Background: APOBEC3G (A3G), which is part of cytidine deaminase family, significantly inhibits miRNA-mediated repression of translation, but the mechanism remains unknown.
Results: A3G competitively binds to the C terminus of MOV10 to inhibit the interaction between AGO2 and MOV10.
Conclusion: A3G affects the normal assembly or maturation of miRISC.
Significance: Our data demonstrate a novel mechanism of the regulation of miRISC activity.
Keywords: Interferon, MicroRNA, RNA Helicase, RNA Silencing, RNA-binding Proteins, APOBEC3G, Ago2, MOV10, miRISC
Abstract
The apolipoprotein-B-mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G or A3G) is a potent restrictive factor for human immunodeficiency virus type 1 (HIV-1) and many other retroviruses. It belongs to the cytidine deaminase family. Recent studies have shown that A3G significantly inhibits microRNA (miRNA)-mediated repression of translation. However, the mechanism underlying this action must be clarified. In this report, we have demonstrated that A3G counteracts miRNA-mediated repression of translation by inhibiting the interaction between moloney leukemia virus 10 (MOV10) protein and Argonaute-2 (AGO2), causing either abnormal assembly or abnormal maturation of miRNA-inducing silencing complex (miRISC). Through a series of MOV10 deletions, we found that A3G binds to a domain at the C terminus in MOV10, where it competitively inhibits the binding of AGO2 to that same domain. The interaction between A3G and MOV10 relies on its association with a small RNA named 7SL RNA. The A3G mutant W127L, which is unable to bind to 7SL RNA, shows significantly incapability to counteract the miRNA-mediated repression of translation. Our data demonstrate a novel mechanism involved in the regulation of miRISC activity. Although both A3G and MOV10 belong to the interferon antiviral system and inhibit HIV-1 and other retroviruses, their opposing effects on the cellular miRNA activity suggest that they play much more complicated regulatory roles in various cellular functions.
Introduction
MicroRNAs (miRNAs)3 are 20 to 22 nucleotides (nts) long. They participate in the regulation of various biological functions in many eukaryotic lineages, including plants, animals, and fungi (1–3). The expression of many miRNAs are usually specific to a tissue or a developmental stage, and the miRNA expression pattern can be altered during the development of many diseases. Mature miRNAs either cleaves mRNAs or inhibit their translation to cause gene silencing. They are typically associated with miRNA-induced silencing complex (miRISC), which is a large molecular mass complex containing Argonaute and several other proteins (4–7). The miRISC that engages in mRNA cleavage requires perfect sequence complementary to the 3′-untranslated region (3′-UTR) of target mRNA. AGO2 plays an important role in this kind of RNA silencing. However, the miRISC-mediated repression of protein translation requires only partial sequence complementary to the 3′-UTR of target mRNA. AGO1 plays a predominant role in this kind of inhibition (8, 9).
Cellular A3G belongs to the APOBEC3 family, members of which have cytidine deaminase activity. Tandem affinity purification (TAP) assay has been used by several groups to examine A3G-associated proteins. Almost all A3G-associated proteins are RNA-binding proteins, which suggests that A3G is involved in RNA processing or metabolism (10–13). A3G also binds to many P-body-related proteins and co-localizes with them in both P-bodies and stress granules (11, 12, 14). It is associated with AGO1, AGO2, and MOV10. These associations are “bridged” by RNAs. Because these proteins are components of the miRISC, A3G can be involved in miRNA-related activities (11, 12, 14). We have previously demonstrated that A3G and other APOBEC3 family members can counteract the inhibition of protein translation by various miRNAs (12). Through various approaches, we here demonstrate that A3G counteracts miRNA-mediated gene silencing by competitively inhibiting the interactions between MOV10 and AGO2.
EXPERIMENTAL PROCEDURES
Plasmids
Wild-type and mutant plasmids expressing A3G were constructed as described previously (12, 15). Human MOV10 with FLAG or HA epitope tag sequences at their 3′ termini were amplified through reverse transcription-polymerase chain reaction (RT-PCR) with the mRNA of 293T cells as the template. The accuracy was confirmed by DNA sequencing. The tagged MOV10 was then inserted into pcDNA3.1 vector. The pλN-AGO1 and pλN-AGO2 were provided by the laboratory of Dr. Hannon of Cold Spring Harbor Laboratory. Human AGO1 and AGO2 with FLAG and HA tag sequences at their N termini were amplified from the pλN-AGO1 and pλN-AGO2 via PCR, and the accuracy was confirmed by DNA sequencing. The tagged AGO1 or AGO2 was then inserted into pcDNA3.1 vector. A series of deletion mutants of MOV10 were generated via PCR-based mutagenesis, as we have described previously (16). The miRNA reporter constructs used in this study (psi-per-mir16 and psi-per-mir25) were generated by direct insertion of the annealed corresponding miRNA-binding sites into the 3′-UTR region of Renilla luciferase gene in psiCHECK-2 vector (Promega) between XhoI and NotI sites. The psi-nonper-mir16 was generated by direct insertion of the annealed corresponding 3′-UTR fragment (1641–1676 nt) of the human CYCLIN E gene into the 3′-UTR region of Renilla luciferase gene in psiCHECK-2 vector (17). The psi-nonper-mir25 was generated by directly inserting the annealed corresponding 3′-UTR fragment (72–108 nt) of human TP53 gene into the 3′-UTR region of Renilla luciferase gene in psiCHECK-2 vector (18). The psi-5BoxB was constructed by direct insertion of the annealed corresponding five 19 nt BoxB hairpins into the 3′-UTR region of Renilla luciferase gene in psiCHECK-2 vector. Plasmids for SRP14 and plasmids expressing shRNA against SRP14 were provided by Dr. Strub of the University of Geneva, Switzerland (19, 20). Forward and reverse synthetic 60 nt nucleotides were used to construct plasmids expressing shRNA against green fluorescence protein gene (gfp) in the pSuper-Retro mammalian expression vector (Oligoengine). The target sequence in SRP14 for shRNA was 5′-AGGGCATACATTTCCTGCT-3′ and the target sequence in gfp for shRNA was 5′-ACGTAAACGGCCACAAGTT-3′.
Cell Culture and Transfection
Human 293T cells were grown at 37 °C in Dulbecco's modified Eagle's medium (DMEM) (Hyclone) supplemented with 10% fetal calf serum (Invitrogen), 100 units/ml of penicillin and 100 μg/ml of streptomycin. Human H9 cells were maintained in RPMI 1640 medium (Hyclone) supplemented with 10% fetal calf serum, 100 units/ml of penicillin and 100 μg/ml of streptomycin. 293T cells were transfected using Lipofectamine 2000 (Invitrogen) for plasmids or siRNAs. H9 cells were transfected using RNAiMax reagent (Invitrogen) for siRNAs. The procedures suggested by the manufacturer were followed.
