Abstract
Patients with primary pancreatic lymphoma (PPL), which is rare, require a different therapeutic approach and have a better prognosis than those with pancreatic cancer. However, conventional imaging modalities alone are not able to differentiate between pancreatic cancer and other rare tumors such as PPL, although the accurate diagnosis of PPL is crucial. The development of new modalities such as F-18 2′-deoxy-2fluoro-D-glucose (FDG) positron emission tomography combined with computed tomography (PET/CT) contributes to the evaluation of lymphoma staging. However, few reports are currently available regarding PET/CT findings in PPL. In this study, a 56-year old man with PPL was examined using FDG PET/CT imaging, which showed the unique intense uptake of FDG in the pancreas with atypical findings of malignancy in the CT scan and magnetic resonance images.
Keywords: pancreas, lymphoma, positron emission tomography combined with computed tomography, magnetic resonance images
Introduction
Both Hodgkin’s and non-Hodgkin’s types of lymphoma exist. Non-Hodgkin’s lymphomas often invade extra-lymphatic organs, while Hodgkin’s lymphomas rarely disseminate to extra-lymphatic organs. More than 25% of non-Hodgkin’s lymphomas originate from extra-lymphatic organs, approximately 30% of which may involve the pancreas (1). Primary pancreatic lymphomas (PPL) are usually non-Hodgkin’s lymphomas and isolated PPL is quite rare (2). Only three cases (1.5%) of pancreatic lymphoma were found in a review of 207 cases of malignant pancreatic tumors (3). Clinically, PPL is likely to be misdiagnosed as pancreatic cancer. However, patients with PPL require a different therapeutic approach and have a better prognosis than those with pancreatic adenocarcinoma. Conventional imaging modalities alone are not able to differentiate between pancreatic adenocarcinoma and PPL, although the accurate diagnosis of PPL is crucial.
The development of a new modality such as F-18 2′-deoxy-2fluoro-D-glucose (FDG) positron emission tomography combined with computed tomography (PET/CT) has contributed to the evaluation of human cancers and the usefulness of PET/CT has been well established for lymphoma staging (4,5). However, few reports are currently available that pertain to PET/CT diagnosing PPL. In this study, a 56-year-old man with PPL was examined using PET/CT imaging. Results showed the unique intense uptake of FDG in the pancreas with atypical findings of malignancy in the CT scan and magnetic resonance images (MRI).
Patient and methods
A 56-year-old, asymptomatic man was admitted to Tokai University Hachioji Hospital for further examination following an abdominal ultrasound study that showed a mass shadow in the pancreas. A physical examination did not reveal any abnormal findings or systemic lymphadenopathy. Blood analysis showed a slight elevation of the serum interluekin-2 receptor (604 ng/ml) without other abnormalities, including tumor markers such as carcinoembryonic antigen and CA19-9. An abdominal CT scan showed a 5-cm tumor located in the head of pancreas, while an enhanced CT scan showed a slight increase in the tumor without encasement of arteries or veins. The MRI showed a mass with homogeneously high signal intensity on T2-weighted images and low signal intensity on T1-weighted images with gadolinium enhancement (Fig. 1). The CT and MRI findings described above suggested not pancreatic cancer but massive pancreatitis. Endoscopic retrograde cholangiopancreatography did not demonstrate either stenosis or obstruction of either the main pancreatic or common bile duct. Informed patient consent was obtained, as well as approval for the study by an ethics committee of our institute.
Figure 1.
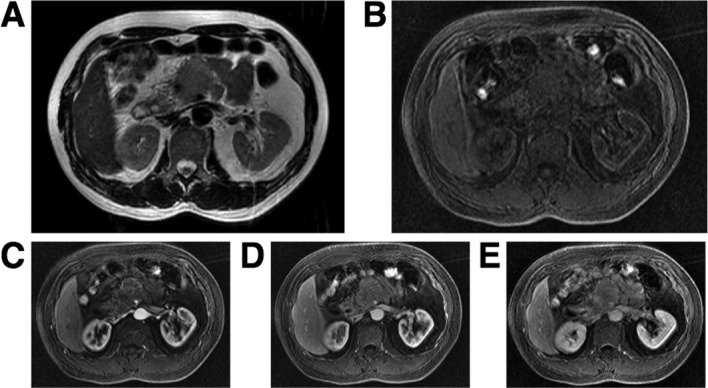
An MRI showed a mass with (A) homogeneously high signal intensity on T2-weighted images and (B) low signal intensity on T1-weighted images with gadolinium enhancement (C, 30sec; D, 60 sec and E, 180 sec).
