Background: Hyaluronan fragments promote innate defense responses in a variety of cell types.
Results: 35-kDa HA fragments induce expression of β-defensin in intestinal epithelium through a TLR4-dependent mechanism.
Conclusion: Specific-sized HA fragments induce β-defensin expression in intestinal epithelium in vitro and in vivo.
Significance: HA fragments may contribute to defense of the intestinal epithelium.
Keywords: Defensins, Epithelium, Hyaluronate, Intestinal Epithelium, Intestine, Innate Barrier Defense, Hyaluronan, beta-Defensin
Abstract
Hyaluronan (HA) is a glycosaminoglycan polymer found in the extracellular matrix of virtually all mammalian tissues. Recent work has suggested a role for small, fragmented HA polymers in initiating innate defense responses in immune cells, endothelium, and epidermis through interaction with innate molecular pattern recognition receptors, such as TLR4. Despite these advances, little is known regarding the effect of fragmented HA at the intestinal epithelium, where numerous pattern recognition receptors act as sentinels of an innate defense response that maintains epithelial barrier integrity in the presence of abundant and diverse microbial challenges. Here we report that HA fragments promote expression of the innate antimicrobial peptide human β-defensin 2 (HβD2) in intestinal epithelial cells. Treatment of HT-29 colonic epithelial cells with HA fragment preparations resulted in time- and dose-dependent up-regulated expression of HβD2 protein in a fragment size-specific manner, with 35-kDa HA fragment preparations emerging as the most potent inducers of intracellular HβD2. Furthermore, oral administration of specific-sized HA fragments promotes the expression of an HβD2 ortholog in the colonic epithelium of both wild-type and CD44-deficient mice but not in TLR4-deficient mice. Together, our observations suggest that a highly size-specific, TLR4-dependent, innate defense response to fragmented HA contributes to intestinal epithelium barrier defense through the induction of intracellular HβD2 protein.
Introduction
The mammalian gastrointestinal tract processes and absorbs the nutrients, water, and electrolytes required to sustain every cell of the body. In addition, the intestine has the added challenge of 1) supporting a large and diverse beneficial bacterial population, the intestinal “microbiome,” while 2) excluding potentially harmful microbes and 3) allowing education of the systemic immune system so that appropriate responses to pathogenic organisms, commensal bacteria, and dietary antigens are developed. A continuous single-cell layer of epithelial cells serves as the interface between host and an intestinal environment containing high concentrations of microbes and ingested foreign substances. Therefore, the integrity of this barrier is of paramount importance to the continued function of the digestive and immune systems and thus to the organism as a whole. Numerous processes, both innate and adaptive, contribute to the continuous maintenance and renewal of the intestinal epithelial barrier (1). Among these, the production of small cationic antimicrobial proteins, the defensins, has an essential role in the preservation of epithelial integrity against persistent microbial challenges.
Hundreds of unique defensins are found throughout the animal kingdom in species as evolutionarily distinct from humans as the horseshoe crab (2), and at least 12 human defensins have been characterized in detail (3). A common characteristic of human defensins is a structure that contains six cysteine residues that form three disulfide bonds, which facilitate folding of the peptide chains into the α-helix or β-sheets that define the protein as an α- or β-defensin (4). Defensins have direct antimicrobial activity against a broad range of human pathogens, including Gram-negative and Gram-positive bacteria, fungi, virus, and protozoa (5), and are expressed by gastrointestinal, urogenital, and pulmonary mucus membranes as well as skin (6) and ocular surfaces (7). Thus, β-defensins contribute to a potent innate defense arsenal against potentially invasive commensal bacteria in organs where the epithelium directly encounters the environment, such as in the mammalian gastrointestinal tract (8).
Whereas β-defensin 1 is constitutively expressed in intestinal epithelial cells (9, 10), β-defensins 2, 3, and 4 are inducible by bacterial stimuli (9, 11–13), cytokine signals (9, 14, 15), and dietary components (16–18). HβD22 has strong antimicrobial effects against several common opportunistic pathogens, including Escherichia coli (19, 20), Pseudomonas aeruginosa (19, 21), and Candida albicans (21, 22). In the intestine, pathogen-associated molecular patterns (PAMPs) act as critical local activators of HβD2 expression through specific interactions with Toll-like receptors (TLRs) and other recognition molecules expressed by epithelial cells (15). The consequence of the interactions between an array of unique PAMPs expressed by a given microbe and specific cell surface receptors on epithelium is the expression of an innate response finely tuned to a distinct microbial challenge. Increased transcription of the gene encoding the HβD2 peptide, DEFB4, has been reported in some intestinal epithelial cell lines following treatment with lipopolysaccharide (LPS) derived from cell membrane of Gram-negative bacteria and by peptidoglycan, a component of the cell wall of Gram-positive bacteria, through TLR4- and TLR2-dependent mechanisms, respectively (13). Salmonella enteritidis flagellin also promotes HβD2 expression (12) via TLR5 (23). Additional PAMPs have been shown to up-regulate HβD2 expression in respiratory epithelial cells, including dsRNA, which binds via TLR3 (24), and CpG (unmethylated C-G dinucleotides more common in bacteria and viral DNA) signaling through TLR9 (25). Fragments of peptidoglycan, muramyl dipeptide, found in the cell wall of intracellular microbes, induce NOD2-dependent HβD2 up-regulation in HEK293 cells (26), enhancing host defenses against invasive microorganisms.
Hyaluronan (HA) is a glycosaminoglycan polymer found in the extracellular matrix of virtually all tissues of the body and is composed of repeating disaccharides of β-glucuronic acid and N-acetylglucosamine covalently bound end-to-end into a simple linear glycosaminoglycan. HA is most commonly found in vivo as high molecular weight polymers (up to 107 Da), but these large polymers can be broken down into fragments by a number of enzymatic and non-enzymatic processes (27). A growing body of evidence implicates HA as an “information-rich” molecule with diverse molecular weight-dependent signaling functions reported in angiogenesis, inflammation, and tumorigenesis among other fields of research (28). Low molecular weight HA is increasingly being characterized as an endogenous danger signal that promotes the expression of immune mediators. Noble et al. (29) were the first to report induction of IL-1β and TNF-α in macrophages following stimulation with HA of indeterminate molecular weight below 105 Da. The same group demonstrated dependence of the response upon the cell surface HA receptor CD44 (29) and later implicated NF-κB in the HA-mediated inflammatory response (30). Termeer et al. (31) demonstrated that HA oligosaccharides of 1.5–2 kDa, but not HA polymers greater than 80 kDa, promote maturation and cytokine release in monocyte-derived dendritic cells (31) via Toll-like receptor 4 (TLR4) (32). Similarly, HA oligosaccharides have been shown to promote TLR4-dependent immune activation of endothelial cells, both in vitro and in vivo (33).
Fragmented HA may also have a role in epithelial defense that is potentially independent of the proinflammatory cytokine release observed in other tissues and cell types following exposure to HA fragments. Intraperitoneal injection of a polydispersed HA preparation of less than 750 kDa suppresses the epithelium-damaging, proinflammatory effects of DSS-induced murine colitis through a TLR4-dependent mechanism (34). Additionally, intermediate molecular mass HA (<200 kDa), but not high molecular mass HA (>1,000 kDa) promotes the expression of HβD2 peptide in human keratinocytes partially through interaction with TLR4 without the accompanying inflammatory cytokine response elicited by LPS (35). Thus, endogenous HA may act as a signal to increase innate epithelial defense without provocation of the potentially damaging proinflammatory mechanisms of immune cells.
In this study, we demonstrate highly size-specific HA-dependent induction of the antimicrobial protein HβD2 in intestinal epithelial cells grown in culture as well as in the colonic mucosa of HA fragment-treated mice. The most potent HA preparation evaluated was 35 kDa in average molecular mass, whereas a panel of both larger and smaller HA fragment preparations had little apparent activity. Importantly, this process also occurred in vivo because oral administration of HA fragments to mice induced increased expression of the murine ortholog of HβD2 in a size-specific manner in the colonic epithelium through a mechanism that is TLR4-dependent.
EXPERIMENTAL PROCEDURES
Cell Culture
HT29 epithelial cells, a line initially derived from a human intestinal tumor, were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS) and incubated at 37 °C in a humidified environment containing 5% CO2. Stock cultures were split at a ratio of 1:20 once per week.
Experimental Cultures
HT29 cells were released from stock cultures by dissociation with solution containing 0.05% trypsin and 0.53 mm EDTA in phosphate-buffered saline for 1.5 min. Collected cells were washed and plated at a 1:15 area/area ratio in 12-well plates (BD Biosciences) and grown until 70–80% confluent (3 days). On the day of the experiment, growth medium was removed, and the HT29 cells were treated with fresh RPMI containing 10% FBS without or with specific molecular weight range HA preparations at the concentrations (0–100 μm) and times (0–24 h) specified in the figure legends. Purified, lyophilized HA was purchased from Lifecore Biomedical, LLC (Chaska, MN). The HA size designations were made on the basis of average molecular mass: 4.7 kDa (HA-4.7), 16 kDa (HA-16), 28.6 kDa (HA-28), 35 kDa (HA-35), 74 kDa (HA-74), and 2000 kDa (HA-2M) (see Fig. 3F for full size spectrum) and suspended in sterile H2O for concentrated stock solutions (10 mg/ml) prior to dilution in medium for cell treatment.
