Background: BRSK2 has never reported to be functional in pancreatic islets.
Results: BRSK2 interacts with PCTAIRE1 and phosphorylates it at Ser-12. Knockdown of BRSK2 augmented low glucose-stimulated insulin secretion.
Conclusion: BRSK2 negatively regulates insulin secretion in β-cells via a PCTAIRE1-dependent mechanism.
Significance: This study reveals a novel function of BRSK2 in insulin secretion and uncovers its related regulation mechanism.
Keywords: Beta Cell, Insulin Secretion, Protein Kinases, Protein Phosphorylation, Protein-Protein Interactions
Abstract
Brain-selective kinase 2 (BRSK2) has been shown to play an essential role in neuronal polarization. In the present study, we show that BRSK2 is also abundantly expressed in pancreatic islets and MIN6 β-cell line. Yeast two-hybrid screening, GST fusion protein pull-down, and co-immunoprecipitation assays reveal that BRSK2 interacts with CDK-related protein kinase PCTAIRE1, a kinase involved in neurite outgrowth and neurotransmitter release. In MIN6 cells, BRSK2 co-localizes with PCTAIRE1 in the cytoplasm and phosphorylates one of its serine residues, Ser-12. Phosphorylation of PCTAIRE1 by BRSK2 reduces glucose-stimulated insulin secretion (GSIS) in MIN6 cells. Conversely, knockdown of BRSK2 by siRNA increases serum insulin levels in mice. Our results reveal a novel function of BRSK2 in the regulation of GSIS in β-cells via a PCTAIRE1-dependent mechanism and suggest that BRSK2 is an attractive target for developing novel diabetic drugs.
Introduction
Brain-selective kinase 2 (BRSK2,4 also known as SAD-A) is a member of the AMP-activated protein kinase (AMPK) subfamily of serine/threonine kinases (1). BRSK2 and closely-related BRSK1 (also named SAD-B) are orthologs of SAD-1 in Caenorhabditis elegans (2, 3). They were originally identified as genes specifically expressed in the brain, with an essential function in neuronal polarization (2). Neurons of SAD-AB−/−-null mutant mice have extended axons, and neurons from hippocampus- and cortex-specific mutant mice also failed to form distinct axons and dendrites in culture (2). Subsequently, BRSK1 was identified to be a novel SV (synaptic vesicle), and active zone cytomatrix-associated protein kinase that is involved in the regulation of neurotransmitter release; it most likely functions by phosphorylating the active zone protein and vesicle priming factor RIM1, among other potential targets in SVs and/or active zones (3).
AMPK has been shown to be a potential therapeutic target for type 2 diabetes (4), largely due to its regulatory function in glucose and lipid metabolism. AMPK undergoes activation at low glucose levels in pancreatic β-cells to regulate the dynamics of insulin-containing secretory vesicles and hence, insulin secretion (5, 6). Activation of AMPK has been reported to impair glucose-induced insulin secretion (GSIS) and survival of pancreatic β-cells and islets (7–9). AMPK is activated by high AMP (and low ATP) concentrations through multiple mechanisms, through modulation of both the intrinsic kinase activity and its phosphorylation and activation by an upstream kinase, AMPK kinase (AMPKK) (10–12). One such AMPKK is LKB1, a tumor suppressor kinase implicated in the pathogenesis of Peutz-Jeghers Syndrome (13–15). LKB1 has been reported to phosphorylate and activate 13 AMPK family members, including BRSK2 (1). Mutation of residue Thr-174 within the T-loop of BRSK2 alters its kinase activity (1). Although BRSK2 belongs to the AMPK family, it has not been shown to play a role in regulating insulin secretion and/or energy metabolism.
PCTAIRE1 is a serine/threonine kinase, which was originally identified as a Cdc2-like kinase (16, 17). As an uncharacterized branch of the cyclin-dependent kinase (CDK) family, PCTAIRE1 has two isoforms in higher organisms, PCTAIRE2 and PCTAIRE3 (17), both of which contain a large N-terminal domain. PCTAIRE kinases are ubiquitously expressed, and it has been found to be predominantly expressed in terminally differentiated cells and transformed cell lines (18, 19). They are not activated by any known cyclins (18) as a result of a serine to cysteine mutation in their conserved cyclin-binding consensus motif. Recently, a novel cyclin CYY-1 was identified and shown to be essential for PCTAIRE1 activity targeting presynaptic components to axons (20). PCTAIRE1 modulates secretory cargo transport by interacting with the COPII complex (21), and regulates secretion of growth hormone from PC12 cells through phosphorylation of residue Ser-569 of the N-ethylmaleimide-sensitive fusion protein (NSF) (22).
In this study, we uncovered new functions of BRSK2 and PCTAIRE1 in pancreatic β-cells. We demonstrated that both kinases are highly expressed in human pancreatic islets and MIN6 murine β-cell line. Importantly, we found that PCTAIRE1 is a substrate of BRSK2 and the phosphorylation of PCTAIRE1 by BRSK2 plays a crucial role in regulating insulin secretion in response to glucose.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies
cDNA encoding full-length human BRSK2 or PCTAIRE1 was subcloned into a BD (pGBKT7)/AD (pGADT7) vector (ClonTech) and a PCMV-Myc/HA expression vector. Full-length and partial cDNA fragments of PCTAIRE1 were subcloned into a pGEX-4T-1 expression vector. Full-length BRSK2 cDNA was subcloned into a pET28a expression vector. The cDNA encoding BRSK2 (K48M and T174E) or PCTAIRE1 (S12A, S12E, S153A, and K194M) mutants were conducted using a Quick Change mutation kit (Stratagene). The antibodies used were as follows: Rabbit anti-PCTAIRE1, PCTAIRE2, PCTAIRE3, or mouse anti-CDC25C (Santa Cruz Biotechnology); Rabbit anti-phospho-CDC25C Ser216 (Cell Signaling); mouse anti-HA, Myc, β-actin/tubulin (Sigma-Aldrich); mouse anti-GST (Novagen); mouse anti-insulin, glucagon (Abcam); rabbit anti-E-cadherin (PTG).
Cell Culture and Transfection
Murine MIN6 pancreatic β-cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 25 mm glucose, supplemented with 15% heat-inactivated fetal calf serum (FCS), 4 mm l-glutamine, and 100 μm β-mercaptoethanol, at 37 °C with 5% CO2 unless specified otherwise. Panc-1, HEK 293T, COS-7, and Hela cell lines were cultured in DMEM with 25 mm glucose, 10% FCS. All transfections were using Lipofectamine 2000TM according to the manufacturer's instructions (Invitrogen).
