Background: Mucroporin-M1 is a scorpion venom-derived peptide.
Results: Mucroporin-M1 peptide activates the MAPK pathway, and then reduces the expression of HNF4α, resulting in the inhibition of HBV replication in vitro and in vivo.
Conclusion: Mucroporin-M1 inhibits HBV replication by activating MAPK pathway and down-regulating HNF4α.
Significance: New-resourced peptide inhibits HBV replication by a novel mechanism.
Keywords: Antiviral Agents, Hepatitis Virus, HNF-4, MAP Kinases (MAPKs), Mouse
Abstract
Hepatitis B virus (HBV) is a noncytopathic human hepadnavirus that causes acute, chronic hepatitis and hepatocellular carcinoma (HCC). As the clinical utility of current therapies is limited, new anti-HBV agents and sources for such agents are still highly sought after. Here, we report that Mucroporin-M1, a scorpion venom-derived peptide, reduces the amount of extracellular HBsAg, HBeAg, and HBV DNA productions of HepG2.2.15 cells in a dose-dependent manner and inhibits HBV capsid DNA, HBV intracellular RNA replication intermediates and the HBV Core protein in the cytoplasm of HepG2.2.15 cells. Using a mouse model of HBV infection, we found that HBV replication was significantly inhibited by intravenous injection of the Mucroporin-M1 peptide. This inhibitory activity was due to a reduction in HBV promoter activity caused by a decrease in the binding of HNF4α to the precore/core promoter region. Furthermore, we confirmed that Mucroporin-M1 could selectively activate mitogen-activated protein kinases (MAPKs) and lead to the down-regulation of HNF4α expression, which explains the decreased binding of HNF4α to the HBV promoter. Moreover, when the protein phosphorylation activity of the MAPK pathway was inhibited, both HNF4α expression and HBV replication recovered. Finally, we proved that treatment with the Mucroporin-M1 peptide increased phosphorylation of the MAPK proteins in HBV-harboring mice. These results implicate Mucroporin-M1 peptide can activate the MAPK pathway and then reduce the expression of HNF4α, resulting in the inhibition of HBV replication in vitro and in vivo. Our work also opens new doors to discovering novel anti-HBV agents or sources.
Introduction
Hepatitis B virus (HBV)3 infection causes acute and chronic liver disease and is a serious health problem worldwide (1). Although it is a hepatotropic, noncytopathic DNA virus, ∼5–10% of adult infections and 90–95% of neonatal infections lead to persistent infection. Persistent infection leads to a high risk of developing chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) (2, 3). Although therapeutic nucleos(t)ide analogs and interferons are used to treat HBV infection, the numbers of HBV-infected individuals and HBV-related deaths continue to increase (4). Thus, there is a vital need for the development of new therapeutic agents and/or candidate sources for such agents.
Hepatocyte nuclear factor 4α (HNF4α) is a member of the nuclear hormone receptor family of transcription factors and binds DNA as a homodimer (5). It plays important roles in regulating the expression and replication of HBV by stimulating the transcription of HBV pregenomic RNA. Overexpression of HNF4α enables replication of the HBV genome even in nonhepatic cell lines (6). A reduction in the expression of HNF4α in liver cells reduces HBV replication in primary human hepatocytes (7) and transgenic mice (8). Therefore, agents that reduce HNF4α expression are potential new anti-HBV sources and can be used for the further development of drugs (9).
The MAPK pathway is known to regulate the expression of HNF4α (10, 11) and lead to the suppression of HBV replication (7). This pathway is apparently responsible for the suppression of HBV replication at the transcriptional level (12). However, sources or agents that activate the MAPK pathway are rarely studied for their anti-HBV effects.
Antimicrobial peptides (AMPs) are important for the antimicrobial efficacy of phagocytes. It has previously been reported that AMPs can activate MAPKs (13). Some AMPs have been shown to be effective against viral pathogens through different mechanisms (14–17). However, little is known about the relationship of the MAPK signaling pathway and AMP antiviral activities.
Here, we found that an antimicrobial peptide, Mucroporin-M1 (16), activated the MAPKs extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) and subsequently inhibited the expression of HNF4α. As a result of HNF4α down-regulation, the transcriptional activity of the HBV promoter was significantly reduced. When the HBV RNA transcript was reduced by the Mucroporin-M1 peptide, production of HBV DNA and proteins also decreased. Using a mouse model of HBV infection (18), we evaluated the expression of the HBV Core antigen in hepatocytes by immunohistochemical staining and determined the presence of HBsAg and HBeAg in the blood by ELISA. The Mucroporin-M1 peptide-treated group showed a lower HBV viral load in both the hepatocytes and blood than did the untreated group. Moreover, we found that the Mucroporin-M1 peptide also activated MAPKs in mouse hepatocytes, similar to the results from human hepatoma cells. These data suggest that a natural animal-derived peptide, Mucroporin-M1, inhibits HBV replication by activating the MAPK pathway and then down-regulating HNF4α expression in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Chemical Synthesis
The Ctri10036, Ctri10033, Ctry2801, Ctriporin, and Mucroporin-M1 peptides were from the scorpion venom peptide library that was recently characterized by our group and were synthesized at purities of >95% by GL Biochem Ltd. (China).
Cell Culture
HepG2.2.15 cells were cultured at 37 °C in a humidified 5% CO2/air atmosphere in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin.
Reagents
Mitogen-activated protein kinases (MAPK) inhibitors PD98059, SB203580, and SP600125 were purchased from Promega (Promega, Madison, WI).
Cytotoxicity
Cytotoxicity was measured by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. HepG2.2.15 cells were seeded in 96-well plates at 104 cells per well and grown to confluence in DMEM containing 10% FCS. Peptides were added to the wells at different concentrations. After 48 h of incubation, 10 μl of MTT solution was added to each well, and the plates were incubated for 2 to 4 h in 5% CO2 at 37 °C. The plates were then gently swirled for 10 min at room temperature to dissolve the precipitate, and the absorbance was measured at a wavelength of 550 nm.
Quantification of HBsAg, HBeAg, and HBV DNA in the Culture Medium
Cells were seeded in 24-well plates at a density of 8 × 104 cells/well in DMEM containing 10% FCS. After 12 h of incubation, the cells were treated with various concentrations of Mucroporin-M1 for 2 days. The HBsAg and HBeAg in the culture medium were measured using an enzyme-linked immunoassay (ELISA) kit. HBV DNA was measured by real-time PCR according to the manufacturer's instructions (Qiagen, Valencia, CA).
HBV RNA, Core Protein, and Replicative DNA Analyses
HepG2 or Hep2.2.15 cells were seeded in 6-well culture plates at a density of 5 × 105 cells per well. At 12 h after seeding, Mucroporin-M1 was added to the cell cultures, and cells were fed with fresh medium for another 2 days. As a control, 10 μm 3TC was added to the cell cultures for 2 days. Cells were collected for Southern, Northern and Western blot analyses for viral DNA, RNA, and protein, respectively. For Southern blot analysis, viral capsid DNA was detected as previously described (19). Radioactively 32P-labeled probes prepared from full-length HBV genomic DNA were generated by using the Redi-prime labeling kit (Amersham Biosciences) as described by the manufacturer. For Northern blot analysis, total RNA was isolated by Trizol (Invitrogen) following the manufacturer's instructions. The prehybridization and hybridization were performed identically to the Southern blot analysis. For Western blot analysis, 40 μg of sample was electrophoresed and transferred to a nitrocellulose membrane (Millipore, Bedford, MA). The membrane was probed using a polyclonal antibody specific for HBV Core antigen (Dako-Cytomation, Carpinteria, CA).
