Background: HLA-B*2705-Asp116 and HLA-B*2709-His-116 associate differently with AS. Both possess an unpaired Cys-67 in the B pocket.
Results: The two molecules cross-present antigens through a proteasome-independent route. Cys-67, although not endorsing this alternative pathway, contributes variously to peptide stabilization.
Conclusion: B27 molecules can load peptides in post-ER compartments. Asp-116/His-116 influences the need of an intact B-pocket.
Significance: Identifying B27 distinctive features might contribute to the understanding of AS pathogenesis.
Keywords: Antigen Presentation, Autoimmunity, Endoplasmic Reticulum (ER), Intracellular Processing, Major Histocompatibility Complex (MHC), HLA-B27 Alleles, Unpaired Cys-67
Abstract
Nascent HLA-class I molecules are stabilized by proteasome-derived peptides in the ER and the new complexes proceed to the cell surface through the post-ER vesicles. It has been shown, however, that less stable complexes can exchange peptides in the Trans Golgi Network (TGN). HLA-B27 are the most studied HLA-class I molecules due to their association with Ankylosing Spondylitis (AS). Chimeric proteins driven by TAT of HIV have been exploited by us to deliver viral epitopes, whose cross-presentation by the HLA-B27 molecules was proteasome and TAP-independent and not restricted to Antigen-Presenting Cells (APC). Here, using these chimeric proteins as epitope suppliers, we compared with each other and with the HLA-A2 molecules, the two HLA-B*2705 and B*2709 alleles differing at residue 116 (D116H) and differentially associated with AS. We found that the antigen presentation by the two HLA-B27 molecules was proteasome-, TAP-, and APC-independent whereas the presentation by the HLA-A2 molecules required proteasome, TAP and professional APC. Assuming that such difference could be due to the unpaired, highly reactive Cys-67 distinguishing the HLA-B27 molecules, C67S mutants in HLA-B*2705 and B*2709 and V67C mutant in HLA-A*0201 were also analyzed. The results showed that this mutation did not influence the HLA-A2-restricted antigen presentation while it drastically affected the HLA-B27-restricted presentation with, however, remarkable differences between B*2705 and B*2709. The data, together with the occurrence on the cell surface of unfolded molecules in the case of C67S-B*2705 mutant but not in that of C67S-B*2709 mutant, indicates that Cys-67 has a more critical role in stabilizing the B*2705 rather than the B*2709 complexes.
Introduction
Because of the strong association with Spondyloarthropathies, HLA-B27 family of alleles is one of the most investigated HLA molecules. Accordingly, much is known about their structure (1), function (2, 3), geographical distribution (4, 5), and disease association, in particular with Ankylosing Spondylitis (AS),2 a chronic rheumatic inflammatory disease. More recently, the interest toward these molecules has been boosted by the observation that they have a protective role in some major viral infections such as AIDS or Hepatitis C (6–11). HLA-B27 includes a number of alleles that differ from each other in one or a few amino acids, and some of them, located in the peptide binding groove, have been proven to be functionally relevant. Noteworthy, some of these alleles such as the B*2709 in Sardinia (12, 13) and the B*2706 in Asia (14), are not associated with AS since they have not been reported, except anecdotically, in patients, and therefore have become a powerful tool to gain insights into disease association. Functional differences between AS-associated and non-AS-associated B27 subtypes (15), B27 transgenic animal models (16) and a recent report showing an association between AS and ERAP1 (17), an ER aminopeptidase trimming the peptides presented by the HLA-class I molecules, strongly suggest a direct role for the HLA-B27 molecules in disease pathogenesis, possibly presenting tissue-specific peptides (18).
It has been recently demonstrated that in TAP-defective cells, HLA-class I molecules can exchange peptides in post-ER vesicles such as in Trans Golgi network (TGN) (19), We have also shown that an HLA-B27-restricted epitope from Influenza virus can be cross-presented to CD8 T cells when exogenously carried to the TGN by chimeric proteins. In this case the presentation was proteasome and TAP-independent. TAP-competent cells such as EBV-B lymphocytes and HeLa cells (19) were also capable of such cross-presentation, allowing to hypothesize that HLA-B27 molecules can routinely exploit this pathway.
It has been demonstrated that B27 molecules can form unstable complexes with peptides longer than nonamers (21). Furthermore, under some circumstances, the presence on the cell surface of misfolded HLA-B27 molecules as well as of B27 oligomers has been documented (22, 23). This suggests that a portion of B27 molecules reach the TGN in partially destabilized three party complexes and are therefore prone to exchange peptides along their way to the cell surface. To further investigate these aspects, we used here TAT-driven carrier proteins in which the original HLA-B27-restricted epitope from the nucleoprotein of Influenza virus, SRYWAIRTR aa 383–391, was replaced with other HLA-B27 or HLA-A2 restricted viral epitopes. These proteins are delivered to the TGN by specific sequences in the C terminus of the influenza virus nucleoprotein (20). We found that cross-presentation by the HLA-A2 molecules required specialized Antigen-Presenting Cells (APC) and was both proteasome and TAP-dependent. Conversely, both B*2705 and B*2709, different from each other in a single amino acid (H116D) that affects binding specificity and T-cell presentation (24–27), used the proteasome and TAP-independent route. Since a hallmark of HLA-B27 molecules is the presence of a highly reactive cysteine at position 67, which influences the shaping of the B pocket and allows homodimer formation (28), this position was mutated in both HLA-B27 (C67S) and in the HLA-A2 molecules (V67C). Expression and antigen presentation were not altered in the case of HLA-A2 while they were dramatically affected in the case of HLA-B27 with striking differences between B*2705 and B*2709 molecules. These findings are further evidence of the functional relevance of the micropolymorphism at amino acid 116 distinguishing the HLA-B27 subtypes differentially associated with AS.
EXPERIMENTAL PROCEDURES
Plasmids
To produce genetic in-frame TAT fusion proteins, DNA sequence encoding for the amino acid region 301–498 of the influenza A virus nucleoprotein (Np) was inserted into the expression vector pTAT-HA (29) as already described (20). The hybrid constructs were generated by double digestion with XhoI and Eco0109I (Fermentas, Burlington, Ontario, Canada) located, respectively, upstream and downstream the NpFlu epitope, to insert the mutated sequences (∼80 bp). The following primers were used: LMP2/RS forward: 5′-CAAGTACTCGAGAACTGAGAAGCAGAAGAAGATGGAGAAGACTCACGGTGAGA, LMP2/RS reverse: 5′-CCGAGGAGGCCCTCTGTTGATTGGTGTTCCCTCCACTTCTCACCGTGAGTCTTCTCCA; NS3–2/RS forward: 5′-CAAGTACTCGAGAACTGAGAAGCAAACTAGTCGCGCTCGGAATCAACGCAGTTA, NS3–2/RS reverse: 5′-CCGAGGAGGCCCTCTGTTGATTGGTGTTCCCTCCACTTCTAACTGCGTTGATTCC; NS3–1/RS forward: 5′-CAAGTACTCGAGAACTGAGAAGCTGCGTTAACGGTGTTTGCTGGACTGTTAGA; NS3–1/RS reverse: 5′-CCGAGGAGGCCCTCTGTTGATTGGTGTTCCCTCCACTTCTAACAGTCCAGCAAAC. EBNA3C/RS forward and reverse primers are in Bettosini et al. (20).