Northern Blot Analysis
Northern blot was performed as previously described (2) using a full-length human BRSK2 cDNA hybridization probe. Human multiple tissue Northern blots, containing 2 μg/lane of poly (A)-purified mRNA normalized for β-actin expression, were purchased from Clontech.
Immunohistochemistry
Human or mice pancreas tissues were fixed with 4% paraformaldehyde and sectioned at 10 μm. Immunostaining was performed with antibodies specific for BRSK2, insulin, glucagon, E-cadherin, or PCTAIRE1, followed by biotin-labeled secondary antibody using 3,3′-diaminobenzidine tetrahydrochloride (DAB/H2O2) or fluorescein isothiocyanate-conjugated goat anti-rabbit/mouse antibody (Alexa Fluor 488 or 555 from Invitrogen). DAPI (Sigma) was also stained for nucleus. Sections were then washed and mounted for confocal microscopy (Leica).
Tissue Source
Human pancreatic tissues were obtained from the pancreatic tumor patients, who had undergone resection at General Surgery Unit of Zhongshan Hospital at Fudan University. We carried out immunostaining in pancreatic tumor tissues that contain a large region of normal pancreatic exocrine and endocrine parts. And these patients were all informed and approved before using their tissues. All tissues were used in accordance with applicable laws and with the Declaration of Helsinki for research involving human tissues.
Mice tissues were isolated from adult BALB/c mice (male, 6-week-old). Mice experiments followed the principles of laboratory animal care and were approved by Fudan University Life Science Ethic Committee.
siRNA Duplexes
Small interfering RNA (siRNA) duplexes were designed and synthesized by GeneChem or GenePharma Biotech. siRNA sequences were as follows: BRSK2: 5′-GCUAGAGCACAUUCAGAAAtt-3′;PCTAIRE1: 5′-GAUCUCCACUGAGGACAUCtt-3′; nonsilence: 5′-UUCUCCGAACGUGUCACGtt-3′ sequence.
Intravenous siRNA Delivery, ELISA Assay, and Glucose Tolerance Tests
6-week-old BALB/c mice (20 g body weight) received tail-vein injections of saline, control siRNA, or siRNA against BRSK2 for one to three consecutive days. Mice were housed with 4–6 animals per cage in a pathogen-free facility on a 12:12 h light/dark cycle. Everyday mice were injected at 4:00 pm and starved at 9:00 pm for 12 hours (water allowed). The siRNAs were administered at a total dose of 1000 μg per mouse. Blood sample collections and islet isolations were performed 18 h after the last injection.
Following an overnight fast, mice were intraperitoneal (IP) injected with 2 mg glucose/g mice weight. Blood glucoses levels were assessed using a freestyle glucometer (Abbott). Serum insulin levels were assayed by a 96-well plate ELISA assay (Linco Research). Serum glucagon levels were assessed by a 96-well plate ELISA assay (ALPCO Diagnostics). Tissues were harvested, snap-frozen, and stored at −80 °C.
Isolated Pancreatic Islet and β-Cell Size Studies
Pancreatic islets were isolated by collagenase perfusion in situ, digested for 28 min, and then purified by single layer Histopaque (Sigma). Isolated islets were cultured in RPMI 1640 medium containing 11 mm glucose, 7.5% FCS, and 10 mm Hepes (Sigma). Islets of BRSK2-RNAi mice and control mice were isolated, spread and photographed using microscopy, followed by analysis using Image J software.
β-Cells were marked by E-cadherin/insulin co-immunostaining. To calculate single β cell size, the area of 50–100 single β cells from five islets of control or BRSK2-RNAi mice were measured using Image J software.
Measurements of Insulin Secretion
MIN6 cells were seeded in 24-well plates and transfected with 200 ng of plasmids encoding Myc-BRSK2, PCTAIRE1 or their mutant forms. 48 h after transfection, cells were washed in PBS and preincubated in glucose-free Krebs-Ringer bicarbonate (KRB) medium (125 mm NaCl, 4.74 mm KCl, 1 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 5 mm NaHCO3, 25 mm HEPES, pH 7.4, with 0.1% BSA) at 37 °C for 30 min. Cells were then incubated in KRB containing 3 mm or 25 mm glucose at 37 °C for 30 min. The amount of insulin released into the incubation medium was assayed using a radioimmunoassay (Linco Research) following the manufacturer's protocol.
Yeast Two-hybrid Assay
A yeast two-hybrid assay was performed following the Matchmaker III system protocol (Clontech). The PCTAIRE1 clone was isolated from the adult human brain two-hybrid cDNA library using human full-length BRSK2 as bait, and identified by DNA sequencing. The interaction of BD (pGBKT7)-BRSK2 and AD (pGADT7)-PCTAIRE1 was verified by re-transformation in yeast.
Western Blot Analysis and Immunoprecipitation
For immunoprecipitation, cells were lysed in chilled lysis buffer (Cell Signaling lysis buffer with complete protease inhibitors). Lysates (200 μg) were collected and incubated with 40 μl protein G-Sepharose beads (Amersham Biosciences) and 1–2 μg corresponding antibody for 4–5 h at 4 °C with gentle rotation. The samples were washed with cold lysis buffer, and subjected to Western analysis or to an in vitro phosphorylation assay (see below).
For Western blot analysis, cell and tissue extracts were prepared and measured using a detergent compatible protein assay kit (Bio-Rad). Samples were equally loaded onto a 4% to 10% or 12% gradient SDS-PAGE gel and transferred onto a nitrocellulose membrane using standard techniques.
In Vitro Phosphorylation Assay
HA-BRSK2 overexpressed in 293T cells and immunoprecipitated with HA antibody were assayed for kinase activity (see immunoprecipitation assay) by incubating with recombinant GST-PCTAIRE1 (full length, deletion mutants and site-directed mutants) as substrates in kinase buffer (20 mm MOPS, PH 7.4, 15 mm MgCl2, 100 μm ATP) containing 1 μCi of [γ-32P]ATP at 30 °C for 30 min. Samples were separated on SDS-PAGE and visualized by autoradiography.