Anti-HBV Activity Analysis in Vivo
All animal studies were approved by the Institutional Animal Care and Use Committee at Wuhan University. A mouse model of acute hepatitis B virus infection was used in this study. A total of 20 μg of pUC-HBV1.3 was injected into the tail veins of 6–9-week-old BALB/c mice in a volume of saline equivalent to 8% of the body mass of each mouse (e.g. 1.6 ml for a mouse of 20 g). The total volume was delivered within 5–8 s. The second day of plasmid injection, Mucroporin-M1 was administered into the tail veins at 12.5 mg/kg. Sera and livers were collected on the third day. Viremia was measured by an ELISA similar to the method used in HepG2.2.15 cells. The HBV Core protein was visualized by immunohistochemical staining of tissues fixed in zinc-buffered formalin using anti-core polyclonal rabbit antibody.
HBV Promoter Luciferase Reporter Assay
The promoter regions of the genes encoding the HBV Core (nucleotides (nt) 1603–1819), X (nt 935–1361), preS (nt 2700–2830), or S (nt 2950–3174) were cloned upstream of the luciferase gene of the pGL3-basic vector. The mutated Core promoter sequence was obtained by converting the 13-nucleotide HNF4 binding site sequence (between 1662 and 1674) from ggactcttggact to cgctagcctcgta as described previously (20). And the mutated Core sequence was constructed into pGL3-basic vector.
HepG2.2.15 cells were transiently transfected with the reporter vector in a 48-well plate by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Twelve hours after transfection, peptides were added to the medium, and cells were incubated for 2 days (similar to an antiviral assay). Transcriptional activity was determined by measuring luciferase activity in a multiwell plate luminometer using the Luciferase Reporter Assay System (Promega).
EMSA
Same as the anti-HBV activity analysis in vitro, Mucroporin-M1 was added to the cell cultures for 2 days in EMSA. Nuclear extracts were prepared with NE-PER extraction reagent and were stored at −80 °C. The protein content was measured with a BCA protein assay. Then, nuclear extracts were incubated with biotin-labeled oligonucleotides in gel-shift binding buffer for 30 min at room temperature. Samples were separated using 5% native polyacrylamide gels followed by chemiluminescent detection. Competition assays were performed by incubating the nuclear extracts with unlabeled oligonucleotides on ice for 25 min before the addition of the biotin-labeled probe. The sequences of the oligomers used were as follows: HNF4, 5′-GAGGACTCTTGGACTCTCA-3′ (nt 1660–1678); HNF3, 5′-TCAAAGACTGTGTGTTTAAGGAC-3′ (nt 1710–1732); FTF, 5′-AATGTCAACGACCGACCTTGAGG-3′ (nt 1681–1703).
Quantification of Gene Expression by Real-time Reverse Transcription Polymerase Chain Reaction
Same as the anti-HBV activity analysis in vitro, Mucroporin-M1 was added to the cell cultures for 2 days in qPCR. Total RNA was extracted using Trizol reagent and was transcribed into cDNA using the First-Strand Synthesis Supermix (Invitrogen). Real-time PCRs were performed using the SYBR green PCR assay and an ABI 7500 system according to the manufacturer's instruction. For mRNA detection, HNF4α and GAPDH primer sets were used: HNF4α forward primer, 5′-GAGTGGGCCAAGTACA-3′; HNF4α reverse primer, 5′-GGCTTTGAGGTAGGCATA-3′; GAPDH forward primer, 5′-CAAGAAGGTGGTGAAGCAG-3′; GADPH reverse primer, 5′-AGGTGGAGGAGTGGGTG-3′).
HNF4α and MAPK Protein Analyses
HNF4α and MAPK pathway proteins were separated by SDS-PAGE and were analyzed by Western blotting. The primary antibodies used were as follows: rabbit polyclonal anti-ERK1/2, rabbit monoclonal anti-phospho-ERK1/2, rabbit monoclonal anti-p38, rabbit polyclonal anti-phospho-p38, mouse monoclonal anti-phospho-SAPK-JNK (Cell Signaling Technology, Beverly, MA), rabbit monoclonal anti-HNF4α (Abcam, Cambridge, UK), rabbit monoclonal anti-β-tubulin and mouse polyclonal anti-β-actin (Santa Cruz Biotechnology).
RESULTS
Screening of Anti-HBV Agents from Scorpion Venom Peptides
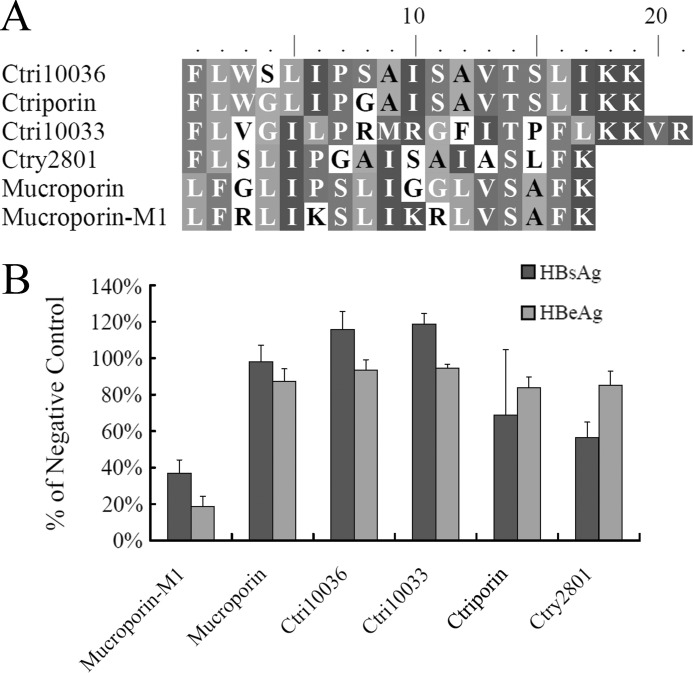
Cationic host defense peptides from scorpion venomous glands were recently characterized by our group. Mucroporin-M1 (21), Ctriporin (22) and other venom-derived peptides were synthesized at a purity of >95% by GL Biochem Ltd. (China). Their molecular weights, as measured by MS, matched the calculated molecular weights of the amidated peptides. Sequence alignments of Mucroporin-M1 with other antimicrobial peptides were performed using ClustalX and BioEdit (Fig. 1A).
FIGURE 1.
Screening of anti-HBV agents from scorpion venom peptides. A, sequence alignments of Mucroporin-M1 and its related peptides. The sequence alignments of Mucroporin-M1 with other antimicrobial peptides were performed using ClustalX and BioEdit. The residues shaded in the same color are highly conserved sites; the residues in similar color are less conserved sites; and the residues without a background color are highly variable sites. B, inhibitory activity of scorpion venom peptides on HBsAg and HBeAg production in HepG2.2.15 cells. Peptides were tested at a concentration of 25 μm for inhibitory activity against HBV replication, as assessed by ELISA.
