Background: Osteoblast-like differentiation of vascular smooth muscle cells (VSMCs) is a mechanism of vascular calcification.
Results: Bone morphogenetic protein endothelial cell precursor-derived regulator (BMPER) was expressed in VSMCs and enhanced osteoblast-like differentiation of VSMCs via NF-κB activation.
Conclusion: BMPER is a novel regulator of osteoblast-like differentiation of VSMCs.
Significance: BMPER may be a potential target for prevention of vascular calcification.
Keywords: Calcification, Differentiation, Gene Expression, NF-κB (NF-κB), Vascular Smooth Muscle Cells
Abstract
Differentiation of vascular smooth muscle cells (SMCs) into osteoblast-like cells is considered to be a mechanism of vascular calcification. However, regulators of osteoblast-like differentiation of vascular SMCs are not fully elucidated. Here, we investigated the expression of bone morphogenetic protein (BMP)-binding endothelial cell precursor-derived regulator (BMPER), a vertebrate homologue of Drosophila crossveinless-2, in vascular SMCs and the role and mode of action of BMPER in osteoblast-like differentiation of human coronary artery SMCs (HCASMCs). BMPER was expressed in cultured human vascular SMCs, including HCASMCs. Silencing of endogenous BMPER expression by an RNA interference technique inhibited osteoblast-like differentiation of HCASMCs, as evaluated by up-regulation of osteoblast markers such as alkaline phosphatase (ALP) and runt-related transcription factor 2 (Runx2), by down-regulation of a SMC marker α-smooth muscle actin (αSMA), and by mineralization. Treatment with recombinant BMPER enhanced, whereas BMP-2 reduced osteoblast-like differentiation. BMPER antagonized BMP-2-induced phosphorylation of Smad 1/5/8, suggesting that the effect of BMPER was mediated by antagonizing the action of BMP. BMPER increased IκBα phosphorylation and NF-κB activity and specific NF-κB decoy oligonucleotides deteriorated osteoblast-like differentiation of HCASMCs by BMPER. In human coronary artery with atherosclerotic plaque containing calcification, the BMPER-positive signals were observed in the neointimal and medial SMCs in the vicinity of the plaque. These findings indicate that BMPER is a novel regulator of the osteoblast-like differentiation of HCASMCs.
Introduction
Coronary artery calcification is an important risk factor for acute coronary syndrome in patients with type II diabetes and chronic kidney disease (1). Epidemiological surveys detected a positive correlation between the degree of coronary artery calcification and the incidence of cardiovascular events (2). Vascular calcification is categorized into two types: intimal calcification, which is characterized by focal calcification in atherosclerotic plaques, and medial calcification, which is known as Mönckeberg's sclerosis. The coexistence of both types of calcification is frequently observed in human coronary arteries. Although protective effects of cardiovascular agents such as statins (3) and renin-angiotensin system inhibitors (4) against vascular calcification were reported, the validity of these agents is still far from satisfactory.
Studies using rodents provided evidence that vascular calcification is an actively regulated and complicated process similar to bone formation (5). The main mechanism underlying vascular calcification is considered to be differentiation of vascular smooth muscle cells (SMCs)2 into osteoblast-like cells (6). Diverse kinds of regulators, such as runt-related transcription factor 2 (Runx2)/Cbfa-1, msh homeobox 2 (MSX-2), and bone morphogenetic proteins (BMPs), have been reported to be involved in this process. However, regulators of osteoblast-like differentiation of vascular SMCs are not fully elucidated.
BMP-binding endothelial cell precursor-derived regulator (BMPER) was originally discovered as a vertebrate homologue of Drosophila crossveinless-2 (7), which acts as a BMP antagonist (8). BMPER is capable of binding to BMP-2, BMP-4 and BMP-6 (9) and is known to exert bidirectional agonistic and antagonistic effects on BMPs (10). BMPER-knock-out mice exhibit aberrant development of bones and cartilages (11). This finding gave us a hint that BMPER might be involved in vascular calcification. Here, we investigated the expression of BMPER in vascular SMCs and the role and mode of action of BMPER in differentiation of human coronary artery SMCs (HCASMCs) into osteoblast-like cells.
EXPERIMENTAL PROCEDURES
Plasmids, Antibodies, and Reagents
The luciferase reporter plasmid pGL3pro containing three NF-κB-binding sites upstream of a luciferase reporter gene (pGL3–3kBpro) (12) was kindly provided by Dr. F. Kambe (Nagoya University, Nagoya, Japan). pTKβ-Gal was purchased from Clontech. cDNAs for the full-length (FL: amino acids 43–685), N-terminal half (NT: amino acids 43–369) and C-terminal half (CT: amino acids 370–685) of human BMPER were obtained by RT-PCR using a cDNA from human pulmonary artery SMCs (HPASMCs) as a template. Expression vectors for FL (pFLAG-CMV1-BMPER(FL)), NT (pFLAG-CMV1-BMPER(NT)), and CT (pFLAG-CMV1-BMPER(CT)) were generated by subcloning the corresponding cDNAs into pFLAG-CMV1. The C terminus of FL and CT was linked to an HA tag. A vector for an uncleavable mutant of BMPER (pFLAG-CMV1-BMPER(UC)), in which G368–P370 was mutated to A368–A370 (10), was generated using a QuikChange site-directed mutagenesis kit (Stratagene). The following antibodies were used: rat anti-BMPER monoclonal antibody (mAb) (R&D Systems); goat anti-BMPER polyclonal antibody (pAb), mouse anti-actin mAb, rabbit anti-Smad 1/5/8 pAb, and anti-ALP pAb (Santa Cruz Biotechnology); mouse anti-α-SMA mAb, and rabbit anti-FLAG pAb (Sigma-Aldrich); mouse anti-GM-130 and anti-EEA1 mAbs (BD Transduction); rabbit anti-clathrin, anti-pSmad 1/5/8, anti-pIκBα, and anti-IκBα pAbs (Cell Signaling); rabbit anti-mitochondrial Hsp70 pAb (Abcam); and mouse anti-HA mAb (Wako Chemical Co.). Horseradish peroxidase-conjugated secondary Abs were purchased from GE Healthcare Bioscience.
Fluorophore (Alexa 488 or 568)-conjugated secondary Abs were purchased from Molecular Probes. Human recombinant BMPER, human BMP-2, human recombinant tumor necrosis factor-α (TNF-α) were purchased from R&D Systems. RIBBON NF-κB Decoy Oligonucleotides Kit was purchased from Gene Design Inc.
Cell Culture and Small Interfering (siRNA) Experiments
Primary cultures of HCASMCs (Lonza) were maintained at 37 °C in Dulbecco's modified Eagle's medium (DMEM) (low glucose) supplemented with 15% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin. Cells between passages 8 and 11 were used for the experiments. Unless otherwise noted, cells were basically cultured in DMEM supplemented with 15% FBS without changing media for the indicated time periods. For siRNA experiments, HCASMCs were transfected with Stealth siRNAs for BMPER (Invitrogen) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. A Stealth siRNA non-silencing negative control (Invitrogen) was used as a control. Forty-eight hours after transfection, HCASMCs were subjected to each experiment. HEK293 cells were maintained at 37 °C in DMEM (high glucose) supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin. At 72 h after transfection of the plasmid vectors encoding the BMPER mutants, the medium was changed to serum-free DMEM, the cells were cultured for the following 48 h and the conditioned media was taken for Western blotting. Primary cultures of human umbilical vein endothelial cells (HUVECs) (Lonza) were maintained at 37 °C in EGM-2 medium (Lonza). After the culture medium was changed to DMEM (low glucose) supplemented with 15% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin, HUVECs were treated with TNF-α, BMPER, or BMP-2.