Fusion Protein and Pull-down Assay
GST-PCTAIRE1, its fragments and/or mutant proteins were expressed in the BL21 (DE3) strain and purified using a glutathione-Sepharose 4B column following the manufacturer's instruction (Amersham Biosciences). GST-tagged fusion proteins or GST proteins were incubated with 40 μl beads and 200 μg lysates from 293T cells expressing HA-BRSK2 for 4 h at 4 °C. Proteins were then subjected to SDS-PAGE and immunoblotted using anti-HA antibody. The fusion proteins were also detected by Western blot using an anti-GST antibody.
Immunofluorescence
MIN6 cells were transfected with PCMV-Myc-BRSK2 and/or the EGFPN1-PCTAIRE1 for 48 h, fixed with 4% paraformaldehyde and permeabilized with Triton X-100. After washes with TBS, cells were stained with DAPI (Sigma) and stained with an anti-Myc antibody followed by fluorescein isothiocyanate-conjugated goat anti-mouse antibody. Images were acquired with a Leica confocal microscope.
RESULTS
Expression and Activity of BRSK2 in Pancreatic Islets and MIN6 β-Cells
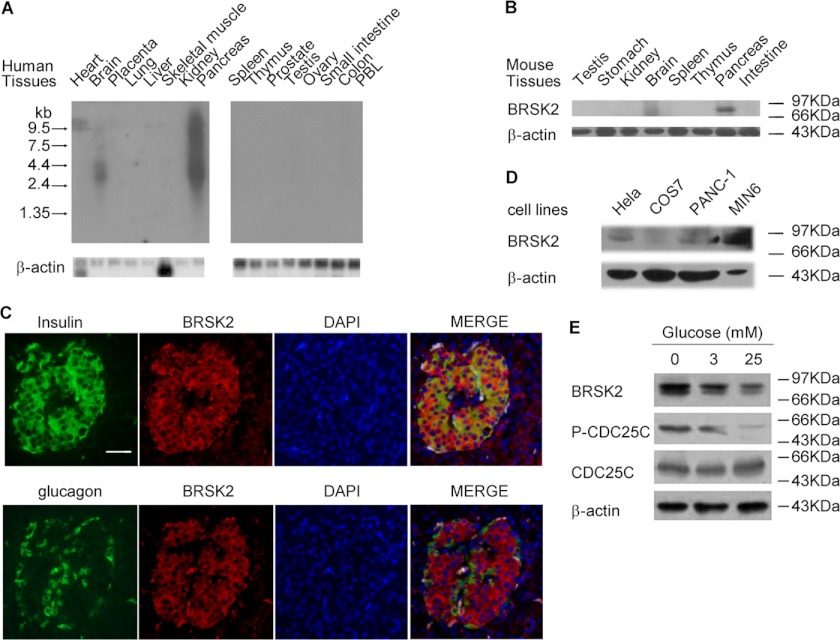
Using Northern blot analysis, we determined the level of mRNA of BRSK2 in a number of human tissues. We were surprised to find that BRSK2 mRNA was expressed at an even higher level in pancreas than in the brain (Fig. 1A) (2, 3). A similar expression pattern was seen at the protein level by Western blot analysis using a newly generated antibody against BRSK2 (Fig. 1B and supplemental Fig. S1, A and B). Immunohistochemical analysis of human pancreatic tissue with BRSK2 antibody also revealed abundant staining in pancreatic islets and ducts (Fig. 2B and supplemental Fig. S1C). Moreover, BRSK2 was found to be specifically co-localized with insulin, but not glucagon (Fig. 1C). In agreement with those observations, we also found that BRSK2 was highly expressed in MIN6, a murine β-cell line (Fig. 1D), and in homogenates of isolated mouse islets (Fig. 4A). Importantly, when MIN6 cells were treated with varying concentrations of glucose for 5 h, the expression of BRSK2 was down-regulated in a dose-dependent manner, which was accompanied by decreases in the phosphorylation of CDC25C (Fig. 1E and supplemental Fig. S1, D and E). These preliminary observations hinted at a potential role of BRSK2 in pancreas, raising the possibility that it might be involved in the regulation of insulin secretion.
FIGURE 1.
Expression and activity of BRSK2 in pancreatic islets and MIN6 β-cells. A, Northern blot analysis using full length BRSK2 probe showed BRSK2 expression in human brain and pancreatic tissues. B, Western blot of BRSK2 with high level in mouse pancreas besides in brain. C, immunostaining of human pancreatic tissues with anti-BRSK2 antibody or/and anti-insulin antibody or anti-glucagon antibody followed by fluorescein isothiocyanate-conjugated goat anti-rabbit/mouse antibody. Scale bar, 25 μm. D, expression of BRSK2 in MIN6 β-cells compared with other cell lines by Western blot with BRSK2 antibody. E, expression and activity of BRSK2 was inhibited by elevated glucose concentrations for 5 h in MIN6 cells as visualized by Western blot using BRSK2, CDC25C, or phosphor-CDC25C Ser-216 antibody. CDC25C F (CDC25C fragment, aa143–253) was phosphorylated on Ser-216 by BRSK2 and used as substrate for BRSK2 activity detection according to our unpublished data (supplemental Fig. S1E). Loading of each lane was controlled by immunolabeling of β-actin.
FIGURE 2.
