Abstract
The present study aimed to investigate the anticancer effect of aloe-emodin, an anthraquinone compound present in the leaves of Aloe vera, on two human colon carcinoma cell lines, DLD-1 and WiDr. Colon carcinoma cells were treated with various concentrations of aloe-emodin for different durations. Cell viability was measured by sodium 3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate assay. DNA fragmentation was analyzed by agarose gel electrophoresis. Nuclear shrinkage was visualized by Hoechst 33258 staining. Western blotting was used to indicate the release of apoptosis-inducing factor and cytochrome c from mitochondria and the phosphorylation of Bid. Caspase-3 and casein kinase II activities were measured by the respective assays. Cell viability analyses showed that aloe-emodin induced cell death in a dose- and time-dependent manner. Notably, the WiDr cells were more sensitive to aloe-emodin than the DLD-1 cells. Aloe-emodin caused the release of apoptosis-inducing factor and cytochrome c from mitochondria, followed by activation of caspase-3 leading to DNA fragmentation, nuclear shrinkage and apoptosis. In addition, exposure of colon carcinoma cells to aloe-emodin suppressed the casein kinase II activity in a time-dependent manner and was accompanied by a reduced phosphorylation of Bid, a downstream substrate of casein kinase II and a pro-apoptotic molecule. These findings showed that the inhibition of casein kinase II activity, the release of apoptosis-inducing factor and cytochrome c, and the caspase-3 activation are involved in aloe-emodin-mediated apoptosis in colon carcinoma cells.
Keywords: aloe-emodin, apoptosis, colon carcinoma cells
Introduction
Aloe-emodin is a natural active compound present in the leaves of Aloe vera (1). Some studies have found that aloe-emodin has numerous biological properties including antiviral, antimicrobial and hepatoprotective activities (2). Aloe-emodin has been reported to exhibit anticancer activity on neuroectodermal tumors, lung squamous cell carcinoma and hepatoma cells (3–5). Aloe-emodin has also been shown to inhibit S-phase progression in both a transformed glia and a human glioma cell line, sensitize HeLa cells to As2O3 via the generation of reactive oxygen species, and affect the anticancer activity of cisplatin by blocking the activation of extracellular signal-regulated kinase (6–8). However, the effect of aloe-emodin on human colon cancer cells has yet to be investigated.
Apoptosis is an actively regulated process of cell death since its intrinsic pathway involves mitochondria (9). Mitochondrial outer membrane permeabilization in response to cell death triggers (e.g., DNA damage) is an important early step which is regulated by Bcl-2 and controls the release of proteins, such as cytochrome c, from the mitochondria to the cytoplasm where they initiate apoptosis, ultimately leading to cell death (10). Apoptosis-inducing factor, another mitochondrial protein that is released into the cytosol and nucleus, induces chromatin condensation and DNA fragmentation (11). Members of the caspase superfamily are produced and activated, a step that hastens the cell death process involving the caspase-dependent apoptotic pathway (10).
Casein kinase II is a conserved and ubiquitous protein serine/threonine kinase engaged in various functions including normal and abnormal cell proliferation (12). Casein kinase II is localized in both the cytoplasm and nuclear compartment of healthy cells, but is predominantly present in the nuclear compartment of cancer cells and has been found to be deregulated in all carcinomas studied thus far (13). Evidence links casein kinase II to apoptosis (14). Casein kinase II plays a role in the modulation of caspase susceptibility with Bid, a pro-apoptotic member of the Bcl-2 family. It was shown that casein kinase II phosphorylates Bid close to the caspase-8 cleavage site, thereby preventing cells from undergoing apoptosis (15). Thus, casein kinase II is an important target for the treatment of cancer, as the disruption of casein kinase II activity prevents the phosphorylation of Bid, thereby allowing the cleavage of Bid by caspases, followed by apoptosis (13,16). Notably, an extensive down-regulation of casein kinase II by antisense casein kinase II in prostate cancer xenografts was found to eventually lead to a complete disappearance of the tumor (13).
To evaluate the anticancer effect of aloe-emodin, we employed two distinct human colon carcinoma cell lines, DLD-1 and WiDr. The observation of DNA fragmentation and nuclear morphology, and the examination of the release of apoptosis-inducing factor and cytochrome c showed the apoptotic activity of aloe-emodin. The role of casein kinase II in aloe-emodin-induced apoptosis was also investigated. This study reports for the first time that the natural compound aloe-emodin induces apoptosis in human colon carcinoma cells.
Materials and methods
Aloe-emodin
Aloe-emodin [1,8-dihydroxy-3-(hydroxymethyl)-anthraquinone; CAS registry no. 481-72-1, EU no. 2075717, purity ≥95%] was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). It was dissolved in dimethylsulfoxide to a concentration of 18.5 mM and stored at −20°C until use.