Peptides from the scorpion venom were screened for the capacity to inhibit HBV replication in the HepG2.2.15 cell line. Peptide was added to the cells to a final concentration of 25 μm, and the amount of HBsAg and HBeAg present in the culture medium were tested using an ELISA. Incubation with the Mucroporin-M1 peptide resulted in an ∼80% reduction in the amount of HBeAg and a 70% reduction in the amount of HBsAg present in the culture medium compared with cultures not exposed to the peptide, and incubation with the Ctry2801 peptide resulted in an ∼40% reduction in the amount of HBsAg in the culture medium. The other peptides had little effect on the amount of secreted HBsAg and HBeAg (Fig. 1B). We found that the Mucroporin-M1 peptide had the most effective inhibitory activity against the production of HBeAg and HBsAg. Accordingly, we chose the Mucroporin-M1 peptide to study further.
Anti-HBV Effects of Mucroporin-M1 at Noncytotoxic Concentrations
The cytotoxicity of the peptide on HepG2.2.15 cells was tested using an MTT assay. The concentration of Mucroporin-M1 that inhibited 50% of cell growth (CC50) was 87 μm. When the peptide concentration was less than 25 μm, the viability of the peptide-treated cells was greater than 90%, indicating that 25 μm or less of the Mucroporin-M1 peptide was minimally cytotoxic to cells.
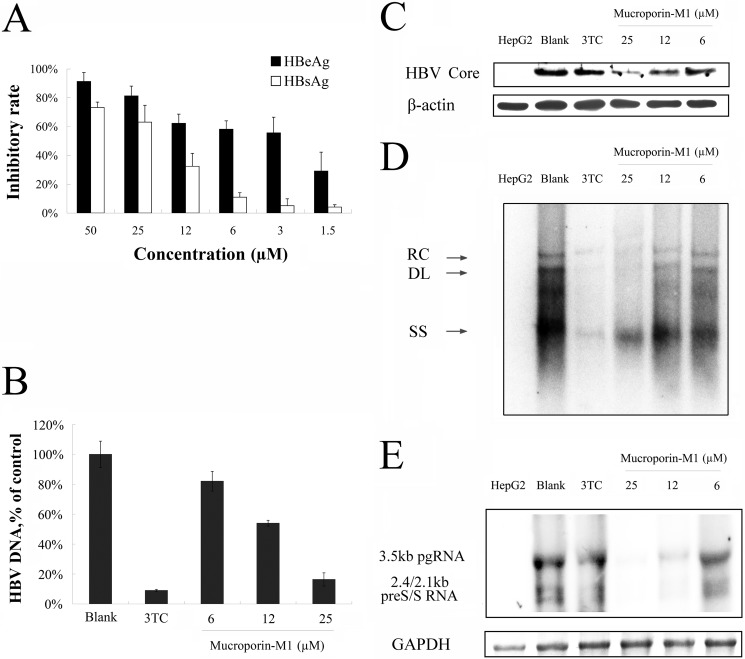
The effect of Mucroporin-M1 on HBV in HepG2.2.15 cells was assessed. HepG2.2.15 is a cell line that has been stably transfected with the HBV genome. The cells were cultured in the presence of 2-fold serial dilutions of the Mucroporin-M1 peptide for 2 days. Inhibitory effects on the production of extracellular HBsAg and HBeAg were determined by ELISA, and the amount of extracellular HBV progeny DNA was assessed by real-time PCR. The data showed that the Mucroporin-M1 peptide inhibited the expression of HBsAg and HBeAg in a dose-dependent manner (Fig. 2A). The IC50 values of Mucroporin-M1 against HBsAg and HBeAg production were 20.6 and 4.9 μm, respectively. The production of HBV progeny DNA was also inhibited in a dose-dependent manner by Mucroporin-M1, with an IC50 of 11 μm (Fig. 2B). Southern, Northern, and Western blot analyses were used for measuring intracellular HBV DNA, RNA, and Core protein levels, respectively, after treatment with the Mucroporin-M1 peptide. The various forms of the HBV intracellular DNA replication intermediates were potently inhibited by Mucroporin-M1 in a concentration-dependent manner, as exemplified in HepG2.2.15 cells. When used as a positive control, 3TC inhibited HBV DNA synthesis effectively (Fig. 2D). HBV RNA expression was also potently inhibited by Mucroporin-M1 in HepG2.2.15 cells. In contrast, viral RNA levels were unchanged after 3TC treatment, as expected (Fig. 2E). HBV Core protein expression was also inhibited in a dose-dependent manner by Mucroporin-M1 in HepG2.2.15 cells. Again, 3TC did not inhibit viral Core protein synthesis, as expected (Fig. 2C). The results showed that the Mucroporin-M1 peptide had anti-HBV activity, and the anti-HBV mechanism of the Mucroporin-M1 peptide was different from that of 3TC.
FIGURE 2.
Anti-HBV activity of Mucroporin-M1 peptide in HepG2.2.15 cells. A, HBsAg and HBeAg expression in the culture medium of HepG2.2.15 after treatment with Mucroporin-M1. Extracellular HBsAg and HBeAg production decreased in a dose-dependent manner after treatment with the indicated concentrations of Mucroporin-M1. B, HBV DNA analysis of the culture medium of HepG2.2.15 after treatment by Mucroporin-M1. Extracellular HBV DNA measured by real-time PCR decreased in a dose-dependent manner after treatment with the different concentrations of Mucroporin-M1. C, Western blot analysis of HBV Core protein synthesis in HepG2.2.15 cells treated by Mucroporin-M1. Mucroporin-M1 inhibited HBV Core protein synthesis, but 3TC did not. D, inhibition of intracellular HBV DNA in HepG2.2.15 cells treated by Mucroporin-M1. Intracellular relaxed circle (RC), double strand (DS), and single strand (SS) DNA synthesis was reduced after treatment with the indicated concentrations of Mucroporin-M1 or 3TC using Southern blot analysis. E, inhibition of HBV RNA synthesis in the HepG2.2.15 cells treated by Mucroporin-M1. Mucroporin-M1 inhibited HBV RNA production in a dose-dependent manner, but 3TC did not.
