Background: The proinflammatory chemokine, CCL5, is a T cell chemoattractant and immunoregulatory molecule.
Results: CCL5 induces glucose uptake in an mTOR-dependent manner, activates the nutrient-sensing kinase AMPK, and enhances intracellular ATP.
Conclusion: CCL5 regulates glucose uptake and ATP generation in activated T lymphocytes in addition to regulating chemotaxis.
Significance: We extend the role of CCL5-mediated signaling beyond lymphocyte chemotaxis.
Keywords: AMP-activated Kinase (AMPK), Chemokines, Glucose, Metabolism, mTOR, Signal Transduction, T Cell
Abstract
Recruitment of effector T cells to sites of infection or inflammation is essential for an effective adaptive immune response. The chemokine CCL5 (RANTES) activates its cognate receptor, CCR5, to initiate cellular functions, including chemotaxis. In earlier studies, we reported that CCL5-induced CCR5 signaling activates the mTOR/4E-BP1 pathway to directly modulate mRNA translation. Specifically, CCL5-mediated mTOR activation contributes to T cell chemotaxis by initiating the synthesis of chemotaxis-related proteins. Up-regulation of chemotaxis-related proteins may prime T cells for efficient migration. It is now clear that mTOR is also a central regulator of nutrient sensing and glycolysis. Herein we describe a role for CCL5-mediated glucose uptake and ATP accumulation to meet the energy demands of chemotaxis in activated T cells. We provide evidence that CCL5 is able to induce glucose uptake in an mTOR-dependent manner. CCL5 treatment of ex vivo activated human CD3+ T cells also induced the activation of the nutrient-sensing kinase AMPK and downstream substrates ACC-1, PFKFB-2, and GSK-3β. Using 2-deoxy-d-glucose, an inhibitor of glucose uptake, and compound C, an inhibitor of AMPK, experimental data are presented that demonstrate that CCL5-mediated T cell chemotaxis is dependent on glucose, as these inhibitors inhibit CCL5-mediated chemotaxis in a dose-dependent manner. Altogether, these findings suggest that both glycolysis and AMPK signaling are required for efficient T cell migration in response to CCL5. These studies extend the role of CCL5 mediated CCR5 signaling beyond lymphocyte chemotaxis and demonstrate a role for chemokines in promoting glucose uptake and ATP production to match energy demands of migration.
Introduction
Chemokines are chemotactic cytokines responsible for orchestrating leukocyte migration. Chemokine binding to specific seven transmembrane-spanning G protein-coupled receptors initiates signaling cascades that promote directional migration through cytoskeletal rearrangement, cell polarization, and integrin activation (1, 2). Indeed, efficient T cell rolling, adhesion, and transmigration through blood vessels are imperative for an effective immune response (2–4). It is now clear that chemokines also regulate numerous migration-unrelated responses, including survival, apoptosis, mRNA translation, angiogenesis, and tumor growth (5–11).
CCL5 (RANTES) is a proinflammatory chemokine that regulates the trafficking of Th1 T cells, macrophages, dendritic cells, and natural killer cells, mediated by activation of the receptors CCR1, CCR3, and/or CCR5 (12–14). CCL5 engagement with its cognate receptor, CCR5, results in the rapid up-regulation of mRNA translation of chemotaxis-related proteins in primary CD4+ T cells, as well as prosurvival factors in MCF-7 breast cancer cells (7, 8). Inhibition studies with the PI3K inhibitor LY294002 and the mTOR2 inhibitor rapamycin have underscored the importance of CCL5 activation of PI3K/mTOR signaling to induce protein synthesis. CCL5-mediated activation of CCR5 leads to the phosphorylation and deactivation of the translational repressor 4E-BP1 in a PI3K/mTOR-dependent manner, which results in the subsequent release of eukaryotic initiation factor-4E (eIF4E) (8). eIF4E binds the mRNA 5′-cap structure together with other initiation factors to form the eIF4F complex, responsible for mRNA unwinding and ribosomal binding during mRNA translation (15). The evolutionarily conserved mTOR is a serine/threonine kinase that exists as two complexes: the mTOR complex 1 (mTORC1), which is rapamycin-sensitive, and mTOR complex 2 (mTORC2), which is rapamycin-insensitive. It is mTORC1 that senses and integrates extrinsic signals to positively regulate cellular proliferation and metabolism in addition to lymphocyte migration and cap-dependent mRNA translation (16–18).
mTORC1 directly regulates the surface expression of a number of nutrient receptors, namely, the amino acid transporter CD98 (4F2HC), the transferrin receptor, and the low-density lipoprotein receptor in response to Akt activation (16). Cytokine-induced glucose uptake is Akt/mTOR-dependent, and rapamycin treatment decreases glycolytic rates in FL5.12 pro-B cells (19). Akt signaling plays a pivotal role in increasing T cell metabolism in response to immune stimulation by increasing glucose and amino acid uptake (16, 20–22). Another important sensor of cellular energy is the AMP-activated protein kinase (AMPK) (23–26). During nutrient deprivation or hypoxia, when intracellular levels of ATP decline and AMP levels rise, AMPK is activated. Active AMPK is responsible for initiating alternative energy-generating processes such as fatty acid oxidation, and inhibition of energy-consuming processes, including cell cycling and biosynthesis (23, 27, 28).
Given that CCL5-CCR5 interactions induce mTOR/4E-BP1 signaling associated with energy-consuming processes such as mRNA translation and chemotaxis (15), we undertook studies to examine whether CCL5/mTOR signaling may contribute to energy generation to support the high energy demands of activated T cells. We provide evidence that at concentrations that support chemotaxis, CCL5 enhances glucose uptake and ATP levels of activated T cells. Specifically, our data indicate that CCL5 simultaneously activates AMPK and mTOR signaling cascades to regulate glucose uptake and chemotaxis in activated T cells. This is the first report that provides evidence for a chemokine, CCL5, regulating metabolic intermediates, glucose and ATP, to facilitate efficient chemotaxis.