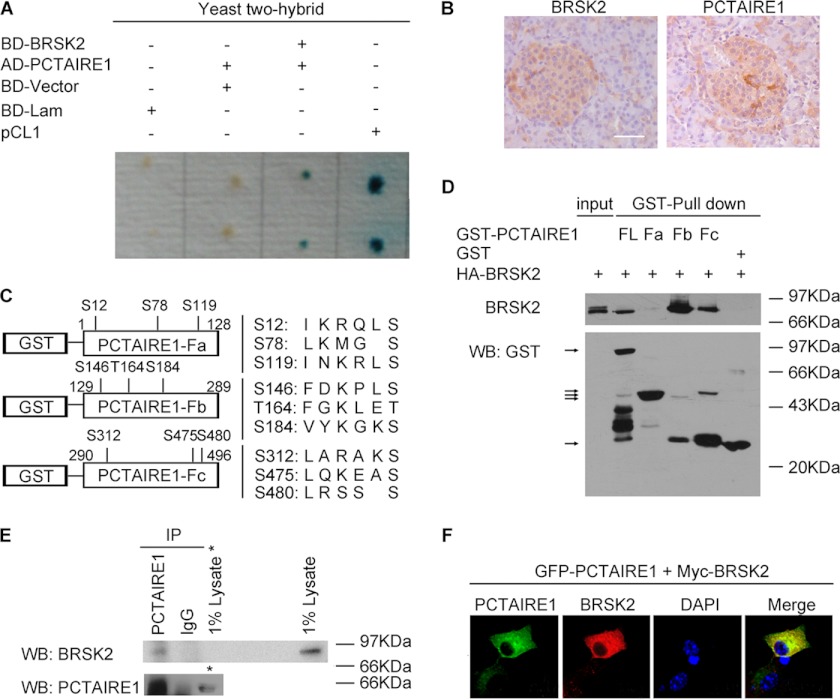
Identification of BRSK2-interacted protein PCTAIRE1. A, interaction of BRSK2 and PCTAIRE1 verified by re-transformation in Yeast. The dots represent yeast colonies. Positive clone showed the blue color. pCL1 was used as positive control, and BD (pGBKT7)-Lam (Lamin) as negative control. B, immunostaining of human pancreatic islets using antibody against BRSK2 or PCTAIRE1, followed by DAB/H2O2 staining. Scale bar, 25 μm. C, three fragments of PCTAIRE1: Fa (aa1–128), Fb (aa129–289), and Fc (aa290–496). All potential phosphorylation sites modified by BRSK2 were pointed out. D, interaction of BRSK2 and PCTAIRE1 full-length (FL) or its three deletion mutants Fa, Fb, and Fc by GST-pull down assay. HA-BRSK2 was expressed in 293T cells. GST-PCTAIRE1 FL, Fa, Fb, Fc, and null GST were expressed in the BL21 (DE3) strain. The Western blot was performed using an antibody against HA (top panel) or GST (bottom panel). The band FL, Fb, and Fc on top panel showed interaction with BRSK2 with input band as control loading 1% HA-BRSK2 cell lysates. The arrows on bottom panel pointed to the bands of GST-PCTAIRE1 full-length (FL) or fragments (Fa, Fb, or Fc) and GST protein. E, association of BRSK2 and PCTAIRE1 in MIN6 cells. Cell lysates were immunoprecipitated (IP) with PCTAIRE1 antibody and immunoblotted with BRSK2 antibody (top panel) or PCTAIRE1 antibody (bottom panel). Rabbit IgG was used as negative control. The right lane on top (1% lysates) or bottom panel (1% lysates *) indicated the BRSK2 or PCTAIRE1 expression in MIN6 cell lysates. F, immunostaining of PCTAIRE1 (green, GFP) and BRSK2 (red, anti-Myc) in MIN6 cells counterstained with DAPI (blue). Magnification, 600×.
FIGURE 4.
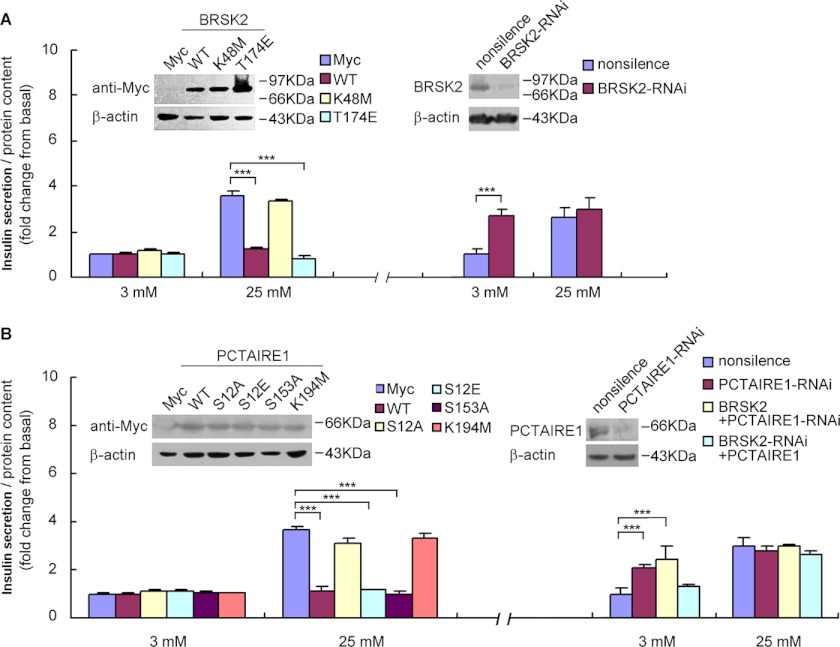
BRSK2 inhibits glucose-stimulated insulin secretion in MIN6 cells. A, insulin secretion in BRSK2 overexpression or BRSK2-RNAi depletion MIN6 cells incubated in 3 mm glucose or 25 mm glucose, respectively. ***, p < 0.001, n = 6/condition. B, insulin secretion in PCTAIRE1 overexpression or PCTAIRE1-RNAi deletion MIN6 cells incubated in 3 mm glucose or 25 mm glucose, respectively. Overexpressing PCTAIRE1 could partly reverse the BRSK2-depletion influence on insulin secretion, while overexpressing BRSK2 had no effect on PCTAIRE1-depletion cells. ***, p < 0.001, n = 6/condition. The transient transfection was performed for 48 h and its expression in MIN6 cells was analyzed by Western blot using Myc, BRSK2, or PCTAIRE1 antibody. PCMV-Myc-null vector and a nonsilence RNAi construct are used as a control for overexpression or RNAi, respectively.
Identification of PCTAIRE1 as a Novel BRSK2-interacting Protein
To gain insights into the function of BRSK2, we searched for its interacting proteins using yeast two-hybrid system and identified PCTAIRE1 as a novel BRSK2-interacting protein. The full-length PCTAIRE1 cDNA was cloned by PCR, and the interaction between PCTAIRE1 and BRSK2 was reconfirmed by the two-hybrid assay (Fig. 2A). Similar to BRSK2 (2, 3), PCTAIRE1 was previously known to regulate neurite outgrowth and secretion of growth hormones (18, 22). Unlike BRSK2, PCTAIRE1 is ubiquitously expressed with the highest abundance in terminally differentiated cells and transformed cell lines (18, 19). Using immunohistochemical staining and Western blot analysis, we found that PCTAIRE1 was highly expressed in both pancreatic islets and MIN6 cells (Fig. 2B and supplemental Fig. S2A). In contrast to PCTAIRE1, the other isoforms, PCTAIRE2 and PCTAIRE3, were not detected in MIN6 cells (supplemental Fig. S2A).