Cell culture and treatments
Human colon carcinoma cell lines, DLD-1 and WiDr, were obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in modified Eagle’s medium (MEM) (Sigma-Aldrich Co.), supplemented with 10% heat-inactivated fetal bovine serum (Moregate BioTech, Bulimba QLD, Australia), 1% MEM non-essential amino acid, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate and 250 ng/ml amphotericin B (all from Sigma-Aldrich Co.). The two cell lines were grown at 37°C in a humidified atmosphere containing 5% CO2. Prior to treatment, the cells were grown to 80–90% confluency and starved by incubation in basal medium (MEM + 1% MEM non-essential amino acid) for 24 h. Various concentrations of aloe-emodin (0–0.37 mM in basal medium) and durations (0, 2, 3, 4, 6, 12, 24 and 48 h) were applied.
Cell viability assay
Cell viability was assessed using the XTT [sodium 3′-[1-(phenylamino-carbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate] assay kit (Sigma-Aldrich Co.), according to the manufacturer’s instructions. The assay was conducted three times, independently.
Lactate dehydrogenase activity assay
At the end of the treatment, the culture medium was centrifuged at 250 × g for 10 min, and the supernatant was saved for the lactate dehydrogenase activity assay. The lactate dehydrogenase released from the lysed cells was detected using the CytoTox 96 Non-Radioactive Cytotoxicity assay (Promega, Madison, WI, USA), according to the manufacturer’s instructions. The assay was conducted three times, independently.
DNA fragmentation assay
Treated cells were centrifuged and lysed in lysis buffer [10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1% SDS, 1 mM EDTA and 2 mg/ml proteinase K] for 1 h at 65°C. Following two successive extractions with phenol/chloroform, the DNA samples were precipitated in ethanol. After washing with 70% ethanol, the DNA samples were resuspended in TE buffer and subjected to 2% agarose gel electrophoresis.
Hoechst 33258 staining
Hoechst 33258 staining was performed as described in a previous study (17). Hoechst 33258-positive nuclei were visualized and photographed using an Olympus fluorescence microscope (Olympus, Tokyo, Japan).
Isolation of mitochondria and extraction of mitochondrial proteins
Mitochondria were isolated using a cell mitochondrial isolation kit (Sigma-Aldrich Co.), according to the manufacturer’s instructions with minor modifications. Briefly, treated cells were harvested, resuspended in lysis buffer containing a protease inhibitor cocktail (Sigma-Aldrich Co.) and incubated on ice for 1 min. Extraction buffer A (1X) containing a protease inhibitor cocktail was added, and the solution was centrifuged at 600 × g for 10 min at 4°C. The supernatant (containing mitochondria) was carefully transferred to a fresh tube and centrifuged at 11,000 × g for 10 min at 4°C. Then, the cytosolic fraction was carefully transferred to a new tube. Mitochondrial proteins were extracted by suspending the pellet in CelLytic M Cell Lysis Reagent (Sigma-Aldrich Co.) with a protease inhibitor cocktail. The samples were stored at −80°C until use. The protein content of the cytosolic and mitochondrial fractions was determined using a BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA) with bovine serum albumin as a standard.
Total protein preparation
Total proteins were extracted with an M-PER mammalian protein extraction reagent (Pierce Biotechnology, Inc.), according to the manufacturer’s instructions. The samples were stored at −80°C until use. The protein concentration was measured as described above.
Immunoblotting
Denatured protein samples were subjected to 15% SDS-PAGE. Proteins were transferred to nitrocellulose membranes. Blocked blots were incubated for 1 h at room temperature with the primary antibodies monoclonal anti-apoptosis-inducing factor (E-1), monoclonal anti-cytochrome c (A-8) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and polyclonal anti-phospho Bid-Ser61 (Bethyl Laboratories, Inc., Montgomery, TX, USA). Monoclonal anti-β-actin (mAbcam 8226) (Abcam Ltd., Cambridge, UK) was used as a control for equal protein loading. Blots were further incubated with secondary antibodies conjugated with alkaline phosphatase for 1 h at room temperature and then incubated with Lumi-Phos WB Chemiluminescent substrate (Pierce Biotechnology, Inc.) and exposed to a Fuji medical X-ray film (Fuji Photo Film Co., Tokyo, Japan). Image processing was performed using the software Fuji Image Gauge. All experiments were conducted three times, independently.
Caspase-3 activity assay
The caspase-3 activity was determined using a caspase-3 assay kit (Sigma-Aldrich Co.). The caspase-3 inhibitor N-acetyl-Asp-Glu-Val-Asp-CHO (Ac-DEVD-CHO; Biomol Research Laboratories, Plymouth, PA, USA) was co-treated with aloe-emodin at a final concentration of 200 μM to inhibit caspase-3 activity in a parallel experiment (18). The assay was conducted three times, independently.