EXPERIMENTAL PROCEDURES
Cells and Reagents
Human peripheral blood (PB)-derived T lymphocytes were isolated from consenting healthy donors, as per a protocol approved by the University Health Network Research Ethics Board. Cells were maintained in RPMI 1640 medium supplemented with 10% dialyzed fetal calf serum (Sigma), 100 units/ml penicillin, 100 mg/ml streptomycin and 2 mm l-glutamine (Invitrogen). CD3+ T cells were purified using the StemSep T cell enrichment mixture according to the manufacturer's specifications (StemCell Technologies). T cells were subsequently activated in Microwell plates coated with 10 μg/ml anti-CD3 antibody (eBiosciences), 5 μg/ml anti-CD28 antibody (eBiosciences), and 5 ng/ml human recombinant IL-12 (Bioshop) for 2 days and further expanded in culture supplemented with 100 units/ml human recombinant IL-2 (Bioshop) every other day for 3 days. To avoid confounding data attributed to IL-2 effects, PB T cells that were used for CCL5 treatment experiments were stimulated with IL-2 on days 2 and 4, and then CCL5 was treated on day 6 without IL-2 stimulation. Cultures that served as positive controls were stimulated with IL-2 on days 2, 4, and 6. T cell purity and CCR5 expression were confirmed on day 6 by flow cytometric analysis using anti-human CD3 antibody, anti-human CD4, anti-human CD8 (eBiosciences) and anti-human CCR5 antibodies (2D7, BD Pharmingen; CD195, BD Bioscience) (supplemental Fig. 1). Antibodies for phospho-AMPK-α (Thr-172), AMPK-α, phospho-GSK-3β (Ser-9), phospho-4E-BP1 (Thr-37/46), and 4E-BP1, were purchased from Cell Signaling Technology. Mouse monoclonal anti-α-tubulin antibody was purchased from R&D Systems. Purified mouse anti-human CD98 (4F2HC) and GLUT-1 antibodies were obtained from Santa Cruz Biotechnology and R&D Systems, respectively. Inhibitors rapamycin and compound C were obtained from Calbiochem. The ATP bioluminescent assay kit, 2-deoxy-d-glucose, and oligomycin were purchased from Sigma. CCL5 was a generous gift from Dr. Amanda Proudfoot (Geneva Research Centre, Merck Serono Intl.). The CCR5 antagonist, TAK-779, was kindly provided by Dr. Clifford Lingwood (University of Toronto, Sickkids Hospital).
Immunoblotting
Cells were incubated with 10 nm CCL5 for the times indicated, washed twice with ice-cold PBS and lysed in 100 μl of lysis buffer (1% Triton X-100, 0.5% Nonidet P-40, 150 mm NaCl, 10 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1 mm EGTA, 0.2 mm PMSF, 10 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin A). For all experiments using inhibitors or activators, cells were pretreated for 1 h with the indicated compound prior to CCL5 treatment. Protein concentration was determined using the Bio-Rad DC protein assay kit (Bio-Rad). 50 μg of each protein lysate was denatured in Laemmli sample reducing buffer, and proteins were resolved by SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane followed by blocking with 5% BSA (w/v) in 1× TBST (0.1% Tween 20) for 1 h at room temperature. Membranes were probed with the specified antibodies overnight in 5% BSA (w/v) in TBST at 4 °C and the respective proteins visualized using the ECL detection system (Pierce).
Flow Cytometric Analysis
1 × 106 cells were incubated with mouse anti-human CCR5 antibody for 30 min on ice and washed twice with ice-cold FACS buffer (PBS/2% FCS). Cells were then incubated with Alexa Fluor 488-conjugated anti-mouse IgG antibody (eBiosciences). As a control, cells were incubated with Alexa Fluor 488-conjugated antibody alone. T cell purity was determined by incubating cells with a phycoerythrin-conjugated anti-human CD3 antibody. As an isotype control, cells were incubated with phycoerythrin-labeled isotype control IgG antibody (eBiosciences). For GLUT-1 and CD98 (4F2HC) surface expression, cells were washed twice with ice-cold FACS buffer and fixed with 2% paraformaldehyde at room temperature for 20 min. Cells were then washed twice with FACS buffer and incubated with mouse anti-human GLUT-1 antibody or mouse anti-human CD98 antibody for 30 min on ice. Cells were then washed twice and incubated with Alexa Fluor 488-conjugated anti-mouse IgG antibody. Cells were analyzed using the FACSCalibur and FlowJo software (BD Biosciences).
Chemotaxis Assay
T cell chemotaxis was assayed using 24-well Transwell chambers with 5-μm pores (Corning). 1 × 105 cells in 100 μl of chemotaxis buffer (RPMI 1640/0.5% BSA) were placed in the upper chambers. CCL5, diluted in 600 μl of chemotaxis buffer, was placed in the lower wells, and the chambers were incubated for 2 h at 37 °C. Cells that migrated to the bottom wells were collected and counted with a hemocytometer. For experiments involving inhibitors, cells were pretreated for 1 h with the indicated inhibitor and then placed in the upper chambers. Cell viability, as measured by propidium iodide staining, was not affected by any of the doses of inhibitors used in this study (data not shown).
Glucose Uptake Assay
3–5 × 106 cells were washed with PBS and resuspended in 500 μl of Krebs-Ringer-HEPES (KRH) (at pH 7.4, 136 mm NaCl, 4.7 mm KCl, 1.25 mm CaCl2, 1.25 mm MgSO4, and 10 mm HEPES). 2-Deoxy-d-[H3] glucose (2 μCi/reaction; PerkinElmer Life Sciences) was added in the presence of CCL5, and the reaction mixture was incubated at 37 °C. Reactions were quenched by the addition of ice-cold KRH containing 200 μm phloretin (Calbiochem), followed by immediate centrifugation through an oil layer (1:1 phthalic acid and dibutlylpthalate from Sigma-Aldrich). Cell pellets were washed and solubilized in 1 m NaOH for 1 h, and radioactivity was measured using a liquid scintillation counter. In experiments involving inhibitors, cells were pretreated for 1 h before the addition of 2-deoxy-d-[3H]glucose and CCL5.
AMPK Signaling Antibody Array
Phosphorylation events in the AMPK signaling pathway were examined using the Full Moon BioSystems Antibody Microarray, according to the manufacturer's specifications (Full Moon BioSystems, Inc.) Briefly, 5 × 106 cells were stimulated with CCL5 for 10 min, washed with ice-cold PBS, and lysed with 200 μl of extraction buffer. Protein samples were biotinylated, then added to a microscope slide chamber with specific antibodies bound to its surface. Cy3-streptavidin was added, and fluorescence was detected using the Axon GenePix 400A Microarray Scanner at PMT voltages between 300–400 (Molecular Devices).
ATP Bioluminescent Assay
Intracellular ATP levels were examined using the ATP bioluminescent assay kit, according to the manufacturer's protocol (Sigma-Aldrich). 2 × 105 cells were either left untreated or pretreated with compound C, 2-DG, or oligomycin prior to stimulation with CCL5. Cells were permeabilized using somatic cell ATP releasing agent and subsequently added to an ATP assay mix containing luciferin. Bioluminescence was measured using a VICTORTM X3 Multilabel Plate Reader (PerkinElmer Life Sciences).
