Background: Ankyrin-B is linked with congenital cardiovascular disease; however, its relevance/regulation in heart failure is unknown.
Results: Ankyrin-B is altered in heart failure, regulated by calcium-dependent pathways, and serves critical roles in cardioprotection in response to myocardial injury.
Conclusion: Ankyrin-B plays a key role in the cardiac response to injury.
Significance: Results define a pathway underlying cardiovascular disease.
Keywords: Adaptor Proteins, Cytoskeleton, Heart, Ion Channels, Membrane Trafficking, Ankyrin, Spectrin
Abstract
Ankyrins (ankyrin-R, -B, and -G) are adapter proteins linked with defects in metazoan physiology. Ankyrin-B (encoded by ANK2) loss-of-function mutations are directly associated with human cardiovascular phenotypes including sinus node disease, atrial fibrillation, ventricular tachycardia, and sudden cardiac death. Despite the link between ankyrin-B dysfunction and monogenic disease, there are no data linking ankyrin-B regulation with common forms of human heart failure. Here, we report that ankyrin-B levels are altered in both ischemic and non-ischemic human heart failure. Mechanistically, we demonstrate that cardiac ankyrin-B levels are tightly regulated downstream of reactive oxygen species, intracellular calcium, and the calcium-dependent protease calpain, all hallmarks of human myocardial injury and heart failure. Surprisingly, βII-spectrin, previously thought to mediate ankyrin-dependent modulation in the nervous system and heart, is not coordinately regulated with ankyrin-B or its downstream partners. Finally, our data implicate ankyrin-B expression as required for vertebrate myocardial protection as hearts deficient in ankyrin-B show increased cardiac damage and impaired function relative to wild-type mouse hearts following ischemia reperfusion. In summary, our findings provide the data of ankyrin-B regulation in human heart failure, provide insight into candidate pathways for ankyrin-B regulation in acquired human cardiovascular disease, and surprisingly, implicate ankyrin-B as a molecular component for cardioprotection following ischemia.
Introduction
Ankyrins and spectrins are cytoskeleton-associated adapter proteins that serve as multifunctional nodes for submembrane protein targeting and scaffolding, regulatory protein docking, and signaling protein modulation in excitable cells. Both classes of proteins are directly or indirectly linked with defects in metazoan physiology including hereditary spherocytosis (1), neurological dysfunction (2), diabetes (3, 4), skeletal muscle disease (5), and cardiovascular phenotypes (reviewed in Ref. 6). In heart, ankyrin-G and βIV spectrin are critical for cardiomyocyte intercalated disc protein targeting, scaffolding, and regulation. Dysfunction in the ankyrin-G pathway is linked with human Brugada syndrome arrhythmia (7–10). Human mutations in a second cardiac ankyrin gene product, ankyrin-B, are directly linked with sinus node dysfunction, atrial fibrillation, ventricular tachycardia following catecholaminergic stress, and susceptibility to sudden cardiac death (11–15). Moreover, ANK2 (encodes ankyrin-B) gene variants are linked with rate corrected QT interval variation in large patient populations (16). In vivo and in vitro data implicate ankyrin-B as critical for the membrane targeting of specific transverse tubule and sarcoplasmic reticulum ion channels and transporters including the Na/Ca exchanger, Na/K-ATPase, Kir6.2, and inositol 1,4,5-trisphosphate receptor, resulting in calcium-handling defects and electrical instability (3, 17–19). Surprisingly, despite over a decade of data linking ankyrin-B dysfunction with cardiovascular disease, the regulation of ankyrin-B in human heart failure has not been investigated. Moreover, the pathways underlying ankyrin-B and spectrin regulation in common forms of heart disease are not established.
Here we provide data for ankyrin-B regulation in human heart failure. Moreover, our new data provide evidence of the role of ankyrin-B in vertebrate cardioprotection. We show that ankyrin-B and downstream membrane-associated partners are coordinately down-regulated in human heart failure. We demonstrate that ankyrin-B regulation is tightly coupled to the activity of reactive oxygen species and intracellular calcium via the calcium-dependent protease calpain. Notably, βII-spectrin, previously thought to mediate ankyrin-dependent modulation in ischemia in heart and brain, is not coordinately regulated with ankyrin-B nor its downstream partners following insult, implicating ankyrin, and not spectrin, as the primary cleavage site for calpain in the heart. Finally, we demonstrate a link between ankyrin-B function and myocardial protection as mouse hearts deficient in ankyrin-B show increased injury and reduced contractility following ischemia reperfusion when compared with the hearts from wild-type littermates. In summary, our findings provide a link of ankyrin-B and βII-spectrin regulation with multiple forms of human heart failure, provide insight into the mechanisms underlying ankyrin-B regulation in cardiac disease, and provide new data on the role of ankyrin-B in cardioprotection following ischemia.
EXPERIMENTAL PROCEDURES
Animals
Age- and sex-matched WT and ankyrin-B (male) littermates were maintained at The Ohio State University animal facility (an Association for Assessment and Accreditation of Laboratory Animal Care-accredited experimental animal facility). Mice were previously backcrossed >20 generations into the C57Bl/6 background. All procedures involving experimental animals were performed in accordance with protocols approved by the Committee for Animal Research and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice were housed in the same facility, consumed the same diet, were provided water ad libitum, and were kept on identical 12-h light/dark cycles.
Neonatal Cardiomyocyte Cultures
Cultures were prepared from post-natal 1 or 2 mouse pups for biochemistry as described (20).
Cell Experiments
Proteolysis of ankyrin-B, βII-spectrin, and Na/K-ATPase was examined using neonatal cardiomyocytes. Myocytes were exposed to 1 μg of rat recombinant m-calpain (Calbiochem) in the presence of different concentrations of CaCl2 at 25 °C for 0, 15, 30, 60, and 90 min. Myocytes were washed, incubated with defined medium for 10 h, and then harvested for immunoblot. In certain experiments, the calpain inhibitor MDL-28170 (Calbiochem) at 10 μmol/liter was preincubated in the medium 10 min before adding calcium and calpain (21–23). For H2O2 experiments, adult or neonatal cardiomyocytes were incubated with 100 μm H2O2 from 0 to 90 min in serum-free medium. Cells were then washed and harvested for immunoblot as described (20, 24).
Immunoblot
Equal quantities of protein lysate (protein concentrations determined using bicinchoninic acid (BCA) protein assay (Pierce)) were analyzed by SDS-PAGE (3–8% Tris acetate gels) and immunoblotting. Protein samples were analyzed on the same gel to allow appropriate quantitative comparison between samples. Antibodies include affinity-purified antibodies to ankyrin-B (1:2000), βII-spectrin (1:1000), and Na/K-ATPase (1:700). Equal protein loading was verified by Coomassie Blue staining. Slight differences in protein loading were corrected using an internal control standard (actin (1:10,000) or GAPDH (1:1000)).
Immunostaining and Confocal Microscopy
Freshly isolated adult mice cardiomyocytes were isolated, immunostained, and imaged as described (24). Images were collected on a Zeiss 510 Meta confocal microscope, using Carl Zeiss Imaging software. Images were imported into Adobe Photoshop Cs for cropping and linear contrast adjustment. For confocal experiments, >20 cardiomyocytes were visualized for each condition.