Casein kinase II activity assay
Casein kinase II activity was measured using a casein kinase assay kit (Sigma-Aldrich Co.) combined with the PKLight HTS protein kinase assay kit (Cambrex Bio Science Rockland, Inc., Rockland, MA, USA). Briefly, after aloe-emodin treatment, casein kinase II substrate α-casein and a reaction buffer containing ATP (both from the casein kinase assay kit) were added to the cell lysates and incubated at 37°C for 15 min. The remaining ATP was then consumed by luciferase, and light was generated from ATP and luciferin (luciferase and luciferin were from the PKLight HTS protein kinase assay kit). Bioluminescence was measured using a luminometer (Berthold Detection Systems, Oak Ridge, TN, USA). The PKLight HTS protein kinase assay kit measures the consumption of ATP and is based on the bioluminescent measurement of the remaining ATP present in the samples after kinase activity. The bioluminescent signal is inversely proportional to the activity of the kinase, that is, a higher value is indicative of an increased amount of remaining ATP and a lower casein kinase II activity in the cells. The assay was conducted three times, independently.
Statistical analysis
The unpaired Student’s t-test was used to identify means that were significantly different from each other (P<0.05).
Results
Aloe-emodin induced cell death in a dose- and time-dependent manner
The effect of aloe-emodin on the viability of DLD-1 and WiDr colon carcinoma cells was examined using the XTT viability assay. Exposure of DLD-1 cells to various concentrations of aloe-emodin (0.04, 0.07, 0.15, 0.22, 0.30 and 0.37 mM) for 24 or 48 h resulted in a dose- and time-dependent decrease in cell viability relative to control cell cultures (Fig. 1A). The IC50 value for 48 h of exposure to aloe-emodin was 0.30–0.37 mM. A similar effect of aloe-emodin was observed after 24 or 48 h of incubation using the WiDr cell line (Fig. 1A). In the latter case, however, the IC50 value for a 48-h exposure to aloe-emodin was 0.15–0.22 mM, suggesting that the WiDr cells were more sensitive to aloe-emodin than the DLD-1 cells. Aloe-emodin-induced cell death was significant at a concentration of 0.37 mM. Thus, this concentration was applied for the rest of the experiments.
Figure 1.
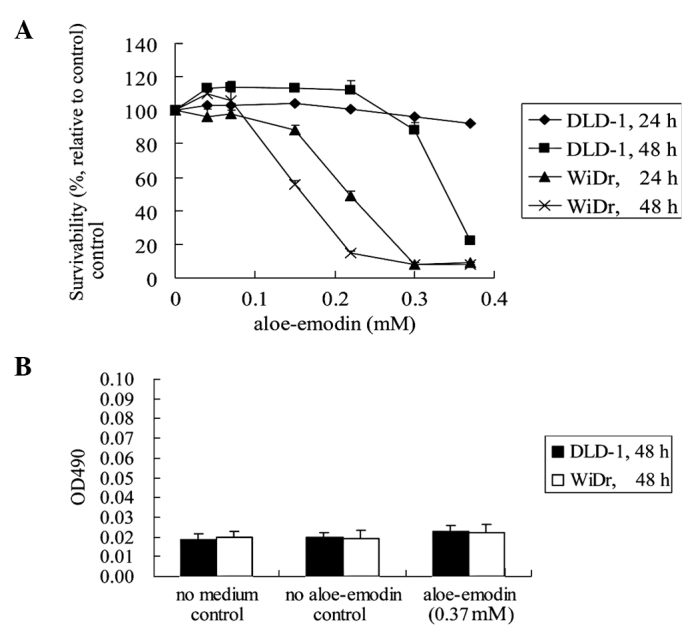
Aloe-emodin is cytotoxic to but does not induce necrosis in the DLD-1 and WiDr colon carcinoma cell lines. The cells were incubated with different concentrations of aloe-emodin for 24 and 48 h. (A) Survival curve of DLD-1 and WiDr cells. The percentage of viable cells was calculated by defining the viability of cells without aloe-emodin treatment as 100%. (B) Lactate dehydrogenase release from DLD-1 and WiDr cells. Data are the results of three independent experiments and are presented as the mean ± SD.
Aloe-emodin did not induce necrosis in DLD-1 and WiDr cells
Treated cells were evaluated for the presence of necrotic cell death by measuring the lactate dehydrogenase release into the medium. Exposure of DLD-1 and WiDr cells to 0.37 mM aloe-emodin for 48 h did not result in the release of lactate dehydrogenase (Fig. 1B).
Aloe-emodin induced apoptosis in DLD-1 and WiDr cells
To investigate whether the aloe-emodin-mediated cell death in DLD-1 and WiDr cells was due to an apoptotic mechanism, DNA fragmentation and nuclear morphological changes that occurred during aloe-emodin treatment were observed. Treatment of DLD-1 and WiDr cells with 0.37 mM aloe-emodin for 24 h resulted in DNA fragmentation (Fig. 2A). Treatment of DLD-1 and WiDr cells with 0.37 mM aloe-emodin for 24 h also resulted in changes in the nuclear morphology as evidenced by the Hoechst 33258 staining (Fig. 2B), a dye often used to label DNA in living cells and to observe morphological and nuclear changes (17,19,20). More fragmented nuclei were observed upon aloe-emodin treatment, both in the DLD-1 and WiDr cells. These observations indicated that aloe-emodin-induced cell death in DLD-1 and WiDr cells involved a typical apoptotic pathway.