Each pair of primers were extended in a Thermal cycler for 1 min at 52 °C followed by 7 min at 72 °C and then digested with XhoI and Eco0109I. The truncated mutants were obtained by deleting the C-terminal region of 98 aa (end) through digestion with Eco0109I and re-ligation of the plasmid. The sequence of all recombinant constructs was confirmed by using BigDye Terminator cycle sequencing kit (Applied Biosystems, Carlsbad, CA) and ABI 377 DNA sequencer (Applied Biosystems).
DNA constructs HLA-B*2705, B*2709 and A*0201 were cloned in the pcDNA3 vector (Invitrogen). The mutated constructs HLA-B*2705Y320F (a gift from S. J. Powis), B*2705C67S, B*2709C67S, and A2V67C were generated through PCR-based mutagenesis of the wild-type cDNAs. To produce the B*2705C67S and B*2709C67S mutants, B*2705 and B*2709 respectively were subjected to site-directed mutagenesis to replace Cys-67 by Ser (C67S), using the primer 5′-GGAGACACAGATCAGCAAGGCCAAGGCC and its respective reverse complement primer. To produce the A2V67C mutant, A2 was subjected to site-directed mutagenesis to replace Val-67 by Cys (V67C), with the primer 5′-GACACGGAAATGCAAGGCCCACTC; and its respective reverse complement primer. All sequences were confirmed by DNA sequencing analysis.
Production, Purification, and Detection of TAT Fusion Proteins
TAT fusion proteins were produced as reported (20), according to Nagahara et al. (29). To purify the TAT recombinant proteins, Ni-nitrilotriacetic acid (Ni-NTA) resin was used according to the protocol provided by Qiagen (Qiagen spa, Milan, Italy). After 24 h of dialysis with 1× phosphate-buffered saline (PBS), the samples were concentrated with an Ultrafree-CL 10 or 30 system (Millipore, Billerica, MA). The proteins were loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and detected by Coomassie Blue staining. For Western blotting analysis, after SDS-PAGE, samples were transferred to nitrocellulose filter and marked with mouse monoclonal immunoglobulin G (IgG) anti-HA (probe F7; Santa Cruz Biotechnology) diluted 1:200 in 1× PBS-0.1% Tween 20–5% dry milk for 1 h and revealed by a goat anti-mouse IgG conjugated to horseradish peroxidase (Pierce).
Synthetic Peptides
The synthetic peptides, RRIYDLIEL (pEBNA3C aa 258–266), RRRWRRLTV (pLMP2 aa 236–244) (PRIMM GmbH, Dubendorf, Zuerich, Switzerland), CVNGVCWTV (pNS3-1 aa 1073–1081) and KLVALGINAV (pNS3–2 aa 1406–1415) (Epimmune Inc., San Diego, CA), were dissolved in dimethyl sulfoxide (DMSO). Concentrations were assayed by BCA assay according to the manufacturer's protocol (Pierce).
Cell Lines
The following cell lines were used in this study: Epstein-Barr virus-transformed B lymphoblastoid cell lines (B-LCLs) (20), TAP-defective CEM 174.T2 cells (ATCC number: CRL-1992™) and HeLa cells (ATCC number: CCL2™) stably transfected with cDNA encoding B*2705, B*2709 (T2 and HeLa), A*0201 wt, and B*2705Y320F, B*2705C67S, B*2709C67S, and A2V67C mutants (HeLa).
B-LCLs were cultured in heat-inactivated 10% fetal calf serum (FCS)-RPMI 1640 medium (Lonza, Basel Switzerland), supplemented with 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin. PBMCs from buffy-coats of anonymous healthy donors were isolated on a gradient of Lymphoprep (Cedarlane Laboratories limited, Hornby, Ontario, Canada). DCs were differentiated from monocytes isolated from PBMCs using Macs Microbeads CD14+ (Miltenyi Biotec, Bergisch Gladbach, Germany) and maintained in 10% FCS-RPMI 1640 medium supplemented with 20 ng/ml human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; Immuno Tools, Friesoythe, Germany) and 2 ng/ml IL-4 (Eurobio, Les Ulis, France).
T2 B*2705 and B*2709 cells were generated by transfection of the CEM 174.T2 cell line with pcDNA3 vector (Invitrogen) in which cDNA encoding for B*2705 and B*2709 had been cloned. Stable transfectants were cultured in complete medium (10% FCS-RPMI 1640 RPMI with 2 mm l-glutamine, 10 units/ml penicillin, and 100 μg/ml streptomycin) supplemented with G418 (800 μg/ml).
HeLa cells transfection was performed with Lipofectamine 2000 Reagent, according to the protocol provided by Invitrogen. After transfection, cells were cultured in Dulbecco's minimal essential medium (DMEM) supplemented with 10% heat-inactivated FCS, 2 mm l-glutamine, 10 units/ml penicillin, and 100 μg/ml streptomycin and selected with G418 (800 μg/ml). NPFLU (aa 383–391), EBNA3C (aa 258–266), and LMP2 (aa 236–244), specific CTLs and NS3–2 (aa 1406–1415) and NS3–1 (aa 1073–1081) specific CTLs were obtained as previously described (20, 30).
Cytotoxicity Assays
Cytotoxic activity of CD8+ T cell lines was assayed by standard 51Cr release assay. CTL were mixed in 96-well U-bottom plates with 51Cr-labeled target cells for 4 h at 37 °C. The effector/target cell ratio was 20:1. Target cells had been previously incubated overnight with synthetic peptides or recombinant proteins or in medium alone. In the inhibition assay, target cells were preincubated with lactacystin (80 μm, Sigma-Aldrich), 45 min before adding the recombinant protein or the synthetic peptides. The percentage of specific lysis was calculated as ×100 [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)].
IFN-γ Production Assay and FACS Analysis
CD8+ T cell clones specific for NS3-2 (KLVALGINAV aa 1406–1415) or NS3–1 (CVNGVCWTV aa 1073–1081) or for the HLA-B27 binding peptide EBNA3C were stimulated with DCs preincubated with the relevant peptide or the recombinant protein in U-bottom microculture wells at 2 × 104 DC/3 × 104 CD8+ T cell/well in 0.2 ml of RPMI 1640 medium supplemented with 10% fetal calf serum (RPMI 1640–10%). DCs loaded with either the peptide or the recombinant protein for 18 h at 37 °C were then washed with RPMI 1640–10% and added to the culture at the DC/T cell ratio of 1:1.5. After 2 h, the cells were further treated with brefeldin-A (10 mg/ml; Sigma-Aldrich) at 37 °C for 18 h. Cells were washed and stained with PerCP-conjugated anti-CD8 (Becton Dickinson, San Jose, CA) for 20 min at 4 °C, fixed, permeabilized using Cytofix/Cytoperm solution (BD Pharmingen) at 4 °C for 20 min, washed with Perm Wash Buffer (BD Pharmingen), intracellular stained with FITC-conjugated anti-IFN-γ antibody (BD Pharmingen) for 30 min at 4 °C, washed, and finally analyzed by the FACSCalibur flow cytometer (Becton Dickinson), using CellQuest software (Becton Dickinson). An isotype-matched mAb was used as negative control.