Statistics
When appropriate, data were analyzed using a two-tailed Student's t test or analysis of variance (Sigma Stat), and values less than p < 0.05 were considered significant. Post hoc comparisons after analysis of variance were performed using the Holm-Sidak test. Values are expressed as the means ± S.E.
RNA Isolation and Reverse Transcription
RNA was isolated from heart tissue as described (25, 26) using the RNeasy kit (Qiagen), and the concentration was quantified by spectrophotometry. cDNA was amplified using SuperScript III reverse transcriptase (Invitrogen), and oligo(dT) was used to prime for cDNA production. Polymerase chain reaction (PCR) was performed in 20-μl reaction volumes. Sense and antisense primers were used at a concentration of 10 μm. PCR was performed using Taq polymerase and 30 cycles of 95 °C for 30 s, 63 °C for 30 s, and 72 °C for 30 s. PCR reactions were run on a 1% agarose gel with no-template controls.
Human Tissue Samples
Left ventricular tissue was obtained from explanted hearts of patients undergoing heart transplantation through The Cooperative Human Tissue Network: Midwestern Division at The Ohio State University. Approval for use of human subjects was obtained from the Institutional Review Board of The Ohio State University. Left ventricular tissue from healthy donor hearts not suitable for transplantation was obtained through the Iowa Donors Network and the National Disease Research Interchange. The investigation conforms to the principles outlined in the Declaration of Helsinki. Age and sex were the only identifying information acquired from tissue providers.
Ischemia Reperfusion Protocol in Langendorff Hearts
Langendorff-perfused mouse hearts were used for measuring myocardial viability and heart function (27). Mice were anesthetized with Avertin, and hearts were quickly excised and rinsed in cold Tyrode's solution containing (in mm): 137 NaCl, 5.4 KCl, 0.5 MgCl2, 0.16 NaH2PO4, 3 NaHCO3, 5 HEPES-NaOH, and 1 mg/10 ml heparin, adjusted to a pH of 7.4 with NaOH. Excess tissue was dissected. Hearts were immediately perfused (retrograde) through the aorta for 1–2 min at room temperature with Hanks' balanced salt solution and mounted on a modified Langendorff apparatus (HSE-HA perfusion systems, Harvard Apparatus) for retrograde aortic perfusion at a constant pressure of 80 mm Hg with carbogen (95% O2, 5% CO2) and Krebs-Henseleit bicarbonate solution consisting of (in mm): 25 NaHCO3, 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 2.5 CaCl2, 0.5 Na-EDTA, 15 glucose, 2 pyruvate, with pH equilibrated to 7.4. The perfused heart was immersed into the water-jacketed bath and was maintained at 36 °C. A polyethylene balloon filled with double distilled H2O was inserted into the cavity of the left ventricle through a left atrial incision. After allowing the heart to stabilize for 10 min, hearts were subjected to 20 min of global ischemia and 30 min of reperfusion.
Measurement of Myocardial Infarction Size
Following ischemia/reperfusion, each heart was removed from the Langendorff perfusion apparatus and immediately frozen. The frozen hearts were cut from apex to base into eight transverse slices of approximately equal thickness (∼0.8 mm). The slices were placed into a small cell culture dish and then incubated in 10% triphenyltetrazolium chloride in phosphate buffer (88 mm Na2HPO4, 1.8 mm NaH2PO4, pH 7.8) at room temperature for 30 min by shaking the dish as described (27). The development of the red formazan pigment in living tissues relies on the presence of lactate dehydrogenase or NADH, whereas failure to stain red indicates a loss of these constituents from necrotic tissue. After staining, the triphenyltetrazolium chloride buffer was replaced by 10% formaldehyde, and the slices were fixed for the next 2 h before the areas of infarct tissue were determined by computer morphometry (MagnaFire SP, with magnification × 6.3 and exposure time 10 ms). The risk area was the sum of total ventricular area. The infarct size was calculated and presented as percentage of risk area.
RESULTS
Ankyrin-B Levels Are Reduced in Human Heart Failure
Despite direct links between ankyrin-B (ANK2) loss-of-function mutations and human arrhythmia phenotypes (sinus node disease, atrial fibrillation, ventricular arrhythmia) (11, 12, 15), there are no data related to ankyrin-B regulation in acquired human cardiovascular disease. We therefore examined ankyrin-B expression levels in human heart failure samples. We first evaluated ankyrin-B expression in left ventricle from failing hearts with a clinical diagnosis of non-ischemic heart disease and in left ventricular free wall tissue from de-identified donors with no clinical history of heart disease (see “Experimental Procedures”). Samples from patients with documented heart failure showed striking reduction in ankyrin-B levels when compared with non-failing samples (Fig. 1, A and B). In fact, ankyrin-B levels were decreased over 60% in left ventricle samples from non-ischemic hearts (decreased 62%, n = 8 non-failing, n = 7 heart failure; p < 0.01). We repeated these analyses for left ventricle samples from de-identified donors with clinical history of ischemic heart failure as well as in non-failing hearts. In agreement with findings in non-ischemic disease, we observed a significant decrease in ankyrin-B expression in ischemic left ventricle samples when compared with non-failing samples (Fig. 1, C and D; n = 8 non-failing, n = 9 heart failure, p < 0.01). We evaluated ankyrin-B (ANK2) mRNA expression in samples of non-failing heart as well as from left ventricular samples of individuals with ischemic and non-ischemic heart failure. We observed no statistical difference in ankyrin-B mRNA samples between non-failing tissue and either ischemic or non-ischemic heart disease samples (Fig. 1E; n = 5/group, not significant for non-failing versus non-ischemic HF3; not significant for non-failing versus ischemic HF), strongly suggesting that differences in ankyrin-B levels observed in Fig. 1, A–D, are due to post-transcriptional mechanisms. Together, these findings provide data identifying ankyrin-B dysregulation in human heart failure.
FIGURE 1.
Ankyrin-B protein expression is reduced in human heart failure. A and B, representative immunoblots and quantitative analysis of ankyrin-B levels in left ventricle samples of patients without heart failure (NF, n = 8) and patients with documented non-ischemic heart failure ((HF (NI)), n = 7; p < 0.01). C and D, representative immunoblots and quantitative analysis of ankyrin-B levels in left ventricle samples of patients without heart failure (n = 8) and patients with documented ischemic heart failure ((HF (Isch), n = 9; p < 0.01). For all experiments, values were adjusted to internal loading control to correct for minor differences in gel loading. E, ankyrin-B (ANK2) mRNA levels were not statistically different between left ventricle samples from non-failing hearts (n = 5) versus ischemic HF samples (n = 5; p = NS). We observed no difference in ANK2 mRNA levels between non-failing heart samples (n = 5) versus non-ischemic HF samples (n = 5; p = NS). All mRNA values were corrected for total RNA as well as actin mRNA expression. A. U., arbitrary units; non-Isch HF, ischemic HF. *, p < 0.05. A.U., arbitrary units.