RT-PCR
Total mRNAs were extracted from tissues of C57BL/6J mice or cultured cells using TRIzol Reagent (Invitrogen). A ReverTra Ace qPCR RT Kit (Toyobo) was used for room temperature. The following primers were used: mouse BMPER: forward, 5′-ATTACCTGCTGCGTCTTGCT-3′ and reverse, 5′-TTCTCTCACGCACTGTGTCC-3′; mouse GAPDH: forward, 5′-GACCCCTTCATTGACCTCAACTAC-3′ and reverse, 5′-TTTCTTACTCCTTGGAGGCCATGT-3′; human BMPER: forward, 5′-AGGACAGTGCTGCCCCAAATG-3′ and reverse, 5′-TACTGACACGTCCCCTGAAAG-3′; human GAPDH: forward, 5′-CTGATGCCCCCATGTTCGTC-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAATTC-3′; human BMP-2: forward, 5′-CCAGCTTCTCCTTTCTCCCT-3′ and reverse, 5′-CCATGGTCGACCTTTAGGAG-3′; human BMP-4 forward, 5′-TCCACAGCACTGGTCTTGAG-3′ and reverse, 5′-CGATCGGCTAATCCTGACAT-3′; and human matrix Gla protein: forward, 5′-TCCAAGAGAGGATCCGAGAA-3′ and reverse, 5′- TACTTATCAATCTGGGGGCG-3′. The PCR products for mouse GAPDH, human BMPER, human GAPDH, human BMP-2, human BMP-4, and human matrix Gla protein were denatured at 96 °C for 2 min, and then subjected to 25 cycles of a three-step amplification comprising denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 1 min. The PCR amplification of mouse BMPER was performed in the same way, except that the annealing temperature was 56 °C.
Human Autopsy Material
Human coronary arteries were obtained from 20 autopsy cases (aged 15–84 years) after informed consent was provided by their families. Formalin-fixed human coronary artery samples were sectioned and subjected to hematoxylin-eosin (H-E) staining, immunohistochemistry, and alizarin red staining.
Immunostaining
Formalin-fixed human coronary artery sections were deparaffinized, hydrated, and subjected to antigen retrieval by treatment with proteinase K (10 mg/ml) at 37 °C for 10 min. Murine carotid arteries were embedded in OCT compound immediately after euthanasia, sectioned, and fixed in 4% paraformaldehyde for 30 min at room temperature. Cultured cells were fixed in 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 30 min and blocked in 5% skim milk in PBS. The nuclei were visualized using DAPI (Molecular Probes). Immunofluorescence microscopy was performed using an LSM 510 META microscope (Carl Zeiss).
Alkaline Phosphatase (ALP) Staining
HCASMCs were cultured in 12-well plates for 10 days without changing the culture medium supplemented with 15% FBS. Recombinant BMPER or BMP-2 was added every 2 days. After the 10-day culture, the cells were washed with PBS, fixed in 4% paraformaldehyde for 5 min. The cells were incubated in substrate working solution (100 mg/ml naphthol AS-MX phosphatase, 600 mg/ml fast red TR salt, 0.5% (v/v) N,N-dimethylformamide, 2 mm MgCl2, and 0.1 m Tris-HCl pH 8.8) at 37 °C for 20 min. The cells were washed until the intense red color became indicative. The ratio of ALP-positive area was calculated from 10 wells, with at least 500 cells counted per well, using ImageJ® software.
Alizarin Red Staining
Mineralization of human coronary arteries, cultured HCASMCs and mouse aortic rings was evaluated by alizarin red staining. For the mineralization assay, HCASMCs and mouse aortic rings were cultured in the mineralization medium (DMEM (high glucose) supplemented with 15% FBS in the presence of 2.5 mm phosphate (13)) for 10 days. After fixation, deparaffinization, and hydration, the sections or cultured cells were rinsed in distilled water and incubated in alizarin red solution (Alizarin red S (Sigma-Aldrich) 1 g dissolved in 100 ml of distilled water, which was adjusted to the pH of 4.1∼4.3 with 10% ammonium hydroxide) for 5∼20 min. After rinsing in distilled water, the sections or cultured cells were dehydrated through a graded alcohol series and xylene, and mounted. The ratio of alizarin red-positive area was calculated in the same way as ALP staining.
Transfection and Reporter Assays
HEK293 cells were plated in 60-mm dishes at a density of 1.0 × 106 cells/dish. The cells were transfected with the plasmid vectors encoding the BMPER mutants using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For HCASMCs, an electroporation method with Amaxa® Basic Nucleofector® Kit Primary Smooth Muscle Cells (Lonza) was used. For reporter assays, HCASMCs were plated in 6-well plates at 1.0 × 105 cells/well. On the following day, the cells were cotransfected with 3 μg of pGL3–3kBpro and 1 μg of pTKβ-Gal. At 6 h after transfection, the cells were washed with PBS, and the medium was changed. The cells were cultured for the following 48 h, and then incubated with recombinant BMPER (50 nm), BMP-2 (300 ng/ml), or TNF-α (10 ng/ml) for 2 h. The cells were lysed with 200 μl of reporter lysis buffer, and the luciferase activities were determined with Pikka Gene (Toyo Ink, Japan) and a luminometer (BioOrbit 1253). β-Galactosidase activities were measured using β-Galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega) according to the manufacturer's instructions. The luciferase activity was normalized by the β-galactosidase activity. For inactivation of endogenous NF-κB in HCASMCs, cells were transfected with NF-κB decoy oligodeoxynucleotides (0.5 μm) every 2 days (14).
Western Blotting
Cells were washed twice with ice-cold PBS and lysed with lysis buffer (20 mm Tris-HCl pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, and 1 mm PMSF). The cell lysates were centrifuged at 12,000 × g at 4 °C for 10 min. The supernatants were mixed with Laemmli sample buffer containing 2-mercaptoethanol and boiled. The boiled samples were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% skim milk, and then sequentially incubated with primary Abs and horseradish peroxidase-conjugated secondary Abs. After incubation with SuperSignal West Pico Chemiluminescent Substrate (Pierce), the signals were detected using an LAS-4000 Mini Imaging System (Fuji Film).
Real-time PCR
mRNA was isolated from tissue samples or cultured cells and subjected to real-time PCR using a 7500 Real-Time PCR System (Applied Biosystems) with a Two Step SYBR® RT-PCR Kit (Takara Bio). Primers for human BMPER, human ALP, human αSMA, human Runx2, human intercellular adhesion molecule-1 (ICAM-1), and human GAPDH were purchased from Takara Bio. GAPDH was used for normalization, and the comparative threshold method was used to assess the relative abundance of the targets.