Statistical Analysis
Statistical significance was analyzed with repeated-measures analysis of variance. A level of p < 0.05 was chosen to identify significant differences. All data are expressed as mean ± S.E.
RESULTS
CCL5 Induces Phosphorylation of Proteins in the AMPK Signaling Pathway
To investigate potential metabolic changes induced by CCL5 in activated T cells, we initially undertook a global screening approach for phosphorylation events examining the energy-sensing, AMPK signaling pathway. We employed an antibody microarray platform that measures the phosphorylation of upstream and downstream substrates of AMPK. At the outset, we confirmed that ex vivo cytokine activation of PB CD3+ T cells induced cell surface expression of CCR5 in a predominant CD4+ cell population (supplemental Fig. 1).
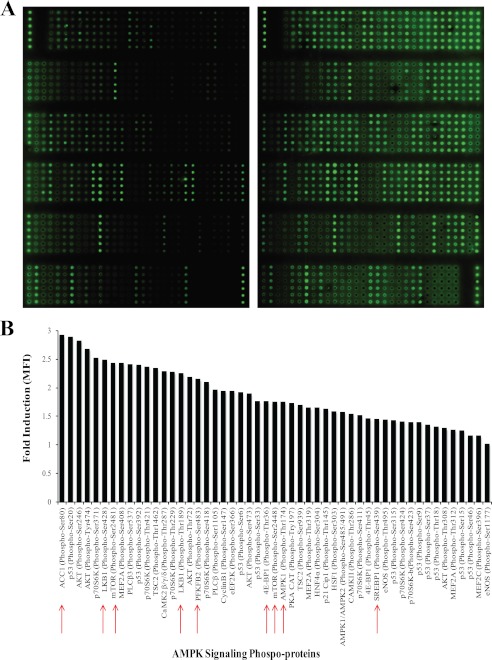
Activated PB T cells were either left untreated or treated with 10 nm CCL5 for 10 min, the cells lysed, and the proteins were biotinylated, as described under “Experimental Procedures.” Biotinylated proteins were then introduced into the microarray slide chambers conjugated with antibodies specific for the AMPK signaling cascade, and T cell-derived proteins were identified using a Cy3-streptavidin detection system. The microarray slide images generated are shown in Fig. 1A and phosphorylation quantitation in Fig. 1B. The data reveal that CCL5 treatment of T cells resulted in the rapid phosphorylation of a number of signaling effectors in the AMPK signaling pathway, as well as effectors in the PI3K/Akt and mTOR/4E-BP1 cascades. Notably, CCL5 induced the phosphorylation of PFKFB-2 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 or PFK-2), a positive regulator of glycolysis, ACC-1 (acetyl-CoA carboxylase 1), an enzyme important for fatty acid synthesis and inhibitor of fatty acid oxidation, and the master regulators of energy status: LKB1, AMPK1/AMPK2, and mTOR.
FIGURE 1.
CCL5 induces phosphorylation of proteins in the AMPK signaling pathway. A, the Full Moon BioSystems AMPK signaling phospho-specific antibody array includes six replicates (vertical columns) of phospho-specific antibodies and their non-phospho pairs, targeted against proteins in the AMPK signaling pathway. Biotinylated protein lysates were added to microscope slide chambers and fluorescence from Cy3-streptavidin was measured with the Axon GenePix 400A microarray scanner. B, the extent of protein phosphorylation (mean fluorescence intensity, MFI) was normalized within each slide and compared between untreated control and cells treated with 10 nm CCL5 for 10 min. The data are represented as fold CCL5-induction relative to untreated controls. Phosphorylated signaling intermediates associated with metabolism are indicated (red arrows).
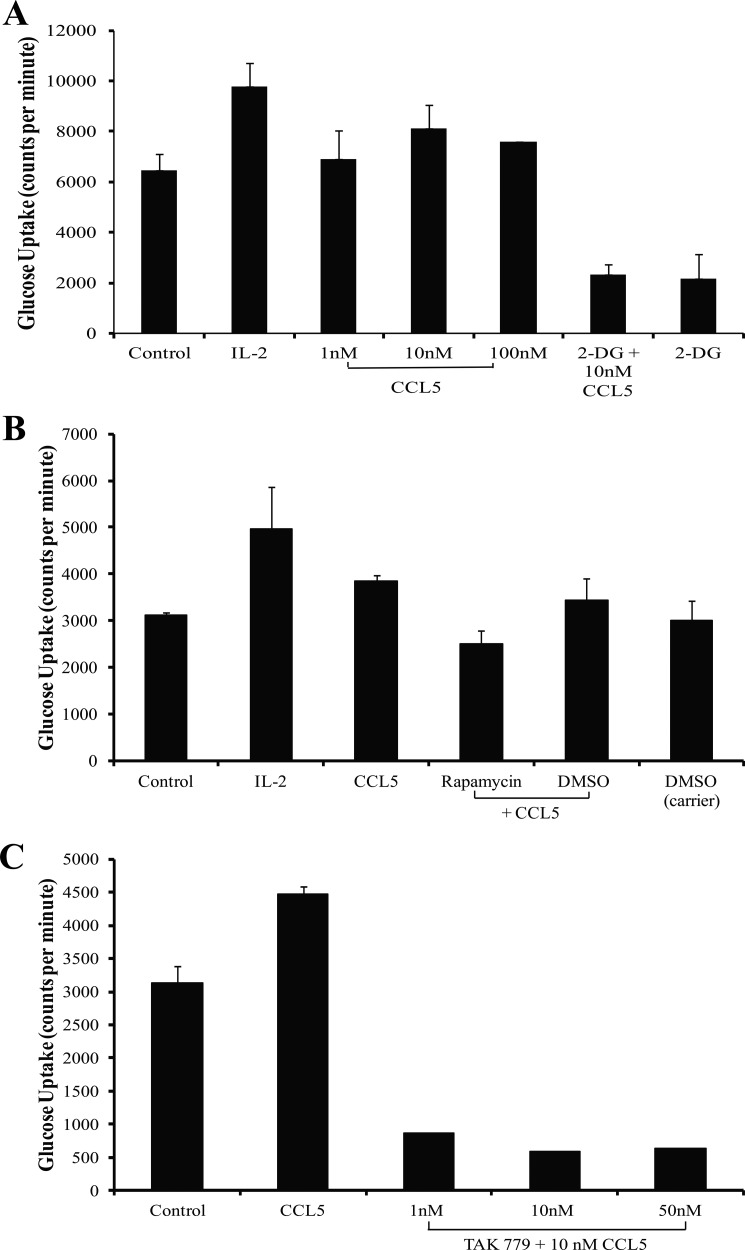
To validate the antibody array findings for AMPK, Western immunoblot time course studies were performed. CCL5 induced maximal phosphorylation of AMPK-α on Thr-172 by 10 min (Fig. 2A). Phosphorylation of Thr-172 is absolutely required for AMPK activation (24, 29). CCL5 also induced the phosphorylation of GSK-3β (glycogen synthase kinase 3β), a downstream substrate of AMPK, on Ser-9, with peak phosphorylation detected at 10 min post-CCL5 treatment (Fig. 2A). GSK-3β is a constitutively active serine/threonine kinase that regulates glycogen synthesis, gene transcription, mRNA translation, and cell proliferation. In its phosphorylated/inactive form, GSK-3β derepresses/releases downstream signaling mediated by glycogen synthase, eIF2B, NF-κB, and other downstream substrates (30–32). Thus, the inhibitory effect of CCL5 treatment on GSK-3β may regulate glycogen storage and other transcriptional events in activated T cells. During energetic stress, active AMPK is able to switch on catabolic processes that generate ATP (33–35). To confirm the effects of CCL5 on AMPK activation and subsequent ATP generation, we evaluated intracellular ATP levels post-CCL5 treatment. Consistent with a maximal activation of AMPK at 10 min post-CCL5 treatment, CCL5 