Abstract
Background
We have previously shown that bortezomib induces a depletion of alloreactive T cells and allows the expansion of T cells with suppressive properties. In the current study, we analyzed the potential synergistic effect of bortezomib in conjunction with sirolimus in order to reduce-graft-versus-host disease without hampering graft-versus-leukemia effect in the allogeneic transplant setting.
Design and Methods
We evaluated the effect of sirolimus, bortezomib or the combination of both in the proliferation and activation of in vitro stimulated T lymphocytes. Pathways involved in this synergy were also analyzed using Western blot assays. Finally, BALB/c mice receiving C57BL/6 allogeneic donor bone marrow with splenocytes were used to measure in vivo the effect of this novel combination on the risk of graft-versus-host disease.
Results
The combination of both drugs synergistically inhibited both activation and proliferation of stimulated T cells. Also, the production of Th1 cytokines (IFN γ, IL-2 and TNF) was significantly inhibited. This effect was due, at least in part, to the inhibition of Erk and Akt phosphorylation. In vivo, the combination reduced the risk of graft-versus-host disease without hampering graft-versus-leukemia effect, as shown in mice receiving graft-versus-host disease prophylaxis with sirolimus plus bortezomib being infused with tumor WEHI cells plus C57BL/6 donor BM and splenocytes.
Conclusions
The current study reveals a synergistic effect of the combination sirolimus and bortezomib to prevent graft-versus-host disease while maintaining the graft-versus-leukemia effect.
Keywords: allogeneic transplant, sirolimus, bortezomib, graft-versus-host, graft-versus-leukemia
Introduction
Graft-versus-host disease (GVHD) represents a major challenge and is the main cause of morbidity and mortality after allogeneic transplantation. Incidence varies greatly depending on the type of donor and the immune suppression used to prevent it. Standard GVHD prophylaxis is currently based on the use of a calcineurin inhibitor plus methotrexate (MTX). In fact, this combination has been used for more than 20 years, and remains the standard of care, although the incidence of acute GVHD is in the range of 20-40% among patients receiving hematopoietic stem cells from a matched, related donor, and this is even higher after unrelated donor transplant.1–3 The incidence of chronic GVHD with the use of a calcineurin inhibitor plus methotrexate (MTX) after peripheral blood stem cell transplantation is in the range of 40–70%, and more than one-third of the patients require immunosuppression for up to four years after transplant.3,4 These data suggest that immune tolerance is not obtained in the long term in an important subset of patients and, in fact, are consistent with those from in vitro studies showing that calcineurin inhibitors block the expansion and function of regulatory T cells (Treg)5–10 which are essential for the generation of a tolerogeneic immune response. The use of in vivo or in vitro T-cell depletion significantly reduces the risk of GVHD but has not led to an improved survival, due to an increase in the incidence of severe infections and relapses.11,12 For this reason, new strategies are being evaluated in an attempt to reduce GVHD without hampering the graft-versus-leukemia (GVL) effect.
Sirolimus is the first commercially available inhibitor of the mammalian target of rapamycin (mTOR). Several studies and clinical trials have confirmed that sirolimus allows the risk of GVHD to be reduced after both related and unrelated donor transplant.13 In addition, an antileukemic effect has been reported in vitro.14,15 Furthermore, using in vitro assays, several studies have shown an increase in the number of Tregs after sirolimus exposure.16–18 Unfortunately, at least using myeloablative conditioning, the use of sirolimus in combination with calcineurin inhibitors may increase the risk of microangiopathy,19 blocks the development of Treg20 and may subsequently compromise tolerance in the long term.21
Bortezomib, a boronic acid dipeptide, is a potent, selective and reversible inhibitor of proteasome.22 The proteasome is a multi-enzyme complex present in all cells. It degrades proteins that regulate cell-cycle progression and causes proteolysis of the ubiquitinated endogenous inhibitor of nuclear factor-κB (NF-κB), IκB. The latter blocks the nuclear translocation and transcriptional activity of NF-κB.23,24 In normal T lymphocytes, NF-κB translocation to the nucleus only occurs after TCR/CD3 and co-stimulatory molecule engagement, while it is not activated in resting T lymphocytes. In our experience, bortezomib induces selective depletion of alloreactive T cells after a mixed lymphocyte culture.25 This could prove to be valuable in the clinical setting for preventing GVHD, as recently shown by Koreth et al. in a clinical trial.26 Interestingly, we have shown that bortezomib does not affect the viability of Treg and allows the expansion of T cells with suppressive properties.27 Accordingly, both sirolimus and bortezomib could favor the development of a tolerogeneic immune response after allogeneic transplantation.
On the basis of these findings, the current study analyzes the potential synergistic effect of sirolimus together with bortezomib in the activation pattern of T cells and in the expansion of Treg. We have evaluated the synergy between both drugs in a murine model28 in order to prevent GVHD.
Design and Methods
Animals
All animal protocols were approved by the University of Salamanca Animal Care and Use Committee. Female BALB/c (H2d) and male C57BL/6 (H2b) mice were purchased from the Charles River Laboratory, France. Animals were kept in specific pathogen-free conditions. Mice were between 8 and 12 weeks of age at the start of the experiments.