Inhibitory Activity of Mucroporin-M1 against HBV in Vivo
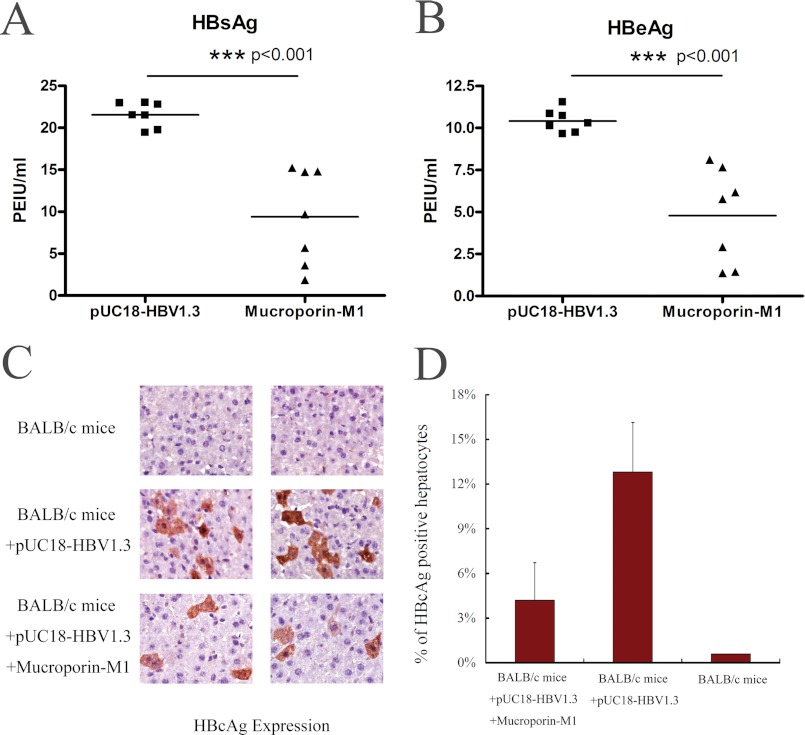
As Mucroporin-M1 had anti-HBV activity in hepatoma cells, we further examined its anti-HBV activity in an HBV infection mouse model. After hydrodynamic injection of the pUC18-HBV1.3 plasmid, the secretion of viral antigens into the blood was monitored at day 2. Three treatments were examined (n = 7 mice per group). HBsAg accumulated to an average concentration of 21.6 PEIU/ml in the untreated mice, whereas the concentration of HBsAg in the blood of the Mucroporin-M1-treated mice was 9.4 PEIU/ml. Similarly to HBsAg production, the amount of HBeAg decreased from 10.4 PEIU/ml in the untreated group to 4.8 PEIU/ml in the Mucroporin-M1-treated group (Fig. 3, A and B). The mice that were not administered pUC18-HBV1.3 did not have detectable viral antigens in their blood.
FIGURE 3.
Anti-HBV activity of Mucroporin-M1 in HBV-infected mice. A, inhibitory activity of Mucroporin-M1 against HBsAg in the blood of the HBV-infected mice. Mucroporin-M1 reduced the expression of HBsAg in the blood of HBV-infected mice by ELISA. B, inhibitory activity of Mucroporin-M1 against HBeAg in the blood of the HBV-infected mice. Mucroporin-M1 reduced the expression of HBeAg in the blood of HBV-infected mice by ELISA. C and D, inhibitory effect on HBV Core protein expression in the hepatocytes of HBV-infected mice treated with Mucroporin-M1. Mucroporin-M1 decreased the expression of HBV Core protein in the hepatocytes of HBV-infected mice treated by Mucroporin-M1, as measured by an immunohistochemical staining assay.
The livers of the mice were also examined for HBV Core protein by immunohistochemical staining. The frequency of HBV Core protein-positive hepatocytes was 4% ± 2% in Mucroporin-M1-treated mice compared with 13% ± 3% in untreated mice (Fig. 3, C and D). Thus, the Mucroporin-M1 peptide inhibited HBV replication in mouse hepatocytes and reduced HBV antigen secretion in mouse blood.
Inhibition of HBV Promoter Activity by Reducing the Interaction of HNF4α with HBV Promoters
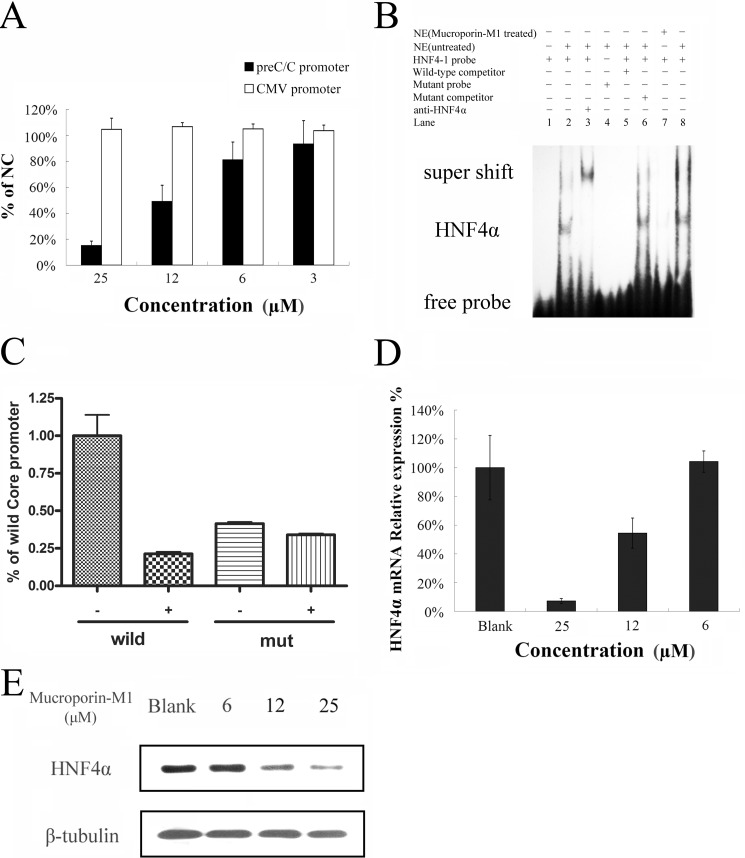
As the results above showed, Mucroporin-M1 reduced the HBV RNA transcript levels, leading to reduced HBV DNA and protein production. These results suggested that the active target of Mucroporin-M1 is the viral RNA transcription step and not the HBV DNA polymerase, unlike other anti-HBV nucleos(t)ide analogs. Therefore, we constructed plasmids containing promoters for the four different HBV transcripts (preC/Cp, Xp, pSp, or Sp) followed by the luciferase reporter gene to examine the effect of Mucroporin-M1 on HBV promoter activity. After transient transfection of the plasmids into HepG2.2.15 cells, Mucroporin-M1 was added to the cell cultures, and viral promoter activity was examined (supplemental data). The data showed that the HBV four promoter activities were partially inhibited by Mucroporin-M1 peptide, where the most significant one was HBV Core promoters. Core/precore promoter-driven luciferase expression decreased to 20% of the negative control level after Mucroporin-M1 peptide treatment, but the CMV promoter was not affected (Fig. 4A).
FIGURE 4.
Inhibitory effect of Mucroporin-M1 on HBV promoter transcriptional activity by reducing the expression of HNF4α. A, inhibitory activity of Mucroporin-M1 on HBV promoter transcription. Mucroporin-M1 selectively inhibited the HBV promoter (preC/Cp) but not the CMV promoter. Data are presented as the means of triplicate experiments (each with duplicate samples) normalized to vehicle-treated cells. Error bars represent standard deviations. NC, vehicle-treated cells. B, EMSA analysis of the interaction between HNF4α and the HBV promoters. Mucroporin-M1 significantly reduced the binding of HNF4α to the HBV promoter (preC/Cp). C, inhibitory activity of Mucroporin-M1 on the mutated Core promoter without HNF4a binding site. The activity of Core promoter with a mutant HNF4 binding site was not affected by 25 μm Mucroporin-M1 peptide. wild, Wild type of Core promoter. mut, Mutant type of Core promoter without HNF4a binding site. −, Not-treated with Mucroporin-M1 peptide. +, Treated with Mucroporin-M1 peptide. D, inhibitory effect of Mucroporin-M1 on HNF4α mRNA expression. The expression of HNF4α in the HepG2.2.15 cells treated with Mucroporin-M1 was quantified by real-time PCR. E, inhibitory effect of Mucroporin-M1 on the expression of the HNF4α protein. The expression of HNF4α in the HepG2.2.15 cells treated with Mucroporin-M1 was quantified by Western blot analysis.