treatment induced maximal intracellular accumulation of ATP by 30 min (Fig. 2B). As a negative control, the AMPK inhibitor, compound C, was used to pretreat PB T cells prior to CCL5 stimulation. As predicted, compound C-treated cells exhibited significantly reduced intracellular ATP production at both 15 and 30 min following CCL5 treatment. These data suggest a role for CCL5 in positively regulating ATP levels in an AMPK-dependent manner. Additionally, to measure the relative CCL5-dependent contributions of oxidative phosphorylation and glycolysis on ATP production, activated PB T cells were pretreated with the inhibitors oligomycin or 2-DG prior to CCL5. A marked reduction in ATP generation induced by 2-DG and not oligomycin suggests that CCL5 inducible ATP generation is predominantly mediated by glycolysis (Fig. 2C).
FIGURE 2.
CCL5 activates the energy-sensing kinase AMPK and the downstream substrate GSK-3β resulting in an increased intracellular ATP levels. A, activated PB T cells were either left untreated or treated with 10 nm CCL5 for the indicated times. Cells were harvested and protein lysates resolved by SDS-PAGE and immunoblotted with anti-phospho-AMPKα (Thr-172) or anti-phospho-GSK-3β (Ser-9) antibodies. Membranes were stripped and reprobed for loading. Relative phosphorylation is shown as signal intensity over loading control. Data are representative of two independent experiments. B, activated PB T cells were either treated with dimethyl sulfoxide or 10 μm compound C for 1 h before treatment with 10 nm CCL5 for the indicated times. Intracellular ATP was measured using a bioluminescent assay. Data are representative of two independent experiments. *, p < 0.01; **, p < 0.05. C, activated PB T-cells were pretreated with dimethyl sulfoxide (DMSO; carrier control), oligomycin (1 μm), or 2-deoxy-glucose (10 mm) for 30 min and then stimulated with IL-2 (20 ng/ml) or CCL5 (10 nm) for 30 min. Intracellular ATP levels were then measured using a bioluminescent assay. Data are representative of two independent experiments. *, p < 0.01.
CCL5 Induces Glucose Uptake That Is mTOR-dependent
AMPK not only functions to initiate ATP regeneration but also induces glucose uptake during energy stress (36). The nutrient-sensitive mTOR likewise responds to nutrient signals to regulate glycolysis (15, 18, 24, 25). As shown in Fig. 3A, CCL5 treatment of activated T cells resulted in a modest increase in glucose uptake in a dose-dependent manner, with maximal uptake at 10 nm of CCL5 (1.2–1.4-fold increase). As anticipated, IL-2 treatment of these activated T cells also resulted in a 1.5–1.9-fold increase in glucose uptake (22). The specific contribution of mTOR signaling to the CCL5-mediated increase in glucose uptake was examined using rapamycin. Inhibition of mTOR by rapamycin effectively reduced CCL5-mediated glucose uptake (Fig. 3B). Finally, to confirm that CCL5 specifically induces glucose uptake through CCR5 activation, the CCR5 antagonist TAK-779 was employed. A marked reduction in glucose uptake was observed in T cells pretreated with TAK-779 (Fig. 3C). These data indicate that CCL5 binding to CCR5, and not CCR1 or CCR3, is required for glucose uptake.
FIGURE 3.
CCL5-mediated glucose uptake is mTOR-dependent. A, activated PB T cells were either left untreated, treated with 20 ng/ml IL-2, or the indicated doses of CCL5 for 2 h. In parallel, cells were pretreated with 10 mm of 2-DG for 1 h prior to treatment with 10 nm CCL5. At time 0, 2 μCi/rxn of 2-deoxy-d-[3H] glucose was added to the cultures. Reactions were quenched and radioactivity measured with a liquid scintillation counter. Data are representative of three independent studies. B, cells were pretreated with either dimethyl sulfoxide (DMSO; carrier) or 50 nm of rapamycin for 1 h prior to treatment with 10 nm CCL5. Tritiated glucose uptake was measured as in A. Data are representative of two independent studies. C, cells were pretreated with CCR5 antagonist, TAK-779 for 1 h prior to treatment with 10 nm CCL5. Tritiated glucose uptake was measured as in A.