Figure 2.
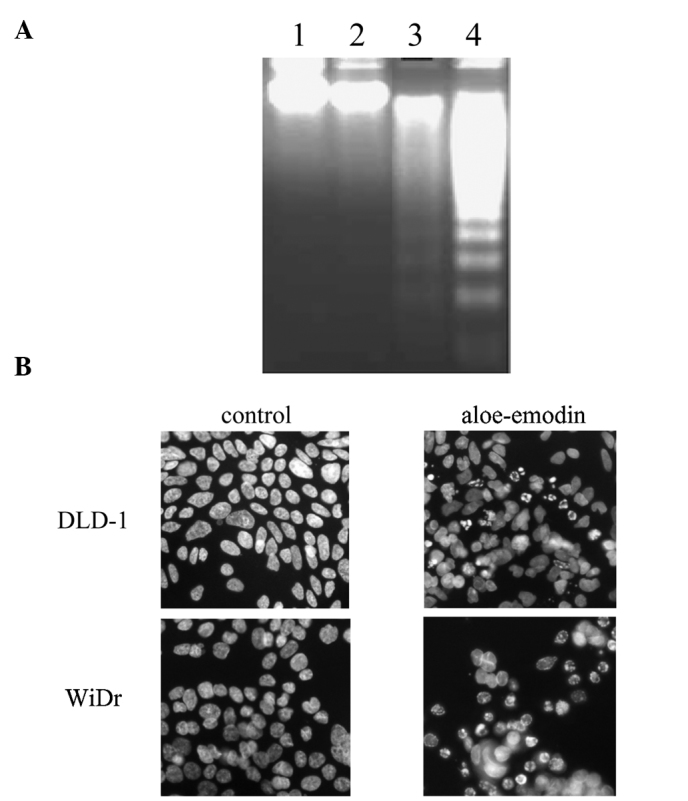
Aloe-emodin induces apoptosis in DLD-1 and WiDr cells. Cells were treated with 0.37 mM aloe-emodin for 24 h. (A) DNA fragmentation was analyzed by 2% agarose gel electrophoresis. Lane 1, untreated DLD-1 cells; lane 2, untreated WiDr cells; lane 3, treated DLD-1 cells and lane 4, treated WiDr cells. (B) Apoptotic cells were detected by Hoechst 33258 staining and examined by fluorescence microscopy. The representative images of three independent experiments are shown (magnification, ×400).
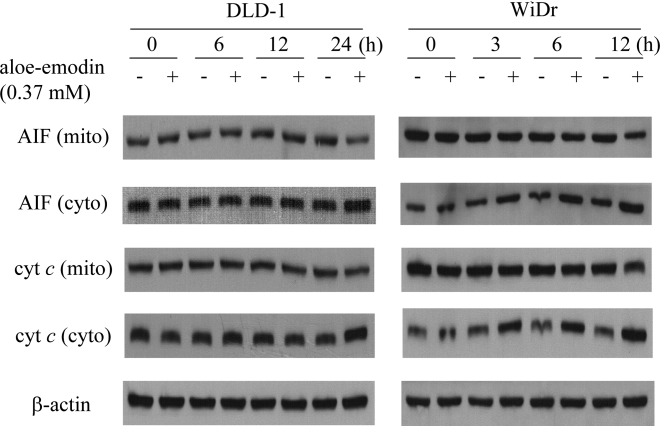
Aloe-emodin induced the release of apoptosis-inducing factor and cytochrome c
The release of apoptosis-inducing factor and cytochrome c in DLD-1 and WiDr cells upon treatment with aloe-emodin was characterized because these processes are typically related to apoptosis (14). An immunoblot analysis of the mitochondrial and cytosolic fractions derived from aloe-emodin-treated DLD-1 and WiDr cells showed a significant decrease in the amount of apoptosis-inducing factor and cytochrome c in mitochondrial fractions. An increase in apoptosis-inducing factor and cytochrome c in the cytosolic fraction for the indicated time intervals was also noted (Fig. 3). Following treatment of the DLD-1 cells, the release of apoptosis-inducing factor and cytochrome c commenced at 24 h, while that of WiDr cells started after 3 h of treatment. The results were consistent with the cell viability data and confirm that WiDr cells are more sensitive to aloe-emodin than DLD-1 cells.
Figure 3.
Aloe-emodin induces the release of apoptosis-inducing factor and cytochrome c (cyt c) in DLD-1 and WiDr cells. Cells were incubated with or without 0.37 mM aloe-emodin for 0, 3, 6, 12 and 24 h. Mitochondrial (mito) and cytosolic (cyto) fractions were analyzed by 15% SDS-PAGE and probed with primary antibodies as described in Materials and methods. Results are representative of three independent experiments.