Synthesis of siRNAs
The siGENOME SMARTpool siRNAs against MOV10 were designed and synthesized by Dharmacon. The target sequences in human MOV10 for siRNAs were as follows: siGENOME SMARTpool siMOV10–1, 5′-GGCGGAACCCAGUCUGUUA-3′; siGENOME SMARTpool siMOV10–2, 5′-GGUCAGAUAUCAGCAAACA-3′; siGENOME SMARTpool siMOV10–3, 5′-GCAGGAAUACCGGGUCUUA-3′; siGENOME SMARTpool siMOV10–4, 5′-CGGCAAGACUGUCACGUUA-3′. The target sequence in Renilla luciferase for siRNA was 5′-CAAGCAAGATCATGCGGAA-3′, the target sequence in A3G for siRNA was 5′-CCATGAAGATCATGAATTA-3′, and the target sequence in gfp for siRNA was 5′-ACGTAAACGGCCACAAGTTC-3′ (21). This last was used as a control.
Expression and Purification of Tagged A3G-containing Complexes
To isolate the proteins that interact with A3G, TAP was used followed by mass spectrum (MS) analysis. Briefly, A3G cDNA was inserted into pcDNA3.1 with the FLAG and HA tags at the N-terminal according to the instructions included in the TAP kit (Sigma). The 293T cells were transfected with FLAG- and HA-tagged A3G and lysed at 48 h post-transfection with a lysis buffer (150 mm NaCl, 50 mm Tris-HCl (pH 7.5), 1 mm EDTA, 1% Triton X-100, and 0.5% Nonidet P-40). The tandem purification of FLAG- and HA-tagged A3G was performed according to the instructions in the TAP kit. Then the A3G-associated proteins were analyzed with SDS-PAGE. To increase the sensitivity of protein detection, a ProteoSilver Plus Silver Stain Kit (Sigma) was used to stain the gel after SDS-PAGE. The visible protein bands were prepared for MS analysis.
Co-immunoprecipitation and Western Blotting
The 60-millimeter (mm)-diameter cultures of 293T cells were transiently transfected with 1.5 μg of A3G-HA-expressing plasmid and 3 μg of plasmid expressing MOV10-FLAG. Twenty-four hours later, cells were collected and disrupted with the lysis buffer containing protease inhibitor mixture (Roche) for 30 min at 4 °C. The cell lysates were then clarified by centrifugation at 18,000 × g for 30 min at 4 °C. After the cell lysates were pre-cleared with agarose beads, anti-HA agarose beads (Sigma) were mixed with the cell lysates and incubated at 4 °C for 4 h. The beads were then washed three times with cold lysis buffer and either eluted in gel loading buffer or treated with the RNase mixture (DNase free, Roche) (20 μg/ml) at 37 °C for 30 min, as described previously (11). The immunoprecipitated samples were analyzed by SDS-PAGE and detected using Western blotting, as described previously (22). Anti-FLAG (rabbit polyclonal, MBL), anti-HA (mouse monoclonal, Covance), anti-β-actin (rabbit polyclonal, CST), anti-GOT2 (mouse monoclonal, Thermo), anti-CREB3L2 (rabbit polyclonal, Proteintech), and anti-MOV10 (rabbit polyclonal, Abcam) were used as primary antibodies. Quantity One (Bio-Rad) was used to quantify the Western blotting results.
To detect the RNA bound to immunoprecipitated proteins, RNase inhibitor at 1000 units/ml (Invitrogen) was added into the immunoprecipitation (IP) lysis buffer. After centrifugation, the RNA-protein complex-associated beads were resuspended in 1 ml of Trizol (Invitrogen), and RNA was extracted according to the manufacturer's instructions.
RNA Detection and Quantification
The eluted and immunoprecipitated RNA samples were first treated with RNase-free DNase (Ambion) and then reverse transcribed using PrimeScript RT reagent kit (TaKaRa) with random hexanucleotide primers. The manufacturer's instructions were followed. The resulting cDNA was either undiluted or serially diluted in diethyl pyrocarbonate (DEPC)-treated water before addition into the real-time reaction to ensure that the amplification was within the linear range of detection. A CFX 96TM Real-Time System (Bio-Rad) was used for quantitative real-time PCR (qRT-PCR) amplification. All primers were synthesized by Invitrogen and qRT-PCR was performed using SYBR green methods as previously described with some modifications (23). The reactions were performed under the following conditions: 95 °C for 10 min followed by 40 cycles of 95 °C for 5 s and 60 °C for 35 s. The primer pairs used to amplify the target sequences (7SL RNA, 5S rRNA, tRNA-Phe, A3G, and GAPDH) were synthesized by Invitrogen as described previously (23).
Knockdown or Restoration of 7SL RNA
Modified versions of previously described procedures were used (19, 20, 24, 25). Briefly, 293T cells were seeded at 50–60% confluence in 60-mm diameter plates and transfected with SRP14-shRNA and gfp-shRNA using Lipofectamine 2000 (Invitrogen). The resulting siRNAs were used for cleaving the mRNAs of human SRP14 and gfp, respectively. The cells were then grown for 24 h in the presence of 3 μg/ml of puromycin (Sigma) to select transfected cells. Then cells were washed three times with phosphate-buffered saline (PBS) and replaced with fresh medium containing puromycin at 0.5 μg/ml. At 5 days post-transfection, the cells expressing shRNAs were replated into 24-well plates and further transfected with A3G-expressing plasmid and miRNA reporter plasmid. Forty-eight hours later, these cells were collected and lysed. Renilla/firefly luciferase activity was measured. For 7SL RNA restoration, 293T cells were transfected and selected as described above. At 96 h post-transfection, cells were plated at equal numbers into 24-well plates and transfected with plasmids expressing SRP14 or with pcDNA3.1 as a control. Twenty-four hours later, cells were further transfected with A3G-expressing plasmid and miRNA reporter plasmid. These cells were collected and lysed at 48 h post-transfection. Renilla/firefly luciferase activity was then measured.
RESULTS
The 7SL RNA Mediates the Interaction between A3G and MOV10
We used a TAP method to isolate A3G-associated proteins. A3G was tagged with a FLAG tag peptide and an HA-tag peptide to the N termini as described in the instruction included in the TAP kit (Sigma). The FLAG-HA-A3G-expressing plasmid was transfected to 293T cells and then A3G-containing protein complexes were extracted from 293T cells using a TAP kit. These proteins were separated by SDS-PAGE and then stained with silver. More than 11 bands were clearly visible in the gel. The 11 principal bands, shown in supplemental Fig. S1A, were excised and analyzed by ESI tandem MS to identify the constituent proteins. As in one previous report, many of the A3G-associated proteins identified in our analysis are known cellular RNA-binding proteins, such as poly (A)-binding protein (PABP1 or PABP2), elongation factor 1 α1 (EF1A1), S20 ribosomal protein and SS-A/Ro (Ro60) (11, 26, 27). We were especially interested in MOV10, which has been described as a novel component of Argonaute-associated RNP complex (9). Because A3G is also the Argonaute-associated protein and can counteract miRNA-mediated protein repression, we studied the interaction between A3G and MOV10 (11, 12). As shown in supplemental Fig. S1B, MOV10 was specifically co-immunoprecipitated with A3G but not with the control protein GFP. After RNase treatment, the interaction between A3G and MOV10 was reduced by ∼63% and only a few MOV10-FLAG proteins remained detectable (supplemental Fig. S1C), indicating that the interaction between MOV10 and A3G is largely dependent on RNA (11).