For the surface expression of wt and mutated HLA molecules, cells were harvested and washed twice with PBS. Staining was performed at 4 °C for 1 h. Cells were first labeled with the nonconjugated primary mAbs: conformational-dependent ME1 specific for HLA-B27 and BB7.2 for HLA-A2; conformational-independent HC10 for HLA-B and HLA-C molecules and HCA2 for HLA-A2, followed by F(ab′)2 of rabbit anti-mouse IgG-FITC (Jackson ImmunoResearch Europe Ltd., Suffolk, UK). Isotype-matched mouse Igs were used as negative controls. The analysis was performed by FACSCalibur (BD Biosciences). For each sample, 10000 events were acquired using forward/side light scatter characteristics and analyzed using Cell Quest software (Becton Dickinson).
Confocal Microscopy
For Confocal Laser Scanning Microscopy (CLSM) analyses, 1 × 105 HeLa cells were grown on cover glasses (diameter 12 mm) for 24 h and then incubated with 0.4 mm TAT fusion proteins. At established times, cells were washed in 1% PBS, fixed by paraformaldehyde 2% in 1× PBS for 20 min at room temperature, and then permeabilized with 0.1% Triton X-100 in PBS containing 1% BSA. For double-staining experiments the cells were incubated in the primary antibody at room temperature for 1 h with the following antibodies: TAT proteins were stained using anti-HA probe-FITC Ab (F7; Santa Cruz Biotechnology; sc7392 FITC) (1:75 in PBS); Furin was stained using rabbit anti-furin Ab (H-220; Santa Cruz Biotechnology; sc20801) (1:25 in PBS). As the fluorescence-labeled secondary antibody, Texas Red-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology; sc2780) (1:200 in PBS) was used. Confocal observations were performed using a Leica DMIRE apparatus (Leica Microsystem, Heidelberg, Germany) equipped with an argon-krypton laser, double-dichroic splitters (488/568 nm) and using a 63× oil immersion lens. Signal from different fluorescent probes were taken in sequential scan settings to prevent cross-talk between the two signals, and co-localization was detected in yellow (pseudo-color). Image acquisition and processing were achieved using the Leica Confocal Software (LCS) (Leica Lasertechnik, Heidelberg, Germany) and Adobe Photoshop software programs (Adobe System, Mountain View, CA). At least 50 cells for each condition, randomly taken from three independent experiments, were analyzed.
Bioinformatics
HotPOINT bioinformatics tool was used to calculate computational hot spots based on conservation, solvent accessibility, and statistical pairwise residue potentials in protein interfaces. TpairPotential feature calculated by HotPOINT algorithm is a statistically significant parameter useful to discriminate hot spots and non-hot-spots (p value: 5.4 × 10−6) and the threshold value (equal to 18.0) was determined by using residues deposited in the Alanine Scanning Energetics database (ASEdb) (31). The modeled complex was analyzed with the LigPlt tool (32) to identify the peptide-protein interactions.
RESULTS
HLA-B27, but Not HLA-A2 Molecules, Exploit a Non-canonical Pathway of Processing and Presentation for CTL Epitopes
Chimeric proteins driven by TAT transduction domain and the nucleoprotein (Np) of influenza A virus (TAT-NpFlu) were used to deliver CTL epitopes restricted for HLA-B27 (20). Here, these proteins were exploited to introduce either HLA-B27- or HLA-A2-restricted viral epitopes. To this purpose, TAT-NpFlu was modified to produce hybrid proteins, in which the HLA-B*2705 NpFlu (SRYWAIRTR, aa 383–391) was replaced with other HLA-B27 restricted epitopes from EBV such as EBNA3C 258–266 (RRIYDLIEL) (20) or LMP2 236–244 (RRRWRRLTV) (Fig. 1). Likewise, two epitopes restricted for HLA-A2, NS3-2 1406–1415 (KLVALGINAV), and NS3-1 1073–1081 (CVNGVCWTV) from NS3 protein of HCV (Fig. 1), were inserted in place of the NpFlu epitope. Two forms of these chimeric proteins, with or without the NpFlu C-terminal sequence (aa 401–498), (reported as end of Np in Fig. 1) were generated. The short and long variants were transduced into B-LCL expressing both HLA-A2 and B*2705 molecules which were then used as targets in standard 51Cr-release assays. The results showed that the two viral peptides pEBNA3C and pLMP2 were properly processed and presented to the specific CTLs provided that the C-terminal portion of Np (end of Np), targeting the protein to the TGN, was present (Fig. 2A). The process was proteasome-independent as shown by the lack of inhibition by lactacystin. In contrast, no lysis by either HLA-A2-restricted NS3-2 or NS3-1 specific CTLs was detectable when the recombinant proteins were transduced into the same HLA-A2 and B*2705 positive B-LCL (Fig. 2A). To ascertain that this was not due to the cell line used here, B-LCLs from three other HLA-A2 positive donors were transduced with the same chimeric proteins and, once again, no specific lysis was observed (data not shown). TAP-deficient T2 cells naturally expressing the HLA-A2 molecules, were also used (Fig. 2B). As expected, wt T2 cells were able to present the pNS3–2 and pNS3–1 epitopes when they were supplied as synthetic peptides, but not when the same epitopes were inserted into the chimeric proteins. In contrast, T2-B*2705 and T2-B*2709 could present both pEBNA3C and pLMP2 B27-restricted epitopes when delivered by the chimeric proteins. The failure to present the HLA-A2-restricted NS3 epitopes was not due to an impairment to cross the cell membrane since the recombinant protein containing the HLA-A2-restricted NS3–2 epitope, like that containing the EBNA3C epitope, colocalizes with furin in the TGN showing that the two proteins follow the same route (Fig. 3). We therefore asked whether the HLA-A2-restricted presentation could be performed by professional APC. Accordingly, Dendritic Cells (DCs) from three different HLA-A2 positive donors were generated, incubated with the chimeric proteins, and used as APC in cross-presentation experiments. In this cellular context, cross-presentation of the same chimeric proteins to the specific CTLs, was successful (Fig. 4). However, the processing was almost completely inhibited by lactacystin, demonstrating that the HLA-A2-restricted epitopes could be excised and processed by specialized APC in a proteasome-dependent pathway. In some experiments, we also noticed a partial inhibition of the production of IFN-γ by CTLs when the exogenous peptides were presented, probably due to the toxic effect of the drug, however the presentation of both NS3–2 and NS3–1 epitopes was virtually suppressed by lactacystin. On the contrary, B*2705 positive DC, although able to cross-present the B27-restricted epitope carried by the TATNpEBNA3C protein, were only partially inhibited by lactacystin, suggesting that the DC exploit the proteasome-independent additional route described in the case of the EBV-B and HeLa cells (Fig. 4, lower panels).
FIGURE 1.