The patient underwent 18F-FDG PET/CT scans. PET/CT imaging was performed with Biograph Duo (Siemens CTI). The Biograph Duo allows for the simultaneous collection of 64 slices over a span of 15.8 cm with a slice thickness of 2.5 mm and a transaxial resolution of 6.3 mm. All data were reconstructed with OSEM image. One hour (early scan) after the intravenous administration of approximately 3.7 Mbq/kg of 18F-FDG, a transmission scan using CT for attenuation correction and anatomical imaging was acquired for 90 sec with a delayed scan after 2 h. To determine the semi-quantitative FDG uptake, regions of interest (ROIs) were placed over the lesion, including the highest uptake area (circular ROI, 1 cm in diameter), and the standardized uptake value (SUV) was calculated. The early PET/CT showed an intense abnormal FDG uptake (SUVmax, 8.67) in the head and body of the pancreas, and a moderately increased uptake in the tail of the pancreas (Fig. 2). The delayed scan demonstrated a more intense FDG uptake (SUVmax, 10.3) in the pancreas. No other areas showed an abnormal uptake of FDG apart from the pancreas. The PET/CT findings strongly suggested malignancy of the pancreas without lymphatic or systemic metastasis.
Figure 2.
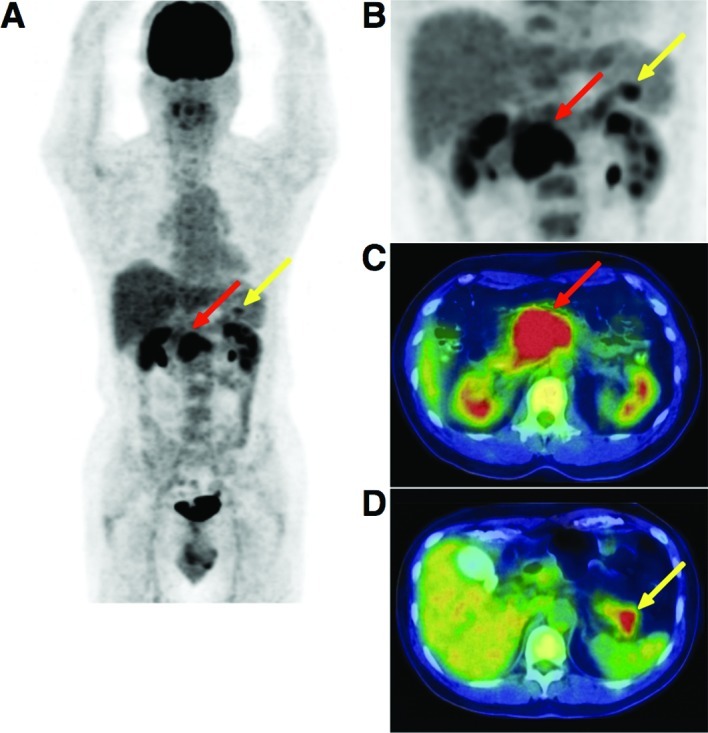
(A) Early PET/CT imaging showed intense abnormal FDG uptake (SUVmax, 8.67) in the head and body of the pancreas (red arrow), and a moderately increased uptake in the tail of the pancreas (yellow arrow). (B–D) A delayed scan demonstrated a more intense FDG uptake (SUVmax, 10.3) in the pancreas.
Results
Histopathologic study
A laparoscopic examination was performed and biopsy specimens were obtained. Microscopic examination showed diffuse infiltrative growth of T-lymphoid cells (Fig. 3). Multiple foci of B-lymphoid cells with small nodular or aggregate features were noted. The B cells showed a medium to relatively large morphology. The cells were embedded in the fibrous or hyalinizing stroma. The immunohistochemical analysis showed that the neoplastic cells were CD79a(+), CD20(+), BCL-2(+), CD3(−) and CD10(−). The MIB1 (Ki67) index was ~70–80%. A histopathological examination confirmed the diagnosis of a low grade B-cell pancreatic lymphoma. The patient underwent an aspiration biopsy of the bone marrow; however, no abnormality was noted. He received chemotherapy after surgery.
Figure 3.
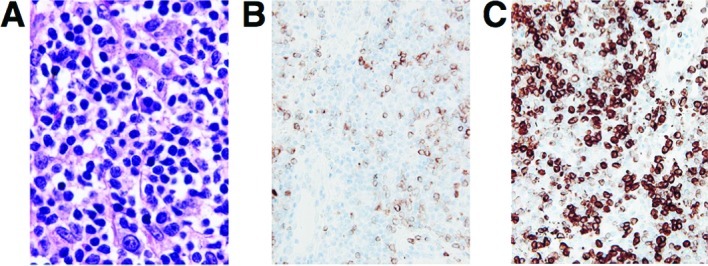
(A) Microscopic examination showed diffuse infiltrative growth of T-lymphoid cells. (B and C) Immunohistochemical analysis showed that the neoplastic cells were positive for CD79a (B) and BCL-2 (C). Original magnification, ×100.