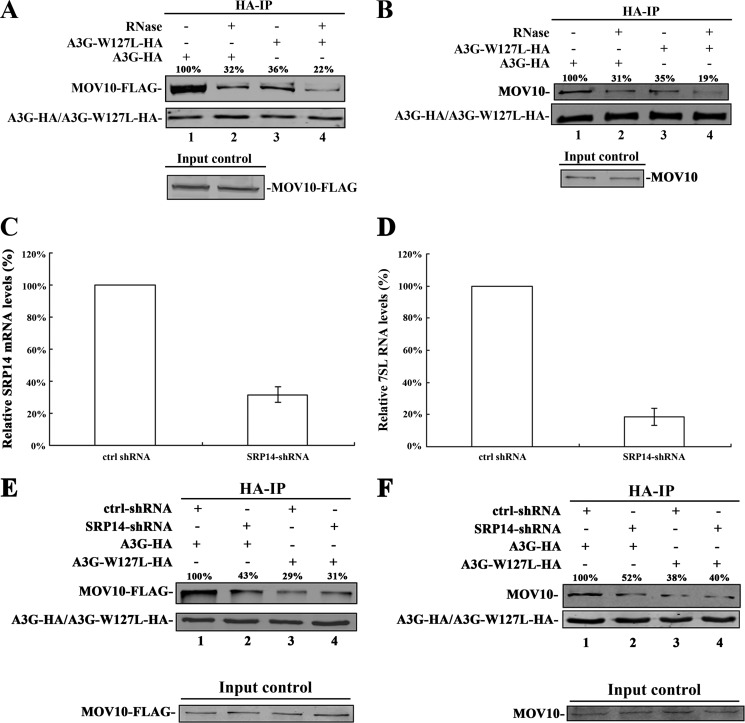
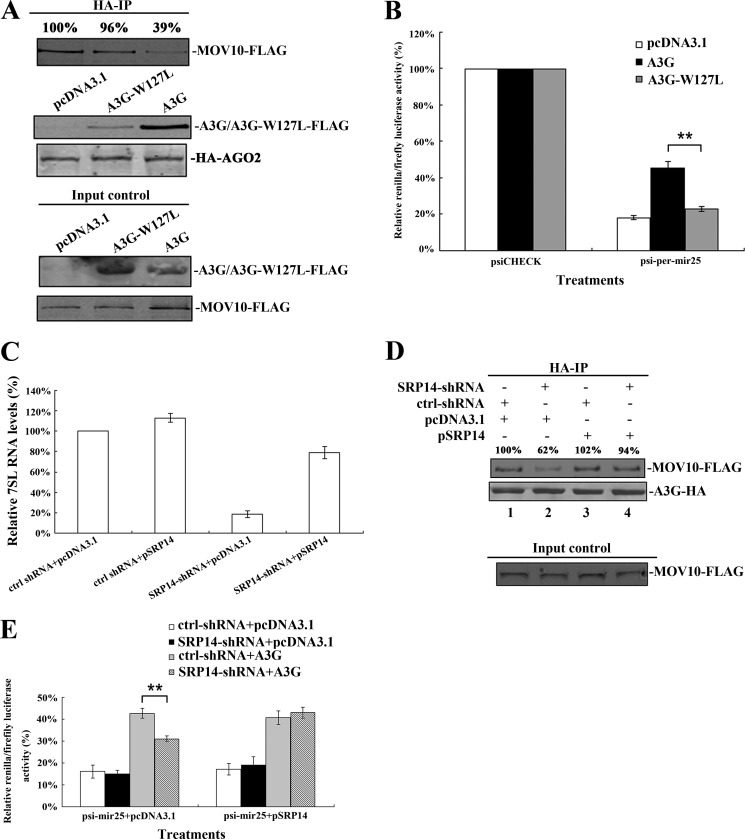
Many studies have found that A3G is associated with various cellular RNAs, including 7SL RNA (23, 24, 28). The 7SL RNA is a ∼300-nt-long RNA and an important component of signal recognition particles (SRP) (23, 24). Importantly, 7SL RNA interacts with A3G, acting as a mediator of A3G packaging into the virions of HIV-1 (23, 28). A single amino acid mutant of A3G, A3G-W127L, showed a ∼80% reduction in 7SL RNA binding relative to that of wild-type A3G. A3G-W127L retained binding affinity to similar to that of various RNAs, such as 5S rRNA, tRNA-Phe, A3G mRNA, and GAPDH mRNA (supplemental Fig. S2) (23, 24). To determine whether W127L mutation in A3G affects its interaction with MOV10, 293T cells were co-transfected with A3G-W127L-HA- (or A3G-HA-) and MOV10-FLAG-expressing plasmids. After immunoprecipitation with anti-HA antibody, the immunoprecipitated samples were treated with or without mixed RNase, followed by washing, analysis with SDS-PAGE, and immunoblotting. We detected a ∼64% reduction in the binding between A3G-W127L and MOV10 relative to the binding of wild-type A3G and MOV10 (Fig. 1A, lanes 1 and 3). The same phenomenon was also observed when the transfected A3G interacted with the endogenous MOV10. This reduction was detected by the addition of anti-MOV10 antibody (Fig. 1B, lanes 1 and 3). These results indicate that 7SL RNA could be key to the interaction between A3G and MOV10. Nevertheless, the RNase treatment does not completely eliminate the interaction between A3G-W127L and MOV10, indicating that A3G could still bind to MOV10 in a 7SL RNA-independent manner (Fig. 1, A, lane 4 and B, lane 4).
FIGURE 1.
The 7SL RNA mediates the interaction between A3G and MOV10. A, 293T cells transfected with A3G-HA- (or A3G-W127L-HA-) and MOV10-FLAG-expressing plasmids were collected and lysed. B, 293T cells transfected with A3G-HA- (or A3G-W127L-HA-) expressing plasmids were collected and lysed. A and B, after immunoprecipitation with anti-HA agarose beads, the samples were treated with or without RNase mixture and then analyzed by immunoblotting using anti-FLAG (A), anti-MOV10 (B), or anti-HA antibody (A and B). C, SRP14 mRNA in 293T cells expressing control-shRNA and SRP14-shRNA was quantified using real-time RT-PCR at 72 h post-transfection. The SRP14 mRNA in cells expressing control-shRNA was defined as 100%. D, 7SL RNA in 293T cells expressing control-shRNA and SRP14-shRNA was quantified by real-time RT-PCR at 6 days post-transfection. The 7SL RNA in cells expressing control-shRNA was defined as 100%. Data in C and D represent mean ± S.D. E, 293T cells expressing control-shRNA or SRP14-shRNA were co-transfected with MOV10-FLAG- and A3G-HA- (or A3G-W127L-HA-) expressing plasmids. F, 293T cells expressing control-shRNA or SRP14-shRNA were transfected with A3G-HA- (or A3G-W127L-HA-) expressing plasmids. E and F, after 24 h, cells were lysed and co-immunoprecipitated with anti-HA agarose beads. The precipitated samples were analyzed by immunoblotting using anti-FLAG (E), anti-MOV10 (F) or anti-HA antibody (E and F). Values in A, B, E, and F represent portions of MOV10-FLAG (or MOV10) normalized against A3G-HA (or A3G-W127L-HA) relative to control values.