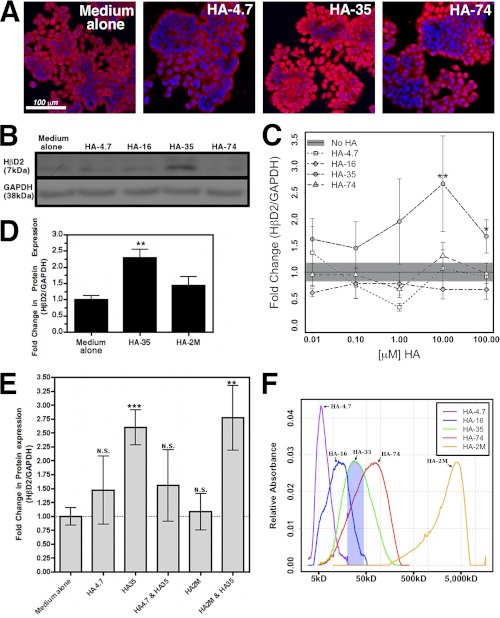
FIGURE 3.
A, representative confocal micrographs of HT-29 cells treated with medium alone or equal mass (100 μg/ml) concentrations of HA-4.7, HA-35, or HA-74 for 6 h. Cells were fluorescently immunostained for HβD2 protein (red), and nuclei were stained with DAPI (blue). B, representative Western blot showing HβD2 protein relative to GAPDH protein expression in whole cell lysates of HT-29 cells treated with HA-4.7, HA-16, HA-35, or HA-74 at equal molar concentrations (10 μm). C, average densitometric quantification of immunoblots from four separate experiments in which HβD2 protein expression was evaluated relative to GAPDH protein expression in whole cell lysates of HT-29 cells. Confluent cultures of HT-29 cells were treated for 9 h with medium alone, or a range of concentrations (0.01–100 μm) of HA fragment preparations of different average molecular weight (HA-4.7, HA-16, HA-35, and HA-74). The solid black horizontal line indicates the mean HβD2/GAPDH ratio in medium-treated cells, and the surrounding gray-shaded region denotes the S.E. among replicate medium-treated samples. D, average densitometric quantification of immunoblots from four separate experiments in which HβD2 protein expression was evaluated relative to GAPDH protein expression in whole cell lysates of HT-29 cells. Confluent cultures of HT-29 cells were treated for 9 h with medium alone or containing HA-35 or HA-2M at equal mass concentrations (350 μg/ml). E, average densitometric quantification of immunoblots from two separate experiments, each with four replicates of each treatment group, in which HβD2 protein expression was evaluated relative to GAPDH protein expression in whole cell lysates of HT-29 cells. Confluent cultures of HT-29 cells were treated for 9 h with medium alone, medium containing equal molar concentrations (10 μm) of HA4.7 alone or HA-35 alone, a combination of HA-4.7 and HA-35 (10 μm each), HA-2M at equal mass concentration (350 μg/ml) relative to HA-35 alone (350 μg/ml = 10 μm for HA-35), or a combination of HA-2M and HA-35 (350 μg/ml each) for 8 h. Significance of differences in normalized HβD2 expression was evaluated by comparison of each treatment and dosage with medium treatment using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). N.S., not significant. F, molecular weight distribution and relative quantity of HA polymers in commercial HA fragment preparations used for in vitro experiments. The blue-shaded region indicates the portion of HA-35 fragment distribution that is enriched relative to other HA fragment preparations evaluated (∼20–45 kDa). Error bars, S.E.
HA Fragment Sizing
Highly purified HA fragment preparations (Lifecore Biomedical, LLC) were electrophoretically separated on a 4–20% gradient polyacrylamide gel (HA-4.7 and HA-16), 5% agarose gel in TBE buffer (HA-35 and HA-74) or on 0.5% agarose in TAE buffer (HA-2M) along with HA standards of known molecular weight (Hyalose LLC, Oklahoma City, OK) to determine the size range of each preparation. Polyacrylamide or agarose gels were stained directly with Stains-All (Sigma), and optical densitometry was used to quantify the polydispersion of Lifecore HA fragments in comparison with HA polymer standards (36).
Detection of HβD2 by Immunoblot (Western) Analysis
Whole cell lysates from HT29 cells were isolated for Western blotting in the following lysis buffer: 300 mm NaCl, 50 mm Tris, pH 8.0, 0.5% Nonidet P-40, 1 mm EDTA, 10% glycerol, protease inhibitor for mammalian tissue P8340 (Sigma). Cell lysate proteins were separated by SDS-PAGE using precast Tricine-based 4–20% gradient gels (Invitrogen). Separated proteins were transferred at 4 °C to PVDF membrane with an electroblotting apparatus (Bio-Rad) at 100 V for 45 min. In cases where analysis of numerous replicate samples or multiple protein targets of differing molecular weight were required, the multistrip Western blotting protocol developed by Aksamitiene et al. (37) was used to increase quantitative output and improve signal consistency. PVDF membranes were air-dried at room temperature for 60 min prior to blocking with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) diluted to 50% concentration in Tris-buffered saline. The membrane was incubated with rabbit polyclonal antibody against HβD2 at 1:750 (Abcam, Cambridge, MA), and the primary antibody was followed by biotin-conjugated anti-rabbit IgG at 1:25,000 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), and finally horseradish peroxidase (HRP)-conjugated streptavidin was added at 1:100,000 (GE Healthcare). Membrane-bound GAPDH protein expression was detected by incubation with rabbit polyclonal antibody against GAPDH at 1:5,000 (Abcam, Cambridge, MA) followed by HRP-conjugated donkey polyclonal anti-rabbit IgG (1:20,000, GE Healthcare). All washing steps were conducted in Tris-buffered saline with 0.1% Tween 20. Protein bands were visualized using ECL plus chemiluminescent development (GE Healthcare) and detection by BioMax XAR scientific imaging film (Carestream Health Inc., Rochester, NY). Differences in chemiluminescent signal intensity were quantified using the NCBI ImageJ software package (38).
Detection of HβD2 by Fluorescence Histochemistry
Descriptions of fluorescence histochemistry and confocal microscopy were provided previously (39). Briefly, HT-29 cells, grown on coverslips, were treated as described in the figure legends, fixed in −20 °C methanol for 5–10 min, and air-dried. For staining, the dry coverslips were incubated in a blocking solution of Hanks' balanced salt solution (HBSS) containing 2% FBS for >30 min. The coverslips were then transferred to a solution containing rabbit polyclonal antibody against HβD2 (Abcam, Cambridge, MA) at 1:500 dilution in HBSS with 2% FBS overnight at 4 °C. Coverslips were washed three times with HBSS before incubation in a solution containing Alexa-568-tagged goat anti-rabbit IgG (1:1,000) (Invitrogen) in HBSS with 2% FBS for 1 h at 25 °C. The coverslips were washed an additional three times in HBSS, inverted, and attached to glass slides with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA) containing 4′,6-diamidino-2-phenylindole (DAPI), which fluorescently labels DNA. For tissue section staining, fixed, paraffin-embedded mouse colon sections were deparaffinized and processed similarly to the cultured cells. Sections were incubated with blocking buffer (HBSS with 2% FBS) for 30 min followed by overnight incubation at 4 °C in a solution of rabbit polyclonal antibody against MuβD3 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:100 dilution in HBSS with 2% FBS. Slides were washed three times in HBSS and incubated in a solution containing Alexa-568-tagged goat anti-rabbit IgG (1:1,000) in HBSS with 2% FBS for 1 h at 25 °C. After washing, Vectashield with DAPI was used to adhere coverslips to antibody-labeled colon sections. Slides were stored at −20 °C until imaged. Confocal images were obtained using a Leica TCS-SP laser-scanning confocal microscope (Leica, Heidelberg, Germany).
Detection of HβD2 by Enzyme-linked Immunosorbent Assay (ELISA)
HT-29 cells were cultured as described above, and replicate wells were treated with medium alone or medium containing HA-35 (10 μm) for 9 h. Culture fluid was then harvested and stored at −80 °C. To remove high molecular weight medium components and to improve the specificity of ELISA detection of the 7-kDa HβD2 protein, thawed medium samples were transferred to 10-kDa cut-off Amicon Ultra-2 centrifugal filter devices (Millipore Corp., Billerica, MA) and centrifuged in a swinging bucket rotor at 4,000 × g for 45 m at 4 °C. HβD2 contents of filtered medium samples were then quantified by an ELISA kit according to the manufacturer's protocol (PeproTech, Rocky Hill, NJ).
Specificity of HA-35 Induction of HβD2 in HT29 Cells
Two experimental strategies were employed to determine whether HA was the active component of the HA-35 preparation for inducing HβD2 protein expression: 1) hyaluronidase digestion of HA-35 and 2) comparison of HA-35 and endotoxin effects on HβD2 expression.
Hylauronidase Digestion of HA-35
The HA-35 fragment preparation was specifically degraded to disaccharides by incubating 1.4 mm HA-35 fragment solution with 0.025 unit/ml Streptococcus dysgalactiae hyaluronidase (Seikagaku Corp.) for 16 h at 37 °C in PBS and subsequently heat-inactivated prior to use as a cell treatment.
Comparison of HA-35 and Endotoxin Effects on HβD2 Expression
To determine whether the increased production of HβD2 by HT29 cells could be due to bacterial endotoxin contamination, the effects of HA-35 preparation were compared with high levels of LPS (1 μg/ml) (Sigma-Aldrich) treatment in the in vitro assays. The LPS preparation positive control activity was determined by its ability to activate T-cells. HT-29 cells were treated with medium alone, medium containing HA-35 (10 μm), or medium containing LPS (1 μg/ml) for 9 h and harvested and analyzed as described above.