Donor mice C57BL/6 were sacrificed by cervical dislocation, and bone marrow (BM) and spleen were harvested by standard techniques. Spleen-cell preparations were obtained by gently crushing the tissues to release the cells. Preparations were filtered to remove debris and washed twice in PBS for injection.
BALB/c (H2d) mice were used as recipients in the graft-versus-host disease (GVHD) model systems. Recipient mice received total body irradiation (TBI) (850cGy divided in two fractions) from a Cs source. The control group received irradiation without stem cell support. Irradiation was followed by the infusion of 5×106 C57BL/6 allogeneic donor BM cells intravenously with or without splenocytes (5–10×106 cells intravenously) as a source of allogeneic T cells. Recipient mice then received RPMI, or sirolimus in RPMI at a dose of 0.25 μg/kg intra-peritoneally on Days 0 to 12 post-transplant and/or bortezomib at a dose of 1 μg/day intravenously on Days 0, 1 and 2 post-transplant and/or cyclosporine A at a dose of 10–15 μg/kg intra-peritoneally on Days 0 to 12 post-transplant. Mice were monitored and weighed twice a week. All moribund mice were sacrified according to the standard practice.
In addition, NOD.CB17-Prkdcscid (NOD/SCID) mice received bone marrow plus splenocytes from BALB/c mice that had stable C57BL/6 hematopoiesis and had not developed GVHD after receiving prior transplantation of BM plus splenocytes and GVHD prophylaxis with sirolimus and bortezomib.
Finally, in order to induce leukemia in the mice, 5×104 WEHI 3b d+ clone 17.3 GFP+ cells (myelo-monocytic leukemic cell line from a BALB/c background) were infused to BALB/c mice together with 5×106 C57BL/6 BM cells (controls) with or without splenocytes and sirolimus plus bortezomib as GVHD prophylaxis either after total body irradiation or on Day 21 after transplantation.
All experiments were performed at least twice and included 4 animals per group. The degree of systemic GVHD was assessed by a standard scoring system that incorporates five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity. Each parameter received a score of 0 (minimum) to 2 (maximum). GVHD score was not blinded. Transplanted mice were ear-punched, and individual weights were obtained and recorded on Day 0 and twice a week thereafter. At the time of analysis, mice from coded cages were evaluated and graded for each criterion. Acute GVHD was also assessed by detailed histopathological analysis of skin, liver and intestine.
Chimerism assays were performed in peripheral blood, spleen and bone marrow at Day 14 day after transplantation. For this purpose, 5 ×105 cells were stained by direct immunofluorescence using monoclonal antibodies (MoAbs) conjugated with the following fluorochromes: fluorescein isothiocyanate (FITC), phyco-erythrin (PE); peridin chlorophyll protein-Cyanin 5.5 (PerCP-Cy5.5) and AlexaFluor. Specific antibodies were purchased from Becton Dickinson Bioscience (BDB) Pharmingen (San Jose, CA, USA). The following combination was used: anti-H2Db-FITC/anti-H2Dd-PE/anti-CD45-PerCP-Cy5.5/anti-CD34-AlexaFluor or anti-H2Db-FITC/anti-H2Dd-PE/anti-CD45-PerCP-Cy5.5/anti-CD3-AlexaFluor. Data were acquired immediately after completion of sample preparation using a FACSCalibur flow cytometer (BDB) equipped with the CellQuestTM program (BDB).