It has been reported that hepatocyte nuclear transcriptional factors, together with viral proteins, bind to the HBV promoters and modulate viral promoter activity (23–26). DNA oligonucleotides corresponding to the HBV precore/core promoter/Enh II sequence HNF4, HNF3, and Fetoprotein transcription factor (FTF) were synthesized and biotin-labeled for electrophoretic mobility-shift assays (EMSAs). Nuclear extracts from HepG2.2.15 cells treated with 25 μm Mucroporin-M1 or left untreated were incubated with the probes to determine whether Mucroporin-M1 alters the binding of nuclear proteins. Binding to the HNF4 probe was significantly decreased after treatment with 25 μm Mucroporin-M1 (Fig. 4B). The binding to the HNF3 DNA probe was decreased after treatment with the Mucroporin-M1 peptide, but the difference was not significant. However, the binding of the nuclear extract to FTF did not yield a distinctive shift (data not shown).
To further confirm whether Mucroporin-M1 specifically reduced HNF4a binding HBV Core promoter, we constructed the mutated Core promoter with a mutant HNF4 binding site. The results of luciferase activities showed that the activity of HBV Core promoter with a mutant HNF4 binding site was almost not inhibited by Mucroporin-M1 treatment (Fig. 4C). The data suggests that the HNF4 binding site specifically played an important role in the HBV inhibitory activity of Mucroporin-M1 peptide.
Mucroporin-M1 Down-regulates HNF4α and Then Inhibits HBV Progeny DNA Expression by Activating MAPKs
To determine whether the decreased binding observed in the EMSAs was due to a reduction in HNF4α, quantitative PCR and Western blot analyses were performed. As shown in Fig. 4, D and E, the reduction in HNF4α expression inevitably resulted in a decrease in the binding of HNF4α to the HBV precore/core promoter, which explained the inhibitory activity of the Mucroporin-M1 peptide on HBV transcription. The decrease in HNF4α expression in HepG2.2.15 cells could also explain the inhibition of other viral protein promoters (pSp, Sp, and Xp) that was observed, because all of these viral protein promoters have HNF4α-binding sites. Taken together, Mucroporin-M1 down-regulated the expression of HNF4α, inhibited HBV promoter activity, and further reduced the levels of the HBV transcript.
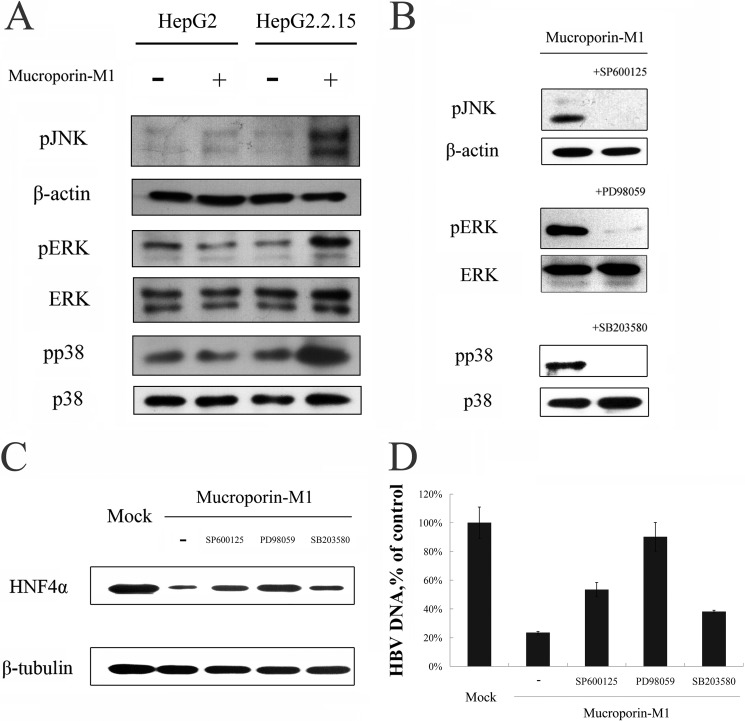
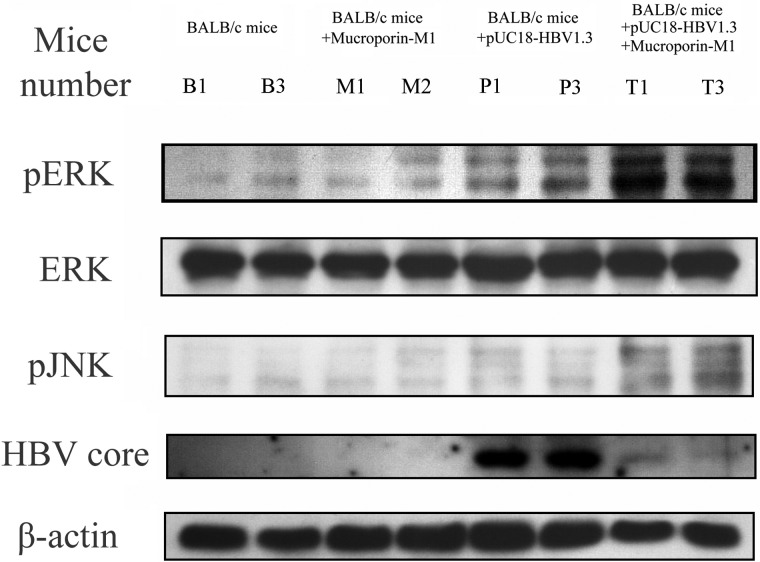
Because the activated MAPK pathway proteins exogenous signal regulated kinase (ERK) 1/2 and Jun N-terminal kinase (JNK) have been reported to control HNF4α expression (10, 11) and because of the reduction in HNF4α expression caused by Mucroporin-M1, we sought to determine whether the MAPK family members ERK1/2, p38, and JNK were activated in HepG2.2.15 cells by the Mucroporin-M1 peptide. The levels of phosphorylated ERK1/2, JNK, and p38 were all increased after treatment with 25 μm peptide, but the MAPK pathways were not activated in HepG2 cells (Fig. 5A). Furthermore, when the upstream inhibitors of the MAPK pathway (PD98059, SP600125, or SB203580), which inhibit ERK1/2, JNK, and p38, respectively, were added to HepG2.2.15 cells prior to Mucroporin-M1 treatment, we found that ERK1/2 phosphorylation was mostly inhibited and that the phosphorylation of p38 and JNK was completely blocked (Fig. 5B). Thus, the activation of the MAPK pathway by Mucroporin-M1 can be blocked by upstream inhibitors of this pathway, which confirmed that Mucroporin-M1 specifically activates the MAPK pathway. Moreover, the inhibition of JNK activation partially overcame Mucroporin-M1-mediated down-regulation of HNF4α, while inhibition of ERK activation completely blocked Mucroporin-M1-mediated down-regulation of HNF4α (Fig. 5C). Similarly, the detection of HBV DNA in the culture medium of HepG2.2.15 by real-time PCR showed that the anti-HBV activity of Mucroporin-M1 was blocked by the inhibition of JNK and ERK activation (Fig. 5D). Although the p38 inhibitor is able to block the activation of p38 that is mediated by Mucroporin-M1, it cannot abolish Mucroporin-M1-mediated down-regulation of HNF4α and the anti-HBV activity of Mucroporin-M1 (Fig. 5, C and D). In HBV-harboring mice, the levels of phosphorylated MAPK proteins were examined by Western blot analysis. Similar to the results in hepatoma cells, Mucroporin-M1 activated ERK1/2 and JNK and reduced HBV Core expression in HBV-harboring mice, but not activated ERK1/2 and JNK in the normal mice (Fig. 6).