In Vivo Induction of Murine HβD2 Ortholog by HA-35
Wild-type adult C57BL/6 mice were provided with conventional drinking water (5 mice), or drinking water supplemented with HA-35 at a final concentration of 1.0 μm (5 mice). Water bottles were changed each day, and the animals were kept on this regimen for 7 days. Mice were sacrificed on day 7, and 0.5-cm sections of the distal colon were harvested and fixed in Histochoice (AMRESCO, Inc., Solon, OH) and paraffin-embedded. Representative cross-sections of the mouse distal colon were deparaffinized and stained for MuβD3, the murine ortholog of HβD2, as described above.
Evaluation of Size-specific Induction of Murine HβD2 Ortholog in Vivo
Male wild-type adult C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed by conventional methods. All treatments were conducted according to Institutional Animal Care and Use Committee-approved protocols. A total of 18 mice were divided equally among six treatment groups: control, HA-4.7, HA-16, HA-35, HA74, or HA-2M. All animals were gavage-fed 0.25 ml of water (control) or 300 μg of HA of the fragment preparation corresponding to the treatment group designation (e.g. HA-4.7 animals received 300 μg of 4.7-kDa HA) suspended in 0.25 ml of water once daily for three consecutive days. Mice were sacrificed 16–18 h after the final gavage treatment, and a 0.5-cm cross-section of the proximal colon descending from the ileocecal junction was excised from each mouse, fixed in Histochoice (AMRESCO, Inc., Solon, OH), and paraffin-embedded. These cross-sections of mouse proximal colon were deparaffinized and stained for MuβD3, the murine ortholog of HβD2, as described above. Replicate staining was quantified in a blinded manner by a scientific panel according to the method described below.
Detection of TLR4 by Fluorescence Histochemistry
Fixed, paraffin-embedded mouse proximal colon sections were deparaffinized, and sections were incubated with blocking buffer (HBSS with 2% FBS) for 30 min followed by overnight incubation at 4 °C in a solution of rabbit polyclonal antibody against murine TLR4 (Abcam, Cambridge, MA) at 1:50 dilution in HBSS with 2% FBS. Slides were washed three times in HBSS and incubated in a solution containing biotin-conjugated anti-rabbit IgG suspended in HBSS with 2% FBS at 1:1,000 (Jackson ImmunoResearch Laboratories Inc.) for 1 h at 25 °C. Slides were subsequently washed three times in HBSS before incubation in a solution containing Alexa-488-tagged streptavidin (Invitrogen) at 1:1,000 for 1 h at 25 °C. After final washing, Vectashield with DAPI was used to adhere coverslips to antibody-labeled and nonspecifically stained control colon sections. Slides were stored at −20 °C until imaged.
Evaluating the Role of TLR4 and CD44 in HA-35-induced HβD2 Expression in Vivo
Male C57/Bl6 (wild-type), B6.129(Cg)-CD44tm1Hbg/J (CD44−/−), and B6.B10ScN-Tlr4lps-del/JthJ (TLR4−/−) mice were purchased from Jackson Laboratory and housed by conventional methods. All treatments were conducted according to Institutional Animal Care and Use Committee-approved protocols. Five mice of each of the three genotypes were fed once per day with tap water alone (250 μl) or a solution containing HA fragments in tap water (300 μg in 250 μl) with an average molecular mass of 28.6 kDa (HA-28, Lifecore Biomedical, LLC) or an equivalent volume of tap water alone by oral gavage once daily for three consecutive days. HA-28 was substituted for the HA-35 used in previous studies due to its nearly identical molecular weight distribution and its availability in sufficient quantity for practical use in animal studies. Mice were sacrificed 16–18 h after the final gavage treatment, and a 2-cm section of the proximal colon descending from the ileocecal junction was excised from each mouse, opened, pressed flat between two layers of paper towel (40), fixed overnight in Histochoice (AMRESCO, Inc., Solon, OH), and paraffin-embedded. Representative longitudinal sections of the mouse proximal colon were deparaffinized and stained for MuβD3 as described above.
Quantification of Histological Observations
Individual sections were labeled in a random fashion to shield the microscopists from knowledge of mouse genotype or HA treatment status. Ideal fluorescent signal exposure times for capturing the complete range of MuβD3 staining intensity were determined at the outset of the experiment by a survey of 10 random sections and were held constant for subsequent image capture. Three MuβD3-stained fields of colonic epithelial structures as well as one unstained control from each animal were digitally captured from longitudinal sections of each of the mouse proximal colon samples. Capture fields were selected on the basis of epithelial tissue morphology as determined by DAPI staining. Each of the images (three stained fields and one unstained field from each mouse) were graded on a 0–4 scale by a panel of four blinded researchers, with a grade of 0 indicating no MuβD3 staining and a grade of 4 corresponding to peak MuβD3 staining intensity (supplemental Fig. 1). The mean scores given by the evaluating panel for each of the images were averaged for each mouse before treatment or genotype status were revealed according to a key.
Statistical Analysis
The statistical difference between treatment groups was evaluated where appropriate by unpaired one-tailed Student's t test, and all error bars were drawn to indicate the S.E. values. Differences were considered significant when p was <0.05. Statistical analysis was performed using R version 2.12.1 for Mac OS X (R Foundation for Statistical Computing; available on the World Wide Web). Graphing was completed using R version 2.12.1 for Mac OS X or GraphPad Prism version 4.0c.
RESULTS
HA-specific Induction of HβD2 Expression in HT-29 Colonic Epithelial Cells Occurs in a Time-dependent Manner
HβD2 is up-regulated in keratinocytes treated with a broadly polydispersed preparation of HA fragments less than 200 kDa in size (35), and we hypothesized that fragmented HA would induce HβD2 expression in intestinal epithelium. Therefore, we first tested the ability of fragmented HA to promote expression of HβD2 protein in the human intestinal epithelial tumor cell line HT-29. Cultured HT-29 cells were treated with or without a polydispersed HA fragment preparation averaging 35 kDa (HA-35) at a concentration of 1 μm for 12 h and fluorescently stained with a specific antibody against HβD2 and observed using confocal microscopy. The micrographs (Fig. 1A) reveal substantially increased intracellular HβD2 staining. Strikingly, HβD2 protein staining has a granular cytoplasmic arrangement after HA-35 treatment (inset).
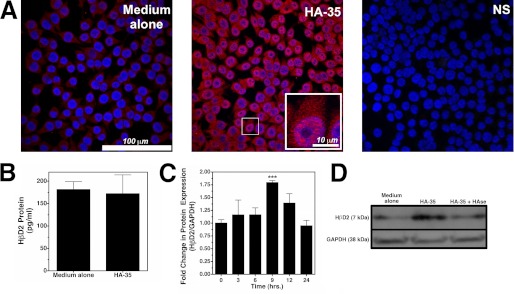
FIGURE 1.
A, representative confocal micrographs show HβD2 expression in confluent cultures of HT-29 cells that were treated with medium alone or containing HA-35 (1 μm) for 12 h. Cells were fluorescently immunostained for HβD2 protein (red), and nuclei were stained with DAPI (blue). NS, immunostaining control in which no α-HβD2 antibody was utilized. B, mean secreted HβD2 as measured by ELISA in the culture medium of HT-29 cells treated for 9 h with medium alone or containing HA-35 (10 μm). No significant difference in secreted HβD2 peptide was detected between media or HA-treated cultures. C, average densitometric quantification of immunoblots from four individual experiments in which the abundance of HβD2 protein relative to GAPDH protein was evaluated in whole cell lysates of HT-29 cells. Replicate cultures were treated with HA-35 (1 μm) at 3-h time intervals (0–24 h) in each experiment. Significance of differences in normalized HβD2 expression was evaluated by comparison of each time point with control treatment using Student's t test (***, p < 0.001). D, representative Western blots demonstrating HβD2 protein expression relative to GAPDH in the whole cell lysates of HT-29 cells treated with medium alone, medium containing HA-35 (10 μm), and medium containing HA-35 (10 μm) that was predigested with Streptococcus dysgalactiae hyaluronidase at 0.025 unit/ml for 16 h at 37 °C. Error bars, S.E.
Defensins, including HβD2, have been principally characterized as secreted peptides produced by the epithelium, which participate in innate extracellular host defense (5). Surprisingly, treatment of HT-29 cells with HA-35 at concentrations up to 10 μm for 9 h resulted in no significant change in secreted HβD2 peptide concentration (p = 0.845 versus medium alone) compared with treatment with medium alone (Fig. 1B).
Induction of intracellular HβD2 protein in HT-29 cells by HA-35 is observed within hours of the initial treatment. Fig. 1C demonstrates that the ratio of HβD2 protein relative to GAPDH protein in whole cell lysates increases with HA-35 treatment, peaking 9 h after the addition of the HA-35 (p = 0.0006 versus 0-h HA-35 treatment), and returning to base-line expression by 24 h. To test whether increases in intracellular HβD2 protein expression were specifically induced by HA, HT-29 cells were treated with medium alone, HA-35 (10 μm), or HA-35 (10 μm) that was specifically degraded into disaccharide components with no known activity by predigestion with Streptococcus dysgalactiae hyaluronidase. Western blot analysis (Fig. 1D) of HT-29 whole cell lysates reveals an increase in intracellular HβD2 protein expression relative to GAPDH protein expression following treatment with 10 μm HA-35 that was entirely reversed by specific destruction of HA-35 with hyaluronidase. Together these findings strongly suggest that fragmented HA promotes expression of intracellular HβD2 protein in colonic epithelium.
