Background: The Salmonella virulence factor SseJ is activated by the small GTPase RhoA.
Results: SseJ contributes to Salmonella virulence by sensing RhoA at the same surface as eukaryotic factors.
Conclusion: SseJ evolved to detect the activation state of host factors by binding the same surface as RhoA endogenous binding proteins.
Significance: SseJ binding to RhoA is important for the pathogenesis of Salmonella.
Keywords: Host-pathogen Interactions, Mutagenesis, NMR, Rho GTPases, RhoA, Salmonella
Abstract
Salmonella enterica serovar typhimurium translocates a glycerophospholipid:cholesterol acyltransferase (SseJ) into the host cytosol after its entry into mammalian cells. SseJ is recruited to the cytoplasmic face of the host cell phagosome membrane where it is activated upon binding the small GTPase, RhoA. SseJ is regulated similarly to cognate eukaryotic effectors, as only the GTP-bound form of RhoA family members stimulates enzymatic activity. Using NMR and biochemistry, this work demonstrates that SseJ competes effectively with Rhotekin, ROCK, and PKN1 in binding to a similar RhoA surface. The RhoA surface that binds SseJ includes the regulatory switch regions that control activation of mammalian effectors. These data were used to create RhoA mutants with altered SseJ binding and activation. This structure-function analysis supports a model in which SseJ activation occurs predominantly through binding to residues within switch region II. We further defined the nature of the interaction between SseJ and RhoA by constructing SseJ mutants in the RhoA binding surface. These data indicate that SseJ binding to RhoA is required for recruitment of SseJ to the endosomal network and for full Salmonella virulence for inbred susceptible mice, indicating that regulation of SseJ by small GTPases is an important virulence strategy of this bacterial pathogen. The dependence of a bacterial effector on regulation by a mammalian GTPase defines further how intimately host pathogen interactions have coevolved through similar and divergent evolutionary strategies.
Introduction
Salmonella enterica serovar typhimurium (S. typhimurium) is a broad host range Gram-negative pathogen that causes acute gastroenterititis, focal, and systemic infections in humans and animals. S. typhimurium pathogenesis is dependent on two specialized secretion systems termed type-III secretion systems, which translocate proteins across the plasma (Salmonella pathogenicity island [SPI1] encoded) and vacuolar (by the Salmonella pathogenicity island 2 [SPI2] encoded system) membranes to alter host cell processes. Many SPI2 effectors localize to the Salmonella-containing vacuole membrane where they function to alter the vacuolar membrane by inducing endosomal tubulation (1–3). In the case of SseJ, its enzymatic activity results in the alteration of the lipid content of the Salmonella-containing vacuole membrane, which among other things may allow endosomal tubulation to occur without loss of the vacuolar membrane structure. The molecular roles of the multiple effector proteins that localize to the vacuolar membrane after translocation and how they alter the endosomal compartment are largely unknown, but this and earlier work define in detail the activity and regulation of SseJ.
SseJ appears to work in cooperation with SifA, an effector protein that is recruited to the Salmonella-containing vacuole and participates in endosomal tubulation. SifA effectively links the vacuole to the microtubule network and motors by binding the host protein SKIP through its amino-terminal domain (4, 5). S. typhimurium lacking sifA lose the vacuolar membrane and escape to the cytoplasm, whereas bacteria that lack both sifA and sseJ remain inside the vacuolar membrane, indicating that membrane disruption is dependent upon SseJ (6). Bacteria lacking sseJ are attenuated in intracellular replication (2, 6). Further, SseJ enzymatic activity is required for full virulence of S. typhimurium in mice, as attempts to complement the ΔsseJ virulence defect with SseJ-containing mutations in the catalytic residues (Ser-151, Asp-247, His-384) failed to restore virulence (2, 6, 7).
SseJ belongs to the GDSL motif lipase family and exhibits phospholipase A1, deacylase, and glycerophospholipid:cholesterol acyltransferase activity; the last of which results in the enzymatic cleavage of phospholipids at the A1 position and the transfer of this acyl chain onto cholesterol (7–9). SseJ forms a complex specifically with the small GTPases RhoA, RhoB, and RhoC, but not with Cdc42, Rac1, or H-Ras. SseJ activity is stimulated upon interaction with the activated form of the eukaryotic small GTPase, RhoA (10). The SseJ-RhoA complex has potent lipase activity as measured by cleavage of the chromogenic substrate, p-nitrophenyl palmitate (pNPP)4. SseJ lipase activity is enhanced mostly by GTP-bound RhoA, the activated form of the GTPase, compared with apo-RhoA (unbound), or GDP-bound RhoA. Additionally, the binding of SseJ to RhoA does not affect the ability of RhoA to cycle between the GDP- and GTP-bound states. Hence, SseJ senses the activation state of RhoA similar to RhoA-activated eukaryotic downstream effector molecules such as ROCK. The requirement of sensing RhoA in regulating SseJ enzymatic activity suggests that tight regulation of SseJ activity may be important for pathogenesis.
RhoA is a member of the Rho GTPase family that functions as major molecular switches that cycle between the GDP-bound inactive state and the GTP-bound active state. The activity of Rho GTPases is regulated by three distinct groups of proteins: guanosine nucleotide dissociation inhibitor (GDI), which interact with the GDP-bound form of RhoA and sequester RhoA from the membrane; guanine nucleotide exchange factor (GEF), which catalyze the exchange of GDP for GTP; and GTPase activating protein (GAP), which stimulate the hydrolysis of GTP to GDP. RhoA specifically regulates a large number of cellular processes including actin reorganization and cell cycle progression, and it mediates these effects through proteins known as effectors, which adopt a conformational active state on binding the activated form of RhoA. Two eukaryotic effector binding domains (PKN1-Hr1a and ROCK-RBD) have been crystallized in a complex with RhoA-GTP (11, 12) and demonstrate that PKN1 and ROCK binding to RhoA is mediated through the RhoA switch regions, which are so named for the conformational change that RhoA undergoes upon binding to either GDP or GTP. The insert helix of RhoA is present only in Rho family GTPases and may be involved in effector binding and specificity (13–15). Because RhoA also regulates SseJ activity in a manner that is dependent on the nucleotide state of RhoA, we investigated the structural interaction between SseJ and RhoA and compared it with interactions between eukaryotic effectors that bind RhoA.