Statistical Analysis
All experiments were performed at least three times, and the results are expressed as means ± S.E. Differences between groups were compared by one-way analysis of variance, followed by Fisher's protected least significant difference post hoc test or Scheffe's post hoc test. Values of p < 0.05 were considered significant.
RESULTS
Expression of BMPER in Murine Tissues and Cultured Human Cells
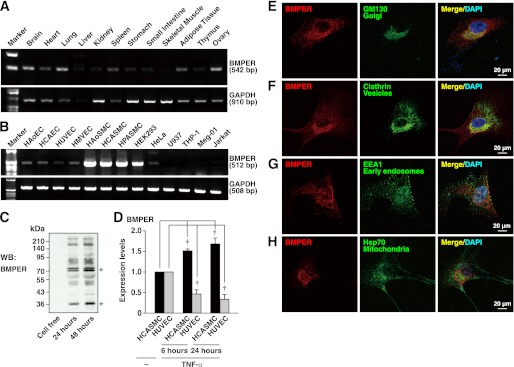
The tissue distribution of BMPER was examined by RT-PCR using cDNAs from murine tissues. BMPER expression was observed in the brain, heart, lung, spleen, adipose tissue, and ovary, and to lesser extents in the stomach, intestine, and skeletal muscle (Fig. 1A). Among cultured human cells, BMPER was most abundantly expressed in vascular SMCs and to lesser extents in endothelial cells and HEK293 cells (Fig. 1B). Faint expression of BMPER was observed in HeLa cells and hematocytes such as U937, THP-1, Meg-01, and Jurkat T cells. We performed Western blotting using the conditioned media of HCASMCs with anti-BMPER pAb and confirmed spontaneous secretion and cleavage of BMPER (Fig. 1C) (9). In endothelial cells, BMPER was reported to be down-regulated by pro-inflammatory cytokines including TNF-α (15). By incubation with TNF-α, the mRNA levels of BMPER were decreased in HUVECs, whereas increased in HCASMCs (Fig. 1D). These results indicate that the regulatory mechanism underlying BMPER expression differs between HUVECs and HCASMCs. The expression of BMPER in vascular smooth muscle was observed in various murine blood vessels including the carotid artery (supplemental Fig. S1), coronary artery, and aorta (results not shown). The subcellular localization of BMPER in HCASMCs was examined by immunofluorescence microscopy. The signal for BMPER was observed in the perinuclear area and co-localized with the signal for the Golgi marker GM130 (Fig. 1E). The signal for BMPER was partly co-localized with that for the membrane vesicle marker clathrin at the Golgi apparatus (Fig. 1F), but not with that for the early endosome marker EEA1 (Fig. 1G) or mitochondrial Hsp70 (Fig. 1H).
FIGURE 1.
Expression of BMPER in murine tissues and cultured human cells. A, mRNA expression of BMPER and GAPDH in murine tissues. B, mRNA expression of BMPER and GAPDH in cultured human cells. HAoEC, human aortic endothelial cell (EC); HCAEC, human coronary artery EC; HUVEC, human umbilical vascular EC; HMVEC, human lung microvascular EC; HAoSMC, human aortic SMC; HPASMC, human pulmonary artery SMC; HEK293, human embryonic kidney 293 cell. C, expression and secretion of BMPER by HCASMCs. The conditioned media of HCASMCs cultured for the indicated time periods were subjected to Western blotting. The leftmost lane was subjected with cell-free media. D, mRNA expression of BMPER in HCASMCs and HUVECs treated with TNF-α (10 ng/ml) for the indicated time periods. Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in the control at day 0 (n = 3). E–H, subcellular localization of BMPER in HCASMCs. HCASMCs were doubly stained with an anti-BMPER pAb and an anti-GM130 mAb (E), an anti-clathrin mAb (F), an anti-EEA1 mAb (G), or an anti-mitochondrial Hsp70 mAb (H). The nuclei were stained with DAPI.
Involvement of BMPER in Spontaneous Osteoblast-like Differentiation of HCASMCs
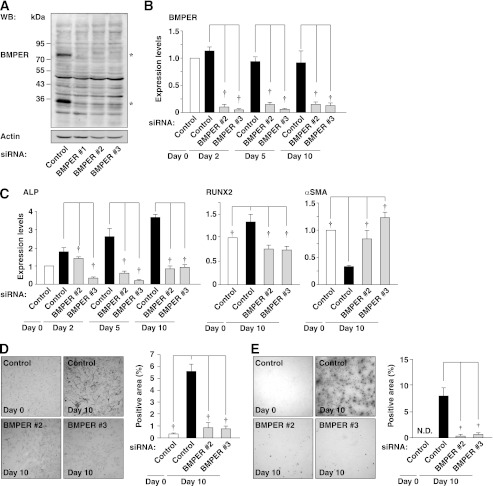
To determine the role of endogenous BMPER in spontaneous osteoblast-like differentiation of HCASMCs, BMPER was silenced by an RNA interference technique. We performed Western blotting using whole cell lysates from HCASMCs. Two bands at ∼80 and ∼35 kDa (asterisks) were detected in control siRNA-transfected cells (Fig. 2A). The ∼80-kDa band corresponded to whole BMPER, whereas the ∼35 kDa band corresponded to a cleaved C-terminal fragment of BMPER (described below). Both bands disappeared in HCASMCs transfected with three different BMPER siRNAs, indicating that the expression of BMPER was effectively knocked down within 48 h after the transfection of each BMPER siRNA. Forty-eight hours after transfection (day 0), culture medium was replaced, and HCASMCs were cultured without changing the medium for another 10 days. Inhibition of BMPER expression by BMPER siRNAs persisted until day 10 as assessed by real-time PCR (Fig. 2B) and ELISA for detection of secreted BMPER in the conditioned medium (supplemental Fig. S2). During the experimental period, the mRNA expression of ALP was spontaneously increased in control siRNA-transfected cells, but significantly down-regulated in BMPER siRNA-transfected cells (Fig. 2C). Similarly, the mRNA expression of Runx2 was increased in control siRNA-transfected cells, while it was down-regulated in BMPER siRNA-transfected cells (Fig. 2C). The mRNA expression of αSMA was spontaneously decreased in control siRNA-transfected cells, while this decrease in the expression of αSMA was prevented in BMPER siRNA-transfected cells (Fig. 2C). The protein expression and activity of ALP were spontaneously increased at day 10 and these increases were significantly suppressed by knockdown of BMPER (supplemental Fig. S3A and Fig. 2D). Mineralization occurred at day 10, which was significantly suppressed by knockdown of BMPER (Fig. 2E). Thus, endogenous BMPER is involved in spontaneous osteoblast-like differentiation of HCASMCs.
FIGURE 2.