Ankyrin-B and Downstream Partners Are Coordinately Altered in Human Heart Failure
Ankyrin-B targets a host of membrane-associated ion channels, transporters, and signaling molecules in heart (26, 28–30). Based on observed decreases in ankyrin-B expression in heart failure, we examined the expression of Na/K-ATPase, a well validated binding partner of ankyrin in heart and other tissue (18). Consistent with the in vivo role of ankyrin-B for Na/K-ATPase targeting and membrane stability, we observed decreased Na/K-ATPase expression in samples of ischemic (p < 0.05, n = 9 versus n = 8 non-failing) and non-ischemic left ventricle when compared with non-failing samples (Fig. 2, A and B; p < 0.01, n = 6 versus n = 8 non-failing). In fact, we observed an approximate ∼56% decrease in Na/K-ATPase expression in non-ischemic samples, nearly mimicking the changes we observed in ankyrin-B with the same samples. Notably, similar to findings for ankyrin-B, Na/K-ATPase mRNA levels were not significantly different between non-failing and non-ischemic HF or non-failing and ischemic HF samples (Fig. 2C; n = 5 from each group, NF for non-failing versus non-ischemic HF; NF for non-failing versus ischemic HF). Thus, ankyrin-B and Na/K-ATPase are coordinately down-regulated by post-transcriptional mechanisms in human heart failure.
FIGURE 2.
Ankyrin-associated proteins are altered in human heart failure. A and B, representative immunoblots and quantitative analysis of Na/K-ATPase levels in left ventricle samples of individuals without heart failure (NF, n = 8) and individuals with documented non-ischemic (A, n = 6; p < 0.01) and ischemic heart failure (B, n = 9, p < 0.01). C, Na/K-ATPase mRNA levels were not statistically different between left ventricle samples from non-failing hearts (n = 5) versus ischemic HF (n = 5; p = NS). We observed no difference in Na/K-ATPase mRNA levels between non-failing heart samples (n = 5) versus non-ischemic HF samples (n = 5; p = NS). All mRNA values were corrected for total RNA as well as actin mRNA expression. A. U., arbitrary units. D and E, representative immunoblots and quantitative analysis of βII-spectrin levels in left ventricle samples of non-failing hearts (n = 8) and individuals with documented non-ischemic (D, n = 7 for each condition; NS) and ischemic heart failure (E, n = 4 for each condition, p < 0.05). Note that we observed no statistical difference for βII spectrin levels in non-ischemic heart samples. For all experiments, values were adjusted to an internal loading control to correct for minor differences in gel loading.*, p < 0.05.
Ankyrin-B “Upstream” Partner Is Altered in Human Heart Failure
The spectrin family of polypeptides plays critical roles for ankyrin function, presumably by targeting ankyrin-based complexes to specific membrane domains (31). This hypothesis, based primarily on the roles of spectrin and ankyrin in the erythrocyte, has been challenged by more recent findings in excitable cells including neurons and cardiomyocytes (32, 33). We examined the regulation of βII-spectrin, the cardiac β-spectrin binding partner for ankyrin-B (32), in human heart failure samples. Notably, we observed decreased βII-spectrin levels in ischemic heart failure samples, consistent with our findings with ankyrin-B and Na/K-ATPase (Fig. 2E; n = 4 ischemic, n = 4 non-failing; p < 0.01). However, although there was a trend for reduced βII-spectrin levels in non-ischemic heart failure samples, we observed no statistical difference when compared with non-failing (Fig. 2D; n = 7 non-ischemic, n = 7 non-failing, NS). These data demonstrate that although βII-spectrin levels are altered in ischemic heart failure, changes in non-ischemic heart failure are less prominent. Notably, these differences in expression patterns relative to changes of ankyrin-B in both forms of heart failure suggest that ankyrin-B and βII-spectrin may be differentially regulated in disease.
Ankyrin-B Levels Are Altered by Reactive Oxygen Species
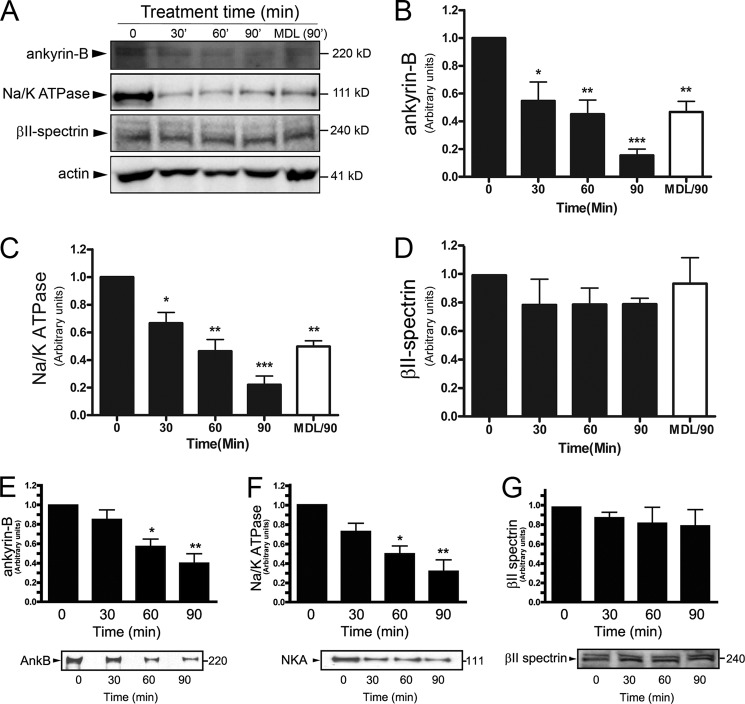
One hallmark of heart failure is elevation of reactive oxygen species (ROS) associated with altered myocyte transcription, apoptosis, and metabolism. We therefore examined whether alterations of ankyrin-B observed in human heart failure were associated with ankyrin-B reduction following activation of ROS (34, 35). In primary adult mouse cardiomyocytes treated with H2O2, we observed significant degradation of ankyrin-B as early as 30 min (Fig. 3, A and B; n = 3/group; p < 0.05). Similarly, we observed that Na/K-ATPase levels reduced significantly at 30 min after H2O2 treatment (Fig. 3, A and C; n = 3/group; p < 0.05). In contrast, identical treatment of adult cardiomyocytes resulted in no difference in ankyrin-B upstream binding partner βII-spectrin, even following 90 min of H2O2 treatment (Fig. 3, A and D; n = 3/group; p = NS). Similar results were obtained in neonatal cardiomyocytes; however, due to the metabolic differences between adult and neonatal myocytes, the time course of cleavage was slightly delayed in the immature cells. For example, although ankyrin-B levels were not altered following 30 min of H2O2 treatment, we observed significant reduction in ankyrin-B levels following 60 and 90 min of treatment (Fig. 3E; n = 4/treatment, p < 0.05). Consistent with findings in human heart failure, we observed decreased expression of Na/K-ATPase with the same time course as observed for ankyrin-B reduction in neonatal cells (Fig. 3F; n = 3/treatment, p < 0.05). However, unlike ankyrin-B or Na/K-ATPase, we observed no difference in βII-spectrin expression following even 90 min of H2O2 treatment (Fig. 3G; n = 4; NS), consistent with experiments in adult myocytes. As discussed in detail later, the reduction of both ankyrin-B and Na/K-ATPase following ROS activation in adult cardiomyocytes was blunted by treatment of myocytes with the membrane-permeable calpain inhibitor MDL28170 (Fig. 3, A–D; n = 3/group; p < 0.05). Together, these findings support ankyrin-B as a target for ROS activity in primary cardiomyocytes and identify a potential upstream molecular pathway for regulation of ankyrin-B levels in heart disease.
FIGURE 3.