FIGURE 5.
MAP kinase pathways involved in the anti-HBV activity of Mucroporin-M1 peptide in vitro. A, activation of the ERK, JNK and p38 pathways by Mucroporin-M1. pERK, ERK, pJNK, JNK, phosphorylated p38 and p38 from HepG2.2.15 cells and HepG2 cells, with or without treatment of Mucroporin-M1, were analyzed by Western blot analysis. −, Not-treated with Mucroporin-M1. +, Treated with Mucroporin-M1. B, inhibitory effect of MAP kinase inhibitors on the MAP kinase activation activity of the peptide Mucroporin-M1. The pERK, pJNK, and phosphorylated p38-producing cells were untreated or treated with 50 μm PD98059 (inhibiting pERK), SP600125 (inhibiting pJNK) or SB203580 (inhibiting phosphorylated p38) for 30 min prior to stimulation with Mucroporin-M1. The MAP kinase inhibitors blocked Mucroporin-M1-induced activation of the MAPKs. C, inhibitory effect of MAP kinase inhibitors on the inhibitory activity of Mucroporin-M1 peptide on HNF4α expression. Twenty-microgram quantities of nuclear proteins from HepG2.2.15 cells (mock) without Mucroporin-M1 treatment or Mucroporin-M1-treated (−) or preincubation with PD98059, SP600125, and SB203580 before Mucroporin-M1 treatment were analyzed by Western blot for HNF4α. D, inhibitory effect of MAP kinase inhibitors on the anti-HBV activity of Mucroporin-M1. HBV progeny DNA in the culture medium of HepG2.2.15 was quantified by real-time PCR.
FIGURE 6.
MAPK pathways involved in the anti-HBV activity of the peptide Mucroporin-M1 in vivo. The ERK and JNK MAP kinase pathways were activated in HBV-infected mice treated with an intravenous injection of Mucroporin-M1. Additionally, the expression of the HBV Core protein was also significantly reduced in mouse hepatocytes after intravenous injection of Mucroporin-M1. Group B, the mice treated by an intravenous injection of saline. Group M, the mice treated by an intravenous injection of Mucroporin-M1 peptide. Group P, the mice treated by both HBV plasmid and saline. Group T, the mice treated by both HBV plasmid and Mucroporin-M1 peptide.
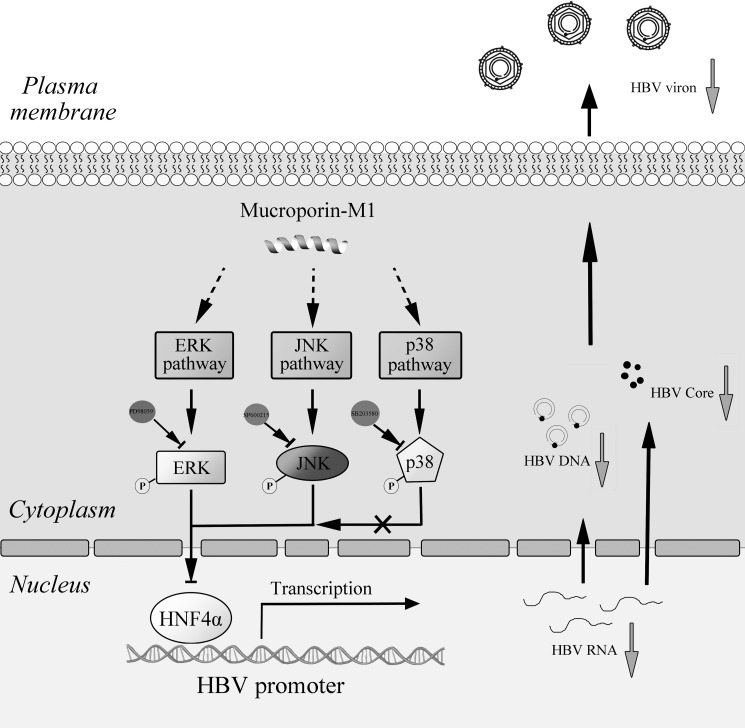
These results demonstrated that the Mucroporin-M1 peptide selectively activated ERK1/2 and JNK and subsequently down-regulated HNF4α expression, leading to the inhibition of HBV replication in both HepG2.2.15 cells and HBV-infected mice (Fig. 7).
FIGURE 7.
Hypothetical model of the anti-HBV activity of the Mucroporin-M1 peptide. Mucroporin-M1 activates the MAPK pathway (ERK, JNK, and p38) and subsequently down-regulates the expression of HNF4α, resulting in a reduction in the interaction between HNF4α and the HBV promoters. Mucroporin-M1 diminishes HBV replication by blocking HBV RNA expression. These findings indicate that the ERK and JNK pathways regulate the expression of HNF4α but that the p38 pathway does not, which suggests that the Mucroporin-M1 peptide may be a good molecular probe for studying the MAPK pathway.
DISCUSSION
As the increase in resistance to nucleos(t)ide analogs renders these drugs less potent, and the use of interferons is limited because of their side effects. The need for potent new anti-HBV agents with different mechanisms of action prompted us to test our scorpion venom-derived peptides for anti-HBV activity. Recently, new compounds targeting different stages of the HBV life cycle have been reported to successfully manage chronic HBV infection. The heteroaryldihydropyrimidines (HAPs) are a new class of antivirals that inhibit the production of HBV virions by binding to the HBV Core protein and causing its degradation, which subsequently inhibits nucleocapsid formation (27). Helioxanthins decrease the amount of host hepatocyte nuclear transcription factors required for the initiation of viral transcription (9, 28). A myristoylated peptide derived from the large HBV envelope protein (Pre S1) blocks virus entry to hepatocytes in vitro and in vivo (29, 30). However, no research has yet reported an agent that activates the MAPK pathway and inhibits HBV replication. Our study examined the Mucroporin-M1 peptide from scorpion venom, which activated MAPKs in HBV-harboring cells, reduced HNF4α expression and abolished HBV replication.