EXPERIMENTAL PROCEDURES
Plasmids and Molecular Techniques
Plasmids used for protein expression of His-RhoA and His-SseJ were described previously (10). The constructs for protein expression of His-RhoA-G14V1–181, His-ROCK-RBD, and His-PKN1-Hr1a were engineered using standard cloning techniques into pET28a. Site-directed mutagenesis of pET28-RhoA-G14V1–181 and pET28-SseJ was carried out using the QuikChange Lightning kit (Stratagene). To produce the expression construct for binding experiments, HA-tagged SseJ was inserted in-frame with an amino-terminal glutathione S-transferase (GST) into pGEX-5x(1) with EcoRI and XhoI. All plasmid constructs and chromosomal point mutants were verified by DNA sequencing. All plasmids used in this study are listed in supplemental Table S1; all strains used in this study are listed in supplemental Table S2.
Expression and Purification of Recombinant Proteins
His-RhoA (human variant) and His-SseJ were expressed in Escherichia coli BL21 (DE3) cells and purified over a 5-ml HisTrap HP column. The His tags were cleaved with thrombin, and the proteins were purified further by gel filtration (HiLoad 16/60 Superdex 200) using an AKTA FPLC system (Amersham Biosciences) as described previously (10). His-ROCK-RBD, His-PKN1-Hr1a, His-RhoA-G14V1-181 point mutants, and His-RhoAΔRas were expressed in E. coli BL21 (DE3) cells, purified over a gravity-flow nickel column, and dialyzed into Tris-buffered saline (TBS), pH 7.6, supplemented with 5 mm MgCl2 and 1 mm DTT. The effector fragment GST-Rhotekin-RBD was purchased from Cytoskeleton Inc. GST-HA-SseJ was expressed in E. coli BL21, purified by gravity-flow over a glutathione-Sepharose column according to the manufacturer's protocol (GE Healthcare), and dialyzed into TBS, pH 7.6, supplemented with 5 mm MgCl2 and 1 mm DTT. All purified proteins were stored in TBS, pH 7.6, supplemented with 10% glycerol, 5 mm MgCl2, and 1 mm DTT at −80 °C.
pNPP Lipase Assays
Lipase activity was determined by hydrolysis of the substrate pNPP and subsequent release of p-nitrophenol, which was detected by measuring the absorbance of the reaction solution at 405 nm. Assays were performed in duplicate in a 96-well plate and repeated at least three separate times. To measure SseJ activity in the presence of effector-binding fragments, in binding buffer, 2.5 μl of 10 mm pNPP dissolved in dimethyl sulfoxide was added to 200 μl of 250 nm SseJ and RhoA with varying concentrations of GST-Rhotekin-RBD, His-ROCK-RBD, and His-PKN1-Hr1a. To measure SseJ activation by RhoA mutants, 2.5 μl of 10 mm pNPP dissolved in dimethyl sulfoxide was added to 200 μl of 250 nm SseJ with varying concentrations of His-RhoA mutants. After mixing, plates were incubated at 37 °C, and increases in absorption at 405 nm were measured on an EnVision Multilabel Reader (PerkinElmer Life sciences) at 1-min intervals.
NMR Spectroscopy
All NMR samples were prepared in 25 mm Tris, pH 7.5, 50 mm NaCl, 5 mm MgCl2, and 1 mm DTT at concentrations of 0.3–0.5 mm, and data were collected at 25 °C. Samples for backbone assignments and titration experiments used 2H,13C,15N-labeled RhoA-GDP and RhoA-GTPγS. Assignment of RhoA backbone resonances was accomplished by analysis of standard triple-resonance experiments (16). All NMR data were collected on Bruker DMX 500 MHz (University of Washington) or Varian Inova 600, 800, and 900 MHz spectrometers (Pacific Northwest National Laboratories). Data were processed and analyzed using NMRPipe (17) and NMRView (18).
Protein Binding
Purified GST-SseJ or GST protein (10 μg) was mixed with 10 μg of His-RhoA in 200 μl of TBS, 5 mm MgCl2, 1 mm DTT and incubated with 20 μl of glutathione-Sepharose beads at 4 °C with rotation. After 3 h, the beads were pelleted, washed five times with phosphate-buffered saline, and resuspended in 40 μl of 1× sample buffer. Samples were boiled, separated by SDS-PAGE, and transferred to nitrocellulose, and His-RhoA was detected using nickel-HRP (KPL). GST and GST-SseJ were detected by staining for proteins directly on the nitrocellulose membranes with Ponceau S.
Transfections and Immunofluorescence Microscopy
Plasmids were purified using EndoFree Plasmid Maxi kits (Qiagen). HeLa cells (American Type Culture Collection) were transfected with plasmids using FuGENE 6 (Roche Applied Science) as recommended and were cultured for 24 h. For the PEDA1 (Invitrogen) experiment, cells were transfected for 22 h then incubated for an additional 2 h with 3.5 μm PEDA1 substrate. If the fluorogenic PEDA1 substrate is cleaved at the sn-1 position, the BODIPY group will exhibit green fluorescence (excitation/emission = 488/530). The cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), and immunostaining was performed. All antibodies were used at 1:200 in Cytoperm buffer. Deconvolution microscopy was performed on an Eclipse TE2000-E or Ti microscope (Nikon) equipped with a Cascade II 1024 EMCCD camera (Photometrics). Images were deconvolved and analyzed with the NIS-Elements image analysis software.
