Abstract
Metastatic breast cancer is the leading cause of cancer-related death in women worldwide and, despite recent therapeutic advances, the disease remains incurable. A critical step in cancer cell metastasis is the degradation of extracellular matrix components by matrix metalloproteinases (MMPs), which permits malignant cells to separate from the primary tumor and access circulatory conduits for seeding distant organs. This study reports a correlation between the elevated secretion of MMP-3 by breast cancer cells and the expression of CCR7 protein, a recently discovered non-classical chemokine receptor that may play a role in metastasis by regulating tumor cell transendothelial migration. MMP-3 secretion is increased in human mammary tumor cells that overexpress CXCR7, and is reduced in mouse breast cancer cells in which the endogenous CXCR7 expression has been knocked down via RNAi. The correlation between CXCR7 and MMP-3 expression in breast cancer may provide additional therapeutic rationale for targeting CXCR7 in order to prevent metastatic disease.
Keywords: chemokines, matrix metalloproteinases, cancer, CXCR7
Introduction
Stage IV or metastatic breast cancer is the most prevalent type of cancer worldwide, and is currently an incurable condition with 20% of patients surviving 5 years or less after diagnosis (1–3). Numerous studies have identified a role for the tumor-expressed chemokine receptor CXCR4 in the regulation of breast cancer metastasis to the axillary lymph node, bone, liver and lung, the four most common metastatic destinations (4). These destinations, in turn, express high levels of the CXCR4 ligand CXCL12 (reviewed in ref. 5). This study showed that a number of primary breast cancer cells and cell lines express CXCR7, a second receptor for CXCL12 (6). CXCR7 also binds CXCL11; however, the binding of either chemokine ligand does not trigger cell migration or intracellular calcium mobilization (7). Recently, a small molecule that blocks CXCL12 binding to CXCR7 and inhibits CXCR4-mediated tumor cell transendothelial migration to CXCL12 was identified (8). Small molecules that selectively target CXCR7 may therefore prevent the metastasis of CXCR4+CXCR7+ breast cancer cells that have gained access to the circulatory system.
A key step in cancer cell metastasis is the degradation of the interstitial matrix and basement membrane by matrix metalloproteinases (MMPs), such as MMP-3. MMP-3 substrates include collagens, gelatin, fibronectin, laminin and aggrecan; MMP-3 is itself proteolytically cleaved and activated by MMP-2, -3, -13 and plasmin (reviewed in ref. 9). Overexpression of an auto-activating MMP-3 variant in mouse mammary epithelium was found to result in neoplastic transformation of the mammary tissue (10,11). Furthermore, high levels of MMP-3 are reported in tumor epithelial cells and the surrounding stromal cells in human breast cancer biopsy samples (12), as well as in the tumor and stroma in xenografts of human breast cancer cell lines implanted in nude mice (13).
The present study found that MMP-3 secretion was increased in human mammary tumor cells that overexpress CXCR7. In a xenograft model, SCID mice implanted with MDA-MB-435s human breast cancer cells transfected with CXCR7 (435-CXCR7) tumor cells showed elevated levels of circulating hMMP-3 relative to mice bearing mock-transfected 435 tumors. Furthermore, in 4T1 mouse mammary tumor cells that express endogenous CXCR7, mMMP-3 secretion was significantly reduced following CXCR7-RNAi knockdown. Together, these data suggest a correlation between CXCR7 expression and MMP-3 secretion in breast cancer that may contribute to metastatic disease.
Materials and methods
Cell lines
Mouse 4T1 (4T1-WT) and human MDA-MB-435s (435-WT) breast cancer cell lines were purchased from American Type Culture Collection and cultured in RPMI-1640 or DMEM with 10% fetal bovine serum (FBS), respectively. 435-CXCR7, 435-empty vector control transfectants, and 4T1- CXCR7-RNAi-985 cell lines were generated and previously characterized (6).
Animal experiments
Animal procedures were approved by the ChemoCentryx Institutional Animal Care Committee. A total of 1 million 435-WT or 435-CXCR7 cells were injected subcutaneously into 6- to 8-week-old female CB17 SCID mice (Charles River Laboratories). Animals were sacrificed when the tumor volumes reached 1,000 mm3 or the animals lost >20% of their initial body weight.