Inhibition of osteoblast-like differentiation of HCASMCs by knockdown of BMPER. A, knockdown of BMPER. Cell lysates of HCASMCs, which were transfected with control or BMPER siRNAs and then cultured for 48 h, were subjected to Western blotting. Two bands of BMPER at ∼80 and ∼35 kDa (asterisks) are detected in control siRNA-transfected cells. B, mRNA expression of BMPER in HCASMCs transfected with control or BMPER siRNAs. Forty-eight hours after transfection of each siRNA, culture media were replaced to new media containing 15% FBS and then HCASMCs were cultured for up to 10 days. Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in the control at day 0 (n = 4). C, mRNA expression of ALP and αSMA in HCASMCs transfected with control or BMPER siRNAs. Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in the control at day 0 (n = 5). D, ALP staining of HCASMCs transfected with control or BMPER siRNAs. Representative images are shown on the left. The right graph shows the percentages of the ALP-positive surface area relative to the total surface area of HCASMCs (n = 5). E, alizarin red staining of HCASMCs transfected with control or BMPER siRNAs. Representative images are shown on the left. The right graph shows the percentages of the alizarin red-positive surface area relative to the total surface area of HCASMCs (n = 5). †p < 0.01.
Enhancement of Osteoblast-like Differentiation of HCASMCs by Recombinant BMPER
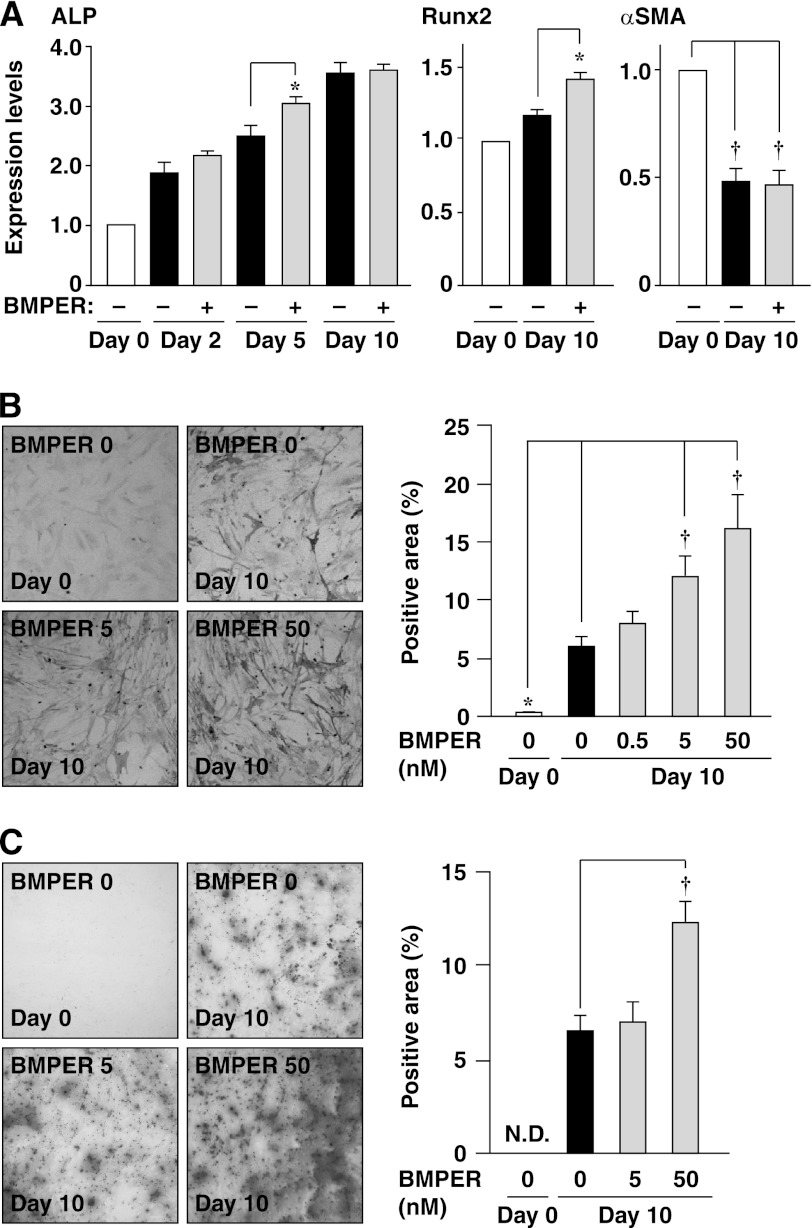
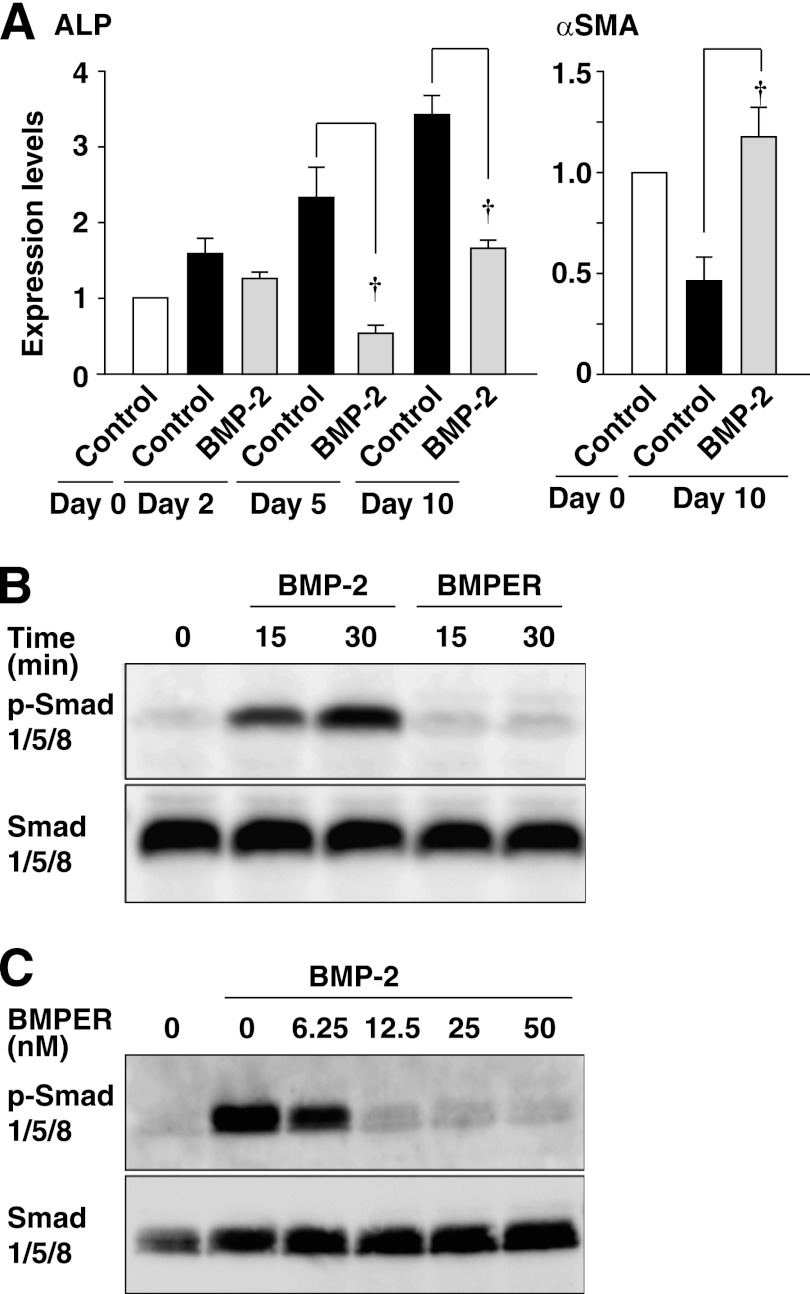
The effects of exogenous addition of BMPER on the expression of osteoblast and SMC markers were examined by real-time PCR. In our preliminary experiments, we measured the plasma concentration of BMPER in humans and found that they are ∼50 nm (mean roughly 5 nm).3 Therefore, we exogenously added BMPER at 5 or 50 nm, although the concentration of BMPER was much greater than that in the conditioned medium (supplemental Fig. S2). The mRNA expression of ALP showed a slight but statistically significant increase by incubation with BMPER at day 5, but not at day 2 or day 10 (Fig. 3A). The mRNA expression of Runx2 was increased by incubation with BMPER at day 10 (Fig. 3A). The mRNA expression of αSMA in the presence of BMPER was equivalent to that in the absence of BMPER at day 10 (Fig. 3A). The expression of ALP in the presence of BMPER was equivalent to that in the absence of BMPER at day 10 (supplemental Fig. S3B). The activity of ALP was increased by BMPER in a concentration-dependent manner (Fig. 3B). However, BMPER did not show any significant effects on the expression of BMP-2, -4, or matrix Gla protein (supplemental Fig. S4). Mineralization was enhanced by BMPER (Fig. 3C). Consistently, mineralization in mouse aortic rings was enhanced by BMPER (supplemental Fig. S5). Taken together, these findings indicate that exogenous BMPER is capable of enhancing osteoblast-like differentiation of HCASMCs.