Oxidative stress alters ankyrin-B and Na/K-ATPase expression. A–G, representative immunoblots and statistical data of ankyrin-B (AnkB), Na/K-ATPase (NKA), and βII-spectrin levels in isolated adult cardiomyocytes (A–D; n = 3/group) and neonatal cardiomyocytes (F–G; n = 4/group) subjected to H2O2 to activate ROS. In B–D, MDL28170 was included in specific experiments as a calpain inhibitor for 90 min of treatment (MDL/90). For all experiments, protein levels were corrected for loading using actin. Unless specifically noted (*, p < 0.05, **, p < 0.01, ***, p < 0.001), differences were nonsignificant.
Ankyrin-B Levels Are Regulated by Alterations in Extracellular Calcium
In addition to elevated ROS, heart failure is characterized by alterations in cardiac calcium resulting in structural, electrical, metabolic, and transcriptional remodeling (36). We therefore examined the regulation of ankyrin-B expression following alterations in calcium concentrations. Lysates from adult mouse hearts were treated for 1 h with low (500 μm) to high (5 mm) extracellular calcium for 1 h. Following treatment, ankyrin-B levels were reduced by low level calcium treatment (500 μm, 1 mm CaCl2; n = 5, p < 0.05), whereas we observed no differences at mid to high [CaCl2] (Fig. 4A; n = 5, NS). We observed similar findings for Na/K-ATPase at low calcium concentrations (Fig. 4B, n = 3, p < 0.05). However, again consistent with a potential alternative mechanism for βII-spectrin regulation in heart failure, we observed differences in CaCl2-dependent regulation of βII-spectrin levels (Fig. 4C). βII-spectrin levels were reduced only at 2.5 mm CaCl2, a concentration where ankyrin-B levels were not significantly altered (Fig. 4C, n = 3, p < 0.05). In summary, ankyrin-B and βII-spectrin expression levels are regulated by alterations in myocyte calcium, albeit likely via potentially unique mechanisms.
FIGURE 4.
Aberrant calcium and calpain alters ankyrin-B, Na/K-ATPase, and βII spectrin expression. A–F, representative immunoblots and statistical data of ankyrin-B (n = 5/treatment), Na/K-ATPase (n = 3/treatment), and βII-spectrin (n = 3/treatment) levels in wild-type mouse left ventricle lysates treated with varying calcium concentrations for 60 min. Although levels of all three proteins were altered, we observed differences in the calcium dependence of proteolysis (*, p < 0.05; **, p < 0.01). G–P, ankyrin-B is a target of cardiac calcium/calpain. Representative immunoblots and statistical data of ankyrin-B (AnkB), Na/K-ATPase, and βII-spectrin levels in adult mouse ventricular cardiomyocytes treated with calcium/calpain for 0–90 min (n = 3/treatment, *, p < 0.05) are shown. Although all three proteins were targeted by calcium/calpain, we observed rapid degradation of ankyrin-B and Na/K-ATPase at 30 min of treatment when compared with βII-spectrin (decreased only at 60 min of treatment). In G–J, MDL28170 was included in specific experiments as a calpain inhibitor for 90 min of treatment (MDL/90). K–P, representative immunoblots and statistical data of ankyrin-B, Na/K-ATPase, and βII-spectrin levels in neonatal mouse ventricular cardiomyocytes treated with calcium/calpain for 0–90 min (n = 4/treatment, *, p < 0.05, **, p < 0.01, ***, p < 0.001). Although all three proteins were targeted by calcium/calpain, we observed rapid degradation of ankyrin-B and Na/K-ATPase at 15 min of treatment when compared with βII-spectrin (decreased only at 90 min of treatment). For all blots, protein levels are corrected for GAPDH expression.
Calpain Alters Cardiac Ankyrin-B Expression
Calpains are calcium-dependent cysteine proteases, downstream from both elevated Ca2+ and ROS, implicated in both structural and electrical modeling in cardiovascular disease, and previously linked with ankyrin- and spectrin-based pathways in both heart and brain, albeit primarily studied downstream of membrane ion channels and transporters (37, 38). Based on altered ankyrin-B expression in response to altered calcium and ROS, we directly examined the sensitivity of cardiac ankyrin-B to calpain in both adult and neonatal cardiomyocytes. Consistent with prior data in rat heart (38), we observed striking remodeling of adult and neonatal myocyte ankyrin-B levels following even a brief treatment with calcium and activated calpain (Fig. 4, G and H and K and L; n = 3/group for adult experiments; n = 4/group for neonatal experiments). In agreement with the role of ankyrin-B in Na/K-ATPase regulation, we observed calcium- and calpain-dependent decreases in Na/K-ATPase expression following a similar time course as ankyrin-B in both adult and neonatal cardiomyocytes (Fig. 4, G and I and M and N, n = 3/group for adult experiments; n = 4/group for neonatal experiments). Notably, the reduction of both ankyrin-B and Na/K-ATPase following calcium/calpain treatment in adult cardiomyocytes was abolished by treatment of myocytes with the membrane-permeable calpain inhibitor MDL28170 (Fig. 4, G and I; n = 3/group; p < 0.05).
Changes in ankyrin-B and Na/K-ATPase expression were accompanied by striking alterations in the subcellular localization of both ankyrin-B and the Na/K-ATPase in isolated adult ventricular cardiomyocytes. Both ankyrin-B and Na/K-ATPase showed reduced and heterogeneous expression at transverse tubule and peripheral sarcolemma membranes following 15 min of calpain cleavage (Fig. 5, A and B). Following exposure for greater than 60 min, we observed loss of organized ankyrin-B and Na/K-ATPase in treated myocytes, with remaining staining in distributed puncta throughout the membrane system (Fig. 5, A and B).
FIGURE 5.
Cellular ankyrin-B and Na/K-ATPase are targeted by calcium/calpain. A and B, expression and localization of ankyrin-B and Na/K-ATPase in wild-type adult mouse cardiomyocytes are altered by calcium/calpain treatment. α-Actinin was also examined as a control for cell viability. Scale bar equals 10 μm.
Calcium/calpain treatment had an effect on the expression of βII-spectrin in cardiomyocytes; however, the effect was blunted relative to ankyrin-B in both adult and neonatal cardiomyocyte experiments (Fig. 4, G and J and O and P, n = 3/group for adult experiments; n = 4/group for neonatal experiments). In fact, we observed a small, yet statistically significant reduction in βII-spectrin only after 90 min of calcium/calpain treatment in neonatal cardiomyocyte experiments (Fig. 4, O and P; n = 4, p < 0.05) when compared with ankyrin-B cleavage at 15 min. Collectively, our new data support ankyrin-B as a target for calpain-mediated cleavage in cardiomyocytes and suggest that Na/K-ATPase levels may be reduced due to decreased levels of expression of ankyrin-B, its upstream regulatory molecule. Additionally, our findings suggest that βII-spectrin, although also targeted by calcium- and calpain-dependent activity in myocytes, appears to be secondary to ankyrin-B degradation.