Effect of aloe-emodin on the activation of caspase-3
The involvement of caspase-3 in aloe-emodin-induced apoptosis in DLD-1 and WiDr cells was evaluated. The activity of caspase-3 was significantly induced 12 h after aloe-emodin treatment in WiDr cells (Fig. 4A). This effect was also observed in DLD-1 cells albeit only significantly after 24 h of aloe-emodin treatment (Fig. 4A). To illustrate the sequence of caspase-3 activation and the release of apoptosis-inducing factor and cytochrome c, a time course of caspase-3 activation after aloe-emodin treatment was performed in WiDr cells. As shown in Fig. 4B, caspase-3 activity was significantly induced after 4 h of aloe-emodin treatment. The results showed that the release of apoptosis-inducing factor and cytochrome c started before caspase-3 activation. To demonstrate whether the activation of caspase-3 is essential for aloe-emodin-mediated apoptosis, the influence of the peptidyl inhibitor of caspase-3, Ac-DEVD-CHO, a generally used caspase-3 inhibitor, was analyzed. Aloe-emodin-induced caspase-3 activation in WiDr cells was blocked when co-treated with Ac-DEVD-CHO (Fig. 4B). These data showed that the activation of caspase-3 was an essential step in aloe-emodin-induced apoptosis in the two cell lines. However, the DLD-1 cells appeared to be less sensitive than the WiDr cells (data not shown).
Figure 4.
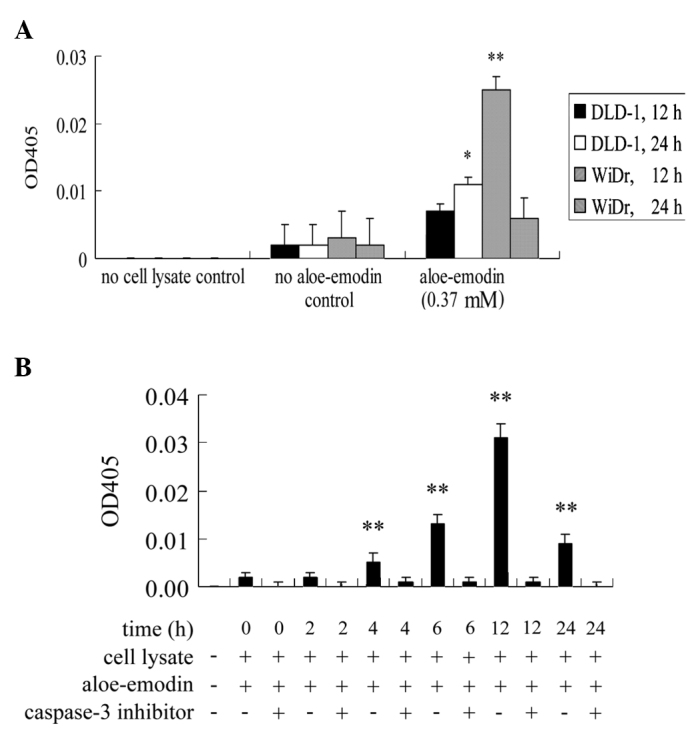
Aloe-emodin activates caspase-3 in DLD-1 and WiDr cells. (A) Cells were incubated with or without 0.37 mM aloe-emodin for 12 and 24 h. (B) WiDr cells were incubated with or without 0.37 mM aloe-emodin for 0, 2, 4, 6, 12 and 24 h. To inhibit caspase-3 activity, a caspase-3 inhibitor, Ac-DEVD-CHO, co-treated with aloe-emodin, was added to WiDr cells at a final concentration of 200 μM. Each bar indicates the value of caspase-3 activity by measuring the release of p-nitroaniline (OD405). Data are the results of three independent experiments and are presented as the mean ± SD. Means are significantly different from the respective control values; *P<0.05 and **P<0.01.
Effect of aloe-emodin on casein kinase II activity and Ser61 phosphorylation of Bid
To investigate the role of casein kinase II in aloe-emodin-induced apoptosis, the activity of casein kinase II was examined, as well as the Ser61 phosphorylation of Bid, a downstream substrate of casein kinase II (Ser61 is one of the phosphorylation sites of Bid). The activity of casein kinase II started to decrease at 3 h of exposure to aloe-emodin and continued to decrease in a time-dependent manner in WiDr cells (Fig. 5A). A similar effect was observed in the DLD-1 cells (Fig. 5A); however, the activity of casein kinase II started to decrease after 12 h of exposure to aloe-emodin. Using anti-phospho Bid-Ser61 polyclonal antibodies, immunoblotting was used to confirm the kinase activity. The aloe-emodin-mediated inhibition of casein kinase II activity caused an inhibition of the Ser61 phosphorylation of Bid (Fig. 5B). These results suggest that aloe-emodin induces apoptosis in DLD-1 and WiDr cells through inhibition of the phosphorylation of Bid.
Figure 5.