CCL5 Prolongs Cell Surface Expression of GLUT-1 and CD98 on Activated T Cells
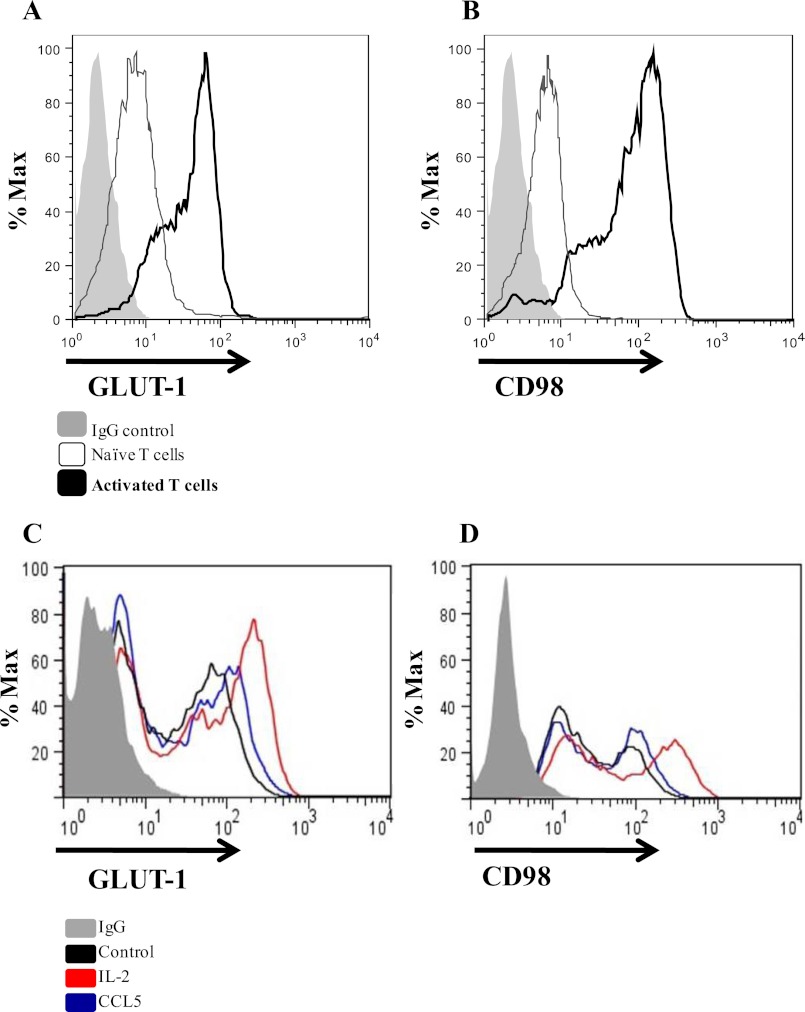
The ability of CCL5 to stimulate glucose uptake may be facilitated through enhanced surface expression of nutrient receptors. Glucose uptake is mediated by a family of facilitative, integral membrane glucose transporters (GLUTs) that are expressed on the cell surface. In lymphocytes, facilitated diffusion is primarily mediated by GLUT-1, a ubiquitously expressed glucose transporter that is up-regulated upon CD3/CD28 ligation (37, 38). Activated lymphocytes also increase expression of the insulin-sensitive GLUT-3 and GLUT-4 receptors, albeit to a lesser degree. Numerous growth signals mediate cell-surface trafficking of GLUT-1 through the PI3K/Akt pathway, thereby increasing glucose uptake and glycolytic flux (22, 37–39). Another key nutrient receptor that is regulated by this pathway is CD98, the heavy chain component of the amino acid-transporter complex (40). Accordingly, we examined the ability of CCL5 to regulate the surface expression of GLUT-1 and CD98.
Whereas naïve T cells express low levels of GLUT-1 and CD98 (Fig. 4, A and B), their cell surface expression is strongly induced upon T cell activation. In time course studies, we observe that CCL5 treatment did not further increase GLUT-1 or CD98 expression at 2, 4, 6, and 8 h post CCL5 treatment (data not shown), with evidence of modest enhanced expression only by 24 h post-treatment (Fig. 4, C and D).
FIGURE 4.
CCL5 prolongs cell surface expression of GLUT-1 and CD98. Activated PB T cells were either left untreated or treated with 10 nm CCL5 or 20 ng/ml IL-2 for 24 h. Cells were fixed with 2% paraformaldehyde and stained for cell surface GLUT-1 (A and C) or CD98 (B and D) expression and analyzed by FACS.
Glucose Uptake and AMPK Signaling Are Required for Efficient CCL5-mediated Chemotaxis
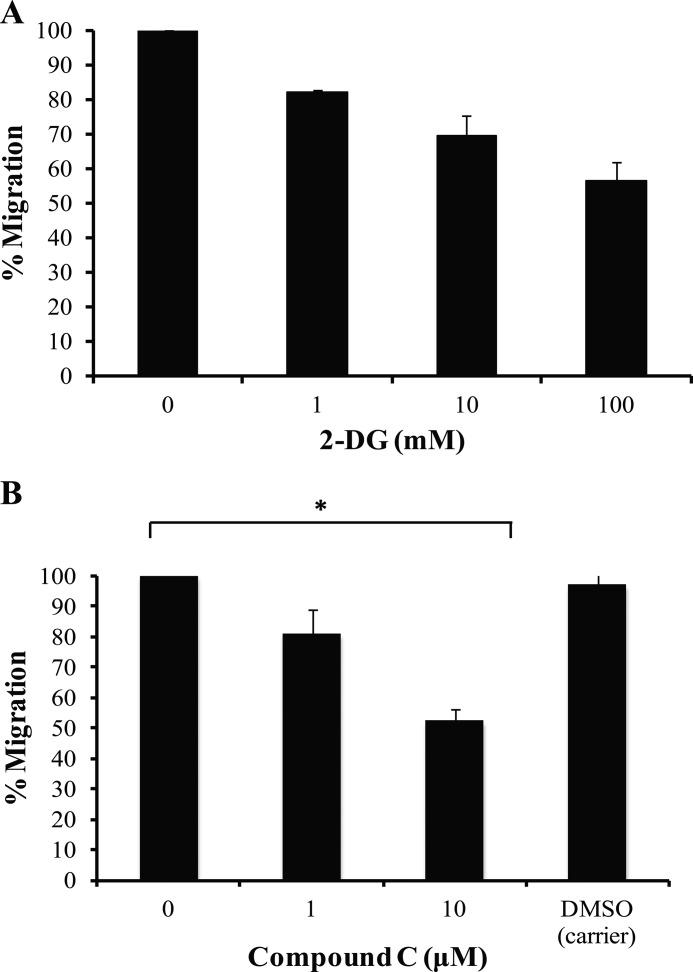
Lymphocyte chemotaxis is an energy-taxing process that requires extensive cytoskeletal rearrangements in response to a migration-promoting agent. To investigate the importance of CCL5-mediated glucose uptake in T cell chemotaxis, inhibition studies were performed using the glucose analog, 2-DG, which effectively inhibits glycolysis. As shown in Fig. 5A, 2-DG pretreatment reduced T cell chemotaxis invoked by CCL5 treatment. These data suggest that efficient chemotaxis requires a steady supply of glucose, which may contribute to CCL5-mediated migration of T lymphocytes. Next, the role of AMPK signaling was evaluated in chemokine-induced chemotaxis. We examined the effects of the AMPK inhibitor, compound C, on CCL5-mediated T cell migration. The data reveal that AMPK inhibition reduced CCL5-inducible T cell chemotaxis (Fig. 5B). The reduction in CCL5-mediated chemotaxis by the inhibitors, 2-DG and compound C, at the doses employed, was not due to any cytotoxic effects (data not shown).