Schematic representation of TAT-NpFlu recombinant proteins. HLA-B*2705-restricted epitope pNpFlu in the TAT-Npflu constructs has been replaced by: the HLA-B27-restricted epitopes pEBNA3C (258–266) or pLMP2 (236–244) from EBV, or by the HLA-A2-restricted pNS3–2 (1406–1415) or pNS3–1 (1073–1081) from the NS3 protein of HCV. The constructs containing or not the C terminus of the nucleoprotein of influenza virus (end of Np) are indicated. The motif Arg/Ser (RS) is included at both the N or the C termini of the epitopes in the hybrid proteins.
FIGURE 2.
HLA-B27 and HLA-A2 presentation of the chimeric epitopes. A,51Chromium release assay in which HLA-A2/B27 double positive B-LCL are incubated overnight with the indicated recombinant proteins (1.4 μm) or with the synthetic peptides (50 μm) or with medium alone (med) and labeled with sodium 51chromate before being mixed with peptide-specific CTLs for 4 h. When indicated, lactacystin was added 45 min before the recombinant protein or the synthetic peptide. B, T2 wt cells which are naturally positive for the HLA-A2 and T2B*2705 and T2B*2709 transfectants were incubated overnight with the recombinant proteins (1.4 μm) or with synthetic peptides (50 μm) or with medium alone (med), and used as antigen-presenting cells in the cytotoxicity assay. Bars represent the mean percentage of lysis ± S.D. of three independent experiments.
FIGURE 3.
The chimeric proteins co-localize with furin in the TGN. TATNpEBNA3C/RSend (top panel on the left) and TAT-NpNS3–2/RSend (bottom panel on the left) are visualized in green within HeLa cells by staining with the anti-HA mAb followed by FITC-conjugated secondary antibody in a confocal microscopy analysis. Middle panels show the TGN depicted in red by staining with an anti α-Furin mAb. Right panels show the co-localization of both chimeric proteins with the convertase α-Furin resident in the TGN as visualized by the overlay.
FIGURE 4.
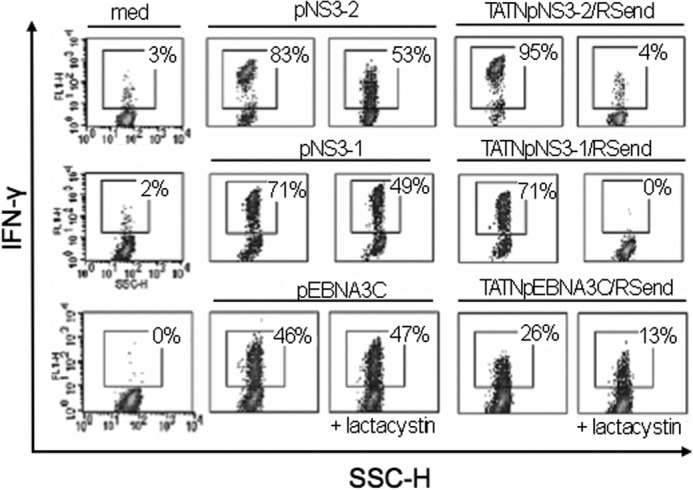
The chimeric proteins are processed and presented by Dendritic Cells. HLA-A2 (upper and middle panel) or B27 (bottom panel) positive DCs were incubated with recombinant proteins (1.4 μm) or synthetic peptides (50 μm) or in medium alone (med). NS3–2 (upper panel), NS3–1 (middle panel) and EBNA3C (bottom panel) specific CTL responses were measured by intracellular staining as % of produced IFN-γ and detected by flow cytometry analysis. % of IFN-γ producing cells are shown in each panel. When indicated, lactacystin has been added 45 min before the synthetic peptides or the recombinant proteins. Data are representative of three independent experiments.
The HLA-B*2705 Y320F Mutant Affecting Recycling Was as Efficient as Wild Type in Presenting the Chimeric Proteins
Once established that the antigen presentation pathway described here was accessible to HLA-B27 but not to HLA-A2 molecules, we asked whether the HLA-B27 molecules that load the excised epitopes could be molecules recycling from the cell membrane. To verify this hypothesis, HLA-B27 cDNA carrying the mutation Y320F which has been shown to impair the endocytosis of HLA-class I molecules (33), was stably transfected into HeLa cells (Fig. 5A) which, like B-LCL, were able to cross-present the epitopes inserted into the chimeric proteins. Cross-presentation experiments were performed as above at different incubation times since the shedding of the molecules from the cell membrane is increased by this mutation. The HLA-B*2705 Y320F mutant was capable to cross-present pLMP2 inserted into the chimeric protein as efficiently as the wt molecules at each time point (4 h or ON) (Fig. 5B).
FIGURE 5.
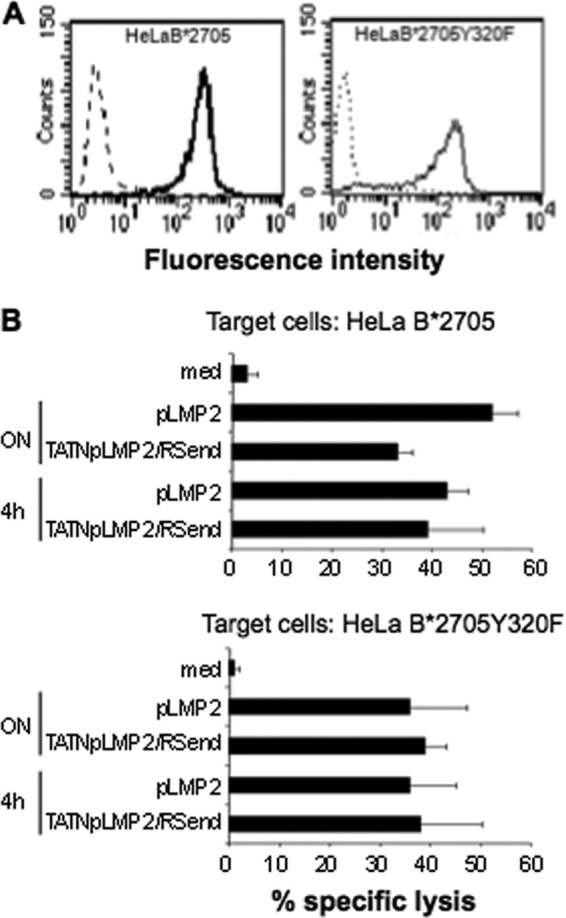
Lack of tyrosine 320 does not impair the presentation of the recombinant proteins by HLA-B27 molecules. A, HeLa B*2705 and B*2705Y320F cells were treated with ME1 (solid line) or with the isotype control IgG1 antibody (dashed line), followed by staining with F(ab')2 of rabbit anti-mouse FITC-conjugated Abs in a flow cytometry analysis. B, cytotoxicity assay in which HeLa expressing B*2705 or B*2705Y320F mutant were incubated overnight or for 4 h with TAT-NpLMP2/RSend recombinant protein (1.4 μm) or pLMP2 synthetic peptide (50 μm) or in medium alone (med) and then mixed with pLMP2-specific CTLs for 4 h. Data are the mean ± S.D. of the percentage of lysis from three separate experiments.