Results
Effect of sirolimus and bortezomib on T-cell activation, proliferation and viability
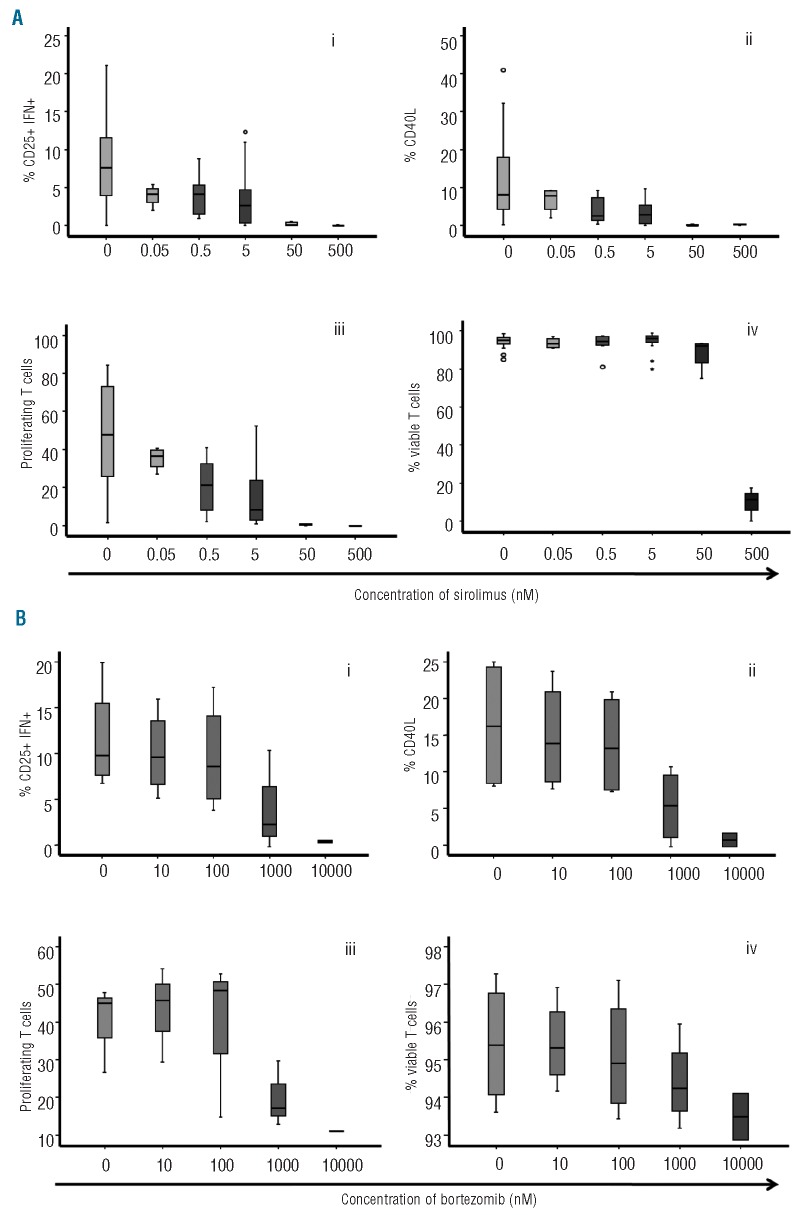
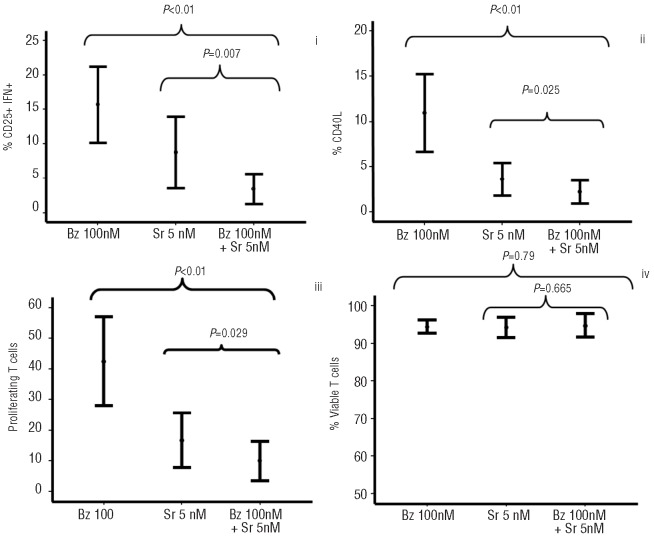
Firstly, we evaluated the effect of sirolimus and then of bortezomib separately on T-cell activation, proliferation and viability. Both drugs induced a dose-response inhibition in T-cell activation and proliferation of T lymphocytes stimulated with anti-CD3 plus anti-CD28 (Figure 1). Although the effect of sirolimus was observed at all the different doses analyzed, the most significant effect was observed for doses of 50 nM or over both for activation and proliferation, while viability was affected at doses of 500 nM or over. A dose response to bortezomib was observed, although its effect on activation and proliferation was not significant for doses of 100 nM or under, while viability was greatly affected at doses over 1000 nM. Considering that a concentration of 0.5–5 nM of sirolimus and of 100 nM of bortezomib did not significantly affect any of the parameters analyzed, we chose these concentrations to evaluate the synergistic effect of the two drugs. Bortezomib and sirolimus synergistically inhibited activation as assessed by the expression of CD25 and production of IFNγ, and expression of CD40L and proliferation assessed as expression of PKH (Figure 2). Calcusyn analysis confirmed a synergistic effect of the combination for all parameters evaluated (Table 1). These effects could not be attributed to a decreased viability of T cells at the concentrations evaluated (Figure 2iv) and were similar in both CD4+ and CD8+ T cells (Online Supplementary Figure S1).
Figure 1.
(A) Box-plot showing the dose-dependent effect of sirolimus on T-cell activation (i,ii) and proliferation (iii). Viability (iv) of T cells only decreased at doses ≥ 500 nM. (B) Box-plot showing the dose-dependent effect of bortezomib on T-cell activation (i,ii), proliferation (iii) and viability (iv). Its effect was not significant for doses ≤ 100 nM for any of the parameters considered. Circles represent values > 95% confidence interval and stars values > 98% CI; 7 cases were analyzed
Figure 2.
Concentrations of sirolimus (5 nM) and bortezomib (100 nM) were used to evaluate the synergistic effect of the two drugs on activation (i, ii), proliferation (iii) and viability (iv) of T-cells; 11 cases were analyzed.
Table 1.
Summary of the activity (fraction affected (Fa)) and combination indexes (CI) of several doses of bortezomib and sirolimus or combinations of both drugs as analyzed by CalcuSyn software.