Discussion
PPL is an extremely rare disease which occurs in the pancreas, with or without the involvement of peripancreatic lymph nodes. The clinical manifestation and imaging result of PPL resembles other pancreatic occupying lesions such as pancreatic carcinoma. However, unlike carcinomas, PPLs are potentially treatable even if not found at the early stage. PPL accounts for fewer than 2% of extra-nodal malignant lymphomas and 0.5% of cases of pancreatic masses (6,7). Fewer than 150 cases of PPL had been reported in the literature until 2006 (8).
Imaging results play a key role in the diagnosis of PPL. Percutaneous ultrasound (US), CT scan and MRI are well-established procedures used in the evaluation of pancreatic masses (9,10). CT is the most common imaging technique used in the detection and characterization of pancreatic tumors. The CT image of PPL resembles that of pancreatic carcinoma, including enlargement of the pancreatic head and density changes. However, fewer signs of invasion of large vessels and metastasis to the liver and spleen are observed. Pancreatic duct dilation is less common in PPL compared to pancreatic cancer. Merkle et al reported that the combination of a bulky localized tumor in the pancreas without significant dilation of the main pancreatic duct supports a diagnosis of pancreatic lymphoma as opposed to adenocarcinoma (11).
MRI findings in PPL usually show homogeneously high signal intensity on T2-weighted images and low signal intensity on T1-weighted images (12). Arcari et al stated that conventional imaging techniques suggest the suspicion of PPL but are unable to distinguish PPL from pancreatic adenocarcinoma (13). PPL is a rare disease with non-specific symptoms, laboratory tests and imaging examination results (14). Therefore, the final diagnosis of PPL should depend on a histopathological examination. The first choice for PPL treatment should be a combination of chemotherapy and radiotherapy as opposed to surgery. The development of FDG-PET/CT contributes to the evaluation of human cancer staging and the usefulness of PET/CT has been well established for lymphoma staging (5), whereas only one report regarding PET findings in PPL was previously published (15). Yoon et al reported that FDG-PET imaging, and not PET/CT, showed round intense FDG uptake in the center of the midabdomen in a patient with PPL. However, results of the PET/CT imaging for a 56-year-old man examined in our study showed a unique intense uptake of FDG in the pancreas as well as atypical findings of malignancy from a CT scan and MRI. The results therefore show that further accumulation and analysis of PET/CT images of PPL may provide additional and novel information to the data currently available from conventional imaging such as US, CT and MRI for the evaluation of pancreatic tumors.
Acknowledgements
We thank Mr. Kenji Kawai for his technical assistance.
References
- 1.Behrns KE, Sarr MG, Strickler JG. Pancreatic lymphoma: is it a surgical disease? Pancreas. 1994;9:662–667. [PubMed] [Google Scholar]
- 2.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Reed K, Vose PC, Jarstfer BS. Pancreatic cancer: 30 year review (1947–1977) Am J Surg. 1979;138:929–933. doi: 10.1016/0002-9610(79)90324-6. [DOI] [PubMed] [Google Scholar]
- 4.Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, Ben-Barak A, Ben-Arie Y, Bar-Shalom R, Israel O. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010;51:25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- 5.Cronin CG, Swords R, Truong MT, Viswanathan C, Rohren E, Giles FJ, O’Dwyer M, Bruzzi JF. Clinical utility of PET/CT in lymphoma. AJR Am J Roentgenol. 2010;194:W91–W103. doi: 10.2214/AJR.09.2637. [DOI] [PubMed] [Google Scholar]
- 6.Zucca E, Roggero E, Bertoni F, Cavalli F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8:727–737. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 7.Boni L, Benevento A, Dionigi G, Cabrini L, Dionigi R. Primary pancreatic lymphoma. Surg Endosc. 2002;16:1107–1108. doi: 10.1007/s00464-001-4247-1. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW. Primary pancreatic lymphomas. J Pancreas. 2006;7:262–273. [PubMed] [Google Scholar]
- 9.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 10.Kelekis NL, Semelka RC. MRI of pancreatic tumors. Eur Radiol. 1997;7:875–886. doi: 10.1007/s003300050221. [DOI] [PubMed] [Google Scholar]
- 11.Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000;174:671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 12.Masui T, Katayama M, Kobayashi S, Shimizu S. MR imaging of primary malignant lymphoma of the pancreas. Radiat Med. 2005;23:213–215. [PubMed] [Google Scholar]
- 13.Arcari A, Anselmi E, Bernuzzi P, Berte R, Lazzaro A, Moroniz CF, Trabacchi E, Vallisa D, Vercelli A, Cavanna L. Primary pancreatic lymphoma. Report of five cases. Haematologica. 2005;90:ECR09. [PubMed] [Google Scholar]
- 14.Lin H, Li SD, Hu XG, Li ZS. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol. 2006;12:5064–5067. doi: 10.3748/wjg.v12.i31.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon SN, Lee MH, Yoon JK. F-18 FDG positron emission tomography findings in primary pancreatic lymphoma. Clin Nucl Med. 2004;29:574–575. doi: 10.1097/01.rlu.0000135269.00531.f8. [DOI] [PubMed] [Google Scholar]