The Effect of Cys-67 on Antigen Presentation
One of the features distinguishing the HLA-B27 from most of the other HLA-class I molecules is the presence of an unpaired and highly reactive cysteine at position 67. The substitution of this residue has been reported to influence, although not dramatically, the peptide repertoire of the HLA-B*2705 molecules (34). We therefore asked whether the presence of Cys-67 was required to cross-present the epitopes embedded into the chimeric proteins. The two subtypes, HLA-B*2705 (Asp-116) and B*2709 (His-116), were mutated at position 67 (C67S) and stably transfected into HeLa cells. The cell surface expression of the C67S mutant was comparable to that of the wt molecules in both B*2705 and B*2709 contexts (Fig. 6, A and B). Interestingly, when the HC10 antibody, which targets a linear epitope of the HLA-B and HLA-C molecules, was used to verify the presence of unfolded B27 heavy chains on the cell surface, the amount of the molecules stained by HC10 was not affected by the C67S mutation in the case of B*2709, while highly increased in the case of B*2705. For comparison, HeLa cells were transfected with either HLA-A2 wt or with a V67C HLA-A2 mutant. The expression of the mutant was similar to that of the wild type as monitored by the specific antibody BB7.2 with a small increase of the HCA2 (linear epitope) reactivity (Fig. 6C). The experiments were repeated with a different set of transfectants and the results were comparable (not shown). The transfectants were then used to present the exogenous peptides or the epitopes embedded into the chimeric proteins to specific CD8+ T cells. The results show that, out of the three HLA-B27-restricted epitopes (pEBNA3C and pLMP2 for both B*2705 and B*2709 and pNpFlu for B*2705, pEBNA3C was the only one to be presented by both mutants (Fig. 7). V67C HLA-A2 mutant was able to present the pNS3–2 peptide as well as wt indicating that this mutation is well tolerated by the HLA-A2 molecules, but it failed, however, to make the HeLa transfectants competent to cross-present the epitope when delivered by the protein (Fig. 7).
FIGURE 6.

Expression of C67S HLA-B27 and V67C HLA-A2 mutants. Expression of wt and C67S B*2705 molecules in A and wt and C67S B*2709 molecules in B) on the cell surface of HeLa cells was evaluated with the mAb ME1 that recognizes conformational molecules (left panels) and mAb HC10 that recognizes unfolded molecules (β2m-free heavy chains) (right panels). C, expression of wt HLA-A2 molecules and V67C mutants was evaluated on HeLa transfectants with BB7.2 mAb that recognizes conformational molecules (left panel), and HCA2 mAb that recognizes unfolded molecules (β2m-free heavy chains) (right panel). One representative staining of three-five independent flow cytometry analysis is shown here.
FIGURE 7.

Effect on antigen presentation of C67S and V67C mutations in respectively HLA-B27 and HLA-A2 molecules. A, antigen-specific CTLs were tested in standard 51Cr-release assays using as target the HeLa cells expressing wt molecules or C67S mutants in the context of B*2705 (upper panels) and B*2709 (bottom panels) and pulsed with the indicated peptides (50 μm) or in medium alone (med). B, HeLa cells stably transfected with HLA-A2 wt or V67C mutant were incubated overnight with TAT-NpNS3–2/RSend recombinant protein (1.4 μm), pNS3–2 synthetic peptide (50 μm), or with medium alone (med) and used as targets for NS3–2 specific CTLs. Data are the mean percentage of lysis ± S.D. of three independent experiments.
To gain insight into the presentation of the EBNA3C epitope by the two HLA-B27 mutants, a dose-response curve was performed using pEBNA3C peptide or the chimeric protein in the two B27 contexts (Fig. 8). The results highlighted an extremely different effect of the mutation C67S in the two B27 contexts: while the C67S B*2705 mutant could present the pEBNA3C at the highest concentration only, the C67S B*2709 mutant was more efficient than wt. Accordingly, the presentation of the chimeric epitopes showed the same trend with a very poor and a very efficient antigen presentation by the C67S B*2705 and the C67S B*2709 mutants respectively thus suggesting that the newly generated epitopes encountering the HLA-B27 molecules in the TGN were likely to be the same as those added as soluble peptides.
FIGURE 8.

Cysteine 67 is crucial for presentation of pEBNA3C by the HLA-B*2705 molecules. Dose-response curves in which HeLa B*2705 and B*2709 transfectants and the corresponding C67S mutants are used as targets in a standard 51Chromium-release assay at the indicated concentration of pEBNA3C or TATNpEBNA3C/RSend recombinant protein. Data show the mean percentage of specific lysis ± S.D. of three independent experiments.
We postulated that such differences in presenting pEBNA3C were due to the only amino acid difference between B*2705 and B*2709 and therefore to the interaction between L9 and Asp-116 or His-116. To better investigate this, the complex between B*2709 protein and the peptide pEBNA3C was built initially using a template-based approach, based on the available structure of the B*2705:pEBNA3C complex (PBD ID: 2BST). The main chain atoms of the B*2709 comparative model and pEBNA3C peptide were optimally superimposed with the main chains of B*2705 and pEBNA3C in the complex. We refined the B*2709:pEBNA3C model using Rosetta FlexPepDock (35), a high resolution of the peptide-protein docking protocol to refine the starting structure of a peptide-protein complex (Fig. 9A). Next, we looked at the interactions and total contact potentials between L9 in the B*2709:pEBNA3C structure by using both LigPlot (32) and HotPOINT algorithm (31). HotPOINT labels His-116 as a hot spot residue at the protein-peptide binding interface, detecting a tpairPotential equal to 26.35. L9 and His-116 are also involved in hydrophobic interactions identified by LigPlot (Fig. 9B); on the opposite HotPOINT predicts Asp-116 in B*2705:pEBNA3C complex as a non-hot spot residue predicting a tpairPotential much below the threshold value and no interactions are detected between L9 and Asp-116 (Fig. 9D). This indicates that the L9 peptide residue is more stably accommodated in the F pocket of the B*2709 than on that of the B*2705 molecules.
FIGURE 9.
Differences in presenting pEBNA3C could be due to the interaction between L9 and Asp-116. A, ribbon representation of the model of B*2709:pEBNA3C complex and C, of solved structure of B*2705:pEBNA3C complex. L9, His-116, and Asp-116 are shown as ball-and-stick, B*2709 and B*2705 peptides are depicted as blue sticks in both complexes. B and D, schemes of the protein-peptide interactions obtained using LigPlot: hydrophobic interactions are represented by arcs with spokes radiating toward the atoms they contact, and the residues involved are shown as ball-and-stick models.
DISCUSSION
The association of Ankylosing Spondylitis with HLA-B27 has been a conundrum for the last 40 years. One of the first hypothesis postulates the existence of HLA-B27-restricted “arthritogenic” peptides as triggers of the disease. This view has now been strengthened by the finding in the HLA-B27-positive but not in the B27 negative patients of a genetic association with ERAP1, an aminopeptidase involved in trimming the HLA-class I epitopes (17). Much is known about the rules governing the HLA-B27 binding properties and it has been recently reported that many peptides derived from cartilage/bone-related proteins are possible ligands for HLA-B27 molecules (18).