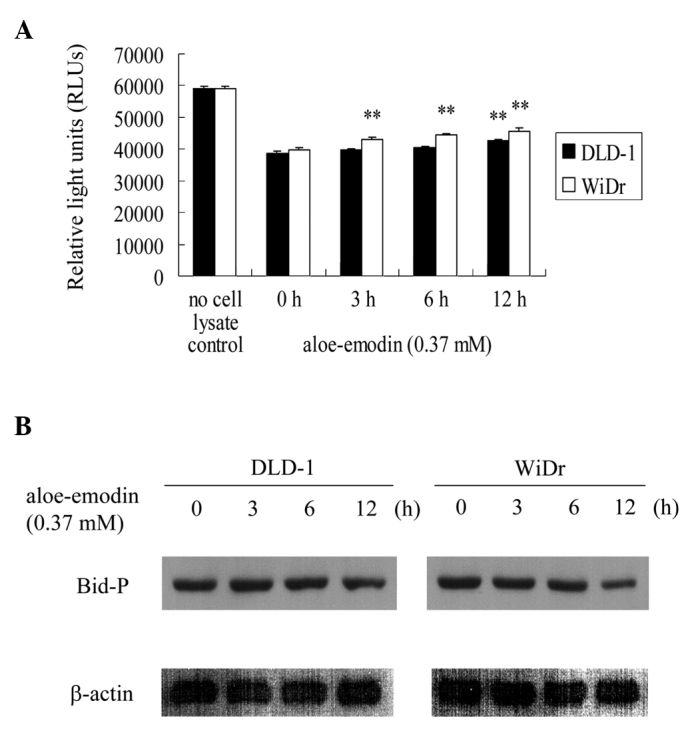
Aloe-emodin inhibits the activity of casein kinase II and the phosphorylation of Bid in DLD-1 and WiDr cells. Cells were incubated with 0.37 mM aloe-emodin for 0, 3, 6 and 12 h. (A) The activity of casein kinase II was measured as described in Materials and methods. Data are the results of three independent experiments presented as the mean ± SD. Means that are significantly different from the respective control values: **P<0.01. (B) Cell lysates were analyzed by 15% SDS-PAGE and probed with primary antibodies as described in Materials and methods. Results are representative of three independent experiments.
Discussion
The present study demonstrated that aloe-emodin exhibits anticancer activity in DLD-1 and WiDr colon carcinoma cells and that the cytotoxic mechanism involves the induction of apoptosis. The latter was characterized by the release of apoptosis-inducing factor and cytochrome c from the mitochondria to the cytosol, activation of caspase 3 and inhibition of the activity of casein kinase II, with the subsequent inhibition of the downstream phosphorylation of Bid. It was reported that, using the human lung squamous carcinoma cell line CH27, apoptosis induced by aloe-emodin was associated with changes in the expression of members of the Bcl-2 family apoptosis regulators (i.e., increase in the Bax level and decrease in Bcl-XL). Aloe-emodin was found to cause cytochrome c release from mitochondria (21). In addition, it was observed that aloe-emodin-induced apoptosis in human hepatocellular carcinoma cell lines HepG2 and Hep3B was accompanied by the induction of p53 and p21 expression (5). Another report suggests that the decrease in the expression of protein kinase Cδ and ɛ isoforms plays a critical role in aloe-emodin-induced apoptosis (21). A similar role of protein kinase C was observed in U-937 glioma cells (6). With the exception of protein kinase Cα, most of the protein kinase C isozymes (protein kinase Cα, βI, γ, δ, ɛ, θ, ξ and μ) were found to decrease upon aloe-emodin treatment. Notably, two hydrophobic residues unique to casein kinase II, Val66 and Ile174, were found to be essential for the interaction with aloe-emodin; a Val66Ala casein kinase II variant exhibited a substantially affected interaction with aloe-emodin (22,23).
In the present study, aloe-emodin-induced apoptosis in colon carcinoma cells involved inhibition of casein kinase II activity and blockage of the phosphorylation of Bid. We also demonstrated that aloe-emodin resulted in the release of apoptosis-inducing factor and cytochrome c from mitochondria, followed by the activation of caspase-3. It is known that phosphorylated Bid is cleaved by caspase-8, and truncated Bid binds to mitochondria to induce the release of apoptosis-inducing factor and cytochrome c from mitochondria (10,24,25). It is also known that, in the cytosol, cytochrome c mediates the allosteric activation of Apf-1, which is required for the proteolytic maturation of caspase-9 and -3 (10,26). Thus, the inhibition of casein kinase II activity following aloe-emodin treatment may lead to the activation of caspase-3 through the phosphorylation of Bid and release of apoptosis-inducing factor and cytochrome c from mitochondria. A similar pathway was described for Radix Paeoniae Alba extract-induced apoptosis in HL-60 leukemic cells and for quercitin-mediated cell apoptosis in murine melanoma B16-BL6 cells (27,28). Ahmad et al found that the down-regulation of casein kinase II by antisense RNA elicited intracellular hydrogen peroxide production, which was associated with caspase-3 activation (29). Although not relative to aloe-emodin, this is another possible pathway between casein kinase II and caspase-3. Thus, further studies are required to elucidate the relationship between casein kinase II and caspase-3 activities.