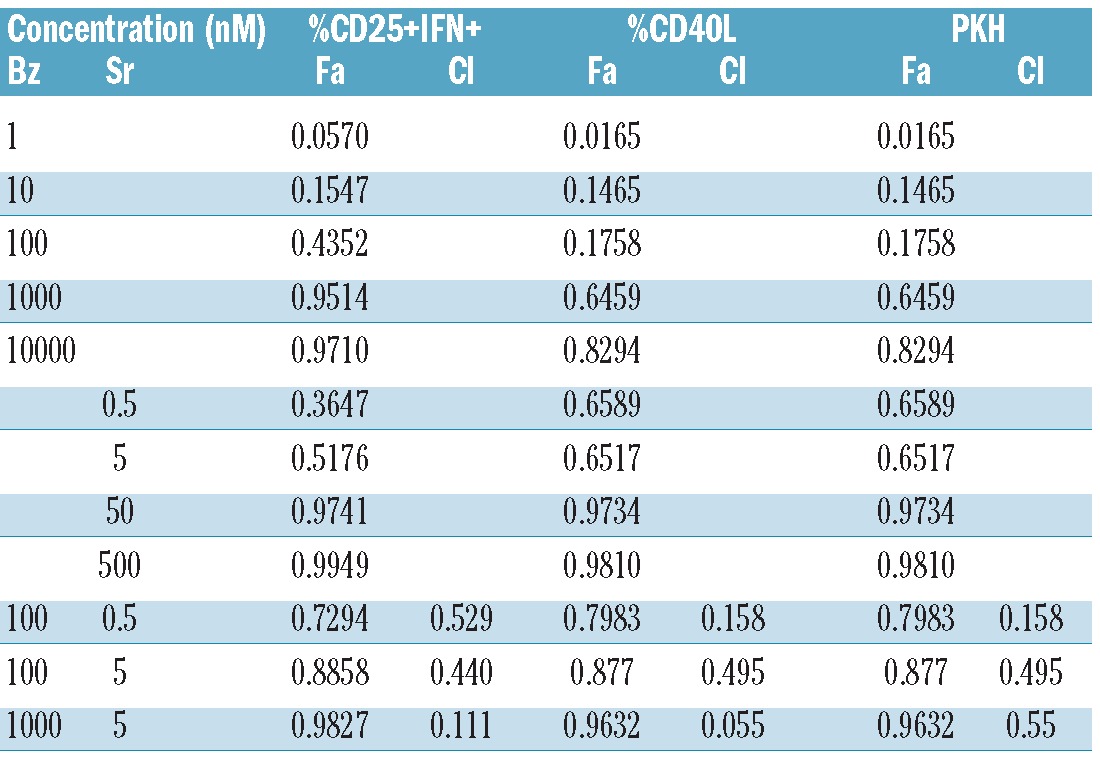
Similar figures were obtained upon adding sirolimus, bortezomib or both after mixed lymphocyte cultures, although the percentage of CD25+IFN+ cells was lower compared to samples stimulated with anti-CD3 plus ant-CD28; the percentage of CD25+IFN+ cells was 7% (range 4–11%) after MLR while this decreased to 2% (range 0–3%) in the presence of bortezomib, 2% (range 0–3%) in the presence of rapamycin at 0.5 nM, and 0.5% (range 0–1%) upon combining both drugs (P<0.01 when compared to untreated samples). Similarly to these effects on human T cells, Online Supplementary Figure S2 shows the effect of Bz, Sr or both after mixed lymphocyte cultures using T cells from C57BL6 and BALB/C mice.
Effect of sirolimus and bortezomib on the cytokine pattern and mechanism involved
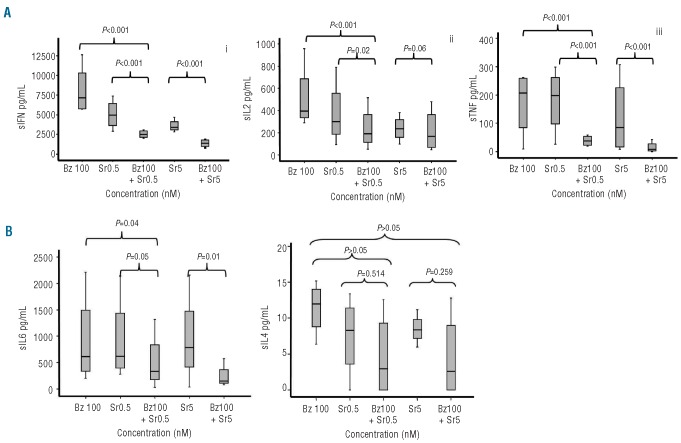
We next analyzed the effect of the drugs, either separately or in combination, on the cytokine pattern of T cells stimulated with antiCD3 plus antiCD28. The combination significantly reduced the production of Th1 cytokines (IFNγ, IL-2 and TNF) as compared to each drug alone while, as far as Th2 cytokines are concerned, only IL-6 significantly decreased on combining the two drugs (Figure 3).