Induction of HβD2 Expression in HT29 Colonic Epithelial Cells Is Specific to HA
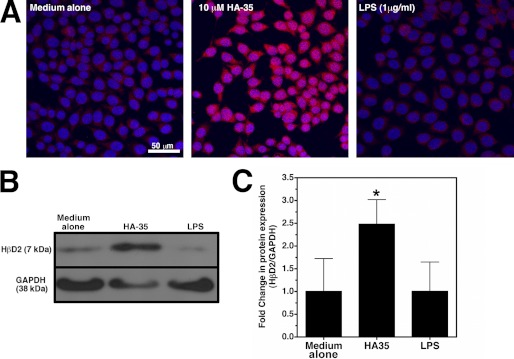
Bacterial lipopolysaccharide (LPS), or endotoxin, is a component of the outer membrane of Gram-negative bacteria that has long been understood to elicit essential innate immune responses through numerous mechanisms (42), including promoting increased transcription of the gene encoding the HβD2 peptide, DEFB4, in certain epithelial cell lines (13). The potential for endotoxin contamination during reagent preparation has long been a caveat to studies on the immune-stimulatory properties of hyaluronan polymers (28), and with this in mind, we compared the induction of HβD2 in HT-29 cells treated with LPS with those cultured in medium containing HA-35. As shown in Fig. 2, A–C, treatment of HT-29 cells with an LPS preparation with demonstrated efficacy in T-cells (data not shown) does not result in increased intracellular HβD2 protein expression compared with treatment with medium alone, even at high concentrations (1 μg/ml). Conversely, treatment with HA-35 (10 μm) consistently resulted in up-regulation of intracellular HβD2 peptide, as evaluated by confocal microscopy of immunostained cultures (Fig. 2A) as well as densitometric Western blot analysis (Fig. 2, B and C). Collectively, our data (Figs. 1D and 2, A–C) indicate that the induction of intracellular HβD2 is HA-specific and is unlikely to be accounted for by inadvertent contamination of HA fragment preparations with stimulatory agents.
FIGURE 2.
A, representative confocal micrographs of HT-29 cell cultures that were treated with medium alone, medium containing HA-35 (10 μm), or medium containing LPS (1 μg/ml) for 9 h. HβD2 protein is immunostained (red), and nuclei are stained with DAPI (blue). B, representative Western blot of HβD2 protein expression relative to GAPDH protein expression in whole cell lysates of HT-29 cells treated with medium alone, media supplemented with HA-35 (10 μm), or media containing LPS (1 μg/ml). C, average densitometric quantification of Western blot results of three experiments in which HT-29 cultures were treated with media alone, HA-35 (10 μm), or LPS (1 μg/ml) for 9 h. HβD2 protein expression is normalized to GAPDH protein in whole cell lysates. Significance of differences in normalized HβD2 expression was evaluated by comparison of each treatment with medium treatment using Student's t test (*, p < 0.05). Error bars, S.E.
Induction of HβD2 in HT29 Cells Is Dependent on HA Size and Concentration
Specific signaling by HA is hypothesized to be polymer size-dependent (28), and therefore we determined the optimal size of HA fragments for induction of HβD2 in intestinal epithelial cells. HT-29 human intestinal epithelial cells were treated with hyaluronan fragments with an average molecular mass of 4.7 kDa (HA-4.7), 16 kDa (HA-16), 35 kDa (HA-35), and 74 kDa (HA-74) at equal molar (Fig. 3, B and C) concentrations ranging from 0.01 to 100 μm or at equal mass concentration (Fig. 3, A and D) for 9 h. Both immunofluorescent staining (Fig. 3A) and Western blot analysis (Fig. 3, B–D) of HT-29 cells treated with HA polymers demonstrate specific induction of HβD2 peptide following treatment with HA-35. The peak intracellular HβD2 peptide expression was observed at a 10 μm (350 μg/ml) concentration of HA-35 (Fig. 3C). Immunofluorescence histochemistry confirmed that HT-29 cells treated with equal mass doses (100 μg/ml) of HA fragments are also optimally induced to express HβD2 protein by HA-35, as seen by red immunofluorescent staining with an HβD2-specific antibody (Fig. 3A). Additionally, the HβD2-inducing properties of HA-35 were compared against an equal mass concentration of a large 2,000-kDa HA polymer (HA-2M) or medium alone. HA-2M had no effect on HβD2 protein expression when compared with medium alone, whereas HA-35 treatment doubled the relative abundance of intracellular HβD2 protein (Fig. 3D). Whether used at equal molar or equal mass concentrations, HA-35 was the most active inducer of HβD2 protein expression among the fragmented HA preparations evaluated. Notably, cell growth rate, as determined by cell counting and trypan blue dye exclusion (data not shown), was unchanged in HT-29 cells treated with HA fragments at the concentrations evaluated (≤100 μm).
Finally, we wanted to evaluate whether the presence of smaller (HA-4.7) or larger (HA-2M) HA fragments could alter the activity of HA-35. HT-29 cells were incubated with media containing equal molar (10 μm) concentrations of HA-4.7 alone, HA-35 alone, or HA-35 and HA-4.7 in combination at 10 μm each or with medium alone for 8 h (Fig. 3E). In addition, HT-29 cells were treated with equal mass concentrations (350 μg/ml) of HA-35 alone, HA-2M alone, or HA-35 and HA-2M in combination at 350 μg/ml each or with medium alone (Fig. 3E). In concordance with our prior experiments, quantitative Western blot analysis of replicate samples revealed a 2.5-fold mean induction of HβD2 protein expression in HT-29 cultures treated with media containing HA-35 alone relative to control-treated cultures. Neither HA4.7 alone nor HA-2M alone promoted significant increases in HβD2 protein expression. Although co-treatment of HT-29 cells with HA-2M and HA-35 at equal mass concentration resulted in HβD2 induction comparable with treatment with HA-35 alone, strikingly, HT-29 cells treated with an equal molar combination of HA-4.7 and HA-35 exhibited no statistically significant increase in HβD2 protein expression relative to control-treated cultures (Fig. 3E). Thus, although the presence of HA-2M has no apparent effect on the activity of HA-35 when HT-29 cells are treated in combination, equal molar concentrations of HA-4.7 are sufficient to interfere with the HβD2-inducing activity of HA-35 fragment preparations.
To further define and compare the distribution of HA fragment sizes in each of the preparations, HA-4.7, HA-16, HA-35, HA-74, and HA-2M were electrophoretically separated by polyacrylamide gel electrophoresis, and the molecular weight distributions were compared against a series of HA polymer standards (Fig. 3F). Predictably, the HA preparation of greatest molecular weight (HA-2M) contained an entirely distinct HA polymer population; however, HA-4.7, HA-16, HA-35, and HA-74 fragment preparations share varying degrees of overlapping distribution with high similarity between HA-35 and HA-74. The HA-35 preparation nonetheless contains the greatest proportion of HA fragments between 20 and 45 kDa of any HA preparation evaluated, and it is this HA fragment population that most distinguishes HA-35 from HA-16 and HA-74 (Fig. 3F), neither of which promote increased HβD2 protein expression in HT-29 cells (Fig. 3, A–C).
In summary, the experiments of Fig. 3 demonstrate that the induction of intracellular HβD2 protein expression by fragmented HA is highly size-specific, with HA-35 emerging as the most potent inducer of HβD2. Smaller (4.7- and 16-kDa) or larger (74- and 2,000-kDa) HA fragments alone do not promote significant intracellular HβD2 protein expression, and comparative electrophoretic polymer sizing analysis suggests that the active HA fragments present in our polydispersed HA-35 preparation are between 20 and 45 kDa in size. Furthermore, the activity of HA-35 is partially inhibited in the presence of equal molar HA-4.7 but not HA-2M.