We have previously shown that HLA-B27 molecules can exploit a proteasome- and TAP-independent pathway for antigen presentation when presenting the HLA-B27-restricted epitope of the nucleoprotein of Influenza virus driven into the cells by TAT transduction domain of HIV. We asked here whether this pathway was accessible to other HLA molecules. Accordingly, the NpFlu 383–391 epitope (R/SRYWAIRTR/S) was replaced with other HLA-B27 or HLA-A2 restricted viral epitopes within the chimeric proteins used as carriers. The results indicated that this route was accessible to both the HLA-B27 alleles analyzed, B*2705 and B*2709, but not to HLA-A2, which however, likewise HLA-B27, co-localizes with the TAT-driven proteins in the TGN. Noteworthy, the embedded HLA-A2-restricted epitopes are successfully processed and presented by professional DC in a canonical, proteasome-dependent pathway indicating these chimeric proteins as good carriers to induce cross-priming through the classical pathway of antigen presentation exploited by DC. The TAT-driven proteins carrying the HLA-A2 or the HLA-B27-restricted epitopes differ only at the antigenic peptide sequence and one of the two HLA-A2-specific epitopes, NS3–2 (RS/KLVALGINAV/RS), possesses N- and a C terminus sequences (RS/RRRWRRLTV/RS), similar to pLMP2 with a Val at P9 and a conservative Lys instead of an Arg at P1. Although the HLA-B27-restricted epitope NpFlu (R/SRYWAIRTR/S) does not possess a canonical furin recognition sequence (RX(K/R)R) at its N terminus, we attempted to inhibit its presentation by using the inhibitor Dec-RVKR-CMK specific for furin or proprotein convertase 7 (PC7). We also used a siRNA strategy to block the expression of the ubiquitous nardylisin (36) also targeting cationic-rich peptide sequences, but we could not observe any inhibition (data not shown). However, although the HLA-B27 epitopes are enriched of Arg at their N terminus, the cut by furin-like proteases would yield too short peptides deprived of the N-terminal anchor. This is most unlikely since the pattern of recognition of both the soluble and the chimeric epitopes by the CD8 T cells is virtually identical. This suggests that other, unknown proteases are involved in the excision of the embedded epitopes, probably targeting the RS epitope-flanking sequences. Given these considerations, we favor the hypothesis that the HLA-B27 molecules, or at least a fraction of them carrying longer or loosely bound peptides, are particularly prone to exchange them in the TGN. This might be favored by the lower pH (5.9) characterizing the Golgi vesicles compared with the ER (pH 7.1) (37, 38) as described for the HLA-B51 molecules in TAP-defective cells (19). Interestingly, both HLA-B51 and HLA-B27 are associated with autoimmune/autoinflammatory diseases, an association rarely found for other HLA-class I molecules. HLA-B*2705 and HLA-B*2709 molecules differentially associated with AS, were both able to present the chimeric epitopes. A feature of the HLA-B27 molecules is the presence of an unpaired, highly reactive cysteine at position 67. This amino acid has been shown to contribute to the shaping and stability of the B pocket where the primary anchor of the bound peptides, almost invariably an Arg, sits and its substitution promotes faster dissociation and modifies the repertoire of the bound peptides (39, 40). Analyzing the C67S B*2705 and B*2709 mutants, we observed a stronger requirement for C67 to stabilize the complexes in the case of B*2705 molecules which, compared with B*2709, display a significant higher ratio between the HC10-reactive (unfolded) and the ME1-reactive (folded) forms on the cell membrane. Accordingly, when we analyzed the antigen presenting competence of the two mutants, we found that neither of them could present pNP-Flu and pLMP2 as soluble peptides or as chimeric proteins, while the presentation of pEBNA3C was dramatically different: very poor by the B*2705 mutant and highly efficient by the C67S B*2709 mutant. A possible explanation for this observation is that B*2705-pEBNA3C and B*2709-pEBNA3C could display distinct conformations whose stability is influenced in different ways by the B pocket. The structure of the pEBNA3C: B*2705 complexes has been solved (41). Therefore, we built a model for this complex and analyzed the interactions and total contact potentials between L9 of pEBNA3C and His-116 or Asp-116 respectively in the B*2709:pEBNA3C three-dimensional model and B*2705 pEBNA3C complex. His-116 was labeled as hot spot residue at the protein-peptide binding interface and we observed hydrophobic interactions between His-116 and L9. On the opposite Asp-116 was predicted as a non-hot spot residue, suggesting that the strong interaction between L9 and His-116 makes the B pocket dispensable for peptide stabilization by the B*2709 but not by the B*2705 molecules. In any case, these results indicate the existence of a subset of peptides differently dependent on B pocket for their stability when bound by the two B27 subtypes. This is also pointed out by the increase of not correctly folded C67S B*2705 molecules on the cell surface. Whether this can be related to disease susceptibility, it cannot be said by this set of experiments. However, the data, once again, show subtle differences between B27 alleles carrying or not the Asp-116 which might bind the same peptides but display different conformations (2, 42). The structural features of this residue clearly influence the strength of binding of some peptides, made here more evident by the structural subversion of the B pocket. This could make the two molecules differently prone to exchange peptides in the TGN where they can encounter a set of tissue-specific peptides different from those generated by the classical proteasomal machinery. Ultimately, the rate of peptide exchange will be influenced by the repertoire of peptides generated in the ER, which in turn depends on the trimming activity of ERAP1 that changes according to the allelic variants, some of which are strongly associated with AS (17, 43).
Acknowledgment
We thank Federica Lucantoni for excellent technical assistance.
This work was supported by the Italian Ministry of Education, University and Research (MIUR) through PRIN (grant n°2008C33HRL-004), by the Istituto Pasteur-Fondazione Cenci Bolognetti, King Abdullah University of Science and Technology (KAUST) (Award No. KUK-11-012-43) Fondazione Roma and by Sapienza through Progetti di Ateneo (Grant n°C26A10CT9F).
- AS
- Ankylosing Spondylitis
- APC
- antigen-presenting cells
- B-LCLs
- EBV-transformed B cells
- DC
- dendritic cells
- EBNA3C
- Epstein-Barr Nuclear Antigen 3C
- LMP2
- latent membrane protein 2
- TAP
- transporters associated with antigen presentation
- NS3
- non-structural protein 3
- HCV
- hepatitis C virus.