Notably, in all of the assays used in the present study, the WiDr colon carcinoma cells were significantly more sensitive to aloe-emodin than the DLD-1 cells. The reason for this difference in sensitivity remains unclear. Controversial data have been analyzed to attempt to correlate differences in response to a given drug with the differentiation state of a given cell line. In an in vitro system using epidermal cells, maturing basal cells were found to undergo apoptosis upon drug treatment, whereas stem cells appeared to be resistant (30). In addition, it has been suggested that undifferentiated keratinocyte stem cells are protected from spontaneous apoptosis (31). Similarly, differentiated human neural crest-derived tumor cells exhibit an increased resistance to potassium ionophore-mediated apoptosis. Cells and human keratinocytes that are undifferentiated are more sensitive to H2O2-induced cell death than their differentiated counterparts (32,33). However, it was shown that the TNF-related apoptosis-inducing ligand triggers apoptosis in undifferentiated but not in differentiated human keratinocytes (34). Microscopy analyses have demonstrated that DLD-1 cells are less differentiated than WiDr cells (data not shown). Thus, our results strongly suggest that the degree of differentiation of colon carcinoma cells affects the cellular response to aloe-emodin. In addition to the degree of differentiation of culture cells, evidence shows that p53-mutant cell lines are less sensitive to aloe-emodin than their p53 wild-type counterparts (35). In the present study, the amount of mutant p53 in the DLD-1 cells was seven times higher than that in the WiDr cells (1.96 and 0.27 ng/ml cell lysate, respectively). Our data are consistent with the above-mentioned study and suggest that the p53 phenotype affects the cellular response to aloe-emodin.
In conclusion, using two human colon carcinoma cell lines, we investigated the anticancer effect of the natural product, aloe-emodin, isolated from Aloe vera leaves. We demonstrated for the first time that aloe-emodin exhibits an anticancer effect against colon carcinoma.
Acknowledgements
The authors would like to thank Dr Sheng-Hsuan Chen, Dr Chun-Chao Chang and Dr Chia-Lang Fang for the helpful comments and review of the manuscript.
References
- 1.Reynolds T. The compounds in Aloe leaf exudates: a review. Bot J Linn Soc. 1985;90:157–177. [Google Scholar]
- 2.Eshun K, He Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries – a review. Crit Rev Food Sci Nutr. 2004;44:91–96. doi: 10.1080/10408690490424694. [DOI] [PubMed] [Google Scholar]
- 3.Pecere T, Gazzola MV, Mucignat C, Parolin C, Vecchia FD, Cavaggioni A, Basso G, Diaspro A, Salvato B, Carli M, Palu G. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000;60:2800–2804. [PubMed] [Google Scholar]
- 4.Lee HZ, Hsu SL, Liu MC, Wu CH. Effects and mechanisms of aloe-emodin on cell death in human lung squamous cell carcinoma. Eur J Pharmacol. 2001;431:287–295. doi: 10.1016/s0014-2999(01)01467-4. [DOI] [PubMed] [Google Scholar]
- 5.Kuo PL, Lin TC, Lin CC. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci. 2002;71:1879–1892. doi: 10.1016/s0024-3205(02)01900-8. [DOI] [PubMed] [Google Scholar]
- 6.MAcevedo-Duncan M, Russell C, Patel S, Patel R. Aloe-emodin modulates PKC isoenzymes, inhibits proliferation and induces apoptosis in U-373MG glioma cells. Int Immunopharmacol. 2004;4:1775–1784. doi: 10.1016/j.intimp.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 8.Mijatovic S, Maksimovic-Ivanic D, Radovic J, Miljkovic D, Kaludjerovic GN, Sabo TJ, Trajkovic V. Aloe emodin decreases the ERK-dependent anticancer activity of cisplatin. Cell Mol Life Sci. 2005;62:1275–1282. doi: 10.1007/s00018-005-5041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penninger JM, Kroemer G. Mitochondria, AIF and caspases – rivaling for cell death execution. Nat Cell Biol. 2003;5:97–99. doi: 10.1038/ncb0203-97. [DOI] [PubMed] [Google Scholar]