FIGURE 5.
Glucose uptake and AMPK signaling are required for efficient CCL5-mediated chemotaxis. A, activated PB T cells were either left untreated or pretreated with 2-DG at the doses indicated for 1 h. A total of 1 × 105 cells in 100 μl of chemotaxis buffer were then placed in the upper chamber of Transwell chambers. CCL5-mediated chemotaxis was measured using 10 nm CCL5. Data are presented as % migration, with the number of migrated cells at 10 nm CCL5 taken as 100%. Data are representative of three independent experiments. B, activated PB T cells were pre-treated with either dimethyl sulfoxide (DMSO; carrier) or different doses of compound C for 1 h. CCL5-mediated chemotaxis was measured as described in A. Data are representative of three independent experiments. *, p < 0.01.
DISCUSSION
T cell migration to sites of infection or inflammation is critical for an effective immune response and is a highly organized process coordinated by chemokines. Inflammatory chemokines bind to the glycosaminoglycans on the surface of endothelial cells and guide recently activated T cells toward the site of infection/inflammation by triggering adhesion and subsequent diapedesis (41–43). In addition to promoting lymphocyte trafficking, chemokine activation of their cognate receptors invokes a variety of signaling cascades in target cells that can result in diverse biological outcomes. Herein, we report on CCL5 inducible signaling events in activated T cells that influence the metabolic intermediates glucose and ATP.
Initial studies investigated the activation of AMPK, the heterotrimeric energy-sensing kinase that is activated under conditions of energy stress. CCL5 induced the rapid phosphorylation/activation of Thr-172 in the AMPK activation loop, in addition to the phosphorylation of a number of downstream substrates including ACC-1, PFKFB-2, and GSK-3β. CCL5 may simultaneously stimulate processes that increase intracellular nutrient and energy levels, while suppressing cell growth and biosynthetic processes through AMPK activation. Certainly, active AMPK acutely inhibits fatty acid and cholesterol synthesis by phosphorylating and inactivating metabolic enzymes ACC-1, SREBP-1, and HMG-CoA reductase in various tissues (28, 44, 45). Active AMPK is also able to stimulate glycolysis through GLUT trafficking and phosphorylation/activation of the glycolytic enzyme, PFKFB-2 (23, 28). Here, CCL5-mediated phosphorylation of ACC-1 may prevent lipid synthesis as a means to conserve energy. In addition, CCL5-mediated increases in intracellular ATP may, in part, be a consequence of increasing Fru-2,6-BP activity and thus glycolysis.
We observed that CCL5 was able to promote ATP accumulation in an AMPK-dependent manner. Consistent with the published literature, changes in intracellular ATP are generally modest. For example, Plas and colleagues (46) report an ∼4% increase in ATP in cells overexpressing Akt compared with cells expressing Bcl-xL, and this small change was attributed to the Akt cells being more metabolically active. Importantly, small changes in metabolic parameters are sufficient to lead to significant physiological changes.
Our studies show that CCL5 is able to promote glucose uptake in an mTOR-dependent manner, although this increase is not accompanied by changes in surface levels of GLUT-1 or CD98. Glucose transport across the plasma membrane of lymphocytes is mediated by specific GLUT proteins: GLUT-1 is responsible for basal glucose transport, whereas GLUT-3 and GLUT-4 regulate glucose uptake in response to insulin stimulation (47). Upon activation, lymphocytes make an important metabolic switch from oxidative phosphorylation to aerobic glycolysis for ATP generation (37, 48, 49). Consistent with this, our data also suggest ATP production to be more dependent upon glycolysis, as indicated by a greater sensitivity to treatment with 2-DG than oligomycin, an inhibitor of oxidative phosphorylation. CD3/CD28 ligation is able to stimulate glucose transport, increase GLUT-1 surface expression, and promote glycolysis via PI3K/Akt signaling. Intriguingly, increased glucose transport can be detected well before increased GLUT-1 expression, suggesting that enhanced nutrient uptake is not necessarily accompanied by a concomitant increase in transporter expression (49). Several studies in muscle cells, adipose tissues, and diabetic models have also demonstrated that hormone-induced changes in glucose uptake can occur without affecting glucose transporter expression and translocation (50–52). The CCL5-stimulated glucose uptake in the absence of enhanced GLUT-1 expression that we observe suggests that CCL5 may promote GLUT-1 intrinsic activity to facilitate glucose uptake.
As mentioned, mTORC1 integrates numerous nutrient signals to regulate metabolism, growth, migration, and protein synthesis (19, 53). Wieman and colleagues (19) demonstrated that IL-3-dependent hematopoietic FL5.12 cells activate the PI3K/Akt/mTOR pathway following IL-3 treatment to stimulate glucose uptake and GLUT-1 trafficking. Interestingly, mTORC1 activity was not required to maintain GLUT-1 surface expression, although inhibition of mTORC1 greatly diminished IL-3-mediated glucose uptake. These data suggest that mTOR signaling may only be required to promote GLUT-1 functionality to enhance glucose uptake. In agreement, we observe that CCL5 is also able to induce glucose uptake in an mTOR-dependent manner. Although rapamycin reduced CCL5-mediated glucose uptake, this reduction was less than that observed for 2-DG. This may be attributed to other mTORC1-independent mechanisms, including the MAP kinases p38, ERK1/2, and other AMPK-signaling effector molecules (51, 54, 55).
To investigate whether glucose uptake was required for CCL5-mediated chemotaxis, the non-metabolized glucose analog, 2-DG, was employed. 2-DG is a potent inhibitor of glycolysis and ATP production and has been examined as a chemotherapeutic agent (56). Prolonged 2-DG treatment in various cancer cell lines interferes with glycolysis, contributing to decreased cell growth, decreased clonogenictiy, and enhanced apoptosis through caspase-3 release. Notably, our chemotactic studies using 2-DG avoided prolonged drug exposure and avoided cell toxicity (data not shown). We provide evidence that glucose uptake inhibition by 2-DG pretreatment reduces CCL5-inducible ATP production and reduced the ability of T cells to migrate toward a CCL5 gradient in a dose-dependent manner. Proliferating lymphocytes depend on growth factor signals to promote glucose uptake to maintain survival. Even in the presence of alternative energy sources, such as glutamine, T cells maintained in glucose-free medium fail to proliferate, underscoring the non-redundant role of glucose in supporting T cell viability (57). In the present study, the inability of effector T cells to take up glucose affected migration as well. Indeed, tumor cell metastasis to secondary sites in response to a chemoattractant is also dependent on active glycolysis (58, 59).