FIGURE 3.
Enhancement of spontaneous osteoblast-like differentiation of HCASMCs by recombinant BMPER. A, mRNA expression of ALP and αSMA analyzed by real-time PCR (n = 3). Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in the absence of BMPER. B, ALP staining of HCASMCs treated with the indicated concentrations of recombinant BMPER for 10 days (n = 5). Representative images are shown on the left. The right graph shows the percentages of the ALP-positive area relative to the total surface area of HCASMCs. C, alizarin red staining of HCASMCs treated with the indicated concentrations of recombinant BMPER for 10 days (n = 5). Representative images are shown on the left. The right graph shows the percentages of the alizarin red-positive area relative to the total surface area of HCASMCs. *, p < 0.05, †, p < 0.01.
Requirement of Both N-terminal and C-terminal Fragments of BMPER for Osteoblast-like Differentiation of HCASMCs
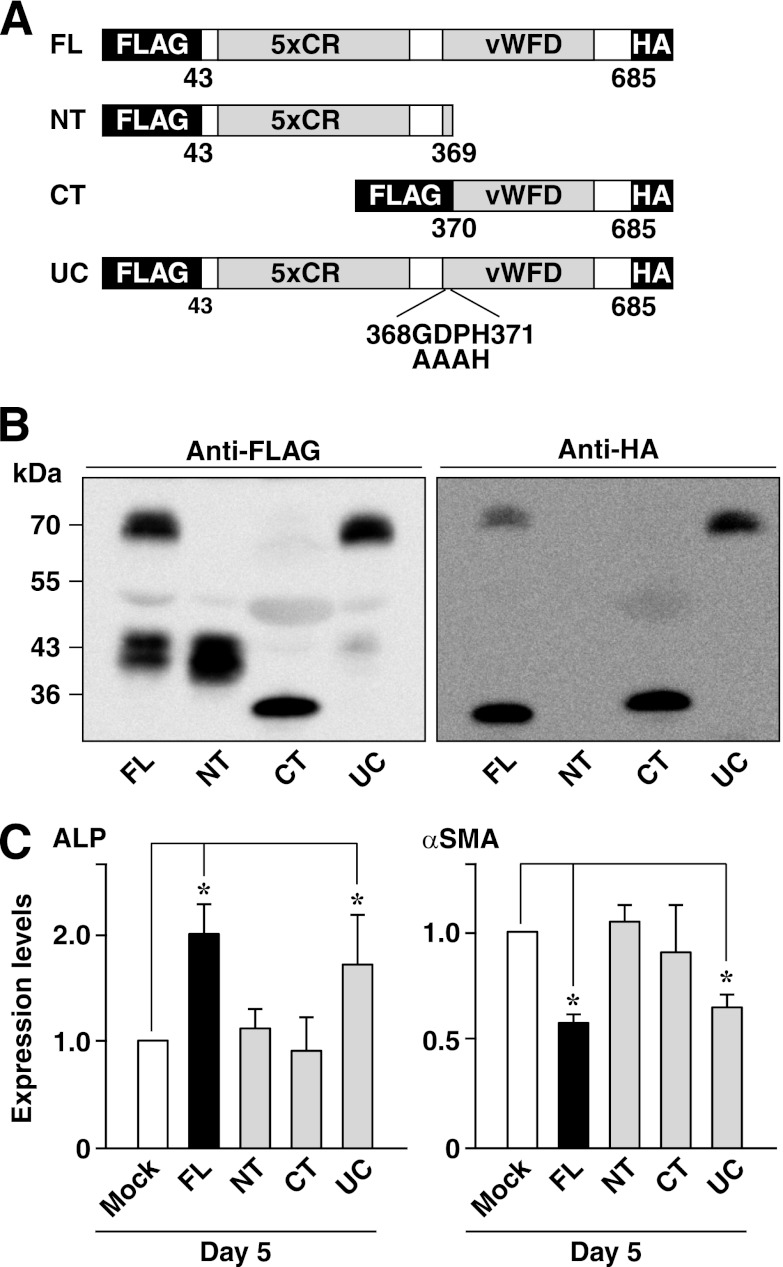
It was reported that BMPER was cleaved enzymatically into N-terminal and C-terminal fragments, which were linked by a disulfide bond and form a heterodimer (10). To clarify which fragment is responsible for the effect of BMPER, we constructed plasmids encoding a full-length (FL), an N-terminal fragment (NT), a C-terminal fragment (CT), and an uncleavable form (UC) of BMPER in which the amino acid sequences at the cleavage site (10) were mutated (Fig. 4A). All of the constructs were tagged by a FLAG epitope at the N terminus, and the full-length, C-terminal fragment and uncleavable form of BMPER were tagged by an HA epitope at the C terminus. Similar amounts of appropriate bands corresponding to the predicted molecular weights were detected in the conditioned medium from HEK293 cells transfected with each plasmid by Western blotting using anti-FLAG or anti-HA mAbs (Fig. 4B), thereby confirming the similar secretion levels and cleavage/non-cleavage of each mutant. Next, the effects of these BMPER mutants on the expression of ALP and αSMA in HCASMCs were examined. To this end, we transfected various BMPER mutants to HCSMCs because the conditioned medium from HEK293 cells transfected with various BMPER mutants did not show any significant effects (results not shown). Indeed, exogenous addition of recombinant BMPER to BMPER-knockdown cells could slightly reverse the effects of knockdown of endogenous BMPER, suggesting that the effect of endogenous BMPER was much more potent than that of exogenous BMPER (supplemental Fig. S6). Transfection of the full-length and uncleavable mutant of BMPER increased the expression of ALP and decreased the expression of αSMA (Fig. 4C). On the other hand, the N-terminal and C-terminal fragments of BMPER did not show any effects on the expression of ALP and αSMC (Fig. 4C). These results indicate that both the N-terminal and C-terminal fragments are indispensable for the effect of BMPER.