Figure 3.
CBA cytokine assays in supernatants after four days of T-cell culture in the presence of sirolimus or bortezomib, either separately or in combination. Box plots show the effect of the drugs on the production of Th1 cytokines (A): INF (i), IL-2 (ii), and TNF (iii). Regarding Th2 cytokines (B), only IL-6 (iv) significantly decreased with the combination of both drugs; 4 cases were analyzed.
In order to explore the mechanisms involved in the effect of sirolimus and bortezomib, Western blot assays were performed to identify pathways involved in T-cell activation and proliferation, such as pAkt and pErk. Sirolimus at a concentration of 5 nM inhibited the phosphorylation of both Akt and Erk while at 100 nM, bortezomib did not inhibit phosphorylation either of Akt or of Erk (Figure 4). Furthermore, an increase in the signal for both pAkt and pErk was observed after exposure to this concentration of bortezomib. By contrast, the combination of the two drugs resulted in a blockade of the activation of both pathways.
Figure 4.
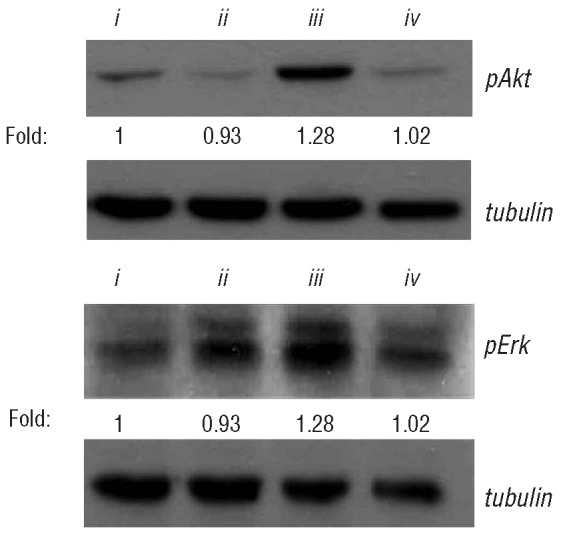
Western blot assays for pAkt and pErk in Jurkat T cells stimulated with anti-CD3 plus anti-CD28 (i) untreated, or treated with (ii) sirolimus 5nM, (iii) bortezomib 100 nM and (iv) sirolimus 5 nM plus bortezomib 100 nM. The numbers shown below the bands indicate the quantitative measurement of the fold change with respect to the untreated sample; data from 4 experiments are shown.
In vivo studies: sirolimus plus bortezomib prevents GVHD
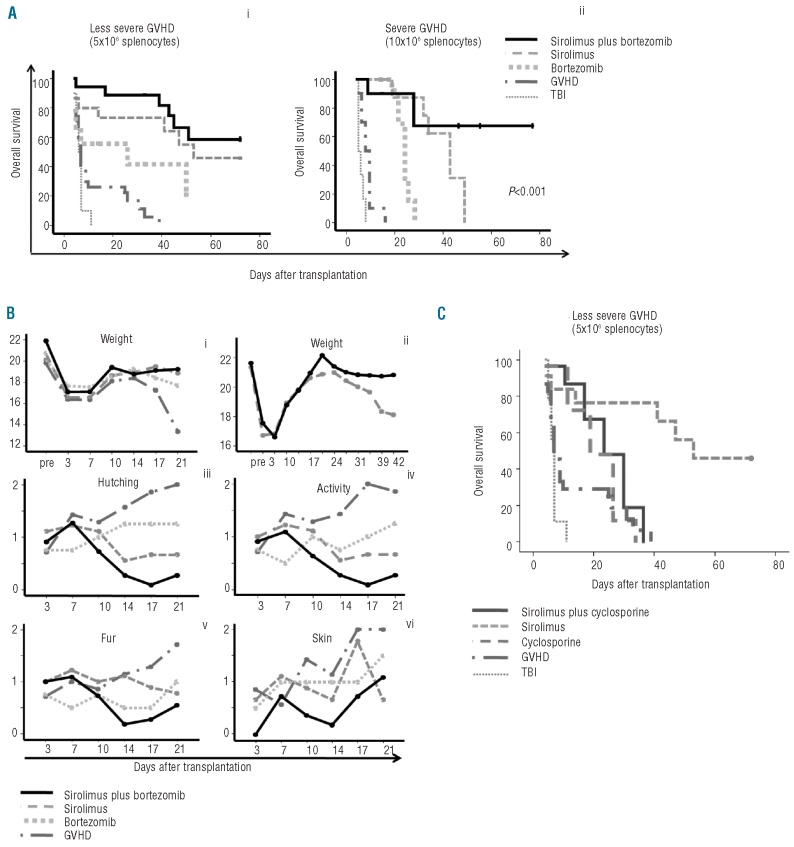
In order to confirm the synergistic effect of sirolimus and bortezomib in vivo, a GVHD mouse model was developed. Mice received bone marrow (5×106 cells) with or without 5×106 splenocytes. Additionally, either sirolimus at a dose of 0.25 μg/kg intra-peritoneally on Days 0 to 12 post-transplant, or bortezomib at a dose of 1 μg/day intravenously on Days 0, 1 and 2 post-transplant, or the combination of both drugs was administered to mice receiving splenocytes. The addition of bortezomib increased survival as compared to mice receiving bone marrow plus splenocytes (Figure 5A). Sirolimus further improved survival while the best results were obtained upon adding both drugs. Also, signs of GVHD significantly improved in the group of mice receiving both drugs as compared to each drug alone (Figure 5B). In order to better determine the effect of the combination of sirolimus and bortezomib on survival, a severe GVHD model was established using 10×106 splenocytes. Survival was again significantly better in the group of mice receiving both drugs, thus confirming in vivo the results previously shown in vitro (Figure 5A). Finally, we investigated whether sirolimus had a similar synergistic effect when used in combination with cyclosporine A (CsA) at 15 μg/kg on Days 0 to 12 post-transplant. CsA marginally improved survival relative to controls, and the combination of sirolimus plus CsA offered no significant improvement in survival (Figure 5C).