Infections
HeLa cells were infected at a multiplicity of infection of 100:1 with S. typhimurium grown from a back dilution from an overnight culture. Cells were washed with 1× phosphate-buffered saline and treated with gentamicin (0.15 mg/ml) for 1 h followed by incubation for 14 h with gentamicin (0.015 mg/ml). The cells were fixed and permeabilized with the Cytofix/Cytoperm kit, and immunostaining was performed. All antibodies were used at 1:200 in Cytoperm buffer. Deconvolution microscopy was performed on an Eclipse TE2000-E microscope equipped with a Cascade II 1024 EMCCD camera. Images were deconvolved and analyzed with the NIS-Elements image analysis software.
Competitive Index Assay
S. typhimurium strains were generated using the λ red recombinase system (19). Mice were ordered from Charles River Laboratories, Inc., and virulence phenotypes were tested by competitive index assay as described previously (2, 7). Six- to eight-week-old female BALB/c mice were inoculated intraperitoneally with a mixture of 5 × 104 organisms each of two serovar typhimurium strains for a total of 105 bacteria in a 0.2-ml volume. Each strain was diluted from cultures grown overnight containing a stable plasmid-based antibiotic marker to allow the strains to be differentiated. The bacterial inoculum contained approximately equal concentrations of both strains, and the ratio of the strains was confirmed by plating dilutions of the inoculum onto selective media. Forty-eight hours after infection, the mice were euthanized by CO2 asphyxiation, the spleens were dissected, and each spleen was homogenized in sterile phosphate-buffered saline. Ratios of each strain in each spleen were calculated from bacterial counts produced by plating aliquots of 1:10 dilutions of homogenized spleen on selective media. The competitive index was calculated by dividing the ratio of bacteria isolated from the spleen by the ratio of bacteria inoculated into the mouse. Competitive index results were determined by calculating the means standard deviations for 10 mice. Statistical significances were determined using PRISM 5 (GraphPad).
RESULTS
SseJ Competes with the Eukaryotic RhoA-activated Proteins, ROCK, PKN1, and Rhotekin, for Binding to RhoA
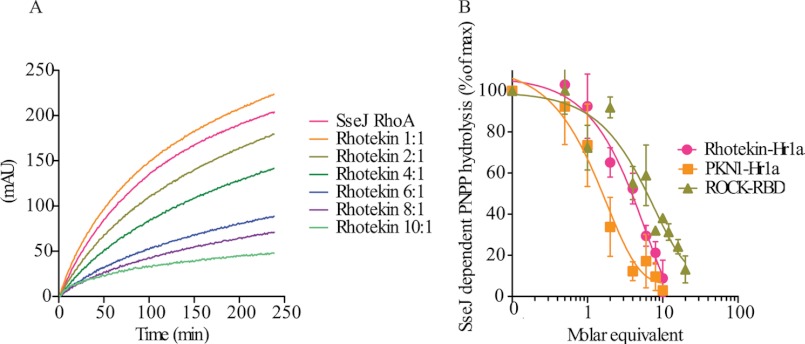
The observation that SseJ is activated upon binding RhoA-GTP demonstrated that SseJ is regulated similarly to eukaryotic effectors that interact with RhoA (10). Whether SseJ evolved to bind a similar RhoA surface as eukaryotic effectors, SseJ must be able to compete effectively with eukaryotic effectors with adequate affinity for binding to RhoA. To test whether SseJ activation by RhoA could occur in the presence of eukaryotic effectors, activation of SseJ lipase activity was measured using the chromogenic substrate, pNPP, in the presence of increasing concentrations of three recombinant RhoA effector binding domains, Rhotekin-Hr1a, ROCK-RBD, or PKN1-Hr1a. SseJ competed with all three effectors with similar kinetics, where at a 1:1 molar ratio of SseJ to eukaryotic effector, the rate of SseJ catalyzed pNPP hydrolysis in the presence of RhoA-GTP remained indistinguishable from when no effector was present (Fig. 1, A and B). However, increasing concentrations up to 10 molar equivalents of eukaryotic effector relative to SseJ resulted in almost complete inability of RhoA to activate SseJ. In contrast, the presence of 10 molar equivalents of a control protein, bovine γ-globulin, had no inhibitory effect on SseJ-RhoA-GTP activity (data not shown). The binding affinity between RhoA-GTP and the binding domains of eukaryotic effectors have been measured for Rhotekin, PKN1, and ROCK, at 147 nm, 150 nm, and 130 nm, respectively (20). Our results suggest that either SseJ has a greater affinity than eukaryotic effectors for RhoA, or that SseJ recognizes a surface of RhoA-GTP that differs from that recognized by the eukaryotic RhoA-binding proteins.
FIGURE 1.
SseJ competes with RhoA eukaryotic effectors for binding to RhoA. SseJ-dependent lipase activation by RhoA was assayed by hydrolysis of pNPP using 250 nm SseJ and RhoA-GTPγS, with (A) increasing equivalents of the RhoA binding domain of Rhotekin and (B) increasing equivalents of the RhoA binding domains of Rhotekin, PKN1, and ROCK. Average of three experiments with S.E. (error bars) plotted demonstrate that SseJ can compete with all three eukaryotic effectors equally.
The RhoA-GDP Solution Structure Demonstrates That SseJ Binding Perturbs the Resonances of a Large Number of Residues on the RhoA Surface
A combination of heteronuclear two-dimensional and three-dimensional NMR was used to examine binding interactions between RhoA and SseJ. The resonances of backbone amides in NMR spectra are sensitive indicators of their environment, and interaction with another protein perturbs the resonances of residues located at the protein interface (21). Resonance assignments for RhoA-GDP and RhoA-GTPγS complexes, containing the first 181 residues and lacking the extreme C-terminal residues that are highly hydrophobic and contain a lipid modification site, have been published previously (22, 23). Multidimensional HNCA and HNCACB NMR spectra were collected for RhoA-GDP and RhoA-GTPγS complexes to verify backbone assignments under new buffer conditions. 122 backbone amide resonances of an expected 169 were assigned for the RhoA-GTPγS complex whereas 150 backbone amide resonances were assigned for the RhoA-GDP complex. Most of the missing assignments in the RhoA-GTPγS complex correspond to residues in the nucleotide binding and switch regions of RhoA. The likely explanation is that the RhoA conformation induced by binding of GTP leads to weak self-association of RhoA-GTP mediated by the switch regions leading to excessive peak broadening and loss of resonances for residues involved in protein self-association.