We then determined whether the knockdown of 7SL RNA affects the interaction between A3G and MOV10. Previous studies have demonstrated that the expression level of 7SL RNA can be affected by human SRP14 depletion and was rescued by overexpression of SRP14 (19, 20, 24, 25). To knock down 7SL RNA, 293T cells were transfected with shRNA targeting human SRP14 (SRP14-shRNA) (Fig. 1C). As indicated in Fig. 1D, 7SL RNA expression was significantly decreased. Then the interaction between A3G and MOV10 decreased significantly (Fig. 1, E and F). These results confirmed that 7SL RNA mediates the interaction between MOV10 and A3G.
MOV10 Is Important for the Function of miRNAs in Human Cells
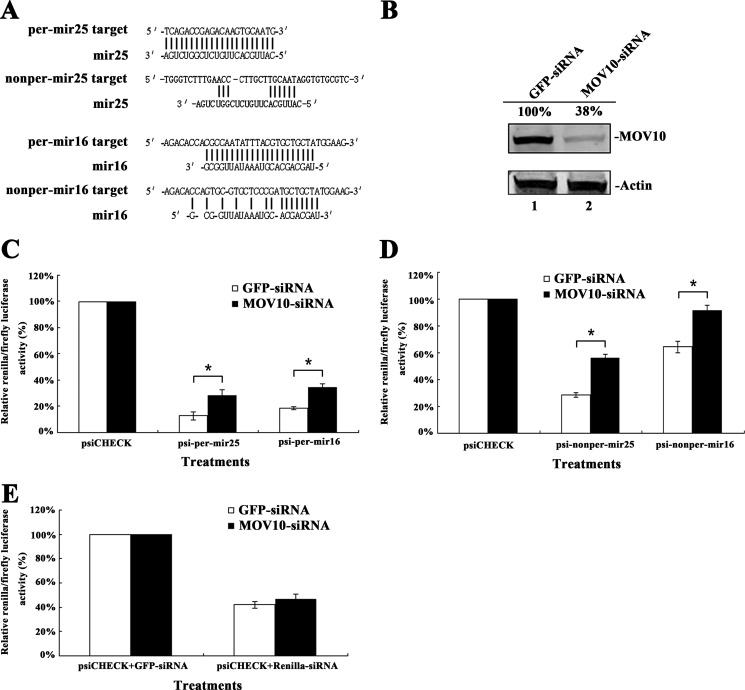
It has been indicated that Armitage, the MOV10 homolog in Drosophila, affects the assembly of siRISC (29). In human cells, MOV10 interacts with AGO protein (9). To determine whether MOV10 affects the efficiency of miRNA-mediated translational repression in human cells, mir25- and mir16-binding sites either perfectly or partially complementary to their corresponding miRNAs were inserted into the 3′-UTR of Renilla luciferase (psiCHECK-2) (Fig. 2A). The 293T cells were first transfected with MOV10-specific siRNA to knock down the expression of endogenous MOV10 (Fig. 2B). These cells were transfected with miRNA reporter plasmids. Fig. 2, C and D show that the presence of mir25- or mir16-binding sites, either perfectly or partially complementary, in the 3′-UTR of Renilla luciferase down-regulated the expression of reporter gene. After depletion of endogenous MOV10, the suppressive effect of miRNAs was inhibited significantly. The same phenotypes were recapitulated with two endogenous genes (GOT2 and CREB3L2) regulated by mir16 or mir25 (supplemental Fig. S3). These results indicate that MOV10 is important for the miRNA-mediated repression of translation in human cells.
FIGURE 2.
The depletion of MOV10 affects miRNA-mediated repression of protein translation in 293T cells. A, sequences of mir25 and mir16 and their target sites used for reporter gene constructs are shown. B, effects of MOV10-specific siRNA on the endogenous MOV10 expression in 293T cells. Values represent portions of MOV10 normalized against actin relative to control values. C and D, 293T cells were first transfected with MOV10-specific siRNA or siRNA for gfp as a control. After 24 h, cells were transfected with a plasmid containing the Renilla luciferase reporter gene with (C) perfect or (D) imperfect binding sites for mir25 or mir16 in the 3′-UTR (the plasmid also contained the firefly luciferase gene as an intraplasmid transfection normalization reporter). psiCHECK-2 was also transfected as a control. At 24 h post-transfection, Renilla/firefly luciferase activities were measured. E, 293T cells were first transfected with MOV10-specific siRNA (gfp-specific siRNA as a control). At 24 h post-transfection, cells were co-transfected with psiCHECK-2 and Renilla luciferase-specific siRNA (gfp-specific siRNA as a control). After 24 h, Renilla/firefly luciferase activity was measured. Data in C, D, and E represent mean ± S.D. (error bars). *, statistically significant, ≤0.05.
We then determined whether MOV10 affects the function of siRNA in human cells. After depleting the expression of MOV10, the 293T cells were co-transfected with Renilla luciferase-specific siRNA and the plasmid containing the Renilla luciferase reporter gene. The Renilla luciferase-specific siRNA reduced the expression of the reporter gene. However, the depletion of MOV10 in 293T cells did not significantly affect the suppressive effect of siRNA (Fig. 2E). This suggests that MOV10 does not play an important role in siRNA-mediated protein repression in human cells, which is inconsistent with the activity of its homolog in Drosophila (29).
A3G Counteracts miRNA-mediated Gene Silencing by Inhibiting the Interaction between MOV10 and AGO2
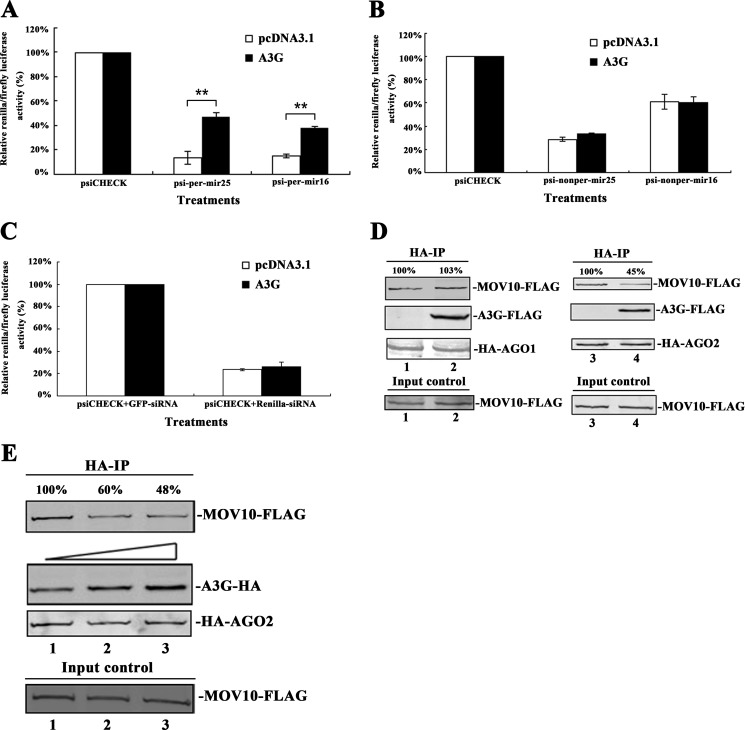
Our previous study has shown that A3G can counteract miRNA-mediated protein inhibition (12). To determine the details of the molecular mechanism, we placed one copy of mir25- and mir16-binding sites with either perfectly or partially complementary to their corresponding miRNAs into the 3′-UTR of the reporter gene. The presence of these binding sites down-regulated the expression of the reporter gene considerably (Fig. 3, A and B). A3G was found to significantly counteract the inhibition of miRNAs on miRNA reporters with single copy of perfect miRNA binding sites but not with single copy of imperfect miRNA binding sites (Fig. 3, A and B). After overexpression of A3G or depletion of endogenous A3G, we recapitulated the same phenotypes with two endogenous genes (GOT2 and CREB3L2), both of which have highly matched binding sites for mir16 or mir25 in the 3′-UTR region (supplemental Fig. S3).