We next determined the specificity of the interaction between BRSK2 and PCTAIRE1 using an in vitro binding assay. Thus, recombinant HA-BRSK2 was purified and incubated with immobilized GST-PCTAIRE1 or GST as a control. HA-BRSK2 was pulled down by GST-PCTAIRE1, but not by GST (Fig. 2D). The BRSK2-PCTAIRE1 interaction was further verified by co-immunoprecipitation using an anti-PCTAIRE1 antibody in MIN6 cells (Fig. 2E). HA-BRSK2 and Myc-PCTAIRE1 were also coimmunoprecipitated (supplemental Fig. S2B). Both proteins were found to be localized in the cytoplasm in MIN6 cells (Fig. 2F). We then employed the GST pull-down assay to determine the domain in PCTAIRE1 that mediates its interaction with BRSK2. We generated three PCTAIRE1 deletion mutants, GST-PCTAIRE1-Fa, Fb, and Fc, corresponding to aa1–128, aa129–289, and aa290–496, respectively (Fig. 2C). As shown in Fig. 2D, BRSK2 was specifically captured by immobilized GST-PCTAIRE1, GST-PCTAIRE1 Fb (aa129–289) and Fc (aa290–496), but not by Fa (aa1–128), indicating that BRSK2 interacts with PCTAIRE1 mainly through its kinase and C-terminal domains, located in the aa129–496 region.
Phosphorylation of PCTAIRE1 by BRSK2 at Ser-12
Analysis by a Scansite Motif Scanner predicted that PCTAIRE1 may be a substrate for the serine/threonine kinase CAMK2 (Ca2+/calmodulin-dependent kinase 2). Because BRSK2 is a serine/threonine kinase belonging to the AMPK subfamily of the CAMK family (23), we investigated whether BRSK2 is capable of phosphorylating PCTAIRE1.
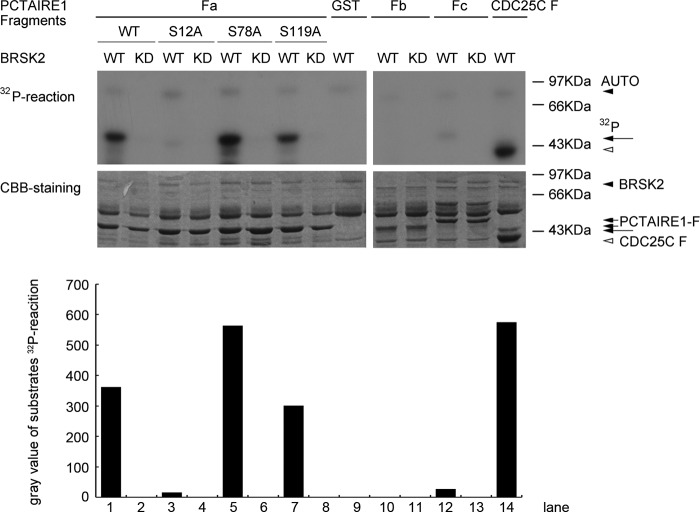
BRSK2 has been reported to preferentially phosphorylate serine/threonine residues within the (L/M/I/F/V)X(R/K/H)XX(S/T) consensus sequence (1). Interestingly, PCTAIRE1 contains 9 BRSK2 phosphorylation consensus sequences, further suggesting that PCTAIRE1 may be a substrate of BRSK2 (Fig. 2C). Using an in vitro kinase assay, we found that BRSK2 phosphorylated PCTAIRE1 while the catalytically inactive mutant did not (Fig. 3). We took advantage of the three deletion mutants of PCTAIRE1, each of which contained three consensus BRSK2 phosphorylation sequences (Fig. 2C). Of the three deletion mutant proteins, GST-PCTAIRE1 Fa (N-terminal domain, aa1–128), but not GST-PCTAIRE1 Fb or Fc (kinase domain and C-terminal domain, aa129–496) was phosphorylated by wild type, but not catalytically inactive BRSK2 (Fig. 3). To further narrow down the site(s) of BRSK2 phosphorylation in PCTAIRE1 Fa domain, we mutated the putative serine residues into alanines and subjected each mutant to the in vitro kinase assay. While BRSK2 phosphorylated S78A and S119A mutants, it failed to phosphorylate the S12A mutant (Fig. 3), suggesting that serine 12 constitutes the main phosphorylation site by BRSK2.
FIGURE 3.
In vitro phosphorylation performance of BRSK2 (wide-type WT or kinase-dead mutant KD) phosphorylation on PCTAIRE1. HA-BRSK2 was expressed in 293T for kinase activity assay. PCTAIRE1 deletion mutants (Fa, Fb, and Fc fragments described in Fig. 2C) and site-directed mutants of Fa (S12A, S78A, and S119A) were used as BRSK2 substrates. The arrowheads in 32P-reaction visualized by autoradiography (top panel) indicated the bands of BRSK2 auto-phosphorylation, PCTAIRE1 fragments phosphorylation and positive control CDC25C F phosphorylation by BRSK2. Corresponding bands using CBB (Coomassie Brilliant Blue) staining (bottom panel) were also arrow-pointed. GST protein showing no BRSK2 phosphorylation signal was used as negative control.
BRSK2 Negatively Regulates Insulin Secretion in MIN6 Cells
The selective expression of BRSK2 in pancreatic tissue and MIN6 cell line prompted us to investigate its potential role in regulating insulin secretion. We used glucose-induced insulin secretion in MIN6 cells as the model system (24). MIN6 cells showed an ∼3-fold increase in insulin secretion when the glucose concentration in the medium was increased from 3–25 mm (Fig. 4A).
We transiently expressed Myc-tagged wild-type BRSK2, a constitutively active mutant T174E or a kinase-dead mutant K48M of BRSK2 in MIN6 cells and measured insulin secretion upon incubation with or without glucose for 36 h. Each of the mutant proteins was found to be expressed at a comparable level by Western blot analysis (Fig. 4A). Overexpression of BRSK2 and the constitutively active T174E mutant had no significant effect on insulin secretion when MIN6 cells were treated with 3 mm glucose, but caused a marked reduction of insulin secretion when glucose concentration was increased to 25 mm glucose (Fig. 4A). In contrast, the inactive K48M form had no effect on insulin secretion in response to 3–25 mm glucose (Fig. 4A).
Reciprocally, down-regulation of BRSK2 by siRNA, as confirmed by Western blot analysis (Fig. 4A), caused a marked increase in insulin secretion from MIN6 cells at 3 mm glucose, but not 25 mm glucose (Fig. 4A). Together, these results strongly suggested that BRSK2 played a negative regulatory role in glucose-stimulated insulin secretion (GSIS) in MIN6 cells.