FIGURE 4.
Requirement of both the N-terminal and C-terminal fragments of BMPER for osteoblast-like differentiation of HCASMCs. A, constructions of the BMPER mutants. All the constructs were tagged by a FLAG epitope at the N terminus, and the full-length, C-terminal fragment and uncleavable form of BMPER were tagged by an HA epitope at the C terminus. FL, full-length; NT, N-terminal fragment; CT, C-terminal fragment; UC, uncleavable form of BMPER in which three amino acids at the cleavage site were mutated as indicated. B, Western blotting of secreted BMPER mutants with anti-FLAG and anti-HA mAbs. Culture media from HEK293 cells transfected with the indicated BMPER mutants or vector alone (Mock) were analyzed. C, mRNA expression of ALP and αSMA in HCASMCs at 48 h after transfection with the indicated BMPER mutants or vector alone (Mock) (n = 5). Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in Mock. *, p < 0.05.
Inhibition of BMP Signaling by BMPER
It was reported that BMP-2 and BMP-4 were involved in vascular calcification (16) and were able to enhance osteoblast-like differentiation of cultured vascular SMCs (17). We examined whether BMPs could be involved in the effect of BMPER. Surprisingly, BMP-2 decreased ALP mRNA expression and increased αSMC mRNA expression (Fig. 5A). Consistently, mineralization was suppressed by BMP-2 (results not shown). These findings indicate that BMP-2 negatively regulates osteoblast-like differentiation of HCASMCs under our experimental conditions. It was shown that BMPER is able to act as both an antagonist and an agonist of BMPs (10). We then examined whether BMPER could inhibit the BMP-induced signaling in HCASMCs. BMP-2, but not BMPER, increased phosphorylation of Smad 1/5/8 (Fig. 5B), which was inhibited by BMPER in a concentration-dependent manner (Fig. 5C), indicating the antagonistic effect of BMPER on BMPs. Vascular calcification is considered a process involving inflammation (18). We examined whether BMPER and BMP-2 could induce inflammatory response (supplemental Fig. S7). In HCASMCs, BMPER increased whereas BMP-2 decreased ICAM-1 mRNA levels. In HUVECs, BMPER, or BMP-2 had no significant effect on ICAM-1 mRNA levels. Thus, the action of BMPER and BMP-2 is the opposite in HCASMCs and differs between HCASMCs and HUVECs. Collectively, these findings suggest that the inhibition of the action of BMPs may be involved in the effect of BMPER on osteoblast-like differentiation of HCASMCs.
FIGURE 5.
Inhibition of BMP signaling by BMPER. A, inhibition of spontaneous osteoblast-like differentiation of HCASMCs by BMP-2. The mRNA expression of ALP and αSMA analyzed by real-time PCR (n = 4). HCASMCs were cultured in the presence or absence of 300 ng/ml BMP-2 for up to 10 days. Each value was standardized by the GAPDH mRNA expression and is presented as the fold increase over that in the control. †, p < 0.01. B and C, inhibition of the BMP-2-induced phosphorylation of Smad 1/5/8 by BMPER. Whole cell lysates of HCASMCs treated with 300 ng/ml BMP-2 or 50 nm (B) or the indicated concentrations (C) of BMPER for the indicated time periods (B) or for 30 min (C) were subjected to Western blotting.
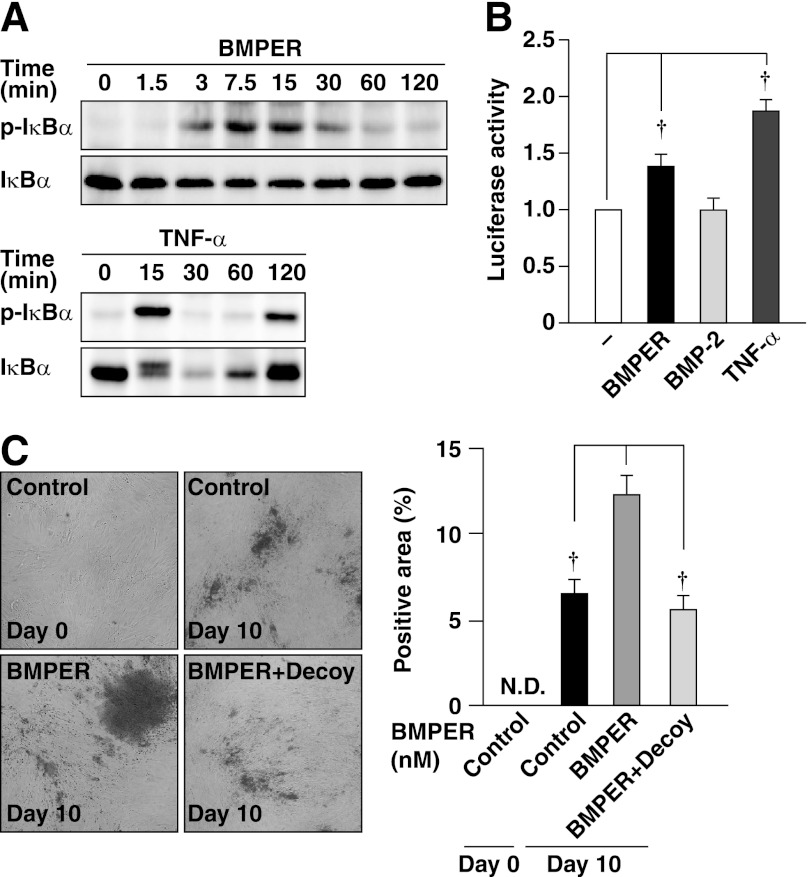
Involvement of the NF-κB Activity in the Effect of BMPER
It was reported that the NF-κB activity plays a critical role in the osteoblastic differentiation of mesenchymal stem cells (19). Therefore, we investigated whether the NF-κB activity was involved in the effect of BMPER. To examine whether BMPER activated the NF-κB, we performed Western blotting to evaluate phosphorylation and degradation of IκB, an upstream regulator of NF-κB, and a luciferase reporter assay for NF-κB. TNF-α was used as a control. BMPER and TNF-α increased phosphorylation of IκBα accompanied by its degradation (Fig. 6A). BMPER and TNF-α, but not BMP-2, significantly increased the NF-κB activity (Fig. 6B). We then examined whether the NF-κB activity was involved in the enhancement of mineralization by BMPER. Treatment of HCASMCs with specific NF-κB decoy oligonucleotides significantly suppressed the BMPER-induced mineralization of HCASMCs (Fig. 6C). Collectively, these findings indicate the involvement of the NF-κB activity in the effect of BMPER.