Consistent with our previous observations that RhoA-GTPγS and SseJ form a stable complex, RhoA-GTPγS resonances observed in 1H-15N-TROSY spectra exhibit a uniform decrease in intensity with increasing concentrations of RhoA-GTPγS. The observed behavior is indicative of the formation of a large complex (>50 kDa) that is in slow exchange on the NMR time scale, making it difficult to study the binding interactions using RhoA-GTPγS (Fig. 2A). However, to identify RhoA residues that directly interact with SseJ, 1H-15N-TROSY spectra were collected on the relatively weaker complex formed between RhoA-GDP and SseJ. We reasoned that the interaction surface would be similar for both forms of RhoA because SseJ binds both RhoA-GTPγS and RhoA-GDP, albeit with different affinities. Binding of RhoA-GDP to SseJ forms a complex that is in the fast-to-intermediate exchange regime on the NMR time scale (Fig. 2B). Under these conditions, RhoA resonances for residues at the binding interface selectively shift or broaden with increasing concentrations of SseJ.
FIGURE 2.
Subset of RhoA residues change chemical environment upon interaction with SseJ. A and B, overlay of 1H,15N-RhoA of (A) 300 μm 15N-RhoA-GTPγS in the absence (black) and presence (red) of 75 μm SseJ and (B) 300 μm 15N-RhoA-GDP in the absence (black) and presence (red) of 75 μm SseJ are shown. A, SseJ caused a uniform loss in peak intensity because of peak broadening in the RhoA-GTPγS spectrum, suggesting tight binding. B, SseJ changed chemical environments of a subset of RhoA-GDP resonances. C, the resonances of 15N-labeled RhoA-GDP residues that broaden (red) or shift (green) upon addition of SseJ are mapped onto the crystal structure of RhoA-GTP (PDB: 1A2B). D, important features of the RhoA-GTP structure are highlighted: switch I (green), switch II (purple), and inset helix (yellow).
As expected, a subset of RhoA-GDP resonances were affected upon addition of SseJ. Resonances of residues of RhoA-GDP that were shifted or that underwent peak broadening were mapped onto the crystal structure of RhoA (Fig. 2C; PDB: 1A2B). For comparison, the locations of switch I and II regions and the insert helix of RhoA are identified on the crystal structure (Fig. 2D). A large number of resonances of residues involved in RhoA nucleotide binding and the switch I and switch II of RhoA was primarily broadened by increasing concentrations of SseJ. In contrast, residues in the insert helix of RhoA were observed to shift when SseJ bound to RhoA-GDP. Little to no changes to RhoA residues on the side opposite of the switch regions were observed. These observations indicated that SseJ binds to a surface of RhoA that includes the RhoA switch regions, similar to other eukaryotic effectors that recognize RhoA. Thus, SseJ likely competes with more abundant eukaryotic effector proteins for binding to the same surface on RhoA.
RhoA Binding and Activation of SseJ Are Mediated Predominantly by Switch II
Two classes of resonances were identified in the NMR titration experiments: resonances in switch I and switch II that broaden and disappear and resonances in the insert helix that shift upon addition of SseJ. Observation of two distinct types of behavior in separate regions of RhoA suggests that binding of SseJ to one site may induce additional secondary affects at a second site. To identify the specific surface of RhoA involved in the binding and activation of SseJ, alanine point mutations were introduced in the surface-exposed residues of the two switch regions of RhoA. These RhoA residues have been mutated and studied previously (13, 24, 25). The RhoA insert helix was replaced with the corresponding loop from Ras (RhoAΔRas) as mutated previously (15). These mutations were introduced into a constitutively active (CA) mutant of RhoA, His-RhoAG14V (CA His-RhoA), and each mutant was affinity-purified and tested for the ability to activate SseJ (Fig. 3A). Activity experiments were performed using 250 nm SseJ with increasing concentrations of CA His-RhoA or each of the 14 CA His-RhoA mutants. Lipase activity of SseJ similar to CA His-RhoA was observed for nine of the mutants generated, including CA His-RhoAΔRas (supplemental Fig. S1). However, five of the CA His-RhoA mutants generated exhibited reduced ability to activate SseJ to varying degrees (Fig. 3A). A summary of these data is shown in supplemental Table S3.
FIGURE 3.
RhoA interacts with SseJ using an overlapping surface as the eukaryotic effectors PKN1 and ROCK. A, lipase assays in the presence of 250 nm SseJ and increasing concentrations of CA His-RhoA, CA His-RhoAF39A, CA His-RhoAR68A, CA His-RhoAL69A, CA His-RhoAL72A, CA His-RhoAF106A, or CA His-RhoAF39A L72A demonstrate residues important for activation of SseJ. B, purified GST-SseJ and CA His-RhoA, CA His-RhoAF39A, CA His-RhoAR68A, CA His-RhoAL69A, CA His-RhoAL72A, CA His-RhoAF106A, or CA His-RhoAF39A L72A were mixed with glutathione beads. Each input and bound sample was blotted with nickel-HRP for the CA His-RhoA and mutants. The bound samples were also evaluated for GST-SseJ by staining of the membrane with Ponceau S. C, residues important for RhoA activation of and binding to SseJ are highlighted on the crystal structure of RhoA-GTP in orange. Residues evaluated but not involved in activation of SseJ (supplemental Fig. S1A) are highlighted in teal. Residues involved in RhoA binding to PKN1 (D) or ROCK (E) are highlighted in yellow. Residues involved in SseJ binding are highlighted in red. Residues involved in binding both PKN1 and SseJ (D) or ROCK and SseJ (E) are highlighted in orange on the RhoA-GTP crystal structure.