BRSK2 Regulates Insulin Secretion in MIN6 Cells via PCTAIRE1 Depending on Its Kinase Activity
Given that BRSK2 interacted with and phosphorylated PCTAIRE1, we asked whether PCTAIRE1 and its kinase activity are critical in mediating the effect of BRSK2 on insulin secretion in MIN6 cells. Myc-tagged wild-type PCTAIRE1, and the phosphorylation-defective PCTAIRE1-S12A and phosphorylation-mimetic S12E mutants were transiently transfected into MIN6 cells. The constitutively active (S153A) and the kinase-dead (K194M) PCTAIRE1 mutants were also employed (18). Similar to BRSK2 and its active T174E form, overexpressing wild type PCTAIRE1 and its active forms S153A mutants or BRSK2 phosphorylation-mimetic S12E mutants markedly inhibited insulin secretion at 25 mm, but not at 3 mm glucose in MIN6 cells (Fig. 4B). In contrast, overexpression of the phosphorylation-defective PCTAIRE1 S12A or the kinase-dead (K194M) mutant did not affect insulin secretion (Fig. 4B). Similar to BRSK2, siRNA down-regulation of PCTAIRE1 (Fig. 4B) significantly stimulated insulin secretion from MIN6 cells at 3 mm glucose, but not at 25 mm glucose (Fig. 4B). These results suggested that BRSK2 phosphorylation of PCTAIRE1 at Ser-12 is required for its regulation of insulin secretion from MIN6 cells.
To verify that BRSK2 lies upstream of PCTAIRE1 in the regulation of glucose-stimulated insulin secretion, PCTAIRE1 was expressed in MIN6 cells in which BRSK2 had been knocked down by RNAi and PCTAIRE1 expression was found to partially reverse the effect of BRSK2 knockdown on insulin secretion (Fig. 4B). In contrast, down-regulation of PCTAIRE1 in MIN6 cells overexpressing BRSK2 failed to inhibit insulin secretion at 25 mm glucose (Fig. 4B). These results suggested that PCTAIRE1 mediated the regulatory effect of BRSK2 on insulin secretion in MIN6 cells. Together, these observations indicated that BRSK2 regulated insulin secretion in MIN6 cells through phosphorylating PCTAIRE1 kinase.
Attenuation of BRSK2 in Mice Islets Increases Serum Insulin Levels
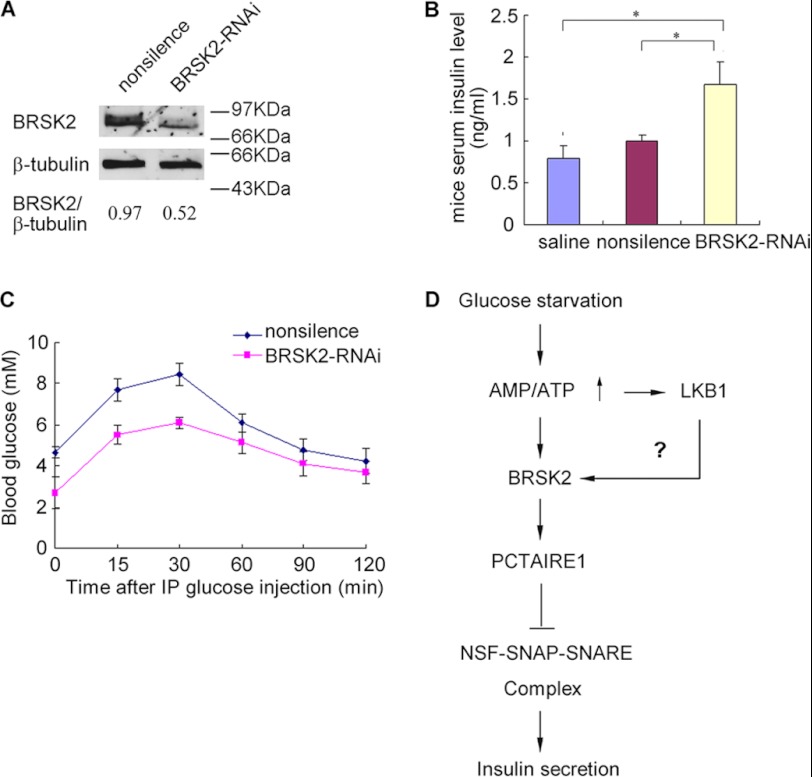
Finally, we attempted to verify the role of BRSK2 in regulating insulin secretion in vivo. It has been shown that intravascular delivery of fluorophoro-labeled siRNA could silence Ins2 expression in the endocrine pancreas (25). We used a similar approach (tail vein injection) to delivering BRSK2 siRNA into mice (BRSK2-RNAi mice), which led to ∼1.8-fold reduction in BRSK2 protein levels in mouse pancreatic islets (Fig. 5A). The down-regulation of BRSK2 resulted in a significant increase (1.5–1.8-fold) in serum insulin levels relative to the control mice (Fig. 5B), but no significant changes in mouse serum glucagon levels (supplemental Fig. S3A). Importantly, BRSK2-RNAi mice displayed improved glucose tolerance (Fig. 5C) and possibly showed a tendency toward enlarged islets and increased β-cell size (supplemental Fig. S3, B and C). These findings suggest a physiological function of BRSK2 in the control of insulin secretion and possibly pancreatic islet homeostasis.
FIGURE 5.
BRSK2-RNAi induces serum insulin level increase and islet area in mice. A, Western blot analysis of BRSK2 levels in mice islet treated with the indicated siRNA duplexes by tail vein injections. B, serum insulin levels in BRSK2-RNAi mice and control mice were measured. *, p < 0.05, n = 3/condition. C, BRSK2-RNAi mice demonstrate enhanced glucose tolerance (GTT) compared with the control mice treated with nonsilence after 2 mg glucose/g mice weight intraperitoneal (IP) injection. n = 6 mice/condition. D, proposed signaling pathway for insulin secretion regulation in pancreatic β-cells.