Calpain Inhibition Blocks Calcium- and ROS-dependent Degradation of Ankyrin-B
Both calcium and ROS may modulate downstream targets via a host of pathways, including calcium-activated calpains. To directly test the role of calpains for both calcium-dependent and ROS-dependent ankyrin-B regulation, we assessed cardiac ankyrin-B expression in response to both elevated calcium and ROS ± pretreatment with the calpain-specific inhibitor MDL28170. As noted above, MDL28170 treatment of adult cardiomyocytes blunted degradation of both ankyrin-B and Na/K-ATPase (Fig. 3, A–C, n = 3/group, p = NS). Consistent with these findings in adult cells, MDL28170 treatment significantly delayed the time course of ankyrin-B degradation following H2O2 treatment (Fig. 6A), and these changes were accompanied by reduced degradation of Na/K-ATPase (Fig. 6B). Reductions in ankyrin-B expression following elevated calcium levels were completely blocked by pretreatment with the calpain inhibitor MDL28170 (Fig. 6C). Finally, MDL28170 treatment blocked calcium/calpain-dependent reductions in ankyrin-B expression in adult and neonatal myocytes by immunoblot (Figs. 4, G and H, and 6D). These changes by immunoblot were paralleled at the level of the single isolated myocyte where we observed no difference in either the levels or the localization of ankyrin-B in calcium/calpain-treated myocytes following pretreatment with the calpain inhibitor MDL28170 (Fig. 6E). The findings in isolated cells add additional support that calpain activation alters ankyrin-B expression in cardiomyocytes independent of effects on βII-spectrin.
FIGURE 6.
MDL28170 blocks ROS-, calcium-, and calcium/calpain-dependent proteolysis of ankyrin-B. Pretreatment of neonatal (A–D) and adult (E) cardiomyocytes with the calpain-specific inhibitor MDL28170 blocks the rapid proteolysis of ankyrin-B and Na/K-ATPase in response to H2O2 (A and B, n = 4; p < 0.05 at 90 min), elevated calcium (C, n = 3, *, p < 0.05), or calcium/calpain treatment (D and E). Scale bar equals 10 μm in E.
Ankyrin-B-deficient Mouse Hearts Show Increased Susceptibility to Ischemia Reperfusion Injury
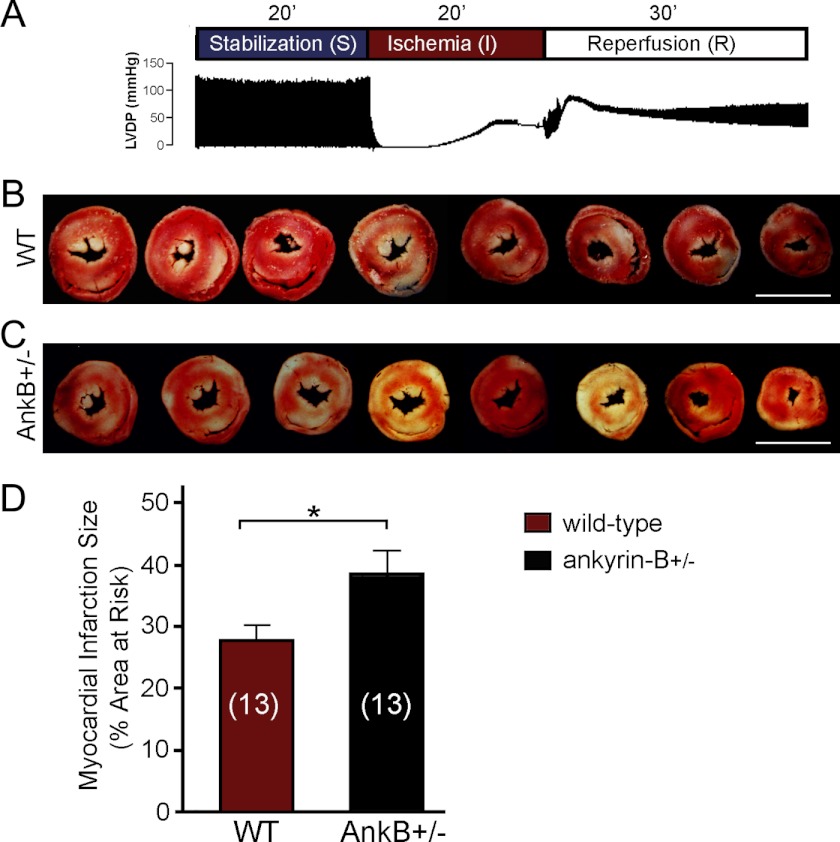
Based on the findings above as well as past work from Inserte et al. (38), we hypothesized that ankyrin-B plays a cardioprotective role in the response of the heart to stress/injury (increased Ca2+, ROS, calpain) and structural, electrical, transcriptional, and metabolic remodeling. As a first step to test this hypothesis, we evaluated the hearts from mice lacking 50% of their ankyrin-B expression (ankyrin-B+/− mice) for response to myocardial ischemia, a common cause of myocardial infarction and death in humans (39). Following reperfusion (Fig. 7, A–D), ischemia-induced infarct size (percentage of area at risk) was assessed as a measure of tissue damage and arrhythmia susceptibility. Cardiac function (left ventricular developed pressure (LVDP) and maximum rates of LVDP rise and fall (±dp/dtmax)) was also assessed in wild-type and ankyrin-B+/− mouse hearts (27) (Fig. 8, A–F, and see “Experimental Procedures”). Notably, ankyrin-B+/− mouse hearts were significantly more sensitive to hypoxic conditions, displaying a nearly 38.8% increase in myocardial infarction size when compared with hearts from wild-type littermates (Fig. 7, B–D; 38.28% ±3.84 and 27.57% ±2.78, respectively, n = 13 mice/genotype; p < 0.05). Consistent with these data, ankyrin-B+/− mouse hearts also displayed reduced LVDP, reduced maximal rates of LVDP rise and fall, and reduced time to onset of ischemia contracture when compared with control mice following ischemia reperfusion (Fig. 8, A–F, p < 0.05, n = 18 WT, n = 17 ankyrin-B+/−).
FIGURE 7.
Ankyrin-B-deficient hearts display increased vulnerability to ischemia reperfusion injury. A, protocol for ischemia-reperfusion in wild-type and ankyrin-B+/− isolated hearts and representative trace demonstrating LVDP and the maximum contractility (+dp/dtmax) and lusitropy (−dp/dtmax) for wild-type heart. B and C, transverse sections from wild-type (B) and ankyrin-B+/− mouse (C) hearts stained with TCC following ischemia-reperfusion protocol show myocardial infarction as pale coloring when compared with viable tissue (red). D, summary data of myocardial infarction size expressed as a percentage of total myocardium for wild-type and ankyrin-B+/− mice hearts in response to ischemia reperfusion. The number of hearts studied in each group is shown in parentheses (n = 13 hearts/genotype; *, p < 0.05). TCC, triphenyltetrazolium chloride.
FIGURE 8.
Ankyrin-B is required for the cardiac response to ischemia reperfusion injury. A–F, data for wild-type and ankyrin-B+/− mice pre- and post-ischemia reperfusion injury documenting differences in LVDP, maximum contractility and lusitropy, recovery of function, and time to onset of ischemia contracture following injury in ankyrin-B+/− mice. The number of hearts studied in each group is shown in parentheses (n = 18 or 17 hearts/genotype; *, p < 0.05).