Orally Administered HA-35 Induces the Expression of the HβD2 Orthologue in Mouse Intestinal Epithelium in Vivo
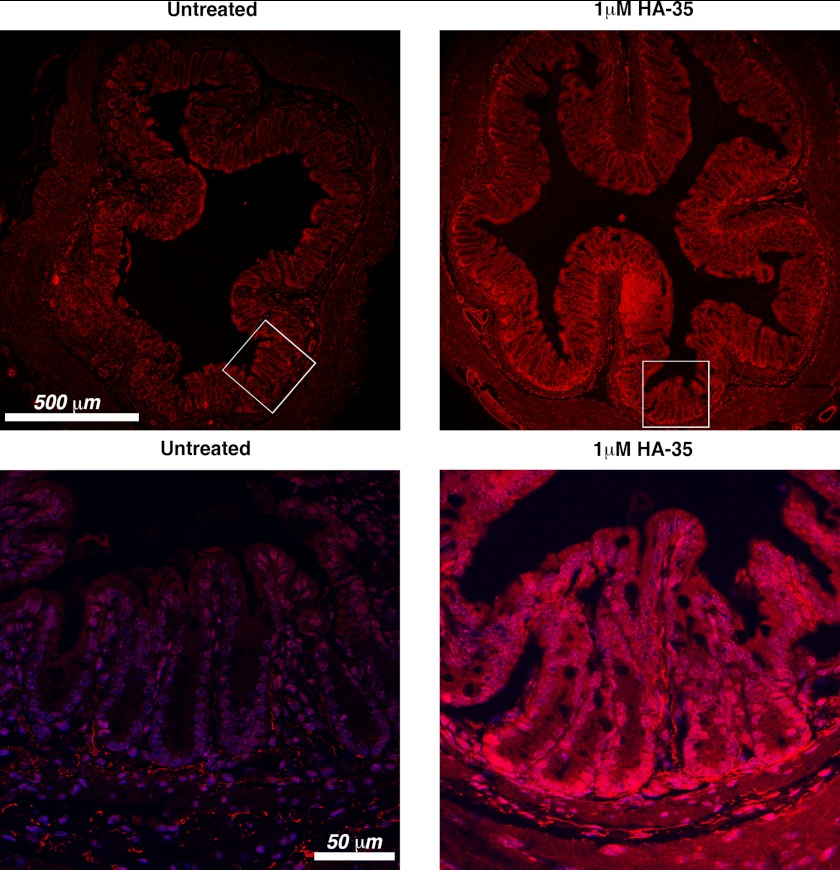
To test whether our in vitro observation, that HA-35 promotes expression of HβD2 protein in intestinal epithelial cells, also occurs with normal intestinal epithelium in vivo, we enriched the drinking water of five adult wild-type C57BL/6 mice with HA-35 (1 μm) and compared them with five age- and sex-matched controls supplied with standard drinking water. Mice were sacrificed after 7 days of ad libitum consumption of HA-supplemented drinking water. Distal colon tissue was excised in cross-sections for immunofluorescent staining. Analysis of cross-sectioned intestinal tissue by confocal microscopy reveals increased expression of murine β-defensin 3 (MuβD3, the mouse ortholog of HβD2 (43)) in the intestinal mucosa of mice consuming HA-supplemented water compared with mice consuming standard water (Fig. 4). Higher magnification reveals increased MuβD3 protein expression localized to the epithelial layer, and in particular the base of the intestinal crypts where putative epithelial stem cells reside (44), in the mucosal tissue of mice consuming HA-35-supplemented water (Fig. 4, bottom panels).
FIGURE 4.
Representative confocal micrographs of distal colon cross-sections from adult mice provided with standard drinking water or drinking water supplemented with HA-35 (1 μm) ad libitum for 7 days. The mouse orthologue of HβD2, MuβD3, is fluorescently immunolabeled (red), and nuclei are stained with DAPI (blue). Bottom panels represent a more highly magnified portion of the lower power (top) field (as indicated by white boxes).
Induction of HβD2 Orthologue in Mouse Intestinal Epithelium by HA Fragments Is Highly Size-specific
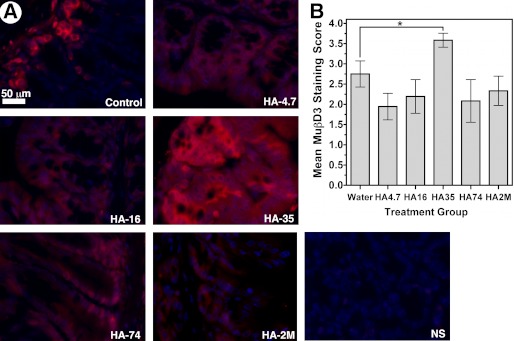
Induction of HβD2 following HA fragment treatment in the HT-29 human intestinal epithelial tumor cell line was found to be highly specific to an HA fragment preparation enriched with HA polymers between 20 and 45 kDa with an average molecular mass of 35 kDa (Fig. 3). Furthermore, supplementation of the drinking water of adult wild-type mice with HA-35 promoted increased MuβD3 protein expression in the colonic mucosa relative to control-fed animals (Fig. 4). However, it remained unclear whether HA size-specific induction of defensin occurred in vivo in a manner similar to our in vitro observations in HT-29 cells. In order to address this question, 18 age- and sex-matched wild-type C57BL/6 mice were segregated equally into the following six treatment groups: control, HA-4.7, HA-16, HA-35, HA74, or HA-2M. All animals were gavage-fed 0.25 ml of water (control) or 300 μg of HA of the fragment preparation corresponding to the treatment group designation (e.g. HA-4.7 animals received 300 μg of 4.7-kDa HA) dissolved in 0.25 ml of water once daily for three consecutive days. Mice were sacrificed within 16–18 h of the final gavage treatment. Proximal colon tissue was fixed and cut in cross-sections for immunofluorescent staining. Analysis of cross-sectioned intestinal tissue by fluorescent microscopy revealed increased epithelial MuβD3 protein expression only in the HA-35-treated animals relative to control-treated animals (Fig. 5). Fig. 5A presents representative high magnification images of murine colonic epithelium from each of the treatment groups immunostained for MuβD3 protein expression. Blinded analysis of fluorescent microscopy imaging revealed significantly increased MuβD3 staining intensity in the epithelial cells of the proximal large intestine of wild-type mice fed HA-35 compared with control-fed wild-type mice (p = 0.0184; Fig. 5B), whereas no significant difference relative to control animals was observed in mice fed HA4.7, HA-16, HA-74, or HA-2M.
FIGURE 5.
A, representative fluorescent micrographs of epithelium of proximal colon sections from adult C57BL/6 wild-type mice. The mice were fed single daily doses of HA-4.7, HA-16, HA-35, HA-74, or HA-2M (300 μg/0.25 ml), and controls were given an equivalent volume of water alone by oral gavage for 3 consecutive days. MuβD3 is immunolabeled (shown in red), and nuclei are blue as a result of DAPI staining. NS, an immunostaining control in which no MuβD3 antibody was utilized. B, average scored MuβD3 staining intensity of proximal colon tissue sections from wild-type mice fed single daily doses of HA-4.7, HA-16, HA-35, HA-74, or HA-2M or an equivalent volume of water alone once daily for 3 consecutive days. Average MuβD3 staining intensity score represents 3 mice/group, with 3 stained sections/mouse, as judged by a blinded panel of four researchers on a scale of 0–4, with a score of 4 corresponding to peak MuβD3 staining for this data set. Significance of differences in mean MuβD3 staining intensity was evaluated using Student's t test in a pair-wise manner as indicated in the figure (*, p < 0.05). Error bars, S.E.
Specific-sized HA Fragment-mediated Induction of HβD2 Orthologue Expression in Mice Is Dependent on TLR4
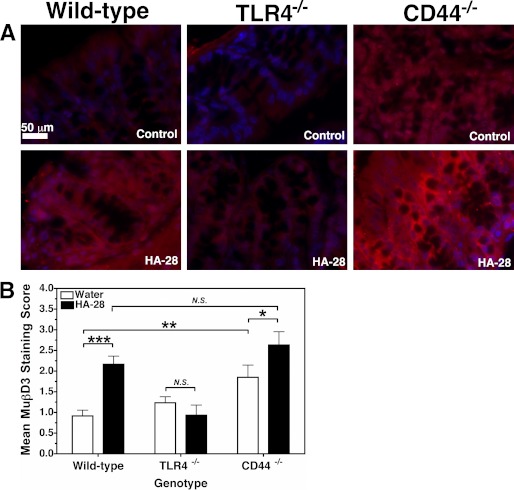
A number of cell surface receptors have been associated with the activation of innate defense responses by HA, including most prominently Toll-like receptor TLR4 (32–35, 45) and CD44, the major HA receptor that is involved in inflammation as well as homeostasis (29, 45–48). Thus, both TLR4 and CD44 were evaluated as candidate HA fragment receptors for the induction of murine HβD2 orthologue in vivo. Adult wild-type, CD44−/−, and TLR4−/− C57BL/6 mice were gavage-fed a solution containing 300 μg of fragmented HA averaging 28.6 kDa (HA-28) dissolved in 0.25 ml of water (∼100 μm) or 0.25 ml of water alone once daily for 3 days. HA-28 was substituted for the HA-35 used in previous studies due to its similar average molecular weight, ability to promote HβD2 protein expression in vitro (supplemental Fig. 2), and commercial availability. Mice were sacrificed at 16–18 h after the final treatment, and the colon tissue was extracted for histological analysis. Wild-type and CD44−/− mice exhibited comparable TLR4 protein expression levels at base line, and TLR4−/− mice did not express TLR4 protein in the intestinal epithelium (supplemental Fig. 3). Fig. 6A presents representative images of colonic epithelium immunostained for MuβD3 protein from each of the treatment groups. Blinded analysis by fluorescent microscopy revealed significantly increased MuβD3 staining intensity in the epithelial cells of the proximal large intestine of wild-type mice fed HA-28 compared with control-fed wild-type mice (p = 0.0003; Fig. 6B). No increase in MuβD3 staining intensity was observed in the colonic epithelium of TLR4−/− mice fed HA-28 relative to control-fed TLR4−/− mice. Increased MμβD3 protein expression was observed in CD44−/−-fed HA-28 relative to control-fed CD44−/− (p = 0.04). Unexpectedly, control-fed CD44−/− mice had significantly higher levels of base-line MuβD3 protein expression in comparison with their wild-type counterparts (p = 0.005; Fig. 6B).
FIGURE 6.
