Atherosclerosis is a principal cause of death in the Westernized world. Previously, it was thought to be a disease primarily involving lipid accumulation in the arterial walls. The inflammatory cells found at the sites of fatty streaks as well as in the more advanced lesions were not considered to be principally responsible for the disease. A different view of atherosclerosis has emerged subsequently. Current concepts of this disease include involvement of the immune system and chronic inflammation as crucial elements in the initiation of endothelial cell dysfunction, in fatty streak formation, and in development of advanced lesions and eventual vessel rupture (for a recent review, see ref. 1). Atherosclerosis can now be viewed as a problem of wound healing and of chronic inflammation. Numerous reports in the past few years have demonstrated clearly that migratory immune cells, including monocytes and T lymphocytes, are key cellular elements at all stages of atherosclerosis. Most exciting and relevant to atherosclerosis as a disease of chronic inflammation are papers by Schönbeck et al. (2) and Lutgens et al. (3) appearing in this issue of PNAS. These research groups clearly show that disruption of the CD40–CD40 ligand (L) system, a key mediator of cell communication in the immune system, prevents progression of established atherosclerotic lesions to more advanced unstable lesions. This evidence, coupled with recent studies showing that disruption of the CD40–CD40L system can retard the initiation of arterial plaque formation, provides compelling evidence for the role of chronic inflammation and elements of the immune response in atherosclerosis. Moreover, these investigations identify CD40–CD40L as key regulators of this process and recognize them as potentially important therapeutic targets.
CD40 is a cell membrane-spanning protein of ≈50 kDa that was found originally on B lymphocytes (see ref. 4 for review). It is responsible for B lymphocyte activation and serves several key functions. Engagement of B lymphocyte CD40 by its ligand (commonly called CD40L but officially termed CD154) helps prevent induction of apoptosis, induces expression of key costimulatory molecules such as B7 for interaction with T lymphocytes, and is crucial for B lymphocytes to undergo Ig class switching. The display of CD40L on T lymphocytes permits a mutual amplification scheme. The result is enhanced B cell survival and plasma cell development with production of high affinity antibody. T cells become activated to produce immunoregulatory cytokines such as IL-4, IFN-γ, IL-6, etc. In human beings, mutations in CD40L lead to an impaired ability of B lymphocytes to undergo Ig class switching, abnormal antibody levels, and an inability to respond to certain bacterial infections (5, 6). Although this concept of the CD40–CD40L system explained much about antigen presentation and B and T lymphocyte biology, it only scratched the surface of the importance of this system in other physiological and pathological systems.
As the CD40–CD40L system was studied, it became clear that its expression was not restricted to the lymphocyte. Key antigen-presenting cells, including macrophages and dendritic cells that can activate T lymphocytes, also expressed CD40. Engagement of CD40 by T lymphocyte CD40L also served to activate these cells to express different functions, such as the production of the type 1 cytokines IL-1, IL-12, and tumor necrosis factor-α (5, 6). Interestingly, further analyses showed that nonhematopoietic cells including fibroblasts, endothelial cells, smooth muscle cells, and certain epithelial cells expressed CD40 and that its display could be markedly up-regulated after exposure to IFN-γ (7–9). It has become clear now that CD40 is a major activation receptor on many cells. Its engagement via CD40L endows these cells with powerful functions. These functions include the production of potent proinflammatory cytokines such as IL-1, IL-6, and IL-8. Moreover, CD40 engagement on fibroblasts and endothelial cells induces synthesis of cyclooxygenase-2 and resultant production of prostaglandins. The signal transduction mechanism has been shown to be mediated by the transcription factor NF-κB (6, 8). A number of proinflammatory cytokines have NF-κB sites in their promoter regions and thus are potential targets of CD40 engagement. Another emergent property, important for atherosclerosis induced by CD40 engagement on endothelial and smooth muscle cells, is the synthesis of matrix metalloproteinases (MMPs; ref. 10). These enzymes degrade collagens and other connective tissue proteins crucial for the stability of atherosclerotic plaques and their fibrous caps. Fig. 1 shows the spectrum of cell activities regulated by CD40–CD40L in both hematopoietic and nonhematopoietic cells.