Protein quantification
The 435-WT or 435-CXCR7 cells were seeded at 200,000 cells/well in 6-well plates and cultured for 2 days in DMEM with 10 or 1% FBS. The conditioned media (CM) were collected and sent to Rules-Based Medicine, Inc. (Austin, TX, USA) for multi-protein analysis via a quantitative and multiplexed immunoassay. Human or murine MMP-3 was measured with ELISA reagents purchased from R&D Systems, Inc. (Minneapolis, MN, USA). In the xenograft model, mouse serum samples were obtained after mice were sacrificed on day 56 or 92, and serum was collected following cardiac puncture. Human and mouse MMP-3 levels were measured in the serum samples. 4T1-WT or 4T1-CXCR7-RNAi-985 cells were seeded at 200,000/well in 6-well plates and cultured for 2-3 days in RPMI with 10% FBS. The CM were collected, and mMMP3 was measured using ELISA.
Results
Secretion of MMP-3 is increased in 435-CXCR7 human breast cancer cells
In mouse tumor models, CXCR7+ breast cancer cells are significantly more aggressive than their CXCR7– counterparts and rapidly form large tumors when implanted in vivo (6). To examine whether CXCR7 overexpression results in the secretion of factors that may enhance tumor growth, we evaluated, using a quantitative and multiplexed immunoassay, the CM of WT MDA-MB-435s human breast cancer cells (435-WT) and cells transfected with CXCR7 (435-CXCR7). Of the 70 analytes examined, we found the largest differences in the levels of MMP-3, which were markedly elevated in 435-CXCR7 CM, when compared with the 435-WT CM (Table I and Fig. 1). This observation was consistent whether the cells were grown in optimal culture conditions (10% FBS) or exposed to environmental stress, such as serum starvation (1% FBS) which the cells may encounter in the tumor microenvironment (Table I and Fig. 1). Complement C3 anaphylatoxin (Compl. C3), CCL5 (RANTES), tissue inhibitor of metalloprotease 1 (TIMP-1), GM-CSF and CCL2 (MCP-1) were also elevated to varying degrees in the 435-CXCR7 CM when compared to the 435-WT CM (Table I and Fig. 1). The manner in which these mediators contribute to tumorigenesis or cell growth has yet to be determined. However, the complement system has recently been found to play a role in promoting the growth of malignant cervical carcinoma in mice through the recruitment of myeloid-derived suppressor cells (reviewed in ref. 14).
Table I.
Evaluation of secreted analytes.a
| Analyte | 435- | 10% FBS (mean ± range) | 1% FBS (mean ± range) |
|---|---|---|---|
| MMP-3 (ng/ml) | X7 | 34.4±13.3 | 12.8±4.8 |
| WT | 2.8±0.6 | 0.6±0.0 | |
| Compl. C3 (ng/ml) | X7 | 6.7±1.0 | 3.0±0.0 |
| WT | 0.7±0.1 | 0.7±0.3 | |
| CCL5 (pg/ml) | X7 | 323.5±13.5 | 206.0±24.0 |
| WT | 56.5±15.7 | 43.3±14.6 | |
| TIMP-1 (ng/ml) | X7 | 205.6±194.5 | 125.7±111.4 |
| WT | 114.7±102.4 | 67.8±53.3 | |
| GM-CSF (pg/ml) | X7 | 16.2±2.7 | 28.7±7.6 |
| WT | 11.6±0.6 | 7.2±0.2 | |
| CCL2 (pg/ml) | X7 | 3145±375 | 1500±40 |
| WT | 2410±230 | 851±54 |
Multiplex immunoassay of the conditioned media from 1 million 435-WT or 435-CXCR7 cells cultured for 48 h with 10 or 1% FBS.
FBS, fetal bovine serum.
Figure 1.