A, representative fluorescent micrographs of epithelium of proximal colon sections from adult C57BL/6 wild-type, TLR4−/−, and CD44−/− mice. The mice were fed single daily doses of HA-28 (300 μg/0.25 ml), and controls were given an equivalent volume of water alone by gavage for 3 consecutive days. MuβD3 is immunolabeled (red), and nuclei are blue. B, average scored MuβD3 staining intensity of proximal colon tissue sections from wild-type, TLR4−/−, and CD44−/− mice fed HA-28 (300 μg) or an equivalent volume of water alone by oral gavage once daily for 3 consecutive days. Average MuβD3 staining intensity score represents 5 mice/group, with 3 stained sections/mouse, as judged by a blinded panel of four researchers on a scale of 0–4, with a score of 4 corresponding to peak MuβD3 staining. The mean staining intensity score of nonspecifically stained sections (1 section/mouse) was 0.24 ± 0.09. Significance of differences in mean MuβD3 staining intensity was evaluated using Student's t test in a pair-wise manner as indicated in the figure (*, p < 0.05; **, p < 0.01; ***, p < 0.001). N.S., not significant. Error bars, S.E.
The experiments summarized in Figs. 4–6 provide compelling evidence that our in vitro observations in HT-29 human intestinal epithelium tumor cells translate to the healthy colonic mucosa of adult mice, with two HA fragment preparations (HA-35 and HA-28) supplied by two distinct methods (prolonged ad libitum consumption or controlled oral administration) reproducibly promoting increased expression of the murine HβD2 orthologue (MuβD3) in the epithelium of both the distal and proximal colon. As with our in vitro studies, this response to fragmented HA is highly size-specific, with only HA-35 and HA-28 promoting significantly increased expression of MuβD3 protein in the colonic epithelium relative to control animals. Furthermore, through the use of gene-specific knock-out mice, we have identified TLR4 as a requisite cell surface receptor for the induction of MuβD3 expression with fragmented HA.
DISCUSSION
Taken together, our data demonstrate that specific-sized HA fragments promote increased intracellular expression of the key innate antimicrobial peptide HβD2 in intestinal epithelial cells, both in vitro and in vivo through induction of murine HβD2 orthologue MuβD3 (43) in both the proximal and distal large intestine. Although activation of innate defense responses by HA fragments has been shown using a variety of cell types (29, 32, 45), little was previously known regarding the effects of HA fragments on intestinal epithelium. Furthermore, we have identified TLR4 as a requisite cell surface receptor for the induction of MuβD3 following oral administration of specific-sized HA in vivo, thus contributing to the mounting evidence that TLR4 is a central mediator of cellular responses to fragmented HA.
Size-specific HA signaling has been reported previously in other cell types (29, 32, 33, 45), often defining HA size broadly as either “high” or “low” molecular weight, with no consensus in the literature on what size range defines either category. Induction of HβD2 in intestinal epithelium requires HA polymers within a relatively narrow molecular mass range; ∼35-kDa HA specifically promotes increased intracellular HβD2 protein accumulation that is not observed following treatment with either smaller (HA-4.7, HA-16) or larger (HA-74, HA-2M) HA polymers when compared at either equal molar or equal mass concentrations (Figs. 3 and 5). HA preparations with an average molecular mass of 28.6 kDa also induce intracellular HβD2 peptide expression comparable to HA-35 in HT-29 cells (supplemental Fig. 2), and we have demonstrated that both HA-28 and HA-35 up-regulate MuβD3 expression in vivo (Figs. 4–6). Equal molar application of HA fragments presumes a ligand-receptor relationship in which one HA fragment or polymer interacts in a specific manner with single receptors, whereas an HA polysaccharide may be capable of interacting with multiple receptors due to its extended molecular conformation and repeating structural motif (49). Therefore, we evaluated the size specificity of HβD2 induction by HA in vitro using both equal molar and equal mass dosing for the HA fragment preparations ≤74 kDa in average molecular mass. Importantly, HA-35 is the most active inducer of HβD2 protein expression using either method of comparison with other HA preparations. Thus, the size range of functionally active HA fragments is defined here as no less than 16 kDa and no greater than 74 kDa. Gel electrophoresis analysis of the HA fragment preparations employed in our studies suggests that the optimal HA fragment(s) for intracellular HβD2 induction is probably in the range of 20–45 kDa (∼53–132 disaccharides) (Fig. 3F). To our knowledge, no signaling activity of any kind has been previously ascribed to an HA polymer within this specific size range. HA found in living tissue assumes a widely polydispersed distribution (28), and it is unclear how HA signaling systems respond to HA fragments within this active range in the presence of larger or smaller HA fragments. Our findings indicate that although high molecular mass HA (2000 kDa) has no impact on the induction of HβD2 by HA-35, equal molar HA-4.7 is sufficient to inhibit HβD2 protein expression during treatment of cultured HT-29 cells with HA-35 (Fig. 3E). This could be accomplished through a number of different mechanisms, including direct competition for receptor binding sites, or through an unknown alternate signaling pathway acting counter to the TLR4-dependent pathway activated by HA-35. Conversely, the TLR4-dependent mechanism of HβD2 induction by HA-35 is unaffected by the presence of an equal mass concentration of HA-2M, indicating selectivity among chemically identical polymers of differing length. Our results contrast with the findings of Campo et al. (50), who proposed that high molecular weight HA displaces small HA oligosaccharides at the cell surface, inhibiting the TLR4-dependent inflammatory response to HA oligosaccharides (∼1.5 kDa) in chondrocytes. Co-receptor complexes incorporating a variety of HA-binding transmembrane proteins, including TLR4, CD44, and others, similar to those proposed by Taylor et al. (45), may confer the size and structural specificity indicated by our experimental findings. However, additional studies are required to clarify the molecular mechanisms of HA selectivity.
Bearing in mind that questions of purity have arisen to challenge several early reports of innate responses to HA (28), we have tested the HA-35 preparation and compared its effect with that of endotoxin (LPS). Predigestion of HA fragments with hyaluronidase fully abrogated the effect of HA treatment in HT-29 cells (Fig. 1D), confirming that HA is the specific stimulatory component of HA-35 required for induction of HβD2 protein expression. Second, despite reports of increased defensin gene transcription following treatment with LPS in certain epithelial cell lines transgenically expressing TLR4 (13), we demonstrated that 35-kDa HA fragments promote intracellular HβD2 peptide accumulation in HT-29 cells, whereas high concentrations of LPS do not (Fig. 2, A–C). Lee et al. (51) have reported that sensitivity to LPS is decreased in HT-29 cells due to low TLR4 expression. Induction of the murine HβD2 orthologue by fragmented HA is TLR4-dependent, as we report in Fig. 5. Differing sensitivity of the TLR4 receptor to the ligands HA and LPS or the presence of ligand-specific co-receptors could account for this discrepancy. In either case, we offer compelling evidence to eliminate inadvertent endotoxin contamination of HA-35 as a contributing factor in the induction of intracellular HβD2 in HT-29 cells.
The effect of intermediate-sized HA on intestinal epithelium appears to be divergent from its effect on skin keratinocytes grown in vitro (35) in that no significant change in secreted HβD2 protein concentration is observed in HT-29 cells following treatment with 35-kDa HA (Fig. 1E). Although defensins are best characterized as secreted effector molecules of innate defense (5), intracellular expression of HβD2 protein has been suggested by positive immunostaining of mucosal tissue (7, 14, 52, 53) and epithelial cell cultures (13, 35) for some time. The function of intracellular HβD2 peptide has not been studied extensively, particularly in epithelium. A recent report by Arnett et al. (54) describes a mechanism by which intracellular defensins inhibit proliferation of the obligate intracellular pathogen Listeria monocytogenes in macrophages. Given that 1) high concentrations of secreted HβD2 cause membrane disruption in human epithelial cells (55), 2) extraordinary numbers of beneficial (56) and protective (57) microbes reside in direct proximity to the colonic epithelium (1), and 3) that intracellular pathogens and their PAMPs are among the most potent inducers of HβD2 expression (12, 24–26), it is possible that intracellular compartmentalization of HβD2 may contribute to the process of epithelial barrier maintenance without causing undue harm to epithelial integrity and commensal flora populations and without promoting unnecessary activation of immune cells (58).
An accumulating body of literature has implicated both TLR4 (32, 33, 35, 45) and CD44 (29, 45–48, 59) as major cell surface receptors in the activation of innate defense responses, as well as homeostatic maintenance, upon exposure to HA of various size ranges. In addition, two previous studies have demonstrated TLR4-dependent mechanisms by which HA contributes to the integrity of the intestinal epithelium in mice (34, 60). However, both of these studies utilized HA polymer preparations predominantly >500 kDa in size. With these reports in mind, TLR4 and CD44 were the natural choices for evaluation as candidate HA fragment receptors for the induction of murine HβD2 ortholog MuβD3 (43) in vivo. Both HA-35 (Figs. 4 and 5) and HA-28 (Fig. 6) promote enhanced expression of MuβD3 in the colonic epithelium of adult C57BL/6 mice, whereas no up-regulation of MuβD3 was observed in HA-28-fed mice deficient in TLR4 (Fig. 6). CD44−/− mice exhibited greater base-line MuβD3 expression in comparison to water-fed wild-type mice that was increased further by HA-28 feeding (Fig. 6). Thus, it was evident that expression of TLR4 is required for the in vivo induction of MuβD3 by HA-28, with CD44 possibly having a regulatory role in base-line MuβD3 expression. We found no evidence of altered TLR4 protein expression between wild-type and CD44−/− mice, suggesting that a compensatory relationship between the HA receptors TLR4 and CD44 is unlikely to account for the observed increase in base-line MuβD3 expression among CD44-deficient animals (supplemental Fig. 3). CD44 is thought to contribute to the maintenance of tissue homeostasis (61), and CD44-deficient mice have been associated with a proinflammatory phenotype in some contexts, including LPS-induced septic shock (62). At least one report places CD44 and TLR4 in opposing regulatory positions, with binding of high molecular weight HA to CD44 attenuating TLR4 signaling in response to LPS (63). One possible explanation for increased background MuβD3 expression in CD44−/− mice could be a relative deficiency in prohomeostatic signaling favoring release of inflammatory cytokines (62, 64), several of which are associated with increased defensin expression (9, 14, 15), in the presence of commensal microbes. Despite the many open questions regarding the nature of specific-sized HA signaling in intestinal epithelium, it is now clear that the induction of HβD2 orthologue MuβD3 by specific-sized HA occurs in the intestinal mucosa of live animals and that TLR4 expression is a necessary component of this innate defense response.