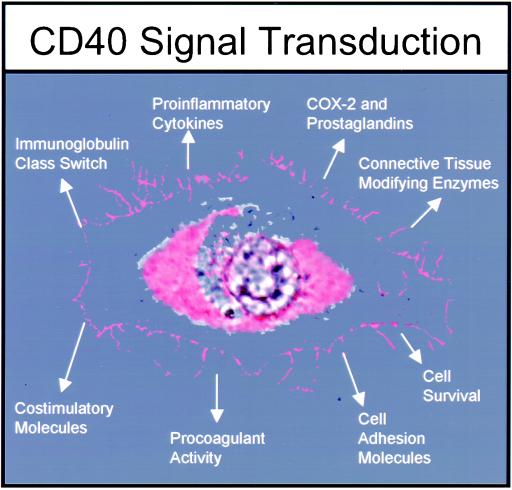
Figure 1.
Spectrum of activities induced after CD40 engagement on hematopoietic and nonhematopoietic cells. Unique to particular types of cells (e.g., lymphocytes, macrophages, and fibroblasts), CD40 engagement sets in motion a pattern of gene expression. The function of CD40 seems to be to “activate” a cell. For example, vascular endothelial cells after CD40 triggering produce cytokines such as IL-1 and IL-8, express cyclooxygenase-2 (COX-2) and MMPs, and display an increased density of cell adhesion molecules. B lymphocytes depend on CD40 for survival, for expression of costimulatory molecules like B7 (to interact with T lymphocytes), and to undergo Ig class switching.
CD40L is obviously a key element in the activation of many types of CD40-expressing cells. Initially, it was thought of as a special molecule expressed only on the surface of T lymphocytes after their activation (4–6). Similar in structure to tumor necrosis factor-α, CD40L is now known to be expressed by activated T cells, eosinophils, mast cells, and most interestingly, by platelets. Platelets have CD40L stored inside them. Once activated by thrombin or other mediators, they expel their stores of CD40L to the environment and also express it on their surface (11). The potential importance of this finding is that a nonnucleated, nonclassical immune cell, in an instant, can incite a cascade of inflammation by interacting with resident connective tissue cells and vascular cells that constitutively display CD40. The result of platelet CD40L interacting with these resident cells would be activation of the vascular cell, resulting in production of cytokines, connective tissue-degrading enzymes, and procoagulant activity as well as the induction or up-regulation of inflammation (see Fig. 2 for how CD40L on blood cells activates cells of the vasculature). To make the scenario even more complex, several other cell types have been shown recently to have CD40L stored within. These include B lymphocytes, macrophages, endothelial cells, and smooth muscle cells (6, 9). The internal CD40L storage system may be a protective mechanism to prevent accidental cell activation (Figs. 1 and 2). It also suggests that amplification mechanisms can be established between cells that coexpress CD40 and CD40L once the internal stores are released. Thus, in the vasculature, it may be possible for both autocrine and paracrine amplification mechanisms to occur via the CD40–CD40L signaling pathway (Fig. 2).
Figure 2.
Involvement of the CD40–CD40L system in atherosclerosis. After an initial inciting event by infectious or physiologic trauma, endothelial cell dysfunction occurs by interaction with platelets and white blood cells. As the mutual activation cascade proceeds by CD40–CD40L interaction, many cells can be involved and produce proinflammatory and regulatory molecules (see Fig. 1). These actions permit cell–cell adhesion and a unique microenvironment to be constructed. If the CD40–CD40L signaling becomes chronic, it may lead to blood vessel dysfunction and occlusion. One reason why disruption of the CD40–CD40L pathway is so effective at stabilizing vessel plaques is that CD40 signaling is an “upstream event.” Blocking the upstream signal (CD40) prevents production of many mediators and chronic activation.