REFERENCES
- 1. Hülsmeyer M., Fiorillo M. T., Bettosini F., Sorrentino R., Saenger W., Ziegler A., Uchanska-Ziegler B. (2004) Dual, HLA-B27 subtype-dependent conformation of a self-peptide. J. Exp. Med. 199, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fiorillo M. T., Maragno M., Butler R., Dupuis M. L., Sorrentino R. (2000) CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J. Clin. Invest. 106, 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nurzia E., Panimolle F., Cauli A., Mathieu A., Magnacca A., Paladini F., Sorrentino R., Fiorillo M. T. (2010) CD8+ T-cell mediated self-reactivity in HLA-B27 context as a consequence of dual peptide conformation. Clin. Immunol. 135, 476–482 [DOI] [PubMed] [Google Scholar]
- 4. Mathieu A., Paladini F., Vacca A., Cauli A., Fiorillo M. T., Sorrentino R. (2009) The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: a unifying hypothesis. Autoimmun. Rev. 8, 420–425 [DOI] [PubMed] [Google Scholar]
- 5. Blanco-Gelaz M. A., López-Vázquez A., García-Fernández S., Martínez-Borra J., González S., López-Larrea C. (2001) Genetic variability, molecular evolution, and geographic diversity of HLA-B27. Hum. Immunol. 62, 1042–1050 [DOI] [PubMed] [Google Scholar]
- 6. McNeil A. J., Yap P. L., Gore S. M., Brettle R. P., McColl M., Wyld R., Davidson S., Weightman R., Richardson A. M., Robertson J. R. (1996) Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. Q. J. M. 89, 177–185 [DOI] [PubMed] [Google Scholar]
- 7. Neumann-Haefelin C., McKiernan S., Ward S., Viazov S., Spangenberg H. C., Killinger T., Baumert T. F., Nazarova N., Sheridan I., Pybus O., von Weizsäcker F., Roggendorf M., Kelleher D., Klenerman P., Blum H. E., Thimme R. (2006) Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology 43, 563–572 [DOI] [PubMed] [Google Scholar]
- 8. Hendel H., Caillat-Zucman S., Lebuanec H., Carrington M., O'Brien S., Andrieu J. M., Schächter F., Zagury D., Rappaport J., Winkler C., Nelson G. W., Zagury J. F. (1999) New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J. Immunol. 162, 6942–6946 [PubMed] [Google Scholar]
- 9. Altfeld M., Kalife E. T., Qi Y., Streeck H., Lichterfeld M., Johnston M. N., Burgett N., Swartz M. E., Yang A., Alter G., Yu X. G., Meier A., Rockstroh J. K., Allen T. M., Jessen H., Rosenberg E. S., Carrington M., Walker B. D. (2006) HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell response against HIV-1. PLoS Med. 3, e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kosmrlj A., Read E. L., Qi Y., Allen T. M., Altfeld M., Deeks S. G., Pereyra F., Carrington M., Walker B. D., Chakraborty A. K. (2010) Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465, 350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elahi S., Dinges W. L., Lejarcegui N., Laing K. J., Collier A. C., Koelle D. M., McElrath M. J., Horton H. (2011) Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 17, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Amato M., Fiorillo M. T., Carcassi C., Mathieu A., Zuccarelli A., Bitti P. P., Tosi R., Sorrentino R. (1995) Relevance of residue 116 of HLA-B27 in determining susceptibility to ankylosing spondylitis. Eur. J. Immunol. 25, 3199–3201 [DOI] [PubMed] [Google Scholar]
- 13. Paladini F., Taccari E., Fiorillo M. T., Cauli A., Passiu G., Mathieu A., Punzi L., Lapadula G., Scarpa R., Sorrentino R. (2005) Distribution of HLA-B27 subtypes in Sardinia and Continental Italy and their association with spondyloarthropathies. Arth. Rheum. 52, 3319–3321 [DOI] [PubMed] [Google Scholar]
- 14. García-Fernández S., Gonzalez S., Miña-Blanco A., Martinez-Borra J., Blanco-Gelaz M., López-Vasquez A., López-Larrea C. (2001) New insights regarding HLA-B27 diversity in the Asian population. Tissue Antigens 58, 259–262 [DOI] [PubMed] [Google Scholar]
- 15. Fiorillo M. T., Rückert C., Hülsmeyer M., Sorrentino R., Saenger W., Ziegler A., Uchanska-Ziegler B. (2005) Allele-dependent similarity between viral and self-peptide presentation by HLA-B27 subtypes. J. Biol. Chem. 280, 2962–2971 [DOI] [PubMed] [Google Scholar]
- 16. Taurog J. D. (2009) Animal models of spondyloarthritis. Adv. Exp. Med. Biol. 649, 245–254 [DOI] [PubMed] [Google Scholar]
- 17. Evans D. M., Spencer C. C., Pointon J. J., Su Z., Harvey D., Kochan G., Oppermann U., Dilthey A., Pirinen M., Stone M. A., Appleton L., Moutsianas L., Leslie S., Wordsworth T., Kenna T. J., Karaderi T., Thomas G. P., Ward M. M., Weisman M. H., Farrar C., Bradbury L. A., Danoy P., Inman R. D., Maksymowych W., Gladman D., Rahman P., Spondyloarthritis Research Consortium of Canada (SPARCC), Morgan A., Marzo-Ortega H., Bowness P., Gaffney K., Gaston J. S., Smith M., Bruges-Armas J., Couto A. R., Sorrentino R., Paladini F., Ferreira M. A., Xu H., Liu Y., Jiang L., Lopez-Larrea C., Díaz-Peña R., López-Vázquez A., Zayats T., Band G., Bellenguez C., Blackburn H., Blackwell J. M., Bramon E., Bumpstead S. J., Casas J. P., Corvin A., Craddock N., Deloukas P., Dronov S., Duncanson A., Edkins S., Freeman C., Gillman M., Gray E., Gwilliam R., Hammond N., Hunt S. E., Jankowski J., Jayakumar A., Langford C., Liddle J., Markus H. S., Mathew C. G., McCann O. T., McCarthy M. I., Palmer C. N., Peltonen L., Plomin R., Potter S. C., Rautanen A., Ravindrarajah R., Ricketts M., Samani N., Sawcer S. J., Strange A., Trembath R. C., Viswanathan A. C., Waller M., Weston P., Whittaker P., Widaa S., Wood N. W., McVean G., Reveille J. D., Wordsworth B. P., Brown M. A., Donnelly P.; Australo-Anglo-American Spondyloarthritis Consortium (TASC); Wellcome Trust Case Control Consortium 2 (WTCCC2) (2011) Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ben Dror L., Barnea E., Beer I., Mann M., Admon A. (2010) The HLA-B*2705 peptidome. Arthritis Rheum. 62, 420–429 [DOI] [PubMed] [Google Scholar]
- 19. Leonhardt R. M., Fiegl D., Rufer E., Karger A., Bettin B., Knittler M. R. (2010) Post-endoplasmic reticulum rescue of unstable MHC class I requires proprotein convertase PC7. J. Immunol. 184, 2985–2998 [DOI] [PubMed] [Google Scholar]