For optimal T cell migration orchestrated by CCL5, we hypothesized that AMPK stimulation of ATP-generating processes may be required. CCL5-mediated T cell chemotaxis was examined following AMPK inhibition by compound C. Herein, compound C pretreatment reduced CCL5-mediated chemotaxis in a dose-dependent manner, suggesting that T cell migration in response to CCL5 is partially dependent on AMPK signaling. Importantly, although AMPK is most well known for its role as an energy sensor, AMPK signaling also regulates cell polarity, actin polymerization, and directional cell migration (60–62). AMPK inhibition by compound C may prevent processes that directly promote CCL5-mediated chemotaxis and indirectly affect ATP generation. Together, these data suggest that both glucose uptake and AMPK signaling have roles in efficient T cell migration.
The present study has identified AMPK as a novel downstream substrate of CCL5 signaling in activated T cells. In addition, we have identified a role for CCL5-mediated mTOR signaling in promoting glucose uptake and for CCL5 in generating ATP production. Collectively, CCL5 may simultaneously induce signaling in both the mTORC1 and AMPK pathways. Intriguingly, the current literature indicates that AMPK activation during energy deprivation indirectly suppresses mTOR activity by phosphorylating/activating TSC2 or directly inactivates mTOR by targeting its Raptor subunit (24). Data generated herein suggest that CCL5 is able to activate both pathways simultaneously in T cells; we infer that mTOR-dependent processes such as mRNA translation and chemotaxis are energy taxing, which may require AMPK signaling to initiate ATP-generating processes.
Supplementary Material
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant 278397.

This article contains supplemental Fig. 1.
- mTOR
- mammalian target of rapamycin
- 2-DG
- 2-deoxy-d-glucose
- ACC-1
- acetyl-CoA carboxylase 1
- AMPK
- AMP-activated protein kinase
- GLUT
- glucose transporter
- GSK-3β
- glycogen synthase kinase-3 β
- PB
- peripheral blood
- PFKFB-2
- 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2.
REFERENCES
- 1. Mellado M., Rodríguez-Frade J. M., Mañes S., Martínez-A C. (2001) Chemokine signaling and functional responses: The role of receptor dimerization and TK pathway activation. Annu. Rev. Immunol. 19, 397–421 [DOI] [PubMed] [Google Scholar]
- 2. Moser B., Wolf M., Walz A., Loetscher P. (2004) Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 25, 75–84 [DOI] [PubMed] [Google Scholar]
- 3. Ward S. G., Bacon K., Westwick J. (1998) Chemokines and T lymphocytes: More than an attraction. Immunity 9, 1–11 [DOI] [PubMed] [Google Scholar]
- 4. Wong M. M., Fish E. N. (2003) Chemokines: Attractive mediators of the immune response. Semin. Immunol. 15, 5–14 [DOI] [PubMed] [Google Scholar]
- 5. Balkwill F. (2004) Cancer and the chemokine network. Nat. Rev. Cancer 4, 540–550 [DOI] [PubMed] [Google Scholar]
- 6. Luther S. A., Cyster J. G. (2001) Chemokines as regulators of T cell differentiation. Nat. Immunol. 2, 102–107 [DOI] [PubMed] [Google Scholar]
- 7. Murooka T. T., Rahbar R., Fish E. N. (2009) CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochem. Biophys. Res. Commun. 387, 381–386 [DOI] [PubMed] [Google Scholar]
- 8. Murooka T. T., Rahbar R., Platanias L. C., Fish E. N. (2008) CCL5-mediated T-cell chemotaxis involves the initiation of mRNA translation through mTOR/4E-BP1. Blood 111, 4892–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murooka T. T., Wong M. M., Rahbar R., Majchrzak-Kita B., Proudfoot A. E., Fish E. N. (2006) CCL5-CCR5-mediated apoptosis in T cells: Requirement for glycosaminoglycan binding and CCL5 aggregation. J. Biol. Chem. 281, 25184–25194 [DOI] [PubMed] [Google Scholar]
- 10. Taub D. D., Turcovski-Corrales S. M., Key M. L., Longo D. L., Murphy W. J. (1996) Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 156, 2095–2103 [PubMed] [Google Scholar]
- 11. Turner L., Ward S. G., Westwick J. (1995) RANTES-activated human T lymphocytes. A role for phosphoinositide 3-kinase. J. Immunol. 155, 2437–2444 [PubMed] [Google Scholar]
- 12. Appay V., Rowland-Jones S. L. (2001) RANTES: A versatile and controversial chemokine. Trends Immunol. 22, 83–87 [DOI] [PubMed] [Google Scholar]
- 13. Duma L., Häussinger D., Rogowski M., Lusso P., Grzesiek S. (2007) Recognition of RANTES by extracellular parts of the CCR5 receptor. J. Mol. Biol. 365, 1063–1075 [DOI] [PubMed] [Google Scholar]
- 14. Juremalm M., Olsson N., Nilsson G. (2002) Selective CCL5/RANTES-induced mast cell migration through interactions with chemokine receptors CCR1 and CCR4. Biochem. Biophys. Res. Commun. 297, 480–485 [DOI] [PubMed] [Google Scholar]
- 15. Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 16. Edinger A. L., Thompson C. B. (2002) Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell 13, 2276–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laplante M., Sabatini D. M. (2009) An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 19, R1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 19. Wieman H. L., Wofford J. A., Rathmell J. C. (2007) Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 18, 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rathmell J. C., Elstrom R. L., Cinalli R. M., Thompson C. B. (2003) Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur. J. Immunol. 33, 2223–2232 [DOI] [PubMed] [Google Scholar]
- 21. Rathmell J. C., Fox C. J., Plas D. R., Hammerman P. S., Cinalli R. M., Thompson C. B. (2003) Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 23, 7315–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wofford J. A., Wieman H. L., Jacobs S. R., Zhao Y., Rathmell J. C. (2008) IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 111, 2101–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujii N., Jessen N., Goodyear L. J. (2006) AMP-activated protein kinase and the regulation of glucose transport. Am. J. Physiol. Endocrinol. Metab. 291, E867–877 [DOI] [PubMed] [Google Scholar]
- 24. Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimura N., Tokunaga C., Dalal S., Richardson C., Yoshino K., Hara K., Kemp B. E., Witters L. A., Mimura O., Yonezawa K. (2003) A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8, 65–79 [DOI] [PubMed] [Google Scholar]
- 26. Shaw R. J. (2009) LKB1 and AMP-activated protein kinase control of mTOR signaling and growth. Acta Physiol. 196, 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buzzai M., Jones R. G., Amaravadi R. K., Lum J. J., DeBerardinis R. J., Zhao F., Viollet B., Thompson C. B. (2007) Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 [DOI] [PubMed] [Google Scholar]