A number of preclinical animal models of chronic inflammation have been shown to involve the CD40–CD40L system. Demonstrating that a remarkable spectrum of chronic inflammatory conditions can be blocked or reduced substantially by disrupting the CD40–CD40L system has generated much excitement. These models include arthritis, graft versus host disease, transplant rejection, lung inflammation, and multiple sclerosis (6, 12, 13). These typically employ either mice with targeted disruption of either CD40 or CD40L genes or the approach used by Lutgens et al. (3) and Schönbeck et al. (2), who used monoclonal anti-CD40L antibodies. The antibodies seem to work by disrupting the communication bridge fashioned by CD40–CD40L. The animals in these preclinical models seem to be no worse for having this system disrupted for months (14). Indeed, disruption of the CD40–CD40L system is being evaluated currently in clinical trials.
Because atherosclerosis is now also considered as a chronic inflammatory disease, much interest has evolved in the potential role of the CD40–CD40L system. Mounting evidence, including the articles in this issue of the PNAS, indicates that the CD40–CD40L pathway is a key element in atherosclerosis. Endothelial cells, macrophages, and smooth muscle cells from human atheroma all express high levels of CD40 (9). Moreover, these cells can be activated in vitro with recombinant forms of CD40L or with T lymphocyte membranes displaying CD40L to express characteristics important in atherosclerosis. These include dramatic up-regulation of connective tissue-degrading MMP (6, 10). MMP production induced by CD40 could destabilize the fibrous plaque cap and allow rupture with obvious consequences. CD40 was described recently as a mechanism of inducing cyclooxygenase-2 and prostaglandin E2 synthesis (15). Cyclooxygenase-2 is highly expressed in human atherosclerotic plaques (16). T lymphocytes, abundant in the plaque, can be activated by prostaglandin E2 to contribute further to the MMP synthesis and tissue remodeling (see Fig. 2). Macrophages, also abundant in the lesions, can be activated via CD40 to produce tissue factor (17). Production of tissue factor would stimulate thrombosis and platelet release of CD40L, thereby enhancing the cycle of chronic inflammation (Fig. 2).
The body of evidence implicating the CD40–CD40L system in chronic inflammation and in atherosclerosis is compelling. This evidence led to initial testing of the hypothesis that disruption of the CD40–CD40L in mouse models of atherosclerosis would down-regulate early disease events. Studies showed, in two different model systems, one with CD40L−/−/ApoE−/− mice and the other with an anti-CD40L antibody treatment of low-density lipoprotein receptor-deficient mice, that initial plaque lesions were smaller and were of a more stable composition (18, 19). The current reports in this issue of PNAS take these initial findings much further. The paper by Schönbeck and colleagues (2) focused on disrupting the CD40–CD40L pathway with a monoclonal anti-CD40L antibody. Low-density lipoprotein receptor-deficient mice were fed a high cholesterol diet, and the question was addressed whether CD40–CD40L disruption could halt the progression or cause regression of established lesions. The key findings were that established lesions failed to progress and that the lesion composition improved such that it was more stable, containing fewer macrophages, less lipid, and more smooth muscle cells and collagen. Lutgens et al. (3) treated ApoE−/− mice with an antibody that disrupts the CD40–CD40L pathway. These authors also demonstrated remarkable changes in plaque composition. Plaques in treated mice failed to progress and had fewer T lymphocytes and macrophages, less lipid, and more collagen. Lutgens et al. (3) also noted an increase in transforming growth factor-β, a cytokine well known for promoting collagen production. These changes are associated with increases important in plaque strength and stability. Both papers conclude that established lesions can be prevented from progressing by disrupting the CD40–CD40L pathway.
In summary, it is now clear that atherosclerosis depends on the immune system and chronic inflammation. The initial cause or causes for the nidus of inflammation and endothelial cell dysfunction are unknown but may be immune (e.g., anti-heat-shock protein antibodies) or infectious or may result from traumas or disordered physiology (1). An emerging body of evidence supports a key role for the CD40–CD40L pathway in atheroma progression. An intriguing study in humans has shown recently that patients with unstable angina have particularly high blood levels of soluble and membrane-bound forms of CD40L (20). These observations suggest that chronic provocation by CD40L and activation of CD40-bearing cells, especially in the plaque microenvironment, could lead to plaque destabilization and rupture. Disruption of the CD40–CD40L pathway in animal models seems to be particularly effective. This efficacy may result from their ability to blunt interactions, not only between classic immune cells, but also between immune and structural cells and between structural cells themselves. Currently, there are several clinical trials being conducted examining disruption of the CD40–CD40L pathway in human disease. Certainly, the studies by Schönbeck et al. (2) and Lutgens et al. (3) highlighted herein suggest that there may be clinical benefit in disrupting the CD40–CD40L pathway in humans with atherosclerosis. Obviously, there is a need for caution, because interference with the CD40–CD40L system is immune suppressive. Much more needs to be learned about the consequences of blocking this pathway, especially for structural cells such as endothelial cells, fibroblasts, and smooth muscle cells in which the role of CD40 is far less understood compared with that in classic immune cells. The utility of blocking CD40 signal transduction may depend on the development of agents that target the CD40 signal transduction conduit in specific cells. In this way, depending on the disease, selective cell targeting can be achieved. In conclusion, study of the CD40–CD40L system has cleared a path for future investigation and intervention into atherosclerosis, the most prevalent cause of death in the Western world.