Our observations regarding induction of HβD2 by specific-sized HA must certainly reflect an endogenous in vivo process maintained and enhanced by natural selection, yet defining the larger physiologic role of fragmented HA in the digestive tract is difficult. One analogy is that of butyrate and the histone deacetylase inhibitor sulforaphane, dietary compounds found in vegetables of the Brassicaceae family, such as broccoli (16), and the F(ab′)2 fragments of immunoglobulin A secreted in human milk (18), all of which have been demonstrated to promote enhanced HβD2 expression and secretion in intestinal epithelium. HA is ubiquitous in vertebrate animals (65) and is almost certainly consumed routinely in the Western world, and further, HA has recently been reported in both human and bovine milk (66). Although digestive processing of fragmented HA has not been fully evaluated, particularly with regard to alterations in polymer sizing, our results suggest that orally ingested HA-35 remains sufficiently intact within the digestive tract to promote MuβD3 expression in the distal colon of mice (Fig. 4). The GI tract may have limited ability to absorb ingested HA; Balogh et al. (67) have demonstrated that radioactivity is almost entirely recovered in the feces of rats fed radiolabeled high molecular weight HA polymers. Induction of defensin expression by dietary HA could conceivably contribute to shifts in microflora species distribution or quantity, as has been shown to follow altered expression of α-defensins (68). Perhaps more importantly, fragmented HA may have therapeutic potential for patients with diseases affecting the integrity of the intestinal epithelial barrier (69). In Crohn disease, deficient induction of HβD2 in the colonic epithelium (53) may contribute to the loss of barrier integrity and vulnerability to invasive, and proinflammatory, mucosal flora (41).
Supplementary Material
Acknowledgments
We thank Dr. Vince Hascall for critical reading of the manuscript, Dr. Judy Drazba for microscopy assistance, and Ripal Amin and Abeer Alsofyani for assisting in HA sizing analysis. David Hill thanks Drs. Vince Hascall, Jean-Paul Achkar, Ed Maytin, and Ed Greenfield for many useful discussions while serving as his doctoral thesis committee.
This work was supported, in whole or in part, by National Institutes of Health Grant HD061918 (to C. A. de la M.). Carol A. de la Motte and Sean P. Kessler are co-inventors on pending and issued patents held by the Cleveland Clinic. Relating to these technologies, they have a financial interest in Lifecore Biomedical, LLC.

This article contains supplemental Figs. 1–3.
- HβD2
- human β-defensin 2
- PAMP
- pathogen-associated molecular pattern
- HA
- hyaluronan
- TLR
- Toll-like receptor
- HA-35
- 35-kDa hyaluronan fragment preparation
- HA-4.7
- 4.7-kDa hyaluronan fragment preparation
- HA-16
- 16-kDa hyaluronan fragment preparation
- HA-74
- 74-kDa hyaluronan fragment preparation
- HA-2M
- 2000-kDa hyaluronan fragment preparation
- HA-28
- 28.6-kDa hyaluronan fragment preparation
- MuβD3
- murine β-defensin 3
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- HBSS
- Hanks' balanced salt solution.
REFERENCES
- 1. Turner J. R. (2009) Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 [DOI] [PubMed] [Google Scholar]
- 2. Underwood M. A., Bevins C. L. (2010) Defensin-barbed innate immunity. Clinical associations in the pediatric population. Pediatrics 125, 1237–1247 [DOI] [PubMed] [Google Scholar]
- 3. Menendez A., Brett Finlay B. (2007) Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 19, 385–391 [DOI] [PubMed] [Google Scholar]
- 4. Seo E. S., Vargues T., Clarke D. J., Uhrín D., Campopiano D. J. (2009) Preparation of isotopically labeled recombinant β-defensin for NMR studies. Protein Expr. Purif. 65, 179–184 [DOI] [PubMed] [Google Scholar]
- 5. Zasloff M. (2002) Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- 6. Selsted M. E., Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 [DOI] [PubMed] [Google Scholar]
- 7. Garreis F., Schlorf T., Worlitzsch D., Steven P., Bräuer L., Jäger K., Paulsen F. P. (2010) Roles of human β-defensins in innate immune defense at the ocular surface. Arming and alarming corneal and conjunctival epithelial cells. Histochem. Cell Biol. 134, 59–73 [DOI] [PubMed] [Google Scholar]
- 8. Bevins C. L., Salzman N. H. (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 [DOI] [PubMed] [Google Scholar]
- 9. O'Neil D. A. (2003) Regulation of expression of β-defensins. Endogenous enteric peptide antibiotics. Mol. Immunol. 40, 445–450 [DOI] [PubMed] [Google Scholar]
- 10. Frye M., Bargon J., Lembcke B., Wagner T. O., Gropp R. (2000) Differential expression of human α- and β-defensins mRNA in gastrointestinal epithelia. Eur. J. Clin. Invest. 30, 695–701 [DOI] [PubMed] [Google Scholar]
- 11. Wada A., Mori N., Oishi K., Hojo H., Nakahara Y., Hamanaka Y., Nagashima M., Sekine I., Ogushi K., Niidome T., Nagatake T., Moss J., Hirayama T. (1999) Induction of human β-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem. Biophys. Res. Commun. 263, 770–774 [DOI] [PubMed] [Google Scholar]
- 12. Ogushi K., Wada A., Niidome T., Mori N., Oishi K., Nagatake T., Takahashi A., Asakura H., Makino S., Hojo H., Nakahara Y., Ohsaki M., Hatakeyama T., Aoyagi H., Kurazono H., Moss J., Hirayama T. (2001) Salmonella enteritidis FliC (flagella filament protein) induces human β-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 276, 30521–30526 [DOI] [PubMed] [Google Scholar]
- 13. Vora P., Youdim A., Thomas L. S., Fukata M., Tesfay S. Y., Lukasek K., Michelsen K. S., Wada A., Hirayama T., Arditi M., Abreu M. T. (2004) β-Defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 173, 5398–5405 [DOI] [PubMed] [Google Scholar]
- 14. O'Neil D. A., Porter E. M., Elewaut D., Anderson G. M., Eckmann L., Ganz T., Kagnoff M. F. (1999) Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 [PubMed] [Google Scholar]
- 15. Froy O. (2005) Regulation of mammalian defensin expression by Toll-like receptor-dependent and -independent signaling pathways. Cell. Microbiol. 7, 1387–1397 [DOI] [PubMed] [Google Scholar]
- 16. Schwab M., Reynders V., Loitsch S., Steinhilber D., Schröder O., Stein J. (2008) The dietary histone deacetylase inhibitor sulforaphane induces human β-defensin-2 in intestinal epithelial cells. Immunology 125, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlee M., Harder J., Köten B., Stange E. F., Wehkamp J., Fellermann K. (2008) Probiotic lactobacilli and VSL#3 induce enterocyte β-defensin 2. Clin. Exp. Immunol. 151, 528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrera G. J., Portillo R., Mijares A., Rocafull M. A., del Castillo J. R., Thomas L. E. (2009) Immunoglobulin A with protease activity secreted in human milk activates PAR-2 receptors, of intestinal epithelial cells HT-29, and promotes β-defensin-2 expression. Immunol. Lett. 123, 52–59 [DOI] [PubMed] [Google Scholar]
- 19. Singh P. K., Jia H. P., Wiles K., Hesselberth J., Liu L., Conway B. A., Greenberg E. P., Valore E. V., Welsh M. J., Ganz T., Tack B. F., McCray P. B. (1998) Production of β-defensins by human airway epithelia. Proc. Natl. Acad. Sci. U.S.A. 95, 14961–14966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antcheva N., Boniotto M., Zelezetsky I., Pacor S., Verga Falzacappa M. V., Crovella S., Tossi A. (2004) Effects of positively selected sequence variations in human and Macaca fascicularis β-defensins 2 on antimicrobial activity. Antimicrob. Agents Chemother. 48, 685–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harder J., Bartels J., Christophers E., Schröder J. M. (1997) A peptide antibiotic from human skin. Nature 387, 861. [DOI] [PubMed] [Google Scholar]
- 22. Feng Z., Jiang B., Chandra J., Ghannoum M., Nelson S., Weinberg A. (2005) Human β-defensins. Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 84, 445–450 [DOI] [PubMed] [Google Scholar]
- 23. Ogushi K., Wada A., Niidome T., Okuda T., Llanes R., Nakayama M., Nishi Y., Kurazono H., Smith K. D., Aderem A., Moss J., Hirayama T. (2004) Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human β-defensin-2 expression in Caco-2 cells. J. Biol. Chem. 279, 12213–12219 [DOI] [PubMed] [Google Scholar]
- 24. Kawai T., Akira S. (2005) Toll-like receptor downstream signaling. Arthritis Res. Ther. 7, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Platz J., Beisswenger C., Dalpke A., Koczulla R., Pinkenburg O., Vogelmeier C., Bals R. (2004) Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J. Immunol. 173, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 26. Voss E., Wehkamp J., Wehkamp K., Stange E. F., Schröder J. M., Harder J. (2006) NOD2/CARD15 mediates induction of the antimicrobial peptide human β-defensin-2. J. Biol. Chem. 281, 2005–2011 [DOI] [PubMed] [Google Scholar]