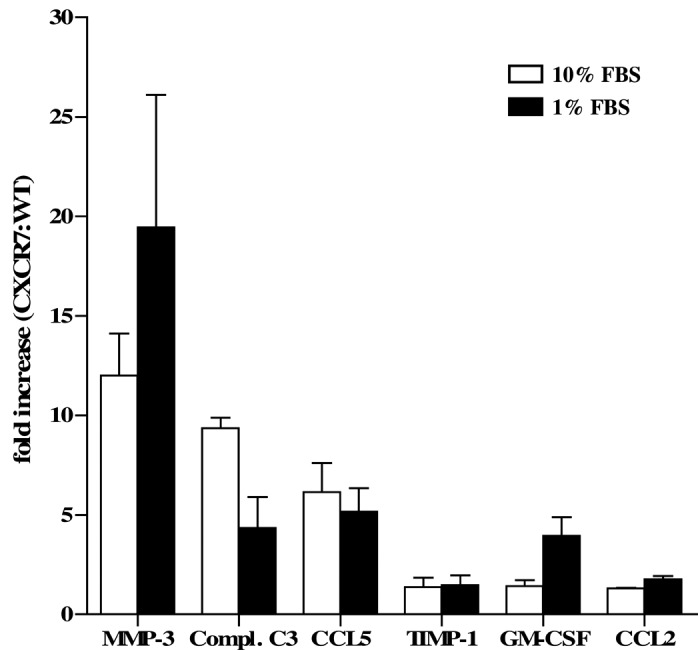
hMMP-3 is selectively upregulated in MDA-MB-435s human breast cancer cells that overexpress CXCR7. The fold increase in analyte concentrations in conditioned media from 435-CXCR7 cells over 435-WT cells is shown. n=2 samples per cell type per culture condition.
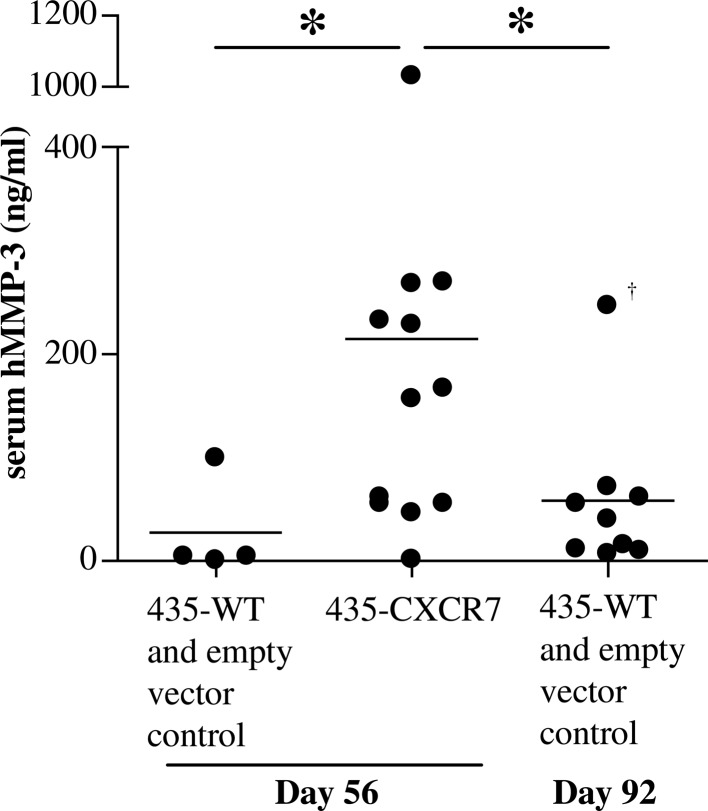
To examine whether the enhanced MMP-3 secretion is maintained in vivo, a xenograft model was used. SCID mice were implanted with 435-CXCR7, 435-WT or 435-empty vector tumor cells. As previously reported, the 435-CXCR7 tumors grew significantly more rapidly than the control tumors (6). Serum from the 435-CXCR7 mice collected at the termination of the study had significantly higher levels of human MMP-3 than serum from the mice implanted with control tumors (Fig. 2). Mouse MMP-3 levels among the groups were below the limits of detection (data not shown). Since 435 cells are the only source of human proteins, the overexpression of CXCR7 correlates with enhanced MMP-3 secretion in breast cancer cells in vitro and in vivo. Since the control tumors were smaller than the 435-CXCR7 tumors on day 56, the control tumors were allowed to grow until day 92, when they reached the average size (~900 mm3) comparable to that of the 435-CXCR7 tumors on day 56 [(6) and data not shown]. These control mice contained lower serum human MMP-3 levels than the 435-CXCR7 tumor-bearing mice (Fig. 2).
Figure 2.
The 435-CXCR7 xenografts express hMMP-3 in vivo. A total of 1 million 435-WT, 435-empty vector control or 435-CXCR7 cells were implanted subcutaneously into SCID mice on day 0. On the indicated days post-implantation, serum was collected and analyzed for hMMP-3 levels using ELISA. Each closed circle represents an individual mouse, and the mean for each group is shown. *p<0.05, Mann-Whitney rank sum test. †The Q-test was used to eliminate the indicated outlier (confidence limit >99%). Representative of two independent experiments.