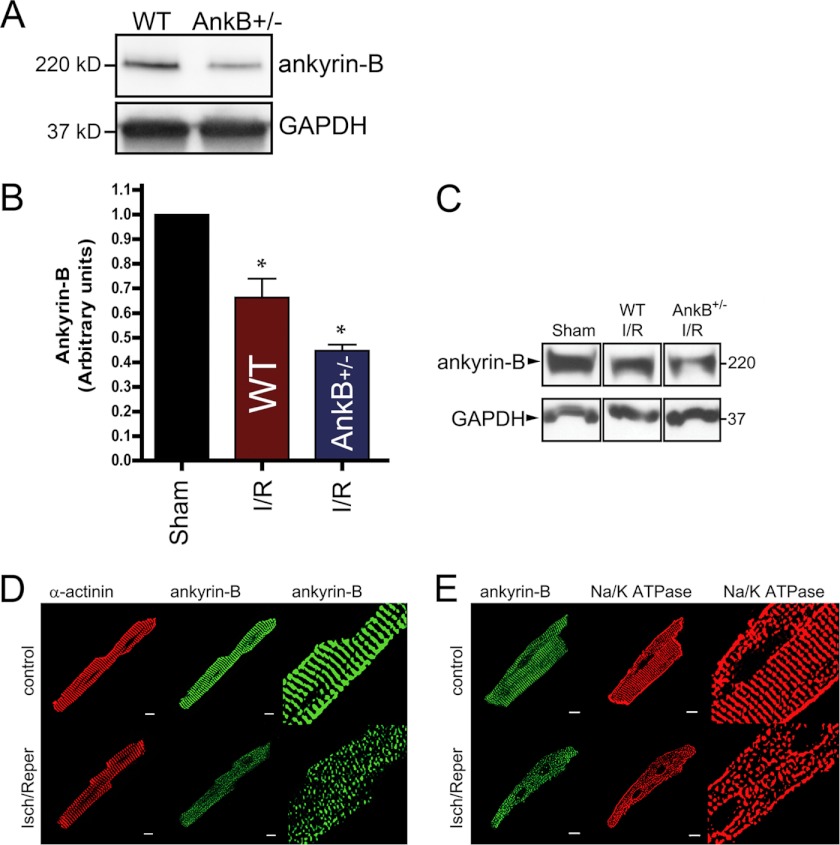
Notably, ankyrin-B levels in wild-type mouse hearts were decreased nearly 34% following ischemia reperfusion in wild-type mouse hearts, consistent with our human data (Fig. 1) and further supporting a key role for ankyrin in cardioprotection. Additionally, ankyrin-B and Na/K-ATPase expression and localization were dramatically altered by the ischemia reperfusion protocol, similar to cellular phenotypes observed following calcium/calpain treatment (Fig. 9, A–E). Ankyrin-B levels in the ankyrin-B+/− mouse were reduced ∼55% at base line (Fig. 9A) and notably were not additionally reduced from this base line following cardiac injury (Fig. 9, A–D). These data support an unanticipated role for ankyrin-B in cardioprotection against ischemia.
FIGURE 9.
Ankyrin-B is targeted in ischemia reperfusion injury. A, ankyrin-B levels are reduced ∼50% in ankyrin-B+/− heart lysates (∼46%, n = 3, *, p < 0.05). B–E, ankyrin-B levels are significantly reduced in wild-type mouse hearts (B and C) and myocytes (D and E) following ischemia reperfusion (I/R) injury (n = 3, *, p < 0.05) We observed coordinate reduction in ankyrin-B and Na/K-ATPase expression and distribution in isolated myocytes following ischemia reperfusion (Isch/Reper) (D and E). Scale bar equals 10 μm.
Ankyrin-B-deficient Mouse Hearts Are Protected from Ischemia Reperfusion Injury by MDL28170
As a final assessment of the link between ankyrin-B, calpain, and cardiac injury, we reassessed the requirement of ankyrin-B for protection against ischemia reperfusion injury in the presence of the calpain inhibitor MDL28170. As shown in Fig. 10, wild-type mouse hearts pretreated with MDL28170 showed a significant improvement in cardiac injury as assessed by myocardial infarction size when compared with untreated mouse hearts following ischemia/reperfusion (p < 0.05; n = 13 WT, n = 12 ankyrin-B+/− mice). Furthermore, consistent with the link between calpain-dependent degradation of ankyrin-B and associated proteins, we observed a decrease in myocardial infarction size in ankyrin-B+/− mouse hearts when pretreated with the calpain inhibitor MDL28170 when compared with untreated ankyrin-B+/− mouse hearts undergoing ischemia/reperfusion. (Fig. 10). Previous work supports that both ankyrin-B and Na/K-ATPase levels are protected from ischemia reperfusion by MDL28170 (38), further supporting our findings. Collectively, our findings demonstrate a role for ankyrin-B as a component of the protective response to cardiac injury.
FIGURE 10.

MDL28170 attenuates ischemia reperfusion damage in ankyrin-B+/− hearts. myocardial infarction size (MI Size, percentage of area at risk) was assessed in wild-type and ankyrin-B+/− mice ± pretreatment with MDL28170. Although wild-type mice showed a small, yet significant improvement in cardiac injury following ischemia/reperfusion, we observed a striking decrease in myocardial infarction size in ankyrin-B+/− mice when pretreated with the calpain inhibitor MDL28170 when compared with untreated ankyrin-B+/− mice undergoing ischemia/reperfusion. In fact, the percentage of area at risk was decreased nearly to the level observed in wild-type mice (*, p < 0.05; n is shown in parentheses for each group).
DISCUSSION
Following acute insult or chronic injury, the heart undergoes complex structural and electrical remodeling processes. This remodeling involves modifications in gene expression, ion channel trafficking, cell viability, and contractility. Although the field has provided exciting discoveries regarding the role of membrane ion channels, receptors and transporters, membrane-associated signaling molecules, transcription factors, and mitochondrial regulatory proteins in these remodeling processes, the role of the myocyte cytoskeleton has remained relatively understudied. Our findings provide data regarding the regulation of ankyrin-B levels in heart failure. Specifically, our work demonstrates significant reductions in ankyrin-B levels in ischemic and non-ischemic human cardiovascular disease. Mechanistically, we demonstrate that multiple signaling mediators of cardiac damage, including elevations in ROS and calpain, as well as alterations in cellular calcium trigger ankyrin-B degradation. Finally, our findings illustrate a requirement of ankyrin-B in cardiac protection against injury.
In heart, ankyrin-B plays multiple roles in cardiac function. At the cardiac Z-line, ankyrin-B targets the Na/Ca exchanger, Na/K-ATPase, and Kir6.2, the α subunit of the KATP channel, to transverse tubule membranes and the inositol 1,4,5-trisphosphate receptor to the sarcoplasmic reticulum membrane (3, 17–19). Additionally, ankyrin-B plays structural roles in the organization of the M-line structure with other cytoskeletal and regulatory components including obscurin and protein phosphatase 2A (30, 40, 41). Both populations in heart are coupled to the underlying actin-based cytoskeleton via spectrin tetramers, of which βII-spectrin associates with ankyrin-B in myocytes. Based on these past data, as well as our new findings, we predict that ankyrin-B plays dual roles in cardiac protection by regulating both electrical and structural features of myocytes. Notably, KATP channels and Na/K-ATPase, both ankyrin-B-associated membrane proteins (and altered in ankyrin-B+/− mice (3, 13, 17)) are well validated components against cardiac damage and are affected in heart failure (27, 42–46). Moreover, the critical role of spectrin and ankyrin function for membrane structure in multiple tissues is clear (47, 48). Ankyrins also likely play additional roles in directly coupling membrane proteins including the KATP channels and Na/K-ATPase (49). Thus, due to its multiple roles in cardiac structural and electrical regulation, alterations in ankyrin-B levels in either acute or chronic cardiac injury appear particularly detrimental to heart function and physiology. Notably, these predictions are supported in the literature by multiple forms of rare monogenic cardiovascular disease that target ankyrin-B, resulting in increased susceptibility to sinus node disease, atrial fibrillation, and ventricular tachycardia (11, 15, 28).