FIGURE 3.
A3G counteracts the interaction between MOV10 and AGO2. A and B, 293T cells were co-transfected with a plasmid expressing A3G and a plasmid containing the Renilla luciferase reporter gene with perfect/non-perfect binding sites for mir25 or mir16 in the 3′-UTR. This plasmid also contains the firefly luciferase gene as an intraplasmid transfection normalization reporter. pcDNA3.1 and psiCHECK-2 were also transfected as controls. At 48 h post-transfection, Renilla/firefly luciferase activity was measured. C, 293T cells were co-transfected with pcDNA3.1-A3G-HA, psiCHECK-2, and Renilla luciferase-specific siRNA. pcDNA3.1 and gfp-specific siRNA were also transfected as controls. At 48 h post-transfection, Renilla/firefly luciferase activity was measured. Data in A, B, and C represent mean ± S.D. (error bars). **, statistically significant, ≤0.01. D, 293T cells were co-transfected with HA-AGO1- (or HA-AGO2-), A3G-FLAG-, and MOV10-FLAG-expressing plasmids. E, 293T cells were co-transfected with HA-AGO2-, MOV10-FLAG- and different amounts of A3G-expressing plasmids. D and E, transfected-cells were lysed and immunoprecipitated with anti-HA antibody at 24 h post-transfection. The precipitated samples were then analyzed by immunoblotting using anti-FLAG and anti-HA antibodies. Values in D and E represent percentage of MOV10-FLAG normalized against HA-AGO1 (or HA-AGO2) and compared with control.
To determine whether A3G affects siRNA function, 293T cells were co-transfected with Renilla luciferase-specific siRNA, the plasmid containing the Renilla luciferase reporter gene and A3G-expressing plasmid. The Renilla luciferase-specific siRNA reduced the expression of the reporter gene significantly. However, the expression of A3G in 293T cells did not counteract the siRNA inhibition, suggesting that A3G does not inhibit the siRNA activity (Fig. 3C).
As noted above, A3G and MOV10 interact with each other, both are components of Argonaute-associated RNP complex and both affect the miRNA activity but have no significant effect on siRNA activity (Figs. 2 and 3, A–C) (9, 11). We therefore hypothesized that A3G could affect the interaction between Argonaute proteins and MOV10 to counteract the miRNA activity. To this end, we co-transfected A3G-FLAG-expressing plasmid, MOV10-FLAG-expressing plasmid and HA-AGO1- (or HA-AGO2-) expressing plasmid into 293T cells simultaneously. We did not detect any change in the quantity of MOV10-FLAG in the HA-AGO1 immunoprecipitated samples either with or without A3G (Fig. 3D). However, we did detect a ∼55% reduction of MOV10-FLAG in the HA-AGO2 immunoprecipitated sample exposed to A3G-expressing plasmid (Fig. 3D). To confirm this, a dose-dependent experiment was performed. The inhibitory effect upon AGO2 and MOV10 interaction was found to correlate to the expression level of A3G (Fig. 3E).
Both A3G and AGO2 Bind to the C Terminus of MOV10
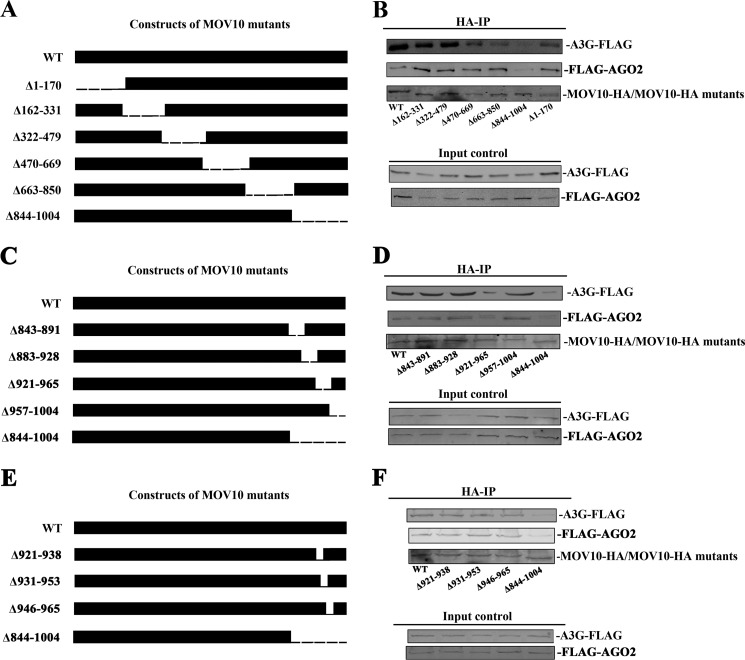
To determine the mechanism by which A3G affects the interaction between MOV10 and AGO2 and to examine the domain of MOV10 that interacts with A3G or AGO2, we generated a series of deletions along the MOV10 1004 amino acids via PCR-based mutagenesis (Fig. 4, A, C, E). The 293T cells were then co-transfected with plasmids expressing A3G-FLAG (or FLAG-AGO2) and a series of MOV10 deletion mutants (Fig. 4, B, D, F). After immunoprecipitation, we found that the MOV10 Δ921–965 mutant had lost much of its ability to bind to either A3G or AGO2 (Fig. 4D). This indicates that the domain (921–965 amino acids) at C terminus of MOV10 is required for MOV10 to interact with either A3G or AGO2. When the data from Figs. 3 and 4 are considered together, it seems likely that A3G and AGO2 bind competitively to the C terminus of MOV10.
FIGURE 4.
A3G and AGO2 both bind to the C terminus of MOV10. A, C, and E, scheme of MOV10 series deletion. A series of MOV10 deletions were generated via PCR-based mutagenesis and all of them were tagged with HA-tag. B, D, and F, 293T cells were co-transfected with A3G-FLAG- (or FLAG-AGO2-) expressing plasmid and a series of MOV10-HA deletion mutants. At 24 h post-transfection, cells were lysed and co-immunoprecipitated with anti-HA-agarose beads. The precipitated samples were then analyzed by Western blotting using anti-FLAG or anti-HA antibody.