- 10.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 11.Susin SA, Lorenzo HK, ZamZami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa U, Mann SK, Firtel RA, Hunter T. Molecular cloning of casein kinase II alpha subunit from Dictyostelium discoideum and its expression in the life cycle. Mol Cell Biol. 1992;12:5711–5723. doi: 10.1128/mcb.12.12.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad KA, Wang G, Slaton J, Unger G, Agmed K. Targeting CK2 for cancer therapy. Anti-Cancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 15.Desagher S, Osen-Sand A, Montessuit A, Magnenat E, Vibois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 16.Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- 17.Lin KY, Wang HH, Lai ST, Pan JP, Chiang AN. β2-glycoprotein I protects J774A.1 macrophages and human coronary artery smooth muscle cells against apoptosis. J Cell Biochem. 2005;94:485–496. doi: 10.1002/jcb.20314. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PAJ, Stewart KN, Cuzzocrea S, Thiemermann C. Differential effects of caspase inhibitors on the renal dysfunction and injury caused by ischemia-reperfusion of the rat kidney. Eur J Pharmacol. 2004;503:173–183. doi: 10.1016/j.ejphar.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Martin RM, Leonhardt H, Cardoso MC. DNA labeling in living cells. Cytometry Part A. 2005;67A:45–52. doi: 10.1002/cyto.a.20172. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Dube A, Bose B. Pharmacokinetics and phototoxicity of purpurin-18 in human colon carcinoma cells using liposomes as delivery vehicles. Cancer Chemother Pharmacol. 2006;57:500–506. doi: 10.1007/s00280-005-0072-x. [DOI] [PubMed] [Google Scholar]
- 21.Lee HZ. Protein kinase C involvement in aloe-emodin- and emodin-induced apoptosis in lung carcinoma cell. Br J Pharmacol. 2001;134:1093–1103. doi: 10.1038/sj.bjp.0704342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarno S, Moro S, Meggio F, Zagotto G, Ben DD, Ghisellini P, Battistutta R, Zanotti G, Pinna LA. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther. 2002;93:159–168. doi: 10.1016/s0163-7258(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 23.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chau DHW, Yuan J, Zhang H, Cheung P, Lim T, Liu Z, Sall A, Yang D. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondria injury and cleavage of eIL4GI but not DAP5/p97/NAT1. Apoptosis. 2007;12:513–524. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- 25.Shulga N, Pastorino JG. Acyl coenzyme A-binding protein augments Bid-induced mitochondrial damage and cell death by activating μ-calpain. J Biol Chem. 2006;281:30824–30833. doi: 10.1074/jbc.M602503200. [DOI] [PubMed] [Google Scholar]
- 26.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 27.Kwon KB, Kim EK, Han MJ, et al. Induction of apoptosis by Radix Paeoniae Alba extract through cytochrome c release and the activations of caspase-9 and caspase-3 in HL-60 cells. Biol Pharm Bull. 2006;29:1082–1086. doi: 10.1248/bpb.29.1082. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XM, Chen J, Xia YG, Xu Q. Apoptosis of murine melanoma B16-BL6 cells induced by quercetin targeting mitochondria, inhibiting expression of PKC-α and translocating PKC-δ. Cancer Chemother Pharmacol. 2005;55:251–262. doi: 10.1007/s00280-004-0863-5. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad KA, Wang G, Ahmed K. Intracellular hydrogen peroxide production is an upstream event in apoptosis induced by down-regulation of casein kinase 2 in prostate cancer cells. Mol Cancer Res. 2006;4:331–338. doi: 10.1158/1541-7786.MCR-06-0073. [DOI] [PubMed] [Google Scholar]
- 30.Morris RJ, Fischer SM, Klein-Szanto AJ, Slaga TJ. Subpopulations of primary adult murine epidermal basal cells sedimented on density gradients. Cell Tissue Kinet. 1990;23:587–602. doi: 10.1111/j.1365-2184.1990.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiberio R, Marconi A, Fila C, et al. Keratinocytes enriched for stem cells are protected from anoikis via an integrin signaling pathway in a Bcl-2 dependent manner. FEBS Lett. 2002;524:139–144. doi: 10.1016/s0014-5793(02)03040-5. [DOI] [PubMed] [Google Scholar]
- 32.Teplova V, Jaaskelainen E, Salkinoja-Salonen M, Saris NE, Serlachius M, Li FY, Andersson LC. Differentiated Paju cells have increased resistance to toxic effects of potassium ionophores. Acta Biochim Pol. 2004;51:539–544. [PubMed] [Google Scholar]
- 33.Zuliani T, Denis V, Nobless E, Schnebert S, Andre P, Dumas M, Ratinaud MH. Hydrogen peroxide-induced cell death in normal human keratinocytes is differentiation dependent. Free Radic Biol Med. 2005;38:307–316. doi: 10.1016/j.freeradbiomed.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Jansen BJ, van Ruissen F, Cerneus S, Cloin W, Bergers M, van Erp PE, Schalkwijk J. Tumor necrosis factor related apoptosis inducing ligand triggers apoptosis in dividing but not in differentiating human epidermal keratinocytes. J Invest Dermatol. 2003;121:1433–1439. doi: 10.1046/j.1523-1747.2003.12636.x. [DOI] [PubMed] [Google Scholar]
- 35.Pecere T, Sarinella F, Salata C, et al. Involvement of p53 in specific anti-neuroectodermal tumor activity of aloe-emodin. Int J Cancer. 2003;106:836–847. doi: 10.1002/ijc.11312. [DOI] [PubMed] [Google Scholar]