Figure 5.
GVHD mouse model. (A) Kaplan-Meier curves representing overall survival of mice after a dose of 5×106 (i) or 10×106 splenocytes (ii) treated with or without sirolimus at a dose of 0.25 mg/kg, intraperitoneally, for 14 days, bortezomib at a dose of 1 μg intravenously on Days 0, +1, +2 post-transplant, or the combination of the two drugs. Control groups receiving only splenocytes without any additional drug to prevent GVHD (so called “GVHD” group), or receiving irradiation without stem cell support (“TBI” group) are shown. Control mice receiving BM without splenocytes survived until the end of the experiment. (B) Evolution of weight loss up to Day 21 (i) or up to Day 42 after transplant (ii), hunching (iii), activity (iv), fur texture (v) and skin integrity (vi) among mice receiving a dose of 5×106 splenocytes, with or without the drugs. GvHD score was not blind. (C) Kaplan-Meier curves representing overall survival in mice receiving a dose of 5×106 splenocytes, with or without sirolimus, cyclosporine or their combinations. The group receiving only sirolimus had the best outcome, so no beneficial effect was observed upon using the combination. The group of mice receiving BM without splenocytes survived until the end of the experiment (> 60 days post-infusion). Five mice per group were included in each experiment. Data from at least 2 experiments are shown.
In vivo studies: graft-versus-leukemia without graft-versus-host disease
We also wanted to evaluate whether the immunosuppressive effect of the combination was unspecific or, by contrast, if it allowed the induction of specific immune tolerance against the host while maintaining the immune response against other antigens. To do this, we infused NOD-SCID mice with 5×106 bone marrow cells plus 5×106 splenocytes from BALB/c mice that had previously received a transplant from C57BL/6 using GVHD prophylaxis with sirolimus and bortezomib and had not developed GVHD. All these BALB/c mice showed 100% C57BL/6 hematopoiesis. Interestingly, all NOD-SCID mice died due to GVHD while, as mentioned above, none of the donors had previously developed GVHD. Although further experiments are required to confirm this finding, these data suggest that the graft had specific immune tolerance against BALB/c but was otherwise able to develop an alloimmune response against histocompatibility antigens from third-party mice.
To further confirm that the combination of sirolimus plus bortezomib inhibited GVHD, thereby enabling the maintenance of the immune response, we infused WEHI (myelo-monocytic leukemic cells) into BALB/c mice after total body irradiation. We observed that all BALB/c mice receiving WEHI plus C57BL/6 donor BM cells died due to leukemic infiltration, while none of those receiving WEHI cells plus C57BL/6 donor BM cells plus splenocytes and GVHD prophylaxis with sirolimus and bortezomib developed leukemic infiltration. Similarly, mice receiving WEHI cells plus C57BL/6 donor BM cells and GVHD prophylaxis with sirolimus and bortezomib but without splenocytes did not develop leukemic infiltration, thus indicating that the anti-leukemic effect could be attributed to either the inoculum and/or the drug combination. We, therefore, exposed WEHI cells to Sr, Bz and the combination of both drugs; either Bz alone or the combination of both drugs had a potent direct cytotoxic effect (Online Supplementary Figure S3). Furthermore, in an attempt to confirm the anti-leukemic effect of the graft, irrespective of the cytotoxic effect of the drugs, we infused the leukemic cells on Day 21 after transplantation, i.e. nine days after the administration of sirolimus and, remarkably, none of the mice that received splenocytes on Day 0 developed either GVHD or leukemic infiltration. This is in contrast to mice receiving WEHI cells on Day 21 after Sr and Bz without having received splenocytes on Day 0 since, in this subgroup, all mice developed leukemic infiltration (n > 8 for all experiments described). Therefore, this approach enabled us to separate the GVHD and GVL effects (Online Supplementary Figure S4).