One initially surprising finding in this study was the lack of coordinate regulation of ankyrin-B and βII spectrin following acute or chronic injury. Notably, in human heart failure samples and in myocytes treated with elevated ROS, calcium, or calpain, we observed either differences in the time course of regulation (with ankyrin degradation preceding spectrin degradation) or lack of spectrin regulation altogether (e.g. elevated ROS; Fig. 3). These findings are in contrast to the traditional role of spectrin molecules in “tethering” ankyrins to the actin-based cytoskeleton. However, more recent findings in excitable cells offer a more complex and collaborative relationship between ankyrins and spectrins. In fact, work in heart, brain, and lung suggests that ankyrins and spectrins are coordinately regulated in tandem for normal vertebrate physiology. Subsequently, animal and cell models lacking ankyrins show loss of spectrin isoform targeting, whereas models lacking specific spectrin gene products display reduced ankyrin expression (2, 24, 27, 32, 33, 50–52). In fact, multiple studies now support differential regulation of ankyrin or spectrin proteolysis depending on the disease state and even the cell type (38, 53). Clearly, additional research will be required to unravel the complex relationships of these functionally related proteins in health and disease.
This work was supported, in whole or in part, by National Institutes of Health Grants HL084583 and HL083422 (to P. J. M.) and HL096805 (to T. J. H.). This work was also supported by grants from the Saving Tiny Hearts Society (to P. J. M.), Pew Scholars Trust (to P. J. M.), American Heart Association Established Investigator Award (to P. J. M.), and Fondation Leducq (Alliance for Calmodulin Kinase Signaling in Heart Disease (to P. J. M. and M. E. A.).
- HF
- heart failure
- NF
- non-failing
- NS
- not significant
- ROS
- reactive oxygen species
- LVDP
- left ventricular developed pressure.
REFERENCES
- 1. Agre P., Orringer E. P., Bennett V. (1982) Deficient red cell spectrin in severe, recessively inherited spherocytosis. N. Engl. J. Med. 306, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 2. Zhou D., Lambert S., Malen P. L., Carpenter S., Boland L. M., Bennett V. (1998) Ankyrin-G is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 143, 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kline C. F., Kurata H. T., Hund T. J., Cunha S. R., Koval O. M., Wright P. J., Christensen M., Anderson M. E., Nichols C. G., Mohler P. J. (2009) Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc. Natl. Acad. Sci. U.S.A. 106, 16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Healy J. A., Nilsson K. R., Hohmeier H. E., Berglund J., Davis J., Hoffman J., Kohler M., Li L. S., Berggren P. O., Newgard C. B., Bennett V. (2010) Cholinergic augmentation of insulin release requires ankyrin-B. Sci. Signal. 3, ra19. [DOI] [PubMed] [Google Scholar]
- 5. Ayalon G., Davis J. Q., Scotland P. B., Bennett V. (2008) An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200 [DOI] [PubMed] [Google Scholar]
- 6. Mohler P. J. (2006) Ankyrins and human disease: what the electrophysiologist should know. J. Cardiovasc. Electrophysiol. 17, 1153–1159 [DOI] [PubMed] [Google Scholar]
- 7. Hund T. J., Mohler P. J. (2011) Differential roles for SUR subunits in KATP channel membrane targeting and regulation. Am. J. Physiol. Heart Circ. Physiol. 300, H33–H35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowe J. S., Palygin O., Bhasin N., Hund T. J., Boyden P. A., Shibata E., Anderson M. E., Mohler P. J. (2008) Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J. Cell Biol. 180, 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohler P. J., Rivolta I., Napolitano C., LeMaillet G., Lambert S., Priori S. G., Bennett V. (2004) Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 101, 17533–17538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato P. Y., Coombs W., Lin X., Nekrasova O., Green K. J., Isom L. L., Taffet S. M., Delmar M. (2011) Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ. Res. 109, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunha S. R., Hund T. J., Hashemi S., Voigt N., Li N., Wright P., Koval O., Li J., Gudmundsson H., Gumina R. J., Karck M., Schott J. J., Probst V., Le Marec H., Anderson M. E., Dobrev D., Wehrens X. H., Mohler P. J. (2011) Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 124, 1212–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohler P. J., Le Scouarnec S., Denjoy I., Lowe J. S., Guicheney P., Caron L., Driskell I. M., Schott J. J., Norris K., Leenhardt A., Kim R. B., Escande D., Roden D. M. (2007) Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation 115, 432–441 [DOI] [PubMed] [Google Scholar]
- 13. Mohler P. J., Schott J. J., Gramolini A. O., Dilly K. W., Guatimosim S., duBell W. H., Song L. S., Haurogné K., Kyndt F., Ali M. E., Rogers T. B., Lederer W. J., Escande D., Le Marec H., Bennett V. (2003) Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421, 634–639 [DOI] [PubMed] [Google Scholar]
- 14. Mohler P. J., Splawski I., Napolitano C., Bottelli G., Sharpe L., Timothy K., Priori S. G., Keating M. T., Bennett V. (2004) A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc. Natl. Acad. Sci. U.S.A. 101, 9137–9142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Scouarnec S., Bhasin N., Vieyres C., Hund T. J., Cunha S. R., Koval O., Marionneau C., Chen B., Wu Y., Demolombe S., Song L. S., Le Marec H., Probst V., Schott J. J., Anderson M. E., Mohler P. J. (2008) Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc. Natl. Acad. Sci. U.S.A. 105, 15617–15622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sedlacek K., Stark K., Cunha S. R., Pfeufer A., Weber S., Berger I., Perz S., Kääb S., Wichmann H. E., Mohler P. J., Hengstenberg C., Jeron A. (2008) Common genetic variants in ANK2 modulate QT interval: results from the KORA study. Circ. Cardiovasc. Genet. 1, 93–99 [DOI] [PubMed] [Google Scholar]
- 17. Li J., Kline C. F., Hund T. J., Anderson M. E., Mohler P. J. (2010) Ankyrin-B regulates Kir6.2 membrane expression and function in heart. J. Biol. Chem. 285, 28723–28730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohler P. J., Davis J. Q., Bennett V. (2005) Ankyrin-B coordinates the Na/K-ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS. Biol. 3, e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohler P. J., Davis J. Q., Davis L. H., Hoffman J. A., Michaely P., Bennett V. (2004) Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J. Biol. Chem. 279, 12980–12987 [DOI] [PubMed] [Google Scholar]
- 20. Abdi K. M., Mohler P. J., Davis J. Q., Bennett V. (2006) Isoform specificity of ankyrin-B: a site in the divergent C-terminal domain is required for intramolecular association. J. Biol. Chem. 281, 5741–5749 [DOI] [PubMed] [Google Scholar]