The 7SL RNA Affects the Activity of A3G by Counteracting miRNA-mediated Repression of Translation
Because A3G binds to MOV10 through 7SL RNA, we determined whether A3G-W127L, an A3G mutant lacking the ability to bind to 7SL-RNA, can inhibit the binding between MOV10 and AGO2. To this end, we transfected 293T cells with A3G-W127L-HA-, MOV10-FLAG- and HA-AGO2-expressing plasmids simultaneously. Unlike wild-type A3G, A3G-W127L was found to lack the ability to inhibit the interaction between MOV10 and AGO2 (Fig. 5A). During the experiment, it lost most of its ability to counteract miRNA-mediated protein repression (Fig. 5B). We then overexpressed SRP14 to restore the 7SL RNA expression level (Fig. 5C) (20). We found that the interaction between A3G and MOV10 was enhanced (Fig. 5D) and the ability of A3G to counteract the miRNA-mediated protein repression was also recovered (Fig. 5E). These results indicate that 7SL RNA mediates the inhibition of A3G upon the depression of miRNA-mediated translation.
FIGURE 5.
The 7SL RNA affects the activity of A3G to counteract miRNA-mediated gene silencing. A, 293T cells were co-transfected with A3G-FLAG- (or A3G-W127L-FLAG-), MOV10-FLAG- and HA-AGO2-expressing plasmids. After 24 h, cells were lysed and co-immunoprecipitated with anti-HA agarose beads. The immunoprecipitated samples were then detected by Western blotting using anti-FLAG and anti-HA antibody. B, 293T cells were co-transfected with pcDNA3.1-A3G-HA (or pcDNA3.1-A3G-W127L-HA) and a plasmid containing the Renilla luciferase reporter gene with binding sites for mir25 in the 3′-UTR (the plasmid also contains the firefly luciferase gene as an intraplasmid control). pcDNA3.1 and psiCHECK-2 were also transfected as controls. At 48 h post-transfection, Renilla/firefly luciferase activity was measured. C, 293T cells expressing control-shRNA or SRP14-shRNA were first transfected with SRP14 expressing plasmid (pcDNA3.1 as a control). After 48 h post-transfection, 7SL RNA in transfected-cells was quantified by real-time RT-PCR. The level of 7SL RNA in cells expressing control-shRNA and pcDNA3.1 was defined as 100%. D, 293T cells expressing control-shRNA and those expressing SRP14-shRNA were first transfected with SRP14 expressing plasmid. At 24 h post-transfection, the transfected cells were then co-transfected with MOV10-FLAG- and A3G-HA- (or A3G-W127L-HA-) expressing plasmids. After 24 h, cells were lysed and co-immunoprecipitated with anti-HA-agarose beads. The precipitated samples were analyzed by immunoblotting using anti-FLAG or anti-HA antibody. E, 293T cells expressing control-shRNA or SRP14-shRNA were first transfected with SRP14 expressing plasmid. After 12 h, the transfected cells were then further transfected with A3G-expressing plasmid and a plasmid containing the Renilla reporter gene with binding sites for mir25 in the 3′-UTR (the plasmid also contains the firefly luciferase gene as an intraplasmid control). At 36 h post-transfection, the cells were harvested and disrupted, and Renilla/firefly luciferase activity was measured. Values in A and D represent portion of MOV10-FLAG normalized against HA-AGO2 (or A3G-HA) and compared with control. Data in B, C, and E represent mean ± S.D. (error bars). **, statistically significant, ≤0.01.
A3G and MOV10 Do Not Affect the Repression of Protein Translation That Is Mediated by Tethered AGO1 or Tethered AGO2
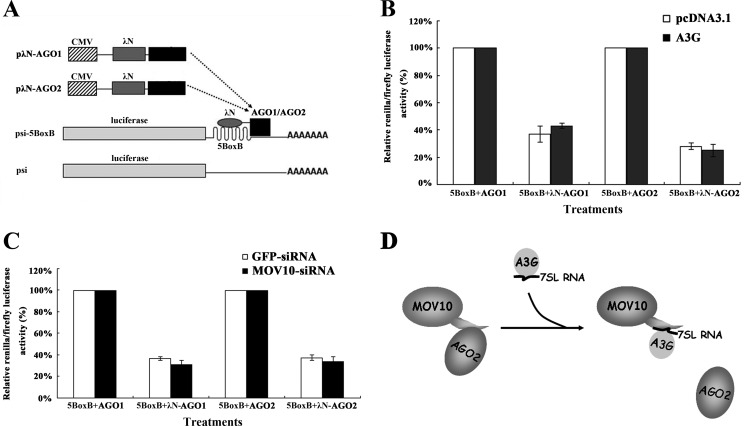
Because A3G, MOV10, and AGO proteins can be found in P-bodies, A3G inhibition of the interaction between AGO2 and MOV10 may occur during or after the binding of AGO proteins to GW182, which is a key component of P-body (9, 11, 30–32). To evaluate this possibility, a tethering assay described in previously published articles was used to examine the effects of mRNA-associated AGO1 and AGO2 on protein translation in Drosophila and human cells (32–35). A fusion protein was constructed with one of these proteins and a λN-peptide, which has a high binding affinity for the five BoxB sites (5-BoxB) in mRNA of reporter gene (Fig. 6A). These proteins were allowed to bind to the mRNA of reporter gene independently of miRNA-mRNA binding. We started the experiments by transfecting 293T cells with A3G-expressing plasmid, pλN-AGO1 (or pλN-AGO2) and psi-5BoxB simultaneously. As shown in Fig. 6B, both λN-AGO1 and λN-AGO2 suppressed the expression of the reporter gene. However, we did not observe significant up-regulation of the expression of the reporter gene expression upon the addition of A3G. The depletion of endogenous MOV10 with MOV10-specific siRNA did not significantly change the repression of the translation of the reporter gene (Fig. 6C). These results show that neither A3G nor MOV10 has any effect on λN-AGO1- (or λN-AGO2-) mediated suppression of protein translation, suggesting that both A3G and MOV10 affect miRNA-mediated repression of translation before AGO protein binds to target mRNA. As indicated in our previous works, A3G does not affect the biogenesis of miRNA (12). These data support the conclusion that A3G affects MOV10-AGO2 binding during the assembly or maturation of miRISC.
FIGURE 6.
A3G and MOV10 do not affect tethered AGO1- or AGO2-mediated down-regulation of protein synthesis. A, scheme of the tethering assay system. psi-5BoxB Renilla mRNA, containing five 19-nt BoxB hairpins in the 3′-UTR of psiCHECK-2, interacting with λN-AGO1 or λN-AGO2. B, 293T cells were co-transfected with A3G-expressing plasmid, pλN-AGO1 (or pλN-AGO2) and psi-5BoxB. pAGO1 (or pAGO2) and pcDNA3.1 were transfected as controls. At 48 h post-transfection, Renilla/firefly luciferase activity was measured. C, 293T cells were first transfected with MOV10-specific siRNA (gfp-specific siRNA as a control). After 24 h, cells were co-transfected with psi-5BoxB and pλN-AGO1 (or pλN-AGO2). pAGO1 (or pAGO2) was transfected as a control. At 24 h post-transfection, Renilla/firefly luciferase activity was measured. Data in B and C represent mean ± S.D. (error bars). D, competitive inhibition of interaction between AGO2 and MOV10 by A3G.