Discussion
The combination of MTX plus calcineurin inhibitors has been widely used since the early pre-clinical studies conducted in the 1980s29,30 and it is still considered the gold standard for GVHD prophylaxis. Nevertheless, this combination is far from ideal. It induces numerous side effects, must be maintained for long periods and, most importantly, is not effective in a significant number of patients who ultimately develop GVHD. Attempts to improve this approach have had mixed results.31,32 Strategies based on in vitro or in vivo T-cell depletion have enabled the risk of GVHD to be reduced but have not improved survival because of an increased risk of relapse and severe infections. Therefore, strategies that could reduce the incidence of GVHD without hampering GVL are urgently required. Other drugs have emerged, such as sirolimus, that have yielded promising results in vitro and in vivo. Indeed, several studies have shown that sirolimus expands regulatory or suppressive T cells in vitro16,17 which could induce tolerance after allogeneic transplantation. On the other hand, clinical trials13 have reported improved overall survival in patients receiving sirolimus plus tacrolimus, although a significant proportion of patients still develop GVHD. One of the major problems with sirolimus is its toxicity profile in combination with calcineurin inhibitors, including the development of microangiopathy or veno-occlusive disease, especially among patients receiving myeloablative conditionings.33,34
We have previously reported that bortezomib induces selective depletion of alloreactive T cells,25 while Treg are resistant to this pro-apoptotic effect. Furthermore, bortezomib allows the expansion of a suppressive T-cell sub-population in vitro.27 In this context, and bearing in mind that sirolimus and bortezomib target different pathways that could synergistically affect T-cell function, in the current study we combined both drugs and confirmed the existence of a very potent synergistic effect in terms of inhibition of both activation and proliferation of T cells. This is not due to increased apoptosis but to a genuine immune-modulatory effect when the two drugs are used in combination, as shown by the effect on the cytokine secretion pattern of stimulated T cells. Interestingly, Th1 cytokines were strongly affected by the combination while Th2 cytokines, with the exception of IL-6, were preserved. This combination may also inhibit the chronic active antibody-mediated rejection that occurs in experimental renal transplantation in the rat35 and could suppress activation of rapamycin-resistant memory T-cells.36 The current study not only confirms the synergy of sirolimus plus bortezomib in a fully mismatched hematopoietic stem cell transplant model, but also shows that it yields better results than those observed with the classical combination of sirolimus plus calcineurin inhibitors which is currently used in the clinical setting. In fact, data from the current study suggest that CsA might inhibit the protective effect of sirolimus. This could be attributed to either the toxicity of the combination or to the effect of CsA abrogating the expansion of Tregs. This is in contrast to the combination with bortezomib since, as previously reported, both drugs allow the expansion of Tregs. In the current study, we failed to demonstrate an increase in the number of Tregs during the first 50 days postransplant (data not shown). Further studies will be performed to confirm these data with a longer follow up but, most importantly, we confirmed that this strategy enabled GVHD to be avoided in the host maintaining the graft-versus-tumor effect that led to an improvement in survival using sirolimus plus bortezomib. Remarkably, the antitumor effect was due to both a direct cytotoxic effect of the drugs on the tumor cells and to a GVL effect of the inoculum. This double mechanism is of great interest in the clinical setting since it would avoid relapses early after transplant until the immune response is fully developed. Furthermore, previous studies have suggested that Bz increases the expression of NK activation receptor ligands in target cells that might also contribute to maintain or even increase the GVL effect without increasing GVHD.37,38
In order to explore the pathways that might account for this synergy, we observed that at doses of 5 nM of sirolimus and 100 nM of bortezomib, the former inhibited Erk and Akt phosphorylation while this effect was not observed for bortezomib. Furthermore, after exposure to 100 nM of bortezomib, the phosphorylation of both substrates was slightly increased. It has previously been reported that the increased expression of pAkt in tumor cell lines could favor resistance to the pro-apoptotic effect of proteosome inhibitors.39 Remarkably, the concomitant use of sirolimus avoids the activation of these pathways in Jurkat T cells. Considering previous studies showing that both in vitro25,27 and in clinical models26 bortezomib significantly decreases the risk of GVHD, the data in the current study on Akt and Erk phosphorylation are remarkable since we demonstrate that the addition of sirolimus blocks pathways the activation of which might allow T cells to escape from the inhibitory effect of Bz on activation and viability of T lymphocytes.
In conclusion, the current study reveals the existence of a potent synergistic effect between sirolimus and bortezomib in terms of inhibition of T-cell activation and proliferation, with a marked decrease in the secretion of Th1 cytokines. These effects are at least partly mediated by the blockade of the Akt and Erk pathways. Finally, this combination allowed GVHD to be prevented while maintaining the GVL effect. On the basis of these findings, a clinical trial using sirolimus plus bortezomib post-transplant is planned.