- 20. Bettosini F., Fiorillo M. T., Magnacca A., Leone L., Torrisi M. R., Sorrentino R. (2005) The C terminus of the nucleoprotein of influenza A virus delivers antigens transduced by Tat to the trans-Golgi network and promotes an efficient presentation through HLA class I. J. Virol. 79, 15537–15546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Urban R. G., Chicz R. M., Lane W. S., Strominger J. L., Rehm A., Kenter M. J., UytdeHaag F. G., Ploegh H., Uchanska-Ziegler B., Ziegler A. (1994) A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc. Natl. Acad. Sci. U.S.A. 91, 1534–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell E. C., Antoniou A. N., Powis S. J. (2012) The multifaceted nature of HLA class I dimer molecules. Immunology 136, 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowness P., Ridley A., Shaw J., Chan A. T., Wong-Baeza I., Fleming M., Cummings F., McMichael A., Kollnberger S. (2011) Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 186, 2672–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fiorillo M. T., Meadows L., D'Amato M., Shabanowitz J., Hunt D. F., Appella E., Sorrentino R. (1997) Susceptibility to ankylosing spondylitis correlates with the C-terminal residue of peptides presented by various HLA-B27 subtypes. Eur. J. Immunol. 27, 368–373 [DOI] [PubMed] [Google Scholar]
- 25. Sesma L., Montserrat V., Lamas J. R., Marina L., Lamas J. R., Marina A., Vázquez J., López de Castro J. A. (2002) The peptide repertoires of HLA-B27 subtypes differentially associated to spondyloarthropathy (B*2704 and B*2706) differ by specific changes at three anchor positions. J. Biol. Chem. 277, 16744–16749 [DOI] [PubMed] [Google Scholar]
- 26. Montserrat V., Martí M., López de Castro J. A. (2003) Allospecific T cell epitope sharing reveals extensive conservation of the antigenic features of peptide ligands among HLA-B27 subtypes differentially associated with spondyloarthritis. J. Immunol. 170, 5778–5785 [DOI] [PubMed] [Google Scholar]
- 27. Rückert C., Fiorillo M. T., Loll B., Moretti R., Biesiadka J., Saenger W., Ziegler A., Sorrentino R., Uchanska-Ziegler B. (2006) Conformational dimorphism of self-peptides and molecular mimicry in a disease-associated HLA-B27 subtype. J. Biol. Chem. 281, 2306–2316 [DOI] [PubMed] [Google Scholar]
- 28. Tran T. M., Satumtira N., Dorris M. L., May E., Wang A., Furuta E., Taurog J. D. (2004) HLA-B27 in transgenic rats forms disulfide-linked heavy chain oligomers and multimers that bind to the chaperone BiP. J. Immunol. 172, 5110–5119 [DOI] [PubMed] [Google Scholar]
- 29. Nagahara H., Vocero-Akbani A. M., Snyder E. L., Ho A., Latham D. G., Lissy N. A., Becker-Hapak M., Ezhevsky S. A., Dowdy S. F. (1998) Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4, 1449–1452 [DOI] [PubMed] [Google Scholar]
- 30. Propato A., Cutrona G., Francavilla V., Ulivi M., Schiaffella E., Landt O., Dunbar R., Cerundolo V., Ferrarini M., Barnaba V. (2001) Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T-cell cross-priming. Nat. Med. 7, 807–813 [DOI] [PubMed] [Google Scholar]
- 31. Tuncbag N., Gursoy A., Keskin O. (2009) Identification of computational hot spots in protein interfaces: combining solvent accessibility and inter-residue potentials improves the accuracy. Bioinformatics 25, 1513–1520 [DOI] [PubMed] [Google Scholar]
- 32. Wallace A. C., Laskowski R. A., Thornton J. M. (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 8, 127–134 [DOI] [PubMed] [Google Scholar]
- 33. Santos S. G., Antoniou A. N., Sampaio P., Powis S. J., Arosa F. A. (2006) Lack of tyrosine 320 impairs spontaneous endocytosis and enhances release of HLA-B27 molecules. J. Immunol. 176, 2942–2949 [DOI] [PubMed] [Google Scholar]
- 34. Alvarez I., Martí M., Vázquez J., Camafeita E., Ogueta S., López de Castro J. A. (2001) The Cys-67 residue of HLA-B27 influences cell surface stability, peptide specificity, and T-cell antigen presentation. J. Biol. Chem. 276, 48740–48747 [DOI] [PubMed] [Google Scholar]
- 35. London N., Raveh B., Cohen E., Fathi G., Schueler-Furman O. (2011) Rosetta FlexPepDock web server–high resolution modeling of peptide-protein interactions. Nucleic Acids Res. 39, W249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kessler J. H., Khan S., Seifert U., Le Gall S., Chow K. M., Paschen A., Bres-Vloemans S. A., de Ru A., van Montfoort N., Franken K. L., Benckhuijsen W. E., Brooks J. M., van Hall T., Ray K., Mulder A., Doxiadis I. I., van Swieten P. F., Overkleeft H. S., Prat A., Tomkinson B., Neefjes J., Kloetzel P. M., Rodgers D. W., Hersh L. B., Drijfhout J. W., van Veelen P. A., Ossendorp F., Melief C. J. (2011) Antigen processing by nardilysin and thimet oligopeptidase generates cytotoxic T cell epitopes. Nat. Immunol. 12, 45–53 [DOI] [PubMed] [Google Scholar]
- 37. Demaurex N., Furuya W., D'Souza S., Bonifacino J. S., Grinstein S. (1998) Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273, 2044–2051 [DOI] [PubMed] [Google Scholar]
- 38. Nakamura N., Tanaka S., Teko Y., Mitsui K., Kanazawa H. (2005) Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 280, 1561–1572 [DOI] [PubMed] [Google Scholar]
- 39. Mear J. P., Schreiber K. L., Münz C., Zhu X., Stevanović S., Rammensee H. G., Rowland-Jones S. L., Colbert R. A. (1999) Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 163, 6665–6670 [PubMed] [Google Scholar]
- 40. Appel H., Kuon W., Kuhne M., Hülsmeyer M., Kollnberger S., Kuhlmann S., Weiss E., Zeitz M., Wucherpfennig K., Bowness P., Sieper J. (2004) The solvent-inaccessible Cys-67 residue of HLA-B27 contributes to T cell recognition of HLA-B27/peptide complexes. J. Immunol. 173, 6564–6573 [DOI] [PubMed] [Google Scholar]
- 41. Stewart-Jones G. B., di Gleria K., Kollnberger S., McMichael A. J., Jones E. Y., Bowness P. (2005) Crystal structures and KIR3DL1 recognition of three immunodominant viral peptides complexed to HLA-B*2705. Eur. J. Immunol. 35, 341–351 [DOI] [PubMed] [Google Scholar]
- 42. Fiorillo M. T., Greco G., Maragno M., Potolicchio I., Monizio A., Dupuis M. L., Sorrentino R. (1998) The naturally occurring polymorphism Asp116–His116, differentiating the ankylosing spondylitis-associated HLA-B*2705 from the non-associated HLA-B*2709 subtype, influences peptide-specific CD8 T cell recognition. Eur. J. Immunol. 28, 2508–2516 [DOI] [PubMed] [Google Scholar]
- 43. Evnouchidou I., Kamal R. P., Seregin S. S., Goto Y., Tsujimoto M., Hattori A., Voulgari P. V, Drosos A. A., Amalfitano A., York I. A., Stratikos E. (2011) Cutting Edge: Coding single nucleotide polymorphisms of endoplasmic reticulum aminopeptidase 1 can affect antigenic peptide generation in vitro by influencing basic enzymatic properties of the enzyme. J. Immunol. 186, 1909–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]