DISCUSSION
The multifunctional MOV10 protein belongs to the RNA helicase superfamily-2. Its homolog in Drosophila, Armitage, is required for the biogenesis of Piwi-interacting RNA (piRNA) (36, 37). MOV10 inhibits the replication of several retroviruses, such as HIV-1 and type C retroviruses in mammalian cells (38–40). It has been shown that its homologs, Armitage in Drosophila and SDE3 in Arabidopsis, interact with AGO proteins and play an important role in siRISC function (29, 41, 42). In neurons, Armitage modulates the RISC-mediated regulation of gene expression that establishes the pattern of synaptic protein synthesis associated with long-term memory (43). It has been proposed that Armitage facilitates the loading of single-stranded siRNA into siRISC, which leads to the maturation of siRISC (29). As reported here, we have confirmed that MOV10 is required for both the miRNA-mediated cleavage of RNA (mediated by the miRNA perfect binding site) and the miRNA-mediated repression of translation (mediated by the miRNA imperfect binding site) in human cells (Fig. 2, A–D) (9, 44). MOV10 is not required for siRNA function in human cells (Fig. 2E). It may exert its effects on siRNA-mediated silencing in a species-specific manner. Because MOV10 has no function on the repression of translation mediated by tethered AGO1 or AGO2, both of which mimic mRNA degradation in P-bodies (Fig. 6C), we suggest that MOV10 exerts its function on miRNA-mediated gene silencing during the assembly or maturation of the miRISC complex. Because MOV10 binds to AGO proteins, we suggest that it is a functional part of miRISC. Its role in miRISC requires further clarification (9).
Our own and previous works have shown that A3G binds to MOV10 in an RNA-dependent manner (supplemental Fig. S1C) (11). We have also demonstrated that the interaction between A3G and MOV10 is mostly mediated by cellular 7SL RNAs (Fig. 1, E and F). Given the close connection between the functions of A3G, MOV10, and miRNA and that neither A3G nor MOV10 significantly affects siRNA function (Figs. 2 and 3, A–C), we hypothesized that A3G could counteract miRNA-mediated gene silencing by interfering with the assembly of MOV10 into miRISC complex, in which AGO protein is the major component. We found that A3G can competitively inhibit the interaction between MOV10 and AGO2 in a dose dependent manner but does not affect the interaction between MOV10 and AGO1 (Fig. 3, D and E). This is in consist with the fact that A3G displays the effect on the perfect miRNA binding site-induced silencing and AGO2 plays an important role in this kind of repression (8, 9). In addition, both A3G and MOV10 have been found to co-localize with AGO1 and AGO2 in P-bodies and stress granules (9, 11). Experiments that have evaluated the suppression of protein translation mediated by tethered AGO1 or AGO2 (Fig. 6, B and C), we have excluded the possibility that the competitive inhibition of A3G on MOV10-AGO2 interaction in P-bodies and therefore suggest that A3G functions during the process of miRISC assembly or maturation. As a result, A3G facilitates the association of miRNA-targeted mRNA with polysomes rather than with P-bodies, as described previously in our laboratory (12).
Through a series of MOV10 deletions, we found that either A3G or AGO2 interacts with the same domain of MOV10 (Fig. 4). A3G disturbs the normal miRISC assembly by competitively binding to a domain of MOV10 and affects the interaction between AGO2 and MOV10. This competitive inhibition causes A3G to counteract miRNA-mediated gene silencing. This hypothesis is further supported by the fact that the A3G mutant A3G-W127L, which loses almost all the ability to interact with MOV10, is not capable of affecting the interaction between AGO2 and MOV10. It also exerts no inhibitory effect upon miRNA function (Fig. 5, A and B). Previous studies have demonstrated that A3G-W127L loses most of its ability to bind with 7SL RNA (23, 24). Our data have also shown that the interaction between A3G and MOV10 is mostly dependent on 7SL RNA (Fig. 1). For this reason, we suggest that 7SL RNA facilitates the interaction between A3G and MOV10 and plays an important role in the inhibitory effect of A3G on miRNA function. Indeed, knockdown and restoration of 7SL RNA have confirmed our hypothesis (Fig. 1, E and F and Fig. 5, D and E).
Our work has demonstrated that A3G interferes with miRISC assembly or maturation by inhibiting the interaction between MOV10 and AGO2 (Fig. 6D). It remains to be further clarified whether this activity is related to its antiviral activities (38–40). Because both A3G and MOV10 belong to interferon antiviral system and both have anti-retroviral activity, our research opens up a new avenue for the study of their complicated regulatory roles in cellular functions. The details of the mechanism underlying the MOV10 effect on the activity of miRISC remain to be clarified.
Supplementary Material
Acknowledgments
We thank Dr. Hannon in Cold Spring Harbor Laboratory, New York for the generous gifts of pλN-AGO1 and pλN-AGO2, Dr. Strub in University of Geneva, Switzerland for the human SRP14 expressing plasmid and the plasmid expressing shRNA against SRP14. We also thank Dr. Mary in University of Geneva, Switzerland for the valuable advice on 7SL RNA knock down experiment.
This work was supported, in whole or in part, National Institutes of Health Grant AI078812 (to H. Z.), Guangdong Innovative Research Team Program (2009010058), National Basic Research Program of China (973 Program, 2010CB912202), Natural Science Foundation of China (30972620, 81101255), Natural Science Foundation of Guangdong (9251008901000022, 10451008901004204), Research Fund for the Doctoral Program of Higher Education of China (20090171110083, 20100171120056), and China Postdoctoral Science Foundation (20100470045).

This article contains supplemental Figs. S1–S3.
- miRNA
- microRNA
- APOBEC3G
- apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G
- MOV10
- Moloney leukemia virus
- miRISC
- miRNA-induced silencing complexes
- AGO
- Argonaute
- nt
- nucleotide
- siRNA
- small interfering RNA
- siRISC
- siRNA-induced silencing complexes
- UTR
- untranslated region
- RT-PCR
- reverse transcription PCR
- P-body
- processing body
- co-IP
- co-immunoprecipitation
- SRP
- signal recognition particle.