- 28. Marsin A. S., Bertrand L., Rider M. H., Deprez J., Beauloye C., Vincent M. F., Van den Berghe G., Carling D., Hue L. (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10, 1247–1255 [DOI] [PubMed] [Google Scholar]
- 29. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doble B. W., Woodgett J. R. (2003) GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham J. R., Tullai J. W., Cooper G. M. (2010) GSK-3 represses growth factor-inducible genes by inhibiting NF-κB in quiescent cells. The Journal of biological chemistry 285, 4472–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jope R. S., Johnson G. V. (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 33. Carling D. (2004) The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem. Sci. 29, 18–24 [DOI] [PubMed] [Google Scholar]
- 34. Hardie D. G., Carling D., Carlson M. (1998) The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 67, 821–855 [DOI] [PubMed] [Google Scholar]
- 35. Hayashi T., Hirshman M. F., Fujii N., Habinowski S. A., Witters L. A., Goodyear L. J. (2000) Metabolic stress and altered glucose transport: Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49, 527–531 [DOI] [PubMed] [Google Scholar]
- 36. Hardie D. G. (2004) AMP-activated protein kinase: A master switch in glucose and lipid metabolism. Rev. Endocr. Metab. Disord. 5, 119–125 [DOI] [PubMed] [Google Scholar]
- 37. Frauwirth K. A., Riley J. L., Harris M. H., Parry R. V., Rathmell J. C., Plas D. R., Elstrom R. L., June C. H., Thompson C. B. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 [DOI] [PubMed] [Google Scholar]
- 38. Maciver N. J., Jacobs S. R., Wieman H. L., Wofford J. A., Coloff J. L., Rathmell J. C. (2008) Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 84, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bentley J., Itchayanan D., Barnes K., McIntosh E., Tang X., Downes C. P., Holman G. D., Whetton A. D., Owen-Lynch P. J., Baldwin S. A. (2003) Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J. Biol. Chem. 278, 39337–39348 [DOI] [PubMed] [Google Scholar]
- 40. Devés R., Boyd C. A. (2000) Surface antigen CD98(4F2): Not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 173, 165–177 [DOI] [PubMed] [Google Scholar]
- 41. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 42. Cyster J. G. (1999) Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 [DOI] [PubMed] [Google Scholar]
- 43. Nieto M., Frade J. M., Sancho D., Mellado M., Martinez-A C., Sánchez-Madrid F. (1997) Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J. Exp. Med. 186, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y., Xu S., Mihaylova M. M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J. Y., Gao B., Wierzbicki M., Verbeuren T. J., Shaw R. J., Cohen R. A., Zang M. (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13, 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brownsey R. W., Boone A. N., Elliott J. E., Kulpa J. E., Lee W. M. (2006) Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 34, 223–227 [DOI] [PubMed] [Google Scholar]
- 46. Plas D. R., Talapatra S., Edinger A. L., Rathmell J. C., Thompson C. B. (2001) Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. The Journal of biological chemistry 276, 12041–12048 [DOI] [PubMed] [Google Scholar]
- 47. Calder P. C., Dimitriadis G., Newsholme P. (2007) Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr. Opin. Clin. Nutr. Metab. Care 10, 531–540 [DOI] [PubMed] [Google Scholar]
- 48. Fox C. J., Hammerman P. S., Thompson C. B. (2005) Fuel feeds function: Energy metabolism and the T-cell response. Nat. Rev. Immunol. 5, 844–852 [DOI] [PubMed] [Google Scholar]
- 49. Frauwirth K. A., Thompson C. B. (2004) Regulation of T lymphocyte metabolism. J. Immunol. 172, 4661–4665 [DOI] [PubMed] [Google Scholar]
- 50. Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. (1991) Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J. Clin. Invest. 87, 2197–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Somwar R., Koterski S., Sweeney G., Sciotti R., Djuric S., Berg C., Trevillyan J., Scherer P. E., Rondinone C. M., Klip A. (2002) A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J. Biol. Chem. 277, 50386–50395 [DOI] [PubMed] [Google Scholar]
- 52. Sweeney G., Keen J., Somwar R., Konrad D., Garg R., Klip A. (2001) High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in l6 rat skeletal muscle cells. Endocrinology 142, 4806–4812 [DOI] [PubMed] [Google Scholar]
- 53. Buller C. L., Loberg R. D., Fan M. H., Zhu Q., Park J. L., Vesely E., Inoki K., Guan K. L., Brosius F. C., 3rd. (2008) A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am. J. Physiol. Cell Physiol. 295, C836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carr E. L., Kelman A., Wu G. S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A. M., Frauwirth K. A. (2010) Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185, 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finlay D., Cantrell D. (2010) Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann. N.Y. Acad. Sci. 1183, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aft R. L., Zhang F. W., Gius D. (2002) Evaluation of 2-deoxy-d-glucose as a chemotherapeutic agent: Mechanism of cell death. Br. J. Cancer 87, 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Greiner E. F., Guppy M., Brand K. (1994) Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J. Biol. Chem. 269, 31484–31490 [PubMed] [Google Scholar]
- 58. Beckner M. E., Stracke M. L., Liotta L. A., Schiffmann E. (1990) Glycolysis as primary energy source in tumor cell chemotaxis. J. Natl. Cancer Inst. 82, 1836–1840 [DOI] [PubMed] [Google Scholar]
- 59. Kroemer G., Pouyssegur J. (2008) Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell 13, 472–482 [DOI] [PubMed] [Google Scholar]
- 60. Nakano A., Kato H., Watanabe T., Min K. D., Yamazaki S., Asano Y., Seguchi O., Higo S., Shintani Y., Asanuma H., Asakura M., Minamino T., Kaibuchi K., Mochizuki N., Kitakaze M., Takashima S. (2010) AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat. Cell Biol. 12, 583–590 [DOI] [PubMed] [Google Scholar]
- 61. Williams T., Brenman J. E. (2008) LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 18, 193–198 [DOI] [PubMed] [Google Scholar]
- 62. Miranda L., Carpentier S., Platek A., Hussain N., Gueuning M. A., Vertommen D., Ozkan Y., Sid B., Hue L., Courtoy P. J., Rider M. H., Horman S. (2010) AMP-activated protein kinase induces actin cytoskeleton reorganization in epithelial cells. Biochem. Biophys. Res. Commun. 396, 656–661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.