- 27. Stern R., Kogan G., Jedrzejas M. J., Soltés L. (2007) The many ways to cleave hyaluronan. Biotechnol. Adv. 25, 537–557 [DOI] [PubMed] [Google Scholar]
- 28. Stern R., Asari A. A., Sugahara K. N. (2006) Hyaluronan fragments. An information-rich system. Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 29. Noble P. W., Lake F. R., Henson P. M., Riches D. W. (1993) Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-α-dependent mechanism in murine macrophages. J. Clin. Invest. 91, 2368–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noble P. W., McKee C. M., Cowman M., Shin H. S. (1996) Hyaluronan fragments activate an NF-κ B/I-κB α autoregulatory loop in murine macrophages. J. Exp. Med. 183, 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Termeer C. C., Hennies J., Voith U., Ahrens T., Weiss J. M., Prehm P., Simon J. C. (2000) Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 165, 1863–1870 [DOI] [PubMed] [Google Scholar]
- 32. Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J. C. (2002) Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor K. R., Trowbridge J. M., Rudisill J. A., Termeer C. C., Simon J. C., Gallo R. L. (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 279, 17079–17084 [DOI] [PubMed] [Google Scholar]
- 34. Zheng L., Riehl T. E., Stenson W. F. (2009) Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology 137, 2041–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gariboldi S., Palazzo M., Zanobbio L., Selleri S., Sommariva M., Sfondrini L., Cavicchini S., Balsari A., Rumio C. (2008) Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. J. Immunol. 181, 2103–2110 [DOI] [PubMed] [Google Scholar]
- 36. Bhilocha S., Amin R., Pandya M., Yuan H., Tank M., LoBello J., Shytuhina A., Wang W., Wisniewski H. G., de la Motte C., Cowman M. K. (2011) Agarose and polyacrylamide gel electrophoresis methods for molecular mass analysis of 5–500-kDa hyaluronan. Anal. Biochem. 417, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aksamitiene E., Hoek J. B., Kholodenko B., Kiyatkin A. (2007) Multistrip Western blotting to increase quantitative data output. Electrophoresis 28, 3163–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 39. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid/polycytidylic acid/inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 163, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peterson E., Miller Y., Atchue K., Margeson L., Yandl E. (2008) Your paper towels can do what? Histologic 41, 38–41 [Google Scholar]
- 41. Wehkamp J., Koslowski M., Wang G., Stange E. F. (2008) Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol. 1, S67–S74 [DOI] [PubMed] [Google Scholar]
- 42. Beutler B., Rietschel E. T. (2003) Innate immune sensing and its roots. The story of endotoxin. Nat. Rev. Immunol. 3, 169–176 [DOI] [PubMed] [Google Scholar]
- 43. Bals R., Wang X., Meegalla R. L., Wattler S., Weiner D. J., Nehls M. C., Wilson J. M. (1999) Mouse β-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67, 3542–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Medema J. P., Vermeulen L. (2011) Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474, 318–326 [DOI] [PubMed] [Google Scholar]
- 45. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 46. de La Motte C. A., Hascall V. C., Calabro A., Yen-Lieberman B., Strong S. A. (1999) Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I·C). J. Biol. Chem. 274, 30747–30755 [DOI] [PubMed] [Google Scholar]
- 47. Siegelman M. H., DeGrendele H. C., Estess P. (1999) Activation and interaction of CD44 and hyaluronan in immunological systems. J. Leukoc. Biol. 66, 315–321 [DOI] [PubMed] [Google Scholar]
- 48. Bourguignon L. Y., Ramez M., Gilad E., Singleton P. A., Man M. Q., Crumrine D. A., Elias P. M., Feingold K. R. (2006) Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J. Invest. Dermatol. 126, 1356–1365 [DOI] [PubMed] [Google Scholar]
- 49. Wolny P. M., Banerji S., Gounou C., Brisson A. R., Day A. J., Jackson D. G., Richter R. P. (2010) Analysis of CD44-hyaluronan interactions in an artificial membrane system. Insights into the distinct binding properties of high and low molecular weight hyaluronan. J. Biol. Chem. 285, 30170–30180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Campo G. M., Avenoso A., Campo S., D'Ascola A., Nastasi G., Calatroni A. (2010) Molecular size hyaluronan differently modulates Toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie 92, 204–215 [DOI] [PubMed] [Google Scholar]
- 51. Lee S. K., Il Kim T., Kim Y. K., Choi C. H., Yang K. M., Chae B., Kim W. H. (2005) Cellular differentiation-induced attenuation of LPS response in HT-29 cells is related to the down-regulation of TLR4 expression. Biochem. Biophys. Res. Commun. 337, 457–463 [DOI] [PubMed] [Google Scholar]
- 52. Lehmann J., Retz M., Harder J., Krams M., Kellner U., Hartmann J., Hohgräwe K., Raffenberg U., Gerber M., Loch T., Weichert-Jacobsen K., Stöckle M. (2002) Expression of human β-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect. Dis. 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wehkamp J., Fellermann K., Herrlinger K. R., Baxmann S., Schmidt K., Schwind B., Duchrow M., Wohlschläger C., Feller A. C., Stange E. F. (2002) Human β-defensin 2 but not β-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 14, 745–752 [DOI] [PubMed] [Google Scholar]
- 54. Arnett E., Lehrer R. I., Pratikhya P., Lu W., Seveau S. (2011) Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell. Microbiol. 13, 635–651 [DOI] [PubMed] [Google Scholar]
- 55. Zhuravel E., Shestakova T., Efanova O., Yusefovich Y., Lytvin D., Soldatkina M., Pogrebnoy P. (2011) Human β-defensin-2 controls cell cycle in malignant epithelial cells. In vitro study. Exp. Oncol. 33, 114–120 [PubMed] [Google Scholar]
- 56. Sharma R., Young C., Neu J. (2010) Molecular modulation of intestinal epithelial barrier. Contribution of microbiota. J. Biomed. Biotechnol. 2010, 305879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stöber H., Maier E., Schmidt H. (2010) Protective effects of Lactobacilli, Bifidobacteria, and Staphylococci on the infection of cultured HT29 cells with different enterohemorrhagic Escherichia coli serotypes are strain-specific. Int. J. Food Microbiol. 144, 133–140 [DOI] [PubMed] [Google Scholar]
- 58. Yang D., Liu Z. H., Tewary P., Chen Q., de la Rosa G., Oppenheim J. J. (2007) Defensin participation in innate and adaptive immunity. Curr. Pharm. Des. 13, 3131–3139 [DOI] [PubMed] [Google Scholar]
- 59. Heldin P., Karousou E., Bernert B., Porsch H., Nishitsuka K., Skandalis S. S. (2008) Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect. Tissue Res. 49, 215–218 [DOI] [PubMed] [Google Scholar]
- 60. Asari A., Kanemitsu T., Kurihara H. (2010) Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J. Biol. Chem. 285, 24751–24758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Entwistle J., Hall C. L., Turley E. A. (1996) HA receptors. Regulators of signaling to the cytoskeleton. J. Cell Biochem. 61, 569–577 [DOI] [PubMed] [Google Scholar]
- 62. Liang J., Jiang D., Griffith J., Yu S., Fan J., Zhao X., Bucala R., Noble P. W. (2007) CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J. Immunol. 178, 2469–2475 [DOI] [PubMed] [Google Scholar]
- 63. Muto J., Yamasaki K., Taylor K. R., Gallo R. L. (2009) Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol. Immunol. 47, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamada S., Sugahara K., Ozbek S. (2011) Evolution of glycosaminoglycans. Comparative biochemical study. Commun. Integr. Biol. 4, 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kawana H., Karaki H., Higashi M., Miyazaki M., Hilberg F., Kitagawa M., Harigaya K. (2008) CD44 suppresses TLR-mediated inflammation. J. Immunol. 180, 4235–4245 [DOI] [PubMed] [Google Scholar]
- 66. Coppa G. V., Gabrielli O., Buzzega D., Zampini L., Galeazzi T., Maccari F., Bertino E., Volpi N. (2011) Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology 21, 295–303 [DOI] [PubMed] [Google Scholar]
- 67. Balogh L., Polyak A., Mathe D., Kiraly R., Thuroczy J., Terez M., Janoki G., Ting Y., Bucci L. R., Schauss A. G. (2008) Absorption, uptake, and tissue affinity of high molecular weight hyaluronan after oral administration in rats and dogs. J. Agric. Food Chem. 56, 10582–10593 [DOI] [PubMed] [Google Scholar]
- 68. Salzman N. H., Hung K., Haribhai D., Chu H., Karlsson-Sjöberg J., Amir E., Teggatz P., Barman M., Hayward M., Eastwood D., Stoel M., Zhou Y., Sodergren E., Weinstock G. M., Bevins C. L., Williams C. B., Bos N. A. (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 11, 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Neill L. A. (2009) A feed-forward loop involving hyaluronic acid and Toll-like receptor-4 as a treatment for colitis? Gastroenterology 137, 1889–1891 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.