One mutation within switch I (CA His-RhoAF39A) of RhoA demonstrated reduced activation of SseJ. At concentrations of 1,000 nm, CA His-RhoAF39A was able to activate SseJ to ∼69% of the level observed for CA His-RhoA. In contrast, three mutations (R68A, L69A, and L72A) within switch II of RhoA significantly reduced the ability of RhoA to activate SseJ. The CA His-RhoAL69A mutant exhibited a modest decrease (52%) in the ability to activate SseJ compared with CA His-RhoA. In contrast, the CA His-RhoAR68A and CA His-RhoAL72A mutants were almost completely unable to activate SseJ enzymatic activity (2 and 17%, respectively). A CA His-RhoAF39A L72A double mutant showed a more dramatic loss, activating SseJ to only 1% of the level of CA His-RhoA at comparable concentrations. Additionally RhoAF106, which is a residue neighboring but not within the switch regions of RhoA, is important for activation of SseJ enzymatic activity because CA His-RhoAF106A activated SseJ to only 6% of that compared with CA His-RhoA at 1,000 nm.
To determine whether the RhoA residues involved in activating SseJ were also those involved in directly binding SseJ, each mutant that showed a decrease in the ability to activate SseJ was tested for the ability to bind GST-SseJ. Purified GST-SseJ was mixed with CA His-RhoA or each of the five mutants CA His-RhoA and glutathione beads. CA His-RhoA pulled down with GST-SseJ, not with GST control. However, each of the five mutants that exhibited reduced ability to stimulate SseJ activity was unable to interact with SseJ in these pulldown assays (Fig. 3B). Thus, all of the mutations identified that altered the ability of RhoA to activate SseJ also decreased the ability of RhoA to bind to GST-SseJ, indicating that the binding and activation surfaces of RhoA for SseJ are the same.
The mutational analysis showed that residues in switch II of RhoA are important for activation of SseJ. These findings were consistent with our results showing that SseJ could effectively compete with eukaryotic effectors for binding to RhoA. A combination of evidence from cocrystal structures of eukaryotic effector binding domains with RhoA and mutational studies has demonstrated the specific residues important in mediating the interaction between these effectors and RhoA (11, 12, 24). Interestingly, two invariant leucines RhoALeu69 and RhoALeu72 that are implicated in most small GTPase contacts with eukaryotic effectors or eukaryotic regulators are also involved in the SseJ interaction with RhoA (26). When residues that are involved in PKN1 (Fig. 3E) or ROCK (Fig. 3D) binding to RhoA are compared with the residues within RhoA that are involved in binding SseJ (Fig. 3C), it is apparent that binding of each effector or SseJ to RhoA must be mutually exclusive because similar residues within RhoA are involved. PKN1, ROCK, and SseJ specifically sense the nucleotide-bound state of RhoA, RhoB, and RhoC and are not activated by other small GTPases such as Cdc42 or Rac1 (10, 24, 26). PKN1 and ROCK both bind to RhoAGlu40 within switch I, and this is the only residue used by these eukaryotic effectors that differs between RhoA and other small GTPases. In contrast, RhoAGlu40 is not critical to the interaction of SseJ with RhoA; the only residue used by SseJ that varies between RhoA and other small GTPases is outside of the switch region in RhoAPhe106, suggesting the possibility that this residue is used by SseJ to distinguish RhoA from other small GTPases. However, SseJ is not unique in recognizing this residue because RhoAPhe106, in addition to RhoAGlu40, appears to be used by another RhoA eukaryotic effector, mDia1, to distinguish RhoA from other small GTPases (13, 15, 25). Therefore, we conclude that SseJ uses a similar surface to bind to RhoA as eukaryotic proteins and likely competes effectively by a relatively increased affinity for this surface.
SseJ Activation by RhoA in HeLa Cells Is Critical for Enzymatic Activity
Six sseJ mutant genes predicted to synthesize proteins with altered RhoA binding surfaces were generated based upon information regarding the SseJ-RhoA binding surface provided by Dr. Jijie Chai (Tsinghua University). Transfected SseJ has been shown to localize to and alter lysosomal-associated membrane protein (LAMP)-1-positive membranous compartments independently of additional RhoA expression, and SseJ recruits endogenous activated RhoA to this location upon transfection (5, 6). This indicates that the presence of endogenous RhoA in cells is sufficient for SseJ activity on endosomes, which can be measured in transfected SseJ by its ability to cleave the fluorescent phospholipase substrate PEDA1 in HeLa cells (27).
The six sseJ mutants were screened for the ability to localize to and alter LAMP-1-positive endosomal compartments by expression of these genes in HeLa cells. HeLa cells were transiently transfected with epitope-tagged SseJ and CA RhoA, and SseJ mutants were monitored for colocalization and recruitment of CA RhoA and for their ability to cleave PEDA1. Five of the SseJ mutants tested (SseJQ172L, SseJR177A, SseJD368L, SseJI373D, and SseJQ387L) demonstrated localization to membranous compartments and colocalization with CA RhoA (Fig. 4A and supplemental Fig. S2A). SseJF121D did not recruit CA RhoA and was diffuse within cells (Fig. 4A). SseJR177A, which recruited RhoA, and SseJF121D that was diffuse in cells were purified and tested for binding to RhoA and activation by RhoA. At 250 nm, there was no activation of deacylase activity for either SseJR177A or SseJF121D in the presence of RhoA-GTPγS (data not shown). Pulldown experiments using purified GST-RhoA and His-tagged SseJR177A or SseJF121D demonstrated that SseJR177A binds to RhoA, whereas SseJF121D does not (data not shown). This result suggests that SseJ must bind to RhoA to localize to endosomal membranes in addition to binding RhoA for potentiating SseJ enzymatic activity.
FIGURE 4.
SseJ point mutants that bind CA-RhoA colocalize with LAMP-1. A, HeLa cells transfected for 24 h with plasmids encoding myc-SseJ, Myc-SseJF121D or Myc-SseJR177A, and CA HA-RhoA stained with anti-Myc (red) and anti-HA (green) antibodies. Scale bar, 5 μm. B, HeLa cells transfected for 24 h with plasmids encoding Myc-SseJ, Myc-SseJ3x, Myc-SseJF121D, or Myc-SseJR177A stained with anti-Myc (red) and anti-LAMP-1 (green) antibodies. Scale bar, 5 μm.