Acknowledgments
We thank Dr. Juan A. Bueren for providing WEHI cells.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding: supported by a grant Rio Hortega (code: CM10/00161) from the Instituto de Salud Carlos III and grant PI080047 from FIS.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Sorror ML, Leisenring W, Deeg HJ, Martin PJ, Storb R. Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graft-versus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant. 2005;11(10):814–5. doi: 10.1016/j.bbmt.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8. [PubMed] [Google Scholar]
- 3.Pérez-Simón JA, Encinas C, Silva F, Arcos MJ, Díez-Campelo M, Sánchez-Guijo FM, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: the national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biol Blood Marrow Transplant. 2008;14(10):1163–71. doi: 10.1016/j.bbmt.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–6. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 5.Coenen JJ, Koenen HJM, Van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not Cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107(3):1018–23. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 6.Segundo DS, Ruiz JC, Izquierdo M, Fernández-Fresnedo G, Gómez-Alamillo C, Merino R, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82(4):550–7. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Zhao L, Sun Z, Sun L, Zhang B, Zhao Y. A potential side effect of cyclosporin A: inhibition of CD4(+) CD25(+) regulatory T cells in mice. Transplantation. 2006;82(11):1484–92. doi: 10.1097/01.tp.0000246312.89689.17. [DOI] [PubMed] [Google Scholar]
- 8.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4(+)CD25(+)FoxP3(+) T cells. Bone Marrow Transplant. 2007;39(9):537–45. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 9.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7(7):1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18(3):1007–18. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 11.Finke J, Behge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Socié G; ATG-Fresenius Trial Group. ATG fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, et al. Gruppo Italiano Trapianto Midollo Osseo (GITMO) Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. 2010;45(2):385–91. doi: 10.1038/bmt.2009.151. [DOI] [PubMed] [Google Scholar]
- 13.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, Antin JH. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–14. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Récher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–34. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 15.Vaysberg M, Balatoni CE, Nepomuceno RB, Krams SM, Martinez OM. Rapamycin inhibits proliferation of Epstein-Barr virus-positive B-cell lymphomas through modulation of cell-cycle protein expression. Transplantation. 2007;83(8):1114–21. doi: 10.1097/01.tp.0000260142.38619.9c. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 17.Uss E, Yong SL, Hooibrink B, van Lier RA, Ten Berge IJ. Rapamycin enhances the number of alloantigen-induced human CD103+CD8+ regulatory T cells in vitro. Transplantation. 2007;83(8):1098–106. doi: 10.1097/01.tp.0000259555.29762.f0. [DOI] [PubMed] [Google Scholar]
- 18.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortin MC, Raymond MA, Madore F, Fugère JA, Pâquet M, St-Louis G, et al. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporine-sirolimus combination. Am J Transplant. 2004;4(6):946–52. doi: 10.1111/j.1600-6143.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 20.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 21.Blaha P, Bigenzahn S, Koporc Z, Schmid M, Langer F, Selzer E, et al. The influence of immunosuppressive drugs on tolerance induction through bone marrow transplantation with costimulation blockade. Blood. 2003;101(7):2886–93. doi: 10.1182/blood-2002-10-3014. [DOI] [PubMed] [Google Scholar]
- 22.Hideshima T, Richardson P, Chauhan D, Palombella V, Elliot E, Adams J, Anderson K. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–6. [PubMed] [Google Scholar]
- 23.Palombella V, Rando O, Goldberg A, Maniatis T. The ubiquitin proteasome pathway is required for processing the NF-kB1 precursor protein and the activation of NF-kB. Cell. 1994;78(5):773–85. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Dou Q. Bax degradation by the ubiquitin/proteasome dependent pathway: involvement in tumor survival and progression. Proc Nat Acad Sci USA. 2000;97(8):3835–55. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107(9):3575–83. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 26.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, Cutler C, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114(18):3956–9. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco B, Perez-Simón JA, Sánchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossío S, Hernández-Campo P, et al. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94(7):975–83. doi: 10.3324/haematol.2008.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke KR, Kobzik L, Martín TR, Brewer J, Delmonte J, Jr, Crawford JM, et al. An Experimental Model of Idiopathic Pneumonia Syndrome After Bone Marrow Transplantation: I. The Roless of Minor H Antigens and Endotoxin. Blood. 1996;88(8):3230–9. [PubMed] [Google Scholar]
- 29.Deeg HJ, Storb R, Weiden PL, Raff RF, Sale GE, Atkinson K, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of intolerance. Transplantation. 1982;34(1):30–5. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Storb R, Kolb HJ, Deeg HJ, Weiden PL, Appelbaum F, Graham TC, et al. Prevention of graft-versus-host disease by immunosuppressive agents after transplantation of DLA-nonidentical canine marrow. Bone Marrow Transplant. 1986;1(2):167–77. [PubMed] [Google Scholar]
- 31.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis for leukemia. N Engl J Med. 1986;314(12):729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 32.Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, et al. Marrow transplantation for severe aplastic anemia: Methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68(1):119–25. [PubMed] [Google Scholar]
- 33.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–31. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(21):1098–105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelbacher R, Meister S, Gückel E, Starke C, Wittmann S, Stief A, et al. Bortezomib and sirolimus inhibit the chronic active antibody-mediated rejection in experimental renal transplantation in the rat. Nephrol Dial Transplant. 2010;25(11):3764–73. doi: 10.1093/ndt/gfq230. [DOI] [PubMed] [Google Scholar]
- 36.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;27;88(12):1349–59. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 37.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, Childs R. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66(14):7317–25. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 38.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113(24):6120–7. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–40. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]