DISCUSSION
In this study, we provide the first line of evidence that BRSK2 and PCTAIRE1 are selectively expressed in high abundance in pancreatic tissues and MIN6 β-cells. At high, but not low concentrations, glucose reduced BRSK2 activity in MIN6 β-cells. Upon activation, BRSK2 interacted with and phosphorylated PCTAIRE1 on its Ser-12 residue, leading to inhibition of insulin secretion in MIN6 cells. Conversely, knockdown of BRSK2 resulted in a significant increase in serum insulin levels and a tendency toward enlarged islets and increased β-cell size in mice. These findings identify a novel signaling pathway involved in the regulation of GSIS in pancreatic β-cells.
It has been previously shown that BRSK2 is activated by the kinase LKB1 (1). LKB1 is a tumor suppressor implicated in Peutz-Jeghers Syndrome (15) and has been implicated in a number of distinct biological processes. Loss of LKB1 in adult β-cells has been recently shown to increase β-cell mass and insulin secretion, and enhance glucose tolerance in mice mainly through the AMPK-mTOR pathway (26, 27). The existence of other pathways by which LKB1 restricts acute insulin secretion in vivo was also suggested. Given that BRSK2 is a downstream phosphorylation target of LKB1 (1), LKB1 may also activate BRSK2 to down-regulate insulin secretion in β-cells.
BRSK2 knockdown by siRNA in mice was found to increase serum insulin levels, improve GTT and show a tendency of increased islet/β-cell size, further supporting a physiological role of BRSK2 in the regulation of insulin secretion and pancreatic homeostasis. This is consistent with the findings by Hezel et al. that Lkb1 deficiency in pancreas reduced expression of BRSK1 and BRSK2 in pancreatic tissues (28), though another group failed to detect BRSK1/2 expression in islets isolated from 12-week-old male C57B1/6 mice (29). While both adult p-Lkb1 mice (28) and transgenic mice lacking LKB1 (29) exhibited improved GTT and p-Lkb1 mice at PD1 (postnatal day 1) have smaller islets (28), the latter showed increased plasma insulin levels and increased β-cell mass with impaired GSIS (29). Whether the increased insulin secretion is due to the augmented islet/β-cell size remains to be verified.
The expression pattern of BRSK2 is also unique and interesting. We noticed that BRSK2 in pancreas tissue was highly abundant. The positive band of BRSK2 mRNA showed distribution from 1.5–10 kB, suggesting that it might have several multi-splice variants in pancreas. According to the Ensembl database, BRSK2 contains about 11 variants which are likely to code proteins. This explains the observations that while we detected BRSK2 as a 75-kDa protein, others have found it to be a 50-kDa protein in islets (26). It also raised the interesting question of whether different BRSK2 variants have distinct functions.
PCTAIRE kinases are structurally related to CDKs, but have not been shown to interact with any known cyclin or cyclin-like proteins (16–18). Hence, their role in cell cycle regulation has remained unclear. More recent studies, however, have demonstrated that PCTAIRE1 modulates the secretory cargo transport (21), phosphorylates NSF (N-ethylmaleimide-sensitive factor) and inhibits growth hormone release from PC12 cells (22). It has been suggested that PCTAIRE1 is a component of the NSF-SNAP-SNARE complex and plays a regulatory role in Ca2+-dependent exocytosis through NSF phosphorylation (22). NSF is an essential factor for vesicular transport and fusion of presynaptic membranes (30, 31), which acts as a molecular chaperone to hydrolyze ATP and alters the conformation of the SNARE complex (32). Complex formation between NSF, SNAP, and SNARE membrane receptors plays an important role in membrane fusion and constitutes an essential mediator of many transport reactions (33). Similar to neurotransmitter release (34), the mechanism underlying insulin secretion in β-cells is also regulated by the SNARE complex (35, 36). The current study demonstrates that PCTAIRE1 functions downstream of BRSK2 to inhibit insulin secretion at low glucose conditions. Importantly, this function of PCTAIRE1 depends on its kinase activity. We speculate that the ability of PCTAIRE1 to phosphorylate and inhibit NSF activity is also a major mechanism by which it regulates insulin secretion.
In summary, we propose a novel signaling pathway regulating insulin secretion in pancreatic β-cells (Fig. 5D). In our model, low levels of glucose activates the kinase activity of BRSK2 possibly by its upstream kinase LKB1. BRSK2, in turn, interacts with and phosphorylates PCTAIRE1 at Ser-12, leading to the inhibition of insulin secretion through phosphorylation of NSF and inactivation of the NSF-SNARE complex, which is required for insulin secretion from pancreatic β-cells. That knockdown of BRSK2 alone caused a significant increase in insulin secretion at low glucose concentration implied that BRSK2 dysfunction might be related to pancreatic β-cell dysfunction. Together with the highly specific expression of BRSK2 in pancreas and the brain, our study also suggests that BRSK2 may serve as a novel target for developing drugs for the treatment of diabetes.
Supplementary Material
Acknowledgments
We thank Dr. Dang Yong-Jun for critically reading the manuscript. We thank Dr. Li Xiaoying at the Rui Jin Hospital for kindly providing the MIN6 cell line.
This work was supported in whole or in part by grants from the National Institutes of Health (R01DK054254 and R01DK083850 (to S. M. N.), and CA124982 (to H. F. D.), by the National 973 Program of China, the 863 Projects of China, and the National Natural Science Foundation of China (30024001).

This article contains supplemental Figs. S1–S3.
- BRSK2
- brain selective kinase 2
- AMPK
- AMP-activated protein kinase
- CDC25C
- cell division cycle 25 homolog c
- CDK
- cyclin-dependent kinase
- GTT
- glucose tolerance test
- LKB1
- liver kinase B1
- mTOR
- mammalian target of rapamycin
- NSF
- N-ethylmaleimide-sensitive fusion protein
- PCTAIRE1
- CDK-related protein kinase
- SV
- synaptic vesicle
- KD
- kinase dead.