SseJ mutant proteins that bind RhoA similar to wild-type cells localized to the endosomal compartment after transfection of HeLa cells. Epitope-tagged SseJ mutants colocalized with LAMP-1 except the SseJF121D mutant that has lost binding to RhoA as this mutant protein demonstrated a diffuse localization pattern consistent with a lack of binding to RhoA (Fig. 4B). Each of the five SseJ mutants that localized to membranous compartments with CA RhoA also colocalized with LAMP-1 but visually did not appear to modify the LAMP-1-positive compartment like wild-type SseJ (Fig. 4B and supplemental Fig. S2B). The SseJ catalytic mutant (SseJ3x) localized to LAMP-1 similarly to SseJR177A. These data were consistent with the requirement of SseJ activity for modification of the endosomal compartment (6, 10). To rule out the possibility that the mutant proteins were altered in folding or stability, each mutant protein was expressed in HeLa cells and examined for stability by Western blotting of Myc-tagged SseJ (supplemental Fig. S3A). Additionally, each SseJ mutant was purified, and its CD spectrum was analyzed and compared with WT SseJ. These data support the idea that these proteins are properly folded and have similar stability as wild-type proteins (supplemental Fig. S3B).
Incubation of HeLa cells transfected with SseJ with PEDA1 results in the formation of fluorescent cholesterol dependent upon SseJ catalytic activity (10). The six SseJ mutants were transiently transfected into HeLa cells, and PEDA1 was added for 2 h to visualize SseJ activity by fluorescence microscopy. Cells transfected with SseJ were able to cleave PEDA1 measured as a statistically significantly increase in fluorescence above levels examined for untransfected cells as demonstrated previously (27). In contrast, no increase in fluorescence was detected for the sseJ mutants tested, indicating that they must alter SseJ activation by RhoA (Fig. 5, A and B). These data demonstrate that SseJ colocalization with LAMP-1 may be dependent on the ability of SseJ to bind RhoA within cells and that alteration of the SseJ binding interface with RhoA can alter SseJ enzymatic activity.
FIGURE 5.
SseJ activation by RhoA is essential for phospholipase activity within HeLa cells. A, HeLa cells transfected with a plasmid encoding Myc-SseJ, Myc-SseJF121D, Myc-SseJQ172L, Myc-SseJR177A, Myc-SseJD368L, Myc-SseJI373D, or Myc-SseJQ387L for 22 h and then incubated for 2 h with 3.5 μm PEDA1 (green) and stained with anti-Myc (red) antibodies. Scale bar, 20 μm. B, mean intensity of cellular BODIPY fluorescence measured using the NIS-Elements image analysis software for 10 fields per condition. Statistical analysis by one-way ANOVA followed by Dunnett's multiple comparison demonstrate that transfection of wild type, but not each of the SseJ mutants tested, resulted in a significant increase in cellular BODIPY fluorescence compared with untransfected cells (p < 0.0001).
S. typhimurium Strains with Mutations in the SseJ Binding Surface with RhoA Are Altered for Systemic Virulence for Mice
To verify that these proteins are expressed and translocated by S. typhimurium, HeLa cells were infected with a ΔsseJ mutant expressing SseJF121D-HA and SseJR177A-HA as examined previously for SseJ-HA (7). SseJF121D-HA was diffusely localized within HeLa cells when delivered by S. typhimurium, whereas SseJR177A-HA was localized to LAMP-1-positive Sifs (supplemental Fig. S4). To determine whether SseJ binding and activation by RhoA are required for S. typhimurium virulence for mice, we tested the virulence phenotype of strains chromosomally expressing SseJ-HA, the catalytic mutant (SseJ3x-HA), SseJF121D-HA, which cannot bind RhoA, and SseJR177A-HA, which can bind RhoA but has no detectable activity in cells (by competitive index). To verify that the plasmid-based antibiotic resistance markers did not influence the competitive index ratios, the chromosomally expressed wild-type SseJ-HA strain containing either a KanR (pWSK129) or AmpR (pWSK29) marker was competed and found to compete equally (0.90 ± 0.14) (Fig. 6). SseJ3x-HA, a catalytic inactive mutant expressed on the chromosome competed against wild-type (SseJ-HA), exhibits a competitive index defect of 0.38 ± 0.18, which is comparable with previous competition results from ΔsseJ strains expressing SseJ3x on a plasmid competed against wild-type (0.44 ± 0.06) (7). When wild-type (SseJ-HA) was competed against the strain expressing either SseJF121D-HA or SseJR177A-HA, phenotypes of 0.42 ± 0.19 and 0.36 ± 0.15 were observed. These competitive indices are not significantly different from the wild-type (SseJ-HA) competed against a strain expressing catalytically inactive SseJ3X-HA. These results demonstrate that SseJ activation by RhoA is required for systemic virulence in mice and that specific interactions at the SseJ binding surface with RhoA are necessary but not sufficient for full virulence.
FIGURE 6.
SseJ activation by RhoA is required for virulence. Female BALB/c mice were inoculated intraperitoneally with a 1:1 mixture of 5 × 104 S. typhimurium expressing (strain 1) wild-type SseJ with pWSK129 (Kan) competed against (strain 2) wild-type SseJ, the catalytic mutant SseJ3X, SseJF121D mutant, or SseJR177A mutant with pWSK29 (Amp50). The competitive index (CI) was calculated by dividing the ratio of strain 2 to strain 1 in the output by the ratio of strain 2 to strain 1 in the input. The graph demonstrates the CI within the spleen of each individual mouse (10 per competition) and denotes the mean ± S.E. (error bars) per group. Statistical analysis by one-way ANOVA followed by Dunnett's multiple comparison demonstrates that the CI value for the wild-type against wild-type competition is significantly different from the values derived for the competition between wild-type against the catalytic mutant SseJ3X, SseJF121D mutant, or SseJR177A mutant.