FIGURE 6.
Involvement of NF-κB in the action of BMPER. A, phosphorylation and degradation of IκBα by BMPER. Cell lysates of HCASMCs treated with 50 nm BMPER or 10 ng/ml TNF-α for the indicated time periods were subjected to Western blotting. B, activation of NF-κB by BMPER. HCASMCs were treated with 50 nm BMPER, 300 ng/ml BMP-2 or 10 ng/ml TNF-α for 2 h. The emission intensity of each sample was standardized by that of the control cells (no stimulation) and is shown as the fold increase over the control value (n = 3). C, alizarin red staining of HCASMCs cultured in the presence or absence of 0.5 μm NF-κB decoy or 50 nm BMPER for 10 days (n = 5). Representative images are shown on the left. The right graph shows the percentages of the alizarin red-positive area relative to the total surface area of HCASMCs. †, p < 0.01.
Expression of BMPER in Human Coronary Arteries
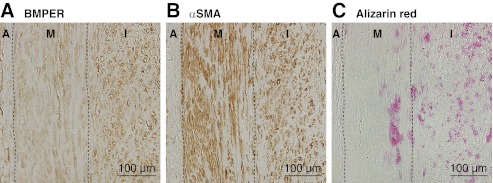
Finally, the expression of BMPER in human atherosclerotic coronary arteries was analyzed. In a coronary artery containing atherosclerotic plaque with microcalcification (Fig. 7), the signal for BMPER was detected in the intimal and medial SMCs in the vicinity of the plaques (Fig. 7, A and B). Microcalcification in the plaque was positive for alizarin red staining (Fig. 7C). We confirmed the expression of BMPER in the intimal SMCs of atherosclerotic coronary artery with microcalcification obtained from other subjects (results not shown). These observations suggest the association of BMPER with human coronary artery calcification.
FIGURE 7.
Expression of BMPER in human atherosclerotic coronary artery. Specimens of a coronary artery containing plaque with microcalcification from a 59-year-old male with diabetes mellitus, hypertension and a smoking habit were subjected to immunostaining of BMPER (A) or αSMA (B), and alizarin red staining (C). A, adventitia; I, intima; M, media.
DISCUSSION
We showed here that BMPER was expressed in cultured human vascular SMCs and spontaneously released by HCASMCs. When HCASMCs were cultured in the serum-containing media, osteoblast-like differentiation spontaneously occurred. Inhibition of endogenous BMPER by siRNAs significantly suppressed, whereas recombinant BMPER enhanced spontaneous osteoblast-like differentiation of HCASMCs. Therefore, it is likely that BMPER spontaneously released by HCASMCs can induce osteoblast-like differentiation in an autocrine or paracrine manner. Our findings indicate that BMPER is a novel regulator of osteoblast-like differentiation of HCASMCs.
BMPER contains five tandem von Willebrand C-like domains at the N-terminal side, which are considered to form a BMP-binding site (20). It was reported that extracellular BMPER bound to BMPs and type I BMP receptors (10) to form a ternary complex. For example, Heinke et al. (21) reported that BMPER biphasically regulated angiogenesis, depending on its concentration, by modifying the binding affinity of BMP-4 to BMP receptors, and thus acted as an agonist or antagonist of BMP-4. In the present study, we consider that the antagonistic action against BMP is likely one of the mechanisms of the BMPER-enhanced osteoblast-like differentiation of HCASMCs because of the following reasons. One is that BMPER acts as an antagonist of BMP-2 because BMPER inhibited the BMP-2-induced phosphorylation of Smad 1/5/8 in HCASMCs. The other is that BMP-2 enhanced osteoblast-like differentiation of HCASMCs. We tried to examine whether the inhibition of BMP signaling could induce mineralization by using dorsomorphin, a specific BMP receptor antagonist (22). However, we failed to examine the effects of this antagonist because HCASMCs could not survive in the presence of the reagent longer than 5 days (results not shown). This is consistent with the aspect of BMPs as a cell survival factor (23).
BMP-2 negatively regulated osteoblast-like differentiation of HCASMCs under our experimental conditions. This result contrasts with the pro-mineralizing action of BMP-2/4 described in previous studies (16, 17, 24). The difference in BMP-2 action may be due to the difference in the type of cells examined. We used primary-cultured HCASMCs, while other groups used calcifying vascular cells (16, 24) or immortalized SMCs (17). Furthermore, cell culture conditions may affect the action of BMP-2.
Compared with the remarkable effects of inhibition of endogenous BMPER by specific siRNAs, the effects of exogenous BMPER were relatively weak. These observations may result from cleavage of recombinant BMPER and/or saturation of the inhibitory action against BMPs by endogenous BMPER. Another possibility is that the endocytotic internalization of exogenous BMPER is not so efficient as other cell types such as mouse endothelial cells (25). The authors showed that BMPER was efficiently internalized by endocytosis in endothelial cells, cardiomyocytes, and fibroblasts, but not in HEK293 or COS7 cells. Furthermore, intracellular accumulation of BMPER may have a more profound effect than the extracellular BMPER.
In addition, we found that BMPER induced the activation of NF-κB in HCASMCs. Cho et al. (19) reported that the activation of NF-κB induced osteoblast differentiation of mesenchymal stem cells derived from human adipose tissue. It is well known that inflammation plays a role in vascular calcification and that pro-inflammatory cytokines such as TNF-α, which induce the activation of NF-κB, are up-regulated in calcifying states such as hyperglycemia, dyslipidemia and uremia (18). Future studies are required to clarify a mechanism of the BMPER-induced phosphorylation of IκB and to determine whether BMPER is involved in the mechanisms of vascular calcification in such pathological states. It was reported that intracellular NF-κB signaling antagonizes Smad signaling induced by BMPs (26). Therefore, inhibition of the BMP-induced Smad signaling by the BMPER-induced activation of NF-κB signaling is likely another mechanism involved in osteoblast-like differentiation of HCASMCs.
We further found that BMPER had a pro-inflammatory effect on HCASMCs. This is in contrast to a previous report demonstrating an anti-inflammatory effect of BMPER in endothelial cells (15). Furthermore, BMPER was increased in HCASMCs, whereas decreased in HUVECs by TNF-α. The action and regulatory mechanism of BMPER differ between HCASMCs and endothelial cells. These findings imply differences among cell types in the role of BMPER in vascular inflammation.
We showed here that BMPER was expressed in human atherosclerotic coronary artery. However, we observed that BMPER was expressed in coronary arteries irrespective of the presence or absence of atherosclerotic plaque or calcification.3 Thus, it is likely that BMPER is expressed in the coronary artery throughout a person's lifetime. A reason why vascular calcification does not occur easily in vivo despite the continuous expression of BMPER in vascular SMCs may be that the balance among inducers like BMPER and repressors is critical for vascular calcification. Indeed, an imbalance among inducers and repressors plays a key role in progressing vascular calcification (5).