Acknowledgments
I thank Patricia Sime, Bradford Berk, and Terry Smith for reviewing this commentary and Beth Graf for help with preparing the figures. The research from this laboratory is supported by National Institutes of Health Grants DE11390, HL56002, EY08976, CA11198, CA11051, and ES01247. Support has also been generously provided by the Pepper Center and the University of Rochester Cancer Center Discovery Fund.
Footnotes
References
- 1.Ross R. N Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Schönbeck U, Sukhova G K, Shimizu K, Mach F, Libby P. Proc Natl Acad Sci USA. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutgens E, Cleutjens K B J M, Heeneman S, Koteliansky V E, Burkly L C, Daemen M J A P. Proc Natl Acad Sci USA. 2000;97:7464–7469. doi: 10.1073/pnas.97.13.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehry M. J Immunol. 1996;156:2345–2348. [PubMed] [Google Scholar]
- 5.Noelle R. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 6.Van Cooten C, Banchereau J. J Leukocyte Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Fries K, Sempowski G, Gaspari A, Blieden T, Looney R, Phipps R P. Clin Immunol Immunopathol. 1995;77:42–51. doi: 10.1016/0090-1229(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 8.Smith R S, Smith T J, Blieden T M, Phipps R P. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 9.Mach F, Schönbeck U, Suhkova G, Bourcier T, Bonnefoy J-Y, Pober J, Libby P. Proc Natl Acad Sci USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schönbeck U, Mach F, Sukhova G, Atkinson E, Levesque E, Herman M, Graber P, Basset P, Libby P. J Exp Med. 1999;189:843–853. doi: 10.1084/jem.189.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henn V, Slupsky J R, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek R A. Nature (London) 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 12.Adawi A, Zhang Y, Baggs R, Finkelstein J, Phipps R P. Am J Pathol. 1998;152:651–657. [PMC free article] [PubMed] [Google Scholar]
- 13.Durie F, Fava R, Foy T, Aruffo A, Ledbetter J, Noelle R. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 14.Adawi A, Zhang Y, Baggs R, Rubin P, Williams J, Finkelstein J, Phipps R P. Clin Immunol Immunopathol. 1998;89:222–230. doi: 10.1006/clin.1998.4606. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Cao H J, Graf B, Meekins H, Smith T J, Phipps R P. J Immunol. 1998;160:1053–1057. [PubMed] [Google Scholar]
- 16.Schönbeck U, Sukhova G, Graber P, Coulter S, Libby P. Am J Pathol. 1999;155:1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mach F, Schönbeck U, Libby P. Atherosclerosis Suppl. 1998;137:S89–S95. doi: 10.1016/s0021-9150(97)00309-2. [DOI] [PubMed] [Google Scholar]
- 18.Mach F, Schönbeck U, Sukhova G, Atkinson E, Libby P. Nature (London) 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 19.Lutgens E, Gorelik L, Daemen M, De Muinck E, Grewal I, Koteliansky V, Flavell R. Nat Med. 1999;5:1313–1316. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 20.Aukrust P, Muller F, Ueland T, Berget T, Aaser E, Brunsvig A, Solum N, Forfang K, Froland S, Gullestad L. Circulation. 1999;100:614–620. doi: 10.1161/01.cir.100.6.614. [DOI] [PubMed] [Google Scholar]