- 21. Liu X., Schnellmann R. G. (2003) Calpain mediates progressive plasma membrane permeability and proteolysis of cytoskeleton-associated paxillin, talin, and vinculin during renal cell death. J. Pharmacol. Exp. Ther. 304, 63–70 [DOI] [PubMed] [Google Scholar]
- 22. Ma W., Han W., Greer P. A., Tuder R. M., Toque H. A., Wang K. K., Caldwell R. W., Su Y. (2011) Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J. Clin. Invest. 121, 4548–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz S. M., Duffy J. Y., Pearl J. M., Goins S., Wagner C. J., Nelson D. P. (2003) Glucocorticoids preserve calpastatin and troponin I during cardiopulmonary bypass in immature pigs. Pediatr. Res. 54, 91–97 [DOI] [PubMed] [Google Scholar]
- 24. Hund T. J., Koval O. M., Li J., Wright P. J., Qian L., Snyder J. S., Gudmundsson H., Kline C. F., Davidson N. P., Cardona N., Rasband M. N., Anderson M. E., Mohler P. J. (2010) A βIV-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J. Clin. Invest. 120, 3508–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gudmundsson H., Curran J., Kashef F., Snyder J. S., Smith S. A., Vargas-Pinto P., Bonilla I. M., Weiss R. M., Anderson M. E., Binkley P., Felder R. B., Carnes C. A., Band H., Hund T. J., Mohler P. J. (2012) Differential regulation of EHD3 in human and mammalian heart failure. J. Mol. Cell Cardiol. 52, 1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gudmundsson H., Hund T. J., Wright P. J., Kline C. F., Snyder J. S., Qian L., Koval O. M., Cunha S. R., George M., Rainey M. A., Kashef F. E., Dun W., Boyden P. A., Anderson M. E., Band H., Mohler P. J. (2010) EH domain proteins regulate cardiac membrane protein targeting. Circ. Res. 107, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J., Marionneau C., Koval O., Zingman L., Mohler P. J., Nerbonne J. M., Anderson M. E. (2007) Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Channels 1, 387–394 [DOI] [PubMed] [Google Scholar]
- 28. Hashemi S. M., Hund T. J., Mohler P. J. (2009) Cardiac ankyrins in health and disease. J. Mol. Cell. Cardiol. 47, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunha S. R., Bhasin N., Mohler P. J. (2007) Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J. Biol. Chem. 282, 4875–4883 [DOI] [PubMed] [Google Scholar]
- 30. Cunha S. R., Mohler P. J. (2008) Obscurin targets ankyrin-B and protein phosphatase 2A to the cardiac M-line. J. Biol. Chem. 283, 31968–31980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett V. (1978) Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J. Biol. Chem. 253, 2292–2299 [PubMed] [Google Scholar]
- 32. Mohler P. J., Yoon W., Bennett V. (2004) Ankyrin-B targets β2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J. Biol. Chem. 279, 40185–40193 [DOI] [PubMed] [Google Scholar]
- 33. Jenkins S. M., Bennett V. (2001) Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 155, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monceau V., Belikova Y., Kratassiouk G., Charue D., Camors E., Communal C., Trouvé P., Russo-Marie F., Charlemagne D. (2004) Externalization of endogenous annexin A5 participates in apoptosis of rat cardiomyocytes. Cardiovasc. Res. 64, 496–506 [DOI] [PubMed] [Google Scholar]
- 35. von Harsdorf R., Li P. F., Dietz R. (1999) Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 99, 2934–2941 [DOI] [PubMed] [Google Scholar]
- 36. Baartscheer A., Schumacher C. A., Belterman C. N., Coronel R., Fiolet J. W. (2003) SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc. Res. 58, 99–108 [DOI] [PubMed] [Google Scholar]
- 37. Brundel B. J., Ausma J., van Gelder I. C., Van der Want J. J., van Gilst W. H., Crijns H. J., Henning R. H. (2002) Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc. Res. 54, 380–389 [DOI] [PubMed] [Google Scholar]
- 38. Inserte J., Garcia-Dorado D., Hernando V., Soler-Soler J. (2005) Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ. Res. 97, 465–473 [DOI] [PubMed] [Google Scholar]
- 39. Janse M. J., Wit A. L. (1989) Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol. Rev. 69, 1049–1169 [DOI] [PubMed] [Google Scholar]
- 40. Bagnato P., Barone V., Giacomello E., Rossi D., Sorrentino V. (2003) Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J. Cell Biol. 160, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhasin N., Cunha S. R., Mudannayake M., Gigena M. S., Rogers T. B., Mohler P. J. (2007) Molecular basis for PP2A regulatory subunit B56α targeting in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 293, H109–H119 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki M., Sasaki N., Miki T., Sakamoto N., Ohmoto-Sekine Y., Tamagawa M., Seino S., Marbán E., Nakaya H. (2002) Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 109, 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gumina R. J., Pucar D., Bast P., Hodgson D. M., Kurtz C. E., Dzeja P. P., Miki T., Seino S., Terzic A. (2003) Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am. J. Physiol. Heart Circ. Physiol. 284, H2106–H2113 [DOI] [PubMed] [Google Scholar]
- 44. Kane G. C., Liu X. K., Yamada S., Olson T. M., Terzic A. (2005) Cardiac KATP channels in health and disease. J. Mol. Cell Cardiol. 38, 937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minami K., Miki T., Kadowaki T., Seino S. (2004) Roles of ATP-sensitive K+ channels as metabolic sensors: studies of Kir6.x null mice. Diabetes 53, Suppl. 3, S176–S180 [DOI] [PubMed] [Google Scholar]
- 46. Gross G. J., Peart J. N. (2003) KATP channels and myocardial preconditioning: an update. Am. J. Physiol. Heart Circ. Physiol. 285, H921–H930 [DOI] [PubMed] [Google Scholar]
- 47. Bennett V. (1982) The molecular basis for membrane-cytoskeleton association in human erythrocytes. J. Cell. Biochem. 18, 49–65 [DOI] [PubMed] [Google Scholar]
- 48. Bennett V. (1992) Ankyrins: adaptors between diverse plasma membrane proteins and the cytoplasm. J. Biol. Chem. 267, 8703–8706 [PubMed] [Google Scholar]
- 49. Haruna T., Horie M., Kouchi I., Nawada R., Tsuchiya K., Akao M., Otani H., Murakami T., Sasayama S. (1998) Coordinate interaction between ATP-sensitive K+ channel and Na+,K+-ATPase modulates ischemic preconditioning. Circulation 98, 2905–2910 [DOI] [PubMed] [Google Scholar]
- 50. Jenkins S. M., Bennett V. (2002) Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc. Natl. Acad. Sci. U.S.A. 99, 2303–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y., Lacas-Gervais S., Morest D. K., Solimena M., Rasband M. N. (2004) βIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier. J. Neurosci. 24, 7230–7240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kizhatil K., Yoon W., Mohler P. J., Davis L. H., Hoffman J. A., Bennett V. (2007) Ankyrin-G and β2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J. Biol. Chem. 282, 2029–2037 [DOI] [PubMed] [Google Scholar]
- 53. Reeves T. M., Greer J. E., Vanderveer A. S., Phillips L. L. (2010) Proteolysis of submembrane cytoskeletal proteins ankyrin-G and αII-spectrin following diffuse brain injury: a role in white matter vulnerability at nodes of Ranvier. Brain Pathol. 20, 1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]