Reduced MMP-3 secretion in CXCR7-RNAi-targeted 4T1 mouse breast cancer cells
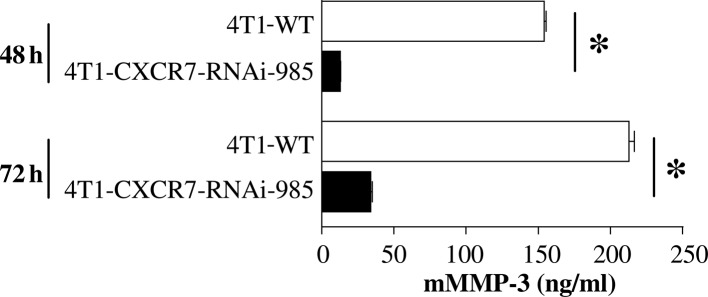
We aimed to ascertain whether inhibiting CXCR7 expression in breast cancer cells results in a reduction in MMP-3 secretion. Since 4T1 mouse mammary tumor cells endogenously express CXCR7 protein (6), we used this cell line to examine the effects of CXCR7-targeted RNAi knockdown on MMP-3 release. Results showed that mMMP-3 secretion was significantly reduced following CXCR7-RNAi knockdown (Fig. 3). Thus, using two complementary approaches, we showed that CXCR7 expression correlates with enhanced in vivo tumor growth (6) and MMP-3 secretion in human and mouse breast cancer cell lines.
Figure 3.
CXCR7 RNAi-knockdown downregulates mMMP-3 in 4T1 mouse mammary cancer cells. A total of 1 million 4T1-WT or 4T1-CXCR7-RNAi-985 cells were cultured in vitro for the indicated times. mMMP-3 levels in the conditioned media were determined by ELISA. The mean ± SEM is shown for triplicate wells. *p<0.05, Student's t-test.
Discussion
Metastatic breast cancer is a leading cause of death in women and is currently incurable (1). MMP-3 is expressed by primary breast cancer cells and/or the underlying stroma (12,13). Additionally, MMP-3 has been implicated as a pro-metastatic factor, both in transforming tumor cells and in degrading the basement membrane for subsequent tumor cell dissemination (10). CXCR7 protein is expressed in breast cancer cells and may contribute to CXCR4-mediated tumor cell transendothelial migration and metastasis (6,8). This study showed that the CXCR7 protein expression correlates with enhanced in vivo tumor growth (6) and the elevated secretion of MMP-3 by breast cancer cells. Therapeutic agents that target CXCR7 may therefore act on multiple pathways to prevent metastatic disease.
Acknowledgements
This work was funded by ChemoCentryx, Inc.
References
- 1.Morris PG, McArthur HL, Hudis CA. Therapeutic options for metastatic breast cancer. Expert Opin Pharmacother. 2009;10:967–981. doi: 10.1517/14656560902834961. [DOI] [PubMed] [Google Scholar]
- 2.Brunello A, Roma A, Falci C, Basso U. Chemotherapy and targeted agents for elderly women with advanced breast cancer. Recent Pat Anticancer Drug Discov. 2008;3:187–201. doi: 10.2174/157489208786242313. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 4.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006;13:191–199. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- 6.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zabel BA, Wang Y, Lewen S, Berahovich RD, Penfold ME, Zhang P, Powers J, Summers BC, Miao Z, Zhao B, Jalili A, Janowska-Wieczorek A, Jaen JC, Schall TJ. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183:3204–3211. doi: 10.4049/jimmunol.0900269. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 10.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haupt LM, Irving RE, Weinstein SR, Irving MG, Griffiths LR. Matrix metalloproteinase localisation by in situ-RT-PCR in archival human breast biopsy material. Mol Cell Probes. 2008;22:83–89. doi: 10.1016/j.mcp.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Haupt LM, Thompson EW, Trezise AE, Irving RE, Irving MG, Griffiths LR. In vitro and in vivo MMP gene expression localisation by in situ-RT-PCR in cell culture and paraffin embedded human breast cancer cell line xenografts. BMC Cancer. 2006;6:18. doi: 10.1186/1471-2407-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markiewski MM, Lambris JD. Unwelcome complement. Cancer Res. 2009;69:6367–6370. doi: 10.1158/0008-5472.CAN-09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]