REFERENCES
- 1. Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kishi M., Pan Y. A., Crump J. G., Sanes J. R. (2005) Mammalian SAD kinase are required for neuronal polarization. Science 307, 929–932 [DOI] [PubMed] [Google Scholar]
- 3. Inoue E., Mochida S., Takagi H., Higa S., Deguchi-Tawarada M., Takao-Rikitsu E., Inoue M., Yao I., Takeuchi K., Kitajima I., Setou M., Ohtsuka T., Takai Y. (2006) SAD: a presynaptic kinase associated with synaptic vesicles and the active zone cytomatrix that regulates neurotransmitter release. Neuron 50, 261–275 [DOI] [PubMed] [Google Scholar]
- 4. Hardie D. G. (2007) AMP-activated protein kinase as a drug target. Annu. Rev. Pharmaco. Toxicol. 47, 185–210 [DOI] [PubMed] [Google Scholar]
- 5. Rajan A. S., Aguilar-Bryan L., Nelson D. A., Yaney G. C., Hsu W. H., Kunze D. L., Boyd A. E., 3rd. (1990) Ion channels and insulin secretion. Diabetes Care 13, 340–363 [DOI] [PubMed] [Google Scholar]
- 6. Tsuboi T., da Silva Xavier G., Leclerc I., Rutter G. A. (2003) 5′-AMP-activated protein kinase controls insulin-containing secretory vesicle dynamics. J. Biol. Chem. 278, 52042–52051 [DOI] [PubMed] [Google Scholar]
- 7. da Silva Xavier G., Leclerc I., Varadi A., Tsuboi T., Moule S. K., Rutter G. A. (2003) Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 371, 761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leclerc I., Woltersdorf W. W., da Silva Xavier G. (2004) Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 286, E1023-E1031 [DOI] [PubMed] [Google Scholar]
- 9. Richards S. K., Parton L. E., Leclerc I., Rutter G. A., Smith R. M. (2005) Overexpression of AMP-activated protein kinase impairs pancreatic β-cell function in vivo. J. Endocrinol. 187, 225–235 [DOI] [PubMed] [Google Scholar]
- 10. Carling D., Clarke P. R., Zammit V. A., Hardie D. G. (1989) Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur. J. Biochem. 186, 129–136 [DOI] [PubMed] [Google Scholar]
- 11. Hawley S. A., Selbert M. A., Goldstein E. G., Edelman A. M., Carling D., Hardie D. G. (1995) 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 270, 27186–27191 [DOI] [PubMed] [Google Scholar]
- 12. Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 377, 421–425 [DOI] [PubMed] [Google Scholar]
- 13. Shaw R. J., Kosmatka M., Bardeesy N. (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenne D. E., Reimann H., Nezu J. (1998) Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18, 38–43 [DOI] [PubMed] [Google Scholar]
- 15. Hemminki A., Markie D., Tomlinson I. (1998) A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391, 184–187 [DOI] [PubMed] [Google Scholar]
- 16. Meyerson M., Enders G. H., Wu C. L. (1992) A family of human cdc2-related protein kinases. EMBO J. 11, 2909–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okuda T., Cleveland J. L., Downing J. R. (1992) PCTAIRE-1 and PCTAIRE-3, two members of a novel cdc2/CDC28-related protein kinase gene family. Oncogene 7, 2249–2258 [PubMed] [Google Scholar]
- 18. Graeser R., Gannon J., Poon R. Y., Dubois T., Aitken A., Hunt T. (2002) Regulation of the CDK-related protein kinase PCTAIRE-1 and its possible role in neurite outgrowth in Neuro-2A cells. J. Cell Sci. 115, 3479–3490 [DOI] [PubMed] [Google Scholar]
- 19. Besset V., Rhee K., Wolgemuth D. J. (1999) The cellular distribution and kinase activity of the Cdk family member Pctaire1 in the adult mouse brain and testis suggest functions in differentiation. Cell Growth Differ. 10, 173–181 [PubMed] [Google Scholar]
- 20. Ou C. Y., Poon V. Y., Maeder C. I. (2010) Two cyclin-dependent kinase pathways are essential for polarized trafficking of presynaptic components. Cell 141, 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmer K. J., Konkel J. E., Stephens D. J. (2005) PCTAIRE protein kinases interact directly with the COPII complex and modulate secretory cargo transport. J. Cell Sci. 118, 3839–3847 [DOI] [PubMed] [Google Scholar]
- 22. Liu Y., Cheng K., Gong K., Fu A. K., Ip N. Y. (2006) Pctaire1 Phosphorylates N-ethylmaleimide-sensitive fusion protein: implications in the regulation of its hexamerization and exocytosis. J. Biol. Chem. 281, 9852–9858 [DOI] [PubMed] [Google Scholar]
- 23. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 24. Calabrese A., Zhang M., Serre-Beinier V., Caton D., Mas C., Satin L. S., Meda P. (2003) Connexin 36 controls synchronization of Ca2+ oscillations and insulin secrection in MIN6 cells. Diabetes 52, 417–424 [DOI] [PubMed] [Google Scholar]
- 25. Bradley S. P., Rastellini C., da Costa M. A. (2005) Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas 31, 373–379 [DOI] [PubMed] [Google Scholar]
- 26. Granot Z., Swisa A., Magenheim J. (2009) LKB1 regulates pancreatic β cell size, polarity, and function. Cell Metabolism 10, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu A., Ng A. C., Depatie C. (2009) Loss of Lkb1 in Adult β Cells Increases β Cell Mass and Enhances Glucose Tolerance in Mice. Cell Metabolism 10, 285–295 [DOI] [PubMed] [Google Scholar]
- 28. Hezel A. F., Gurumurthy S., Granot Z. (2008) Pancreatic Lkb1 deletion leads to acinar polarity defects and cystic neoplasms. Mol. Cell. Biol. 28, 2414–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun G., Tarasov A. I., McGinty J. A. (2010) LKB1 deletion with the RIP2. Cre transgene modifies pancreatic β cell morphology and enhances insulin secretion in vivo. Am. J. Physiol. Endocrinol. Metab. 298, E1261–E1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. (1988) Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl. Acad. Sci. U.S.A. 85, 7852–7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beckers C. J., Block M. R., Glick B. S., Rothman J. E., Balch W. E. (1989) Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature 339, 397–398 [DOI] [PubMed] [Google Scholar]
- 32. Morgan A., Burgoyne R. D. (2004) Membrane traffic: controlling membrane fusion by modifying NSF. Curr. Biol. 14, 968–970 [DOI] [PubMed] [Google Scholar]
- 33. Woodman P. G. (1997) The roles of NSF, SNAPs and SNAREs during membrane fusion. Biochim. Biophys. Acta 1357, 155–172 [DOI] [PubMed] [Google Scholar]
- 34. Wheeler M. B., Sheu L., Ghai M. (1996) Characterization of SNARE protein expression in β cell lines and pancreatic islets. Endocrinology 137, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 35. Barg S., Ma X., Eliasson L. (2001) Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic B cells. Biophys. J. 81, 3308–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barg S., Eliasson L., Renstrom E., Rorsman P. A. (2002) A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse β-cells. Diabetes 51, S74–S82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.