Recently, our group found that Mucroporin-M1 inhibited RNA viruses, including measles, SARS-CoV and influenza H5N1 viruses (16). Additionally, it has been reported that similar peptides inhibit other RNA viruses (14, 17, 31). These antiviral compounds were thought to function by disturbing the viral membrane. Thus, this antiviral mechanism was not effective against DNA viruses and virally infected cells. As a starting point of our study, we found that the Mucroporin-M1 peptide inhibited HBV replication in HepG2.2.15 cells, which is a cell line stably transfected with the HBV genome. This result suggested that Mucroporin-M1 uses a different strategy to inhibit HBV replication. As a similar venom-derived peptide (from bee venom) has been reported to activate MAPKs (13, 32), a pathway that directly participates in the inhibition of HBV replication (7, 12), we sought to determine the relationships between the Mucroporin-M1 peptide, the MAPK pathway and HBV replication. Our data clearly demonstrated that Mucroporin-M1 inhibited HBV replication by activating MAPKs. First, Mucroporin-M1 increased the levels of phosphorylated ERK1/2 and JNK and simultaneously inhibited HBV replication. Second, when the specific inhibitors of ERK1/2 and JNK were added, both ERK1/2 and JNK phosphorylation and the anti-HBV activity of Mucroporin-M1 were blocked. Furthermore, the levels of phosphorylated MAPK proteins were also increased in HBV-infected mice with Mucroporin-M1 treatment. Following MAPK activation in mouse hepatocytes, HBV replication was also inhibited by Mucroporin-M1. These data proved that the Mucroporin-M1 peptide inhibited HBV replication by activating MAPKs. It is well known that the MAPK pathway plays an important role in innate immunity. Pathogens stimulate and activate the MAPK pathway, which induces innate immune responses. Some AMPs participate in the activation of the MAPK pathway (33, 34). Therefore, MAPK pathway activation by AMPs may be a key part of the innate immune response against pathogens.
In this study, we also found that HBV promoter activity was reduced by Mucroporin-M1 peptide treatment. This was due to a decrease in the binding of HNF4α to HBV promoters. When the wild type Core promoter was mutated to the mutant Core promoter without HNF4 binding site, we found that the mutant promoter activity was not reduced by Mucroporin-M1 peptide. Furthermore, the Western blot and quantitative PCR results demonstrated that the expression of HNF4α was inhibited by Mucroporin-M1. The inhibition of HNF4α expression by Mucroporin-M1 treatment was blocked by the specific inhibitors of ERK1/2 or JNK, which suggested that Mucroporin-M1 inhibited the expression of HNF4α by activating ERK1/2 or JNK. This result confirmed that the expression of HNF4α was regulated by the ERK and JNK pathways (10, 35). Interestingly, p38 was also activated by Mucroporin-M1, but it did not impact HNF4α expression. These data may suggest that the p38 cascade does not participate in the regulation of HNF4α expression. Another group has also reported that a p38 inhibitor does not regulate the expression of HNF4α, regardless of the activation state of p38 (35).
HNF4α binds the proximal regulatory element of the nucleocapsid promoter and induces the expression of the 3.5-kb HBV pregenomic RNA in cell culture. When we artificially reduced HNF4α by treatment with Mucroporin-M1, HBV RNA transcripts, DNA replication intermediates, and protein production were decreased in vitro. This phenomenon has also been observed in mice (8, 36) and PHH (7). HNF4α modulates hepatocyte gene expression in hepatoma cells and has been utilized by HBV replication in patients (6). The expression of HNF4α was significantly higher in patients with severe hepatitis B than in those with chronic hepatitis B (37). Down-regulation of HNF4α expression may be used as a method or target for reducing acute liver damage caused by HBV infection. Although knocking out HNF4α affects embryonic viability in mice and HNF4α has been found to disrupt the expression of many genes involved in most aspects of mature hepatocyte function in adults (38), HNF4α is still considered to be an anti-HBV target (9). In our study, Mucroporin-M1 peptide could not activate the phosphorylation of ERKs, p38, and JNK in normal mice, consistent with the results of the cultured HepG2 cells. This selective activation of MAPK pathways contributes to reduce the adverse effect of persistent activation of MAPK pathway. Moreover, we tested that a triple intravenous dose of the Mucroporin-M1 peptide did not influence mouse survival (data not shown). Clearly, further evaluation of the safety of peptide administration is needed. Recently, a gene chip-based study found that HNF4α might be involved in the regulation of the inflammatory response in the liver (39). Thus, a modest reduction in HNF4α expression in hepatocytes might not only reduce HBV replication but also improve the prognosis of HBV infection.
This work was supported by grants from National Key Basic Research Program in China (2010CB529800 and 2010CB530100), National Natural Science Foundation of China (31071942 and 30971500), China Specific Project for Developing New Drugs (2011ZX09401-302 and 2011ZX09102-001-32), and Fundamental Research Funds for the Central Universities of China (1102001).

This article contains supplemental data.
- HBV
- hepatitis B virus
- AMPs
- antimicrobial peptides
- EMSA
- electrophoretic mobility shift assay
- ERK
- extracellular signal-regulated kinase
- HCC
- hepatocellular carcinoma
- HBsAg
- hepatitis B surface antigen
- HBeAg
- hepatitis B early antigen
- HNF
- hepatocyte nuclear factor.
REFERENCES
- 1. McMahon B. J. (2005) Epidemiology and natural history of hepatitis B. Seminars Liver Disease 25, 3–8 [DOI] [PubMed] [Google Scholar]
- 2. Torres H. A., Davila M. (2012) Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat. Rev. Clin. Oncol. 9, 156–166 [DOI] [PubMed] [Google Scholar]
- 3. Bertoletti A., Gehring A. J. (2006) The immune response during hepatitis B virus infection. J. Gen. Virol. 87, 1439–1449 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC) (2012) Surveillance for chronic hepatitis B virus infection-New York City, June 2008–November 2009. Morb. Mortal. Wkly. Rep. 61, 6–9 [PubMed] [Google Scholar]
- 5. Dhe-Paganon S., Duda K., Iwamoto M., Chi Y. I., Shoelson S. E. (2002) Crystal structure of the HNF4α ligand binding domain in complex with endogenous fatty acid ligand. J. Biol. Chem. 277, 37973–37976 [DOI] [PubMed] [Google Scholar]
- 6. Long Y., Chen E., Liu C., Huang F., Zhou T., He F., Liu L., Liu F., Tang H. (2009) The correlation of hepatocyte nuclear factor 4α and 3β with hepatitis B virus replication in the liver of chronic hepatitis B patients. J. Viral Hepatitis 16, 537–546 [DOI] [PubMed] [Google Scholar]
- 7. Hösel M., Quasdorff M., Wiegmann K., Webb D., Zedler U., Broxtermann M., Tedjokusumo R., Esser K., Arzberger S., Kirschning C. J., Langenkamp A., Falk C., Büning H., Rose-John S., Protzer U. (2009) Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 50, 1773–1782 [DOI] [PubMed] [Google Scholar]
