–76 [DOI] [PubMed] [Google Scholar]
- 33. Zhang J., Ma Y., Taylor S. S., Tsien R. Y. (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc. Natl. Acad. Sci. U.S.A. 98, 14997–15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nichols C. B., Rossow C. F., Navedo M. F., Westenbroek R. E., Catterall W. A., Santana L. F., McKnight G. S. (2010) Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 107, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iwatsubo K., Minamisawa S., Tsunematsu T., Nakagome M., Toya Y., Tomlinson J. E., Umemura S., Scarborough R. M., Levy D. E., Ishikawa Y. (2004) Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J. Biol. Chem. 279, 40938–40945 [DOI] [PubMed] [Google Scholar]
- 36. Yan L., Vatner D. E., O'Connor J. P., Ivessa A., Ge H., Chen W., Hirotani S., Ishikawa Y., Sadoshima J., Vatner S. F. (2007) Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130, 247–258 [DOI] [PubMed] [Google Scholar]
- 37. Okumura S., Takagi G., Kawabe J., Yang G., Lee M. C., Hong C., Liu J., Vatner D. E., Sadoshima J., Vatner S. F., Ishikawa Y. (2003) Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc. Natl. Acad. Sci. U.S.A. 100, 9986–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang T., Gao M. H., Lai N. C., Firth A. L., Takahashi T., Guo T., Yuan J. X., Roth D. M., Hammond H. K. (2008) Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117, 61–69 [DOI] [PubMed] [Google Scholar]
- 39. Mattick P., Parrington J., Odia E., Simpson A., Collins T., Terrar D. (2007) Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea pig sinoatrial node cells and modulates the If pacemaker current. J. Physiol. 582, 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang B., Zhao J., Lei Z., Shen S., Li D., Shen G. X., Zhang G. M., Feng Z. H. (2009) miR-142–3p restricts cAMP production in CD4+CD25− T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 10, 180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu L., Das S., Losert W., Parent C. A. (2010) mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell 19, 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Willoughby D., Cooper D. M. (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87, 965–1010 [DOI] [PubMed] [Google Scholar]
- 43. Sadana R., Dessauer C. W. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knock-out and overexpression studies. NeuroSignals 17, 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Antoni F. A., Barnard R. J., Shipston M. J., Smith S. M., Simpson J., Paterson J. M. (1995) Calcineurin feedback inhibition of agonist-evoked cAMP formation. J. Biol. Chem. 270, 28055–28061 [DOI] [PubMed] [Google Scholar]
- 45. Paterson J. M., Smith S. M., Simpson J., Grace O. C., Sosunov A. A., Bell J. E., Antoni F. A. (2000) Characterization of human adenylyl cyclase IX reveals inhibition by Ca2+/calcineurin and differential mRNA polyadenylation. J. Neurochem. 75, 1358–1367 [DOI] [PubMed] [Google Scholar]
- 46. Cumbay M. G., Watts V. J. (2004) Novel regulatory properties of human type 9 adenylate cyclase. J. Pharmacol. Exp. Ther. 310, 108–115 [DOI] [PubMed] [Google Scholar]
- 47. Hacker B. M., Tomlinson J. E., Wayman G. A., Sultana R., Chan G., Villacres E., Disteche C., Storm D. R. (1998) Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9). Genomics 50, 97–104 [DOI] [PubMed] [Google Scholar]
- 48. Cumbay M. G., Watts V. J. (2005) Gαq potentiation of adenylate cyclase type 9 activity through a Ca2+/calmodulin-dependent pathway. Biochem. Pharmacol. 69, 1247–1256 [DOI] [PubMed] [Google Scholar]
- 49. Ghosh S., Nunziato D. A., Pitt G. S. (2006) KCNQ1 assembly and function are blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ. Res. 98, 1048–1054 [DOI] [PubMed] [Google Scholar]
- 50. Shamgar L., Ma L., Schmitt N., Haitin Y., Peretz A., Wiener R., Hirsch J., Pongs O., Attali B. (2006) Calmodulin is essential for cardiac IKs channel gating and assembly: impaired function in long-QT mutations. Circ. Res. 98, 1055–1063 [DOI] [PubMed] [Google Scholar]
- 51. Matavel A., Lopes C. M. (2009) PKC activation and PIP2 depletion underlie biphasic regulation of IKs by Gq-coupled receptors. J. Mol. Cell. Cardiol. 46, 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sampson K. J., Kass R. S. (2010) Molecular mechanisms of adrenergic stimulation in the heart. Heart Rhythm 7, 1151–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]