The results of the present study suggest that strategies for suppressing the action of BMPER may have clinical benefits for preventing or treating vascular calcification, especially in coronary arteries. To explore this idea, it is necessary to clarify the regulatory mechanism of the expression of BMPER. In addition, we showed here that BMPER was secreted by HCASMCs. It would be interesting to investigate whether measurement of the serum concentration of BMPER in humans could be useful for screening for the risk of vascular calcification. Collectively, our findings provide the new idea that BMPER might be a potential target for prevention of vascular calcification.
Supplementary Material
Acknowledgments
We thank Dr. Kambe (Nagoya University) for providing the luciferase reporter plasmid pGL3–3κBpro. We thank Michiyo Nakata for excellent technical assistance in the immunohistochemical staining.
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (to Y. R.), and grants from the Global Center of Excellence (to Y. R. and K. H.), Takeda Science Foundation, and Mitsui Life Social Welfare Foundation (to Y. R.).

This article contains supplemental Figs. S1–S7.
Y. Rikitake, unpublished observation.
- SMC
- smooth muscle cell
- ALP
- alkaline phosphatase
- αSMA
- α-smooth muscle actin
- BMP
- bone morphogenetic protein
- BMPER
- BMP-binding endothelial cell precursor-derived regulator
- HCASMC
- human coronary artery smooth muscle cell
- H-E
- hematoxylin-eosin
- HSPG
- heparan sulfate proteoglycan
- HUVEC
- human umbilical vein endothelial cell
- ICAM-1
- intercellular adhesion molecule-1
- MSX-2
- msh homeobox 2
- Runx2
- runt-related transcription factor 2
- TNF-α
- tumor necrosis factor-α.
REFERENCES
- 1. van der Zee S., Baber U., Elmariah S., Winston J., Fuster V. (2009) Cardiovascular risk factors in patients with chronic kidney disease. Nat. Rev. Cardiol. 6, 580–589 [DOI] [PubMed] [Google Scholar]
- 2. Rennenberg R. J., Kessels A. G., Schurgers L. J., van Engelshoven J. M., de Leeuw P. W., Kroon A. A. (2009) Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc. Health Risk Manag. 5, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Callister T. Q., Raggi P., Cooil B., Lippolis N. J., Russo D. J. (1998) Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N. Engl. J. Med. 339, 1972–1978 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong Z. B., Boughner D. R., Drangova M., Rogers K. A. (2011) Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc Res. 90, 165–170 [DOI] [PubMed] [Google Scholar]
- 5. Persy V., D'Haese P. (2009) Vascular calcification and bone disease: the calcification paradox. Trends Mol. Med. 15, 405–416 [DOI] [PubMed] [Google Scholar]
- 6. Shanahan C. M., Cary N. R., Salisbury J. R., Proudfoot D., Weissberg P. L., Edmonds M. E. (1999) Medial localization of mineralization-regulating proteins in association with Mönckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation 100, 2168–2176 [DOI] [PubMed] [Google Scholar]
- 7. Conley C. A., Silburn R., Singer M. A., Ralston A., Rohwer-Nutter D., Olson D. J., Gelbart W., Blair S. S. (2000) Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127, 3947–3959 [DOI] [PubMed] [Google Scholar]
- 8. Binnerts M. E., Wen X., Canté-Barrett K., Bright J., Chen H. T., Asundi V., Sattari P., Tang T., Boyle B., Funk W., Rupp F. (2004) Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem. Biophys. Res. Commun. 315, 272–280 [DOI] [PubMed] [Google Scholar]
- 9. Moser M., Binder O., Wu Y., Aitsebaomo J., Ren R., Bode C., Bautch V. L., Conlon F. L., Patterson C. (2003) BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol. Cell Biol. 23, 5664–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serpe M., Umulis D., Ralston A., Chen J., Olson D. J., Avanesov A., Othmer H., O'Connor M. B., Blair S. S. (2008) The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 14, 940–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikeya M., Kawada M., Kiyonari H., Sasai N., Nakao K., Furuta Y., Sasai Y. (2006) Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development 133, 4463–4473 [DOI] [PubMed] [Google Scholar]
- 12. Kikumori T., Kambe F., Nagaya T., Imai T., Funahashi H., Seo H. (1998) Activation of transcriptionally active nuclear factor-κB by tumor necrosis factor-α and its inhibition by antioxidants in rat thyroid FRTL-5 cells. Endocrinology 139, 1715–1722 [DOI] [PubMed] [Google Scholar]
- 13. Nakano-Kurimoto R., Ikeda K., Uraoka M., Nakagawa Y., Yutaka K., Koide M., Takahashi T., Matoba S., Yamada H., Okigaki M., Matsubara H. (2009) Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am. J. Physiol. Heart Circ. Physiol. 297, H1673–H1684 [DOI] [PubMed] [Google Scholar]
- 14. Shimizu H., Nakagami H., Tsukamoto I., Morita S., Kunugiza Y., Tomita T., Yoshikawa H., Kaneda Y., Ogihara T., Morishita R. (2006) NFκB decoy oligodeoxynucleotides ameliorates osteoporosis through inhibition of activation and differentiation of osteoclasts. Gene Therapy 13, 933–941 [DOI] [PubMed] [Google Scholar]
- 15. Helbing T., Rothweiler R., Ketterer E., Goetz L., Heinke J., Grundmann S., Duerschmied D., Patterson C., Bode C., Moser M. (2011) BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood 118, 5040–5049 [DOI] [PubMed] [Google Scholar]
- 16. Boström K., Watson K. E., Horn S., Wortham C., Herman I. M., Demer L. L. (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 91, 1800–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li X., Yang H. Y., Giachelli C. M. (2008) BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 199, 271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shao J. S., Cheng S. L., Sadhu J., Towler D. A. (2010) Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension 55, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho H. H., Shin K. K., Kim Y. J., Song J. S., Kim J. M., Bae Y. C., Kim C. D., Jung J. S. (2010) NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J. Cell Physiol. 223, 168–177 [DOI] [PubMed] [Google Scholar]
- 20. Zhang J. L., Qiu L. Y., Kotzsch A., Weidauer S., Patterson L., Hammerschmidt M., Sebald W., Mueller T. D. (2008) Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell 14, 739–750 [DOI] [PubMed] [Google Scholar]
- 21. Heinke J., Wehofsits L., Zhou Q., Zoeller C., Baar K. M., Helbing T., Laib A., Augustin H., Bode C., Patterson C., Moser M. (2008) BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ. Res. 103, 804–812 [DOI] [PubMed] [Google Scholar]
- 22. Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D., Peterson R. T. (2008) Nat. Chem. Biol. 4, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashique A. M., Fu K., Richman J. M. (2002) Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development 129, 4647–4660 [DOI] [PubMed] [Google Scholar]
- 24. Zebboudj A. F., Shin V., Boström K. (2003) Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J. Cell Biochem. 90, 756–765 [DOI] [PubMed] [Google Scholar]
- 25. Kelley R., Ren R., Pi X., Wu Y., Moreno I., Willis M., Moser M., Ross M., Podkowa M., Attisano L., Patterson C. (2009) A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 184, 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamazaki M., Fukushima H., Shin M., Katagiri T., Doi T., Takahashi T., Jimi E. (2009) Tumor necrosis factor α represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-κB. J. Biol. Chem. 284, 35987–35995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.