- 8. Li L., Oropeza C. E., Sainz B., Jr., Uprichard S. L., Gonzalez F. J., McLachlan A. (2009) Developmental regulation of hepatitis B virus biosynthesis by hepatocyte nuclear factor 4α. PloS ONE 4, e5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ying C., Li Y., Leung C. H., Robek M. D., Cheng Y. C. (2007) Unique antiviral mechanism discovered in anti-hepatitis B virus research with a natural product analogue. Proc. Natl. Acad. Sci. U. S. A. 104, 8526–8531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatzis P., Kyrmizi I., Talianidis I. (2006) Mitogen-activated protein kinase-mediated disruption of enhancer-promoter communication inhibits hepatocyte nuclear factor 4α expression. Mol. Cell. Biol. 26, 7017–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Boussac H., Ratajewski M., Sachrajda I., Köblös G., Tordai A., Pulaski L., Buday L., Váradi A., Arányi T. (2010) The ERK1/2-hepatocyte nuclear factor 4α axis regulates human ABCC6 gene expression in hepatocytes. J. Biol. Chem. 285, 22800–22808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng Y., Li J., Johnson D. L., Ou J. H. (2003) Regulation of hepatitis B virus replication by the ras-mitogen-activated protein kinase signaling pathway. J. Virol. 77, 7707–7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang C., Chen T., Zhang N., Yang M., Li B., Lü X., Cao X., Ling C. (2009) Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IκBα kinase-NFκB. J. Biol. Chem. 284, 3804–3813 [DOI] [PubMed] [Google Scholar]
- 14. VanCompernolle S. E., Taylor R. J., Oswald-Richter K., Jiang J., Youree B. E., Bowie J. H., Tyler M. J., Conlon J. M., Wade D., Aiken C., Dermody T. S., KewalRamani V. N., Rollins-Smith L. A., Unutmaz D. (2005) Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol. 79, 11598–11606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorin C., Saidi H., Belaid A., Zairi A., Baleux F., Hocini H., Bélec L., Hani K., Tangy F. (2005) The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334, 264–275 [DOI] [PubMed] [Google Scholar]
- 16. Li Q., Zhao Z., Zhou D., Chen Y., Hong W., Cao L., Yang J., Zhang Y., Shi W., Cao Z., Wu Y., Yan H., Li W. (2011) Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides 32, 1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan R., Zhao Z., He Y., Wu L., Cai D., Hong W., Wu Y., Cao Z., Zheng C., Li W. (2011) A new natural α-helical peptide from the venom of the scorpion Heterometrus petersii kills HCV. Peptides 32, 11–19 [DOI] [PubMed] [Google Scholar]
- 18. Yang P. L., Althage A., Chung J., Chisari F. V. (2002) Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A. 99, 13825–13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng H., Beck J., Nassal M., Hu K. H. (2011) A SELEX-screened aptamer of human hepatitis B virus RNA encapsidation signal suppresses viral replication. PloS ONE 6, e27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raney A. K., Johnson J. L., Palmer C. N., McLachlan A. (1997) Members of the nuclear receptor superfamily regulate transcription from the hepatitis B virus nucleocapsid promoter. J. Virol. 71, 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dai C., Ma Y., Zhao Z., Zhao R., Wang Q., Wu Y., Cao Z., Li W. (2008) Mucroporin, the first cationic host defense peptide from the venom of Lychas mucronatus. Antimicrobial. Agents Chemother. 52, 3967–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Z., Cao L., He Y., Hu J., Di Z., Wu Y., Li W., Cao Z. (2011) Ctriporin, a new anti-methicillin-resistant Staphylococcus aureus peptide from the venom of the scorpion Chaerilus tricostatus. Antimicrobial Agents Chemotherapy 55, 5220–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng Y., Li J., Ou J. H. (2004) Regulation of hepatitis B virus core promoter by transcription factors HNF1 and HNF4 and the viral X protein. J. Virol. 78, 6908–6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu X., Mertz J. E. (2003) Distinct modes of regulation of transcription of hepatitis B virus by the nuclear receptors HNF4α and COUP-TF1. J. Virol. 77, 2489–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang H., McLachlan A. (2001) Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc. Natl. Acad. Sci. U. S. A. 98, 1841–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishida H., Ueda K., Ohkawa K., Kanazawa Y., Hosui A., Nakanishi F., Mita E., Kasahara A., Sasaki Y., Hori M., Hayashi N. (2000) Identification of multiple transcription factors, HLF, FTF, and E4BP4, controlling hepatitis B virus enhancer II. J. Virol. 74, 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deres K., Schröder C. H., Paessens A., Goldmann S., Hacker H. J., Weber O., Krämer T., Niewöhner U., Pleiss U., Stoltefuss J., Graef E., Koletzki D., Masantschek R. N., Reimann A., Jaeger R., Gross R., Beckermann B., Schlemmer K. H., Haebich D., Rübsamen-Waigmann H. (2003) Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 299, 893–896 [DOI] [PubMed] [Google Scholar]
- 28. Li Y., Fu L., Yeo H., Zhu J. L., Chou C. K., Kou Y. H., Yeh S. F., Gullen E., Austin D., Cheng Y. C. (2005) Antiviral Chem. Chemother. 16, 193–201 [DOI] [PubMed] [Google Scholar]
- 29. Gripon P., Cannie I., Urban S. (2005) Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 79, 1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen J., Dandri M., Mier W., Lütgehetmann M., Volz T., von Weizsäcker F., Haberkorn U., Fischer L., Pollok J. M., Erbes B., Seitz S., Urban S. (2008) Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat. Biotechnol. 26, 335–341 [DOI] [PubMed] [Google Scholar]
- 31. Kovalchuk L. V., Gankovskaya L. V., Gankovskaya O. A., Lavrov V. F. (2007) Herpes simplex virus: treatment with antimicrobial peptides. Adv. Exp. Med. Biol. 601, 369–376 [DOI] [PubMed] [Google Scholar]
- 32. Chen H. S., He X., Qu F., Kang S. M., Yu Y., Liao D., Lu S. J. (2009) Differential roles of peripheral mitogen-activated protein kinase signal transduction pathways in bee venom-induced nociception and inflammation in conscious rats. J. Pain 10, 201–207 [DOI] [PubMed] [Google Scholar]
- 33. Niyonsaba F., Ushio H., Nagaoka I., Okumura K., Ogawa H. (2005) The human β-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 175, 1776–1784 [DOI] [PubMed] [Google Scholar]
- 34. Kim C., Gajendran N., Mittrücker H. W., Weiwad M., Song Y. H., Hurwitz R., Wilmanns M., Fischer G., Kaufmann S. H. (2005) Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. U. S. A. 102, 4830–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mogilenko D. A., Dizhe E. B., Shavva V. S., Lapikov I. A., Orlov S. V., Perevozchikov A. P. (2009) Role of the nuclear receptors HNF4α, PPARα, and LXRs in the TNFα-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry 48, 11950–11960 [DOI] [PubMed] [Google Scholar]
- 36. Wang S. H., Yeh S. H., Lin W. H., Yeh K. H., Yuan Q., Xia N. S., Chen D. S., Chen P. J. (2012) Estrogen receptor-α represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4α. Gastroenterology 142, 989–998 [DOI] [PubMed] [Google Scholar]
- 37. Chen E. Q., Sun H., Feng P., Gong D. Y., Liu C., Bai L., Yang W. B., Lei X. Z., Chen L. Y., Huang F. J., Tang H. (2012) Study of the expression levels of Hepatocyte nuclear factor 4α and 3β in patients with different outcome of HBV infection. Virol. J. 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. (2001) Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Z., Bishop E. P., Burke P. A. (2011) Expression profile analysis of the inflammatory response regulated by hepatocyte nuclear factor 4α. BMC Genomics 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]