REFERENCES
- 1. Ambros V., Chen X. (2007) The regulation of genes and genomes by small RNAs. Development 134, 1635–1641 [DOI] [PubMed] [Google Scholar]
- 2. Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 3. Rana T. M. (2007) Illuminating the silence: understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 8, 23–36 [DOI] [PubMed] [Google Scholar]
- 4. Carmell M. A., Xuan Z., Zhang M. Q., Hannon G. J. (2002) The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 16, 2733–2742 [DOI] [PubMed] [Google Scholar]
- 5. Caudy A. A., Myers M., Hannon G. J., Hammond S. M. (2002) Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 16, 2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gregory R. I., Chendrimada T. P., Cooch N., Shiekhattar R. (2005) Human RISC couples microRNA biogenesis and post-transcriptional gene silencing. Cell 123, 631–640 [DOI] [PubMed] [Google Scholar]
- 7. Liu J., Carmell M. A., Rivas F. V., Marsden C. G., Thomson J. M., Song J. J., Hammond S. M., Joshua-Tor L., Hannon G. J. (2004) Argonaute2 is the catalytic engine of mammalian RNAi. Science 305, 1437–1441 [DOI] [PubMed] [Google Scholar]
- 8. Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. (2004) Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197 [DOI] [PubMed] [Google Scholar]
- 9. Meister G., Landthaler M., Peters L., Chen P. Y., Urlaub H., Lührmann R., Tuschl T. (2005) Identification of novel argonaute-associated proteins. Curr. Biol. 15, 2149–2155 [DOI] [PubMed] [Google Scholar]
- 10. Chiu Y. L., Witkowska H. E., Hall S. C., Santiago M., Soros V. B., Esnault C., Heidmann T., Greene W. C. (2006) High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition. Proc. Natl. Acad. Sci. U.S.A. 103, 15588–15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallois-Montbrun S., Kramer B., Swanson C. M., Byers H., Lynham S., Ward M., Malim M. H. (2007) Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules. J. Virol. 81, 2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J., Liang Z., Yang B., Tian H., Ma J., Zhang H. (2007) Derepression of microRNA-mediated protein translation inhibition by apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) and its family members. J. Biol. Chem. 282, 33632–33640 [DOI] [PubMed] [Google Scholar]
- 13. Kozak S. L., Marin M., Rose K. M., Bystrom C., Kabat D. (2006) The anti-HIV-1 editing enzyme APOBEC3G binds HIV-1 RNA and messenger RNAs that shuttle between polysomes and stress granules. J. Biol. Chem. 281, 29105–29119 [DOI] [PubMed] [Google Scholar]
- 14. Wichroski M. J., Robb G. B., Rana T. M. (2006) Human retroviral host restriction factors APOBEC3G and APOBEC3F localize to mRNA processing bodies. PLoS Pathog. 2, e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H., Yang B., Pomerantz R. J., Zhang C., Arunachalam S. C., Gao L. (2003) The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H., Dornadula G., Alur P., Laughlin M. A., Pomerantz R. J. (1996) Amphipathic domains in the C terminus of the transmembrane protein (gp41) permeabilize HIV-1 virions: a molecular mechanism underlying natural endogenous reverse transcription. Proc. Natl. Acad. Sci. U.S.A. 93, 12519–12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pei Y., Wang X., Zhang X. (2009) Predicting the fate of microRNA target genes based on sequence features. J. Theor. Biol. 261, 17–22 [DOI] [PubMed] [Google Scholar]
- 18. Kumar M., Lu Z., Takwi A. A., Chen W., Callander N. S., Ramos K. S., Young K. H., Li Y. (2011) Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 30, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lakkaraju A. K., Luyet P. P., Parone P., Falguières T., Strub K. (2007) Inefficient targeting to the endoplasmic reticulum by the signal recognition particle elicits selective defects in post-ER membrane trafficking. Exp. Cell Res. 313, 834–847 [DOI] [PubMed] [Google Scholar]
- 20. Lakkaraju A. K., Mary C., Scherrer A., Johnson A. E., Strub K. (2008) SRP keeps polypeptides translocation-competent by slowing translation to match limiting ER-targeting sites. Cell 133, 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng G., Lei K. J., Jin W., Greenwell-Wild T., Wahl S. M. (2006) Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 203, 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang S., Sun Y., Zhang H. (2001) The multimerization of human immunodeficiency virus type I Vif protein: a requirement for Vif function in the viral life cycle. J. Biol. Chem. 276, 4889–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang T., Tian C., Zhang W., Luo K., Sarkis P. T., Yu L., Liu B., Yu Y., Yu X. F. (2007) 7SL RNA mediates virion packaging of the antiviral cytidine deaminase APOBEC3G. J. Virol. 81, 13112–13124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bach D., Peddi S., Mangeat B., Lakkaraju A., Strub K., Trono D. (2008) Characterization of APOBEC3G binding to 7SL RNA. Retrovirology 5, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mary C., Scherrer A., Huck L., Lakkaraju A. K., Thomas Y., Johnson A. E., Strub K. (2010) Residues in SRP9/14 essential for elongation arrest activity of the signal recognition particle define a positively charged functional domain on one side of the protein. Rna 16, 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore M. J. (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309, 1514–1518 [DOI] [PubMed] [Google Scholar]
- 27. Wolin S. L., Steitz J. A. (1983) Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell 32, 735–744 [DOI] [PubMed] [Google Scholar]
- 28. Tian C., Wang T., Zhang W., Yu X. F. (2007) Virion packaging determinants and reverse transcription of SRP RNA in HIV-1 particles. Nucleic Acids Res. 35, 7288–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomari Y., Du T., Haley B., Schwarz D. S., Bennett R., Cook H. A., Koppetsch B. S., Theurkauf W. E., Zamore P. D. (2004) RISC assembly defects in the Drosophila RNAi mutant armitage. Cell 116, 831–841 [DOI] [PubMed] [Google Scholar]
- 30. Eystathioy T., Chan E. K., Tenenbaum S. A., Keene J. D., Griffith K., Fritzler M. J. (2002) A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13, 1338–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eystathioy T., Jakymiw A., Chan E. K., Séraphin B., Cougot N., Fritzler M. J. (2003) The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. Rna 9, 1171–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J., Rivas F. V., Wohlschlegel J., Yates J. R., 3rd, Parker R., Hannon G. J. (2005) A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7, 1261–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. (2006) mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20, 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gehring N. H., Neu-Yilik G., Schell T., Hentze M. W., Kulozik A. E. (2003) Y14 and hUpf3b form an NMD-activating complex. Mol. Cell 11, 939–949 [DOI] [PubMed] [Google Scholar]
- 35. Pillai R. S., Artus C. G., Filipowicz W. (2004) Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. Rna 10, 1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haase A. D., Fenoglio S., Muerdter F., Guzzardo P. M., Czech B., Pappin D. J., Chen C., Gordon A., Hannon G. J. (2010) Probing the initiation and effector phases of the somatic piRNA pathway in Drosophila. Genes Dev. 24, 2499–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zamore P. D. (2010) Somatic piRNA biogenesis. EMBO J. 29, 3219–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burdick R., Smith J. L., Chaipan C., Friew Y., Chen J., Venkatachari N. J., Delviks-Frankenberry K. A., Hu W. S., Pathak V. K. (2010) P body-associated protein Mov10 inhibits HIV-1 replication at multiple stages. J. Virol. 84, 10241–10253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furtak V., Mulky A., Rawlings S. A., Kozhaya L., Lee K., Kewalramani V. N., Unutmaz D. (2010) Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity. PLoS One 5, e9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X., Han Y., Dang Y., Fu W., Zhou T., Ptak R. G., Zheng Y. H. (2010) Moloney leukemia virus 10 (MOV10) protein inhibits retrovirus replication. J. Biol. Chem. 285, 14346–14355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cook H. A., Koppetsch B. S., Wu J., Theurkauf W. E. (2004) The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell 116, 817–829 [DOI] [PubMed] [Google Scholar]
- 42. Lim A. K., Kai T. (2007) Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 104, 6714–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ashraf S. I., McLoon A. L., Sclarsic S. M., Kunes S. (2006) Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124, 191–205 [DOI] [PubMed] [Google Scholar]
- 44. Chendrimada T. P., Finn K. J., Ji X., Baillat D., Gregory R. I., Liebhaber S. A., Pasquinelli A. E., Shiekhattar R. (2007) MicroRNA silencing through RISC recruitment of eIF6. Nature 447, 823–828 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.