DISCUSSION
This study has demonstrated the structural basis by which SseJ is activated by the nucleotide-bound state of RhoA. SseJ was shown to have properties similar to eukaryotic small GTPase-activated proteins that bind to the conformational sensitive switch regions. Activation of SseJ by RhoA is mutually exclusive of binding eukaryotic effectors, and SseJ can compete effectively by likely having a greater affinity for a similar binding surface on activated RhoA. NMR experiments coupled with mutational studies examining the effect of SseJ binding to RhoA demonstrate that in solution SseJ perturbs a large number of residues on the RhoA surface but that only a subset of these residues is on the activation surface. Examination of two SseJ mutants in the binding interface with RhoA revealed that a specific interaction between SseJ and RhoA is required for enzymatic activation and systemic virulence for mice and that simple targeting of RhoA to the endosome does not by itself complement the virulence defect from abrogating SseJ enzymatic activity.
Eukaryotic RhoA-binding proteins that preferentially sense either the GTP- or GDP-bound state of RhoA specifically recognize the switch regions of RhoA for activation. Here, we show through RhoA protein NMR and mutational analysis of RhoA that SseJ also specifically recognizes the switch regions of RhoA. Amino acid substitutions of two residues whose conformations are nucleotide-dependent, RhoAPhe39 within switch I or RhoAArg68 within switch II, dramatically decrease the ability of RhoA to interact with and activate SseJ. Consistent with SseJ having a similar binding surface on RhoA as eukaryotic effectors, SseJ activation by RhoA can be competed by three eukaryotic effectors, PKN1, ROCK, and Rhotekin, implying mutually exclusive binding (Fig. 1). Many eukaryotic effectors including PKN1, ROCK, and Rhotekin utilize coil-coiled motifs to interact with a few residues within the RhoA switch regions (26), whereas SseJ does not have a recognizable predicted Rho binding domain (RBD) and interacts with a large surface of RhoA including the switch regions. This predicted convergent evolution of SseJ to recognize a similar surface of RhoA as eukaryotic effectors adds to the paradigm that bacterial effectors evolved very different mechanisms to mimic the behavior of eukaryotic proteins (28).
We determined previously using commercially available RhoB that SseJ was unable to bind RhoB in vitro (10). Because all residues involved in RhoA interaction with SseJ are invariant with RhoC and RhoB, and overexpression of CA RhoA, CA RhoB, and CA RhoC with SseJ can induce endosomal tubulation within HeLa cells (5), we reassessed SseJ-RhoB binding using RhoB that we purified and determined that SseJ is able to bind to and be activated by RhoB (data not shown). A number of eukaryotic effectors has been characterized extensively for concurrent binding to RhoA, RhoB, and RhoC, and it is difficult to establish which protein is the true binding partner of these effectors (29). Similarly, it is still unknown whether SseJ preferentially binds RhoA, RhoB, or RhoC in vivo. RhoB could be the preferred binding partner of SseJ because it is an endosomal GTPase (30) and has been demonstrated to recruit the RhoA effector protein PKN1 to the endosomal compartment (31). SseJ has been demonstrated to specifically bind to the RhoABC family but not to Cdc42 or Rac1 (10), suggesting that specific residues important for interaction must differ between RhoA and Cdc42 and Rac1. We identified a single amino acid, Phe-106, that is variable between RhoA and Cdc42 or Rac1 that is involved in the RhoA interaction with SseJ, which is located outside of the switch regions of RhoA but is critical for RhoA binding and activation of SseJ. This residue, Phe-106, is not utilized by the two effectors crystallized with RhoA, PKN1, or ROCK (11, 12) but is required by mDia1, which has been crystallized with RhoC (25) and has been demonstrated to bind RhoA also. These results indicate that SseJ uses conserved residues to differentiate the nucleotide-bound state of RhoA as eukaryotic effectors and distinguishes RhoABC from other Rho GTPases in a similar manner to at least one eukaryotic effector. It is tempting to speculate that the larger binding surface allows SseJ to compete effectively with eukaryotic effectors in vitro; although in vivo, the relatively large excess of RhoA may make this possibility not relevant.
SseJ localization to the endosomal compartment was demonstrated previously to be independent of enzymatic activity and was presumed to be independent of binding RhoA because expression of SseJ within host cells results in recruitment of RhoA to the endosomal compartment. A mutation in the SseJ binding interface, SseJF121D, indicates that SseJ binding to RhoA is required for SseJ localization to the endosomal compartment because SseJF121D is diffusely localized upon transient expression within HeLa cells. This suggests that SseJ localization to the endosome is dependent on at least the nature of and possibly the formation of the SseJ-RhoA complex. Because RhoA is not normally localized to the endosomal compartment, we speculate that an unidentified host factor is involved in recruitment of the complex to the endosomal vacuole. This factor is unlikely to be RhoB given the results herein and the results that RhoB is at best equivalent to RhoA in binding and activation of SseJ. This discovery suggests that SseJ activity, in addition to being temporally regulated by the signaling state of RhoA, is also spatially regulated, further indicating how very specific regulation of SseJ enzymatic activity is important for virulence. We demonstrated a similar virulence defect in mice for S. typhimurium expressing catalytically inactive SseJ3x or SseJF121D. These results show that specific SseJ binding to RhoA is necessary for virulence. However, binding is not sufficient for virulence, consistent with the idea that recruitment alone of RhoA by SseJ is not a virulence mechanism. Instead, this requirement is because of in vivo activation of SseJ glycerophospholipid:cholesterol acyltransferase activity by sensing of the RhoA activation state at a very specific time in the endosomal maturation of the Salmonella-containing vacuole.
Supplementary Material
Acknowledgments
NMR experiments were performed, in part, in the Environmental Molecular Sciences Laboratories at the Pacific Northwest National Laboratories. We thank R. Klevit and B. Kulasekara for discussions and critical reading of this manuscript.
This project was supported, in whole or in part, by National Institutes of Health Grant R01 AI048683.

This article contains supplemental Figs. S1–S4 and Tables S1–S3.
- pNPP
- p-nitrophenyl palmitate
- RBD
- Rho binding domain
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- CA
- constitutively active
- TROSY
- transverse relaxation optimized spectroscopy.
REFERENCES
- 1. Kuhle V., Hensel M. (2002) SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell. Microbiol. 4, 813–824 [DOI] [PubMed] [Google Scholar]
- 2. Freeman J. A., Ohl M. E., Miller S. I. (2003) The Salmonella enterica serovar typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 71, 418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brumell J. H., Goosney D. L., Finlay B. B. (2002) SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic 3, 407–415 [DOI] [PubMed] [Google Scholar]
- 4. Boucrot E., Henry T., Borg J. P., Gorvel J. P., Méresse S. (2005) The intracellular fate of Salmonella depends on the recruitment of kinesin. Science 308, 1174–1178 [DOI] [PubMed] [Google Scholar]
- 5. Ohlson M. B., Huang Z., Alto N. M., Blanc M. P., Dixon J. E., Chai J., Miller S. I. (2008) Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tabulation. Cell Host Microbe 4, 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruiz-Albert J., Yu X. J., Beuzón C. R., Blakey A. N., Galyov E. E., Holden D. W. (2002) Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol. Microbiol. 44, 645–661 [DOI] [PubMed] [Google Scholar]
- 7. Ohlson M. B., Fluhr K., Birmingham C. L., Brumell J. H., Miller S. I. (2005) SseJ deacylase activity by Salmonella enterica serovar typhimurium promotes virulence in mice. Infect. Immun. 73, 6249–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lossi N. S., Rolhion N., Magee A. I., Boyle C., Holden D. W. (2008) The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid:cholesterol acyltransferase activity. Microbiology 154, 2680–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nawabi P., Catron D. M., Haldar K. (2008) Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol. Microbiol. 68, 173–185 [DOI] [PubMed] [Google Scholar]
- 10. Christen M., Coye L. H., Hontz J. S., LaRock D. L., Pfuetzner R. A., Megha, Miller S. I. (2009) Activation of a bacterial virulence protein by the GTPase RhoA. Sci. Signal. 2, ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maesaki R., Ihara K., Shimizu T., Kuroda S., Kaibuchi K., Hakoshima T. (1999) The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol. Cell 4, 793–803 [DOI] [PubMed] [Google Scholar]
- 12. Dvorsky R., Blumenstein L., Vetter I. R., Ahmadian M. R. (2004) Structural insights into the interaction of ROCKI with the switch regions of RhoA. J. Biol. Chem. 279, 7098–7104 [DOI] [PubMed] [Google Scholar]
- 13. Lammers M., Meyer S., Kühlmann D., Wittinghofer A. (2008) Specificity of interactions between mDia isoforms and Rho proteins. J. Biol. Chem. 283, 35236–35246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker S. J., Brown H. A. (2002) Specificity of Rho insert-mediated activation of phospholipase D1. J. Biol. Chem. 277, 26260–26267 [DOI] [PubMed] [Google Scholar]
- 15. Zong H., Kaibuchi K., Quilliam L. A. (2001) The insert region of RhoA is essential for Rho kinase activation and cellular transformation. Mol. Cell. Biol. 21, 5287–5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sattler M., Schleucher J. R., Griesinger C. (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Res. Spectroscopy 34, 93–158 [Google Scholar]
- 17. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 18. Johnson B. A., Blevins R. A. (1994) NMR view: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 19. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenstein L., Ahmadian M. R. (2004) Models of the cooperative mechanism for Rho effector recognition: implications for RhoA-mediated effector activation. J. Biol. Chem. 279, 53419–53426 [DOI] [PubMed] [Google Scholar]
- 21. Zuiderweg E. R. (2002) Mapping protein-protein interactions in solution by NMR spectroscopy. Biochemistry 41, 1–7 [DOI] [PubMed] [Google Scholar]
- 22. Cierpicki T., Bielnicki J., Zheng M., Gruszczyk J., Kasterka M., Petoukhov M., Zhang A., Fernandez E. J., Svergun D. I., Derewenda U., Bushweller J. H., Derewenda Z. S. (2009) The solution structure and dynamics of the DH-PH module of PDZRhoGEF in isolation and in complex with nucleotide-free RhoA. Protein Sci. 18, 2067–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasmi-Seabrook G. M., Marshall C. B., Cheung M., Kim B., Wang F., Jang Y. J., Mak T. W., Stambolic V., Ikura M. (2010) Real-time NMR study of guanine nucleotide exchange and activation of RhoA by PDZ-RhoGEF. J. Biol. Chem. 285, 5137–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutchinson C. L., Lowe P. N., McLaughlin S. H., Mott H. R., Owen D. (2011) Mutational analysis reveals a single binding interface between RhoA and its effector, PRK1. Biochemistry 50, 2860–2869 [DOI] [PubMed] [Google Scholar]
- 25. Rose R., Weyand M., Lammers M., Ishizaki T., Ahmadian M. R., Wittinghofer A. (2005) Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435, 513–518 [DOI] [PubMed] [Google Scholar]
- 26. Dvorsky R., Ahmadian M. R. (2004) Always look on the bright site of Rho: structural implications for a conserved intermolecular interface. EMBO Rep. 5, 1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christen M., Coye L. H., Hontz J. S., LaRock D. L., Pfuetzner R. A., Megha, Miller S. I. (2009) Activation of a bacterial virulence protein by the GTPase RhoA. Sci. Signal. 2, ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galán J. E. (2009) Common themes in the design and function of bacterial effectors. Cell Host Microbe 5, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler A. P., Ridley A. J. (2004) Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 301, 43–49 [DOI] [PubMed] [Google Scholar]
- 30. Adamson P., Paterson H. F., Hall A. (1992) Intracellular localization of the P21ρ proteins. J. Cell Biol. 119, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mellor H., Flynn P., Nobes C. D., Hall A., Parker P. J. (1998) PRK1 is targeted to endosomes by the small GTPase, RhoB. J. Biol. Chem. 273, 4811–4814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.