Background: Despite the importance of CD163 as a hemoglobin scavenger receptor, precisely how CD163 is regulated remains unknown.
Results: α1-Acid glycoprotein (AGP) activates the toll-like receptor-4/CD14-dependent autocrine regulatory loops of IL-6 and/or IL-10, leading to an enhancement in CD163.
Conclusion: AGP protects against hemoglobin-induced oxidative stress via the up-regulation of CD163 during hemolysis.
Significance: AGP is a novel regulator of CD163.
Keywords: Atherosclerosis, Hemoglobin, Macrophages, Oxidative Stress, Toll-like Receptors (TLR), CD163, α1-Acid Glycoprotein, Hemolysis
Abstract
CD163, a scavenger receptor that is expressed at high levels in the monocyte-macrophage system, is a critical factor for the efficient extracellular hemoglobin (Hb) clearance during hemolysis. Because of the enormous detrimental effect of liberated Hb on our body by its ability to induce pro-inflammatory signals and tissue damage, an understanding of the molecular mechanisms associated with CD163 expression during the acute phase response is a central issue. We report here that α1-acid glycoprotein (AGP), an acute phase protein, the serum concentration of which is elevated under various inflammatory conditions, including hemolysis, up-regulates CD163 expression in both macrophage-like differentiated THP-1 (dTHP-1) cells and peripheral blood mononuclear cells in a time- and concentration-dependent manner. Moreover, the subsequent induction of Hb uptake was also observed in AGP-treated dTHP-1 cells. Among representative acute phase proteins such as AGP, α1-antitrypsin, C-reactive protein, and haptoglobin, only AGP increased CD163 expression, suggesting that AGP plays a specific role in the regulation of CD163. Consistently, the physiological concentrations of AGP induced CD163, and the subsequent induction of Hb uptake as well as the reduction of oxidative stress in plasma were observed in phenylhydrazine-induced hemolytic model mice, confirming the in vivo role of AGP. Finally, AGP signaling through the toll-like receptor-4 (TLR4) and CD14, the common innate immune receptor complex that normally recognizes bacterial components, was identified as a crucial stimulus that induces the autocrine regulatory loops of IL-6 and/or IL-10 via NF-κB, p38, and JNK pathways, which leads to an enhancement in CD163 expression. These findings provide possible insights into how AGP exerts anti-inflammatory properties against hemolysis-induced oxidative stress.
Introduction
The release of hemoglobin (Hb) is a physiological phenomenon associated with intravascular and intraplaque hemolysis. The liberated Hb has potent pro-inflammatory properties and, under appropriate conditions, can cause tissue damage and the oxidation of low density lipoproteins (1–3), because free radicals and altered Hb products are generated during the reaction between Hb-heme and physiological oxidants such as H2O2 (4). Therefore, the body has developed a system that efficiently scavenges Hb liberated from erythrocytes. Haptoglobin (Hp),2 an acute phase plasma protein, provides the first line of defense against the effects of liberated Hb (5). When Hb binds to Hp, Hb remains sequestered within the intravascular compartment, thus attenuating Hb-mediated vasoactivity, oxidative toxicity, and tissue peroxidation (6, 7). Upon complex formation, Hp transforms the Hb αβ-dimer into a high affinity ligand that interacts with the Hb scavenger receptor CD163, which then clears the Hb-Hp complex from the circulation (8). In addition, after the depletion of plasma Hp or a massive Hb release after erythrocyte extravasation as the result of a tissue injury, Hp-independent, low affinity Hb binding to CD163 can be observed (9). Thus, because CD163 plays a critical role in protecting against Hb-induced tissue damage during hemolysis, it is important to understand the factors that are responsible for regulating CD163.
CD163 is exclusively expressed on circulating monocytes and tissue macrophages (10). In addition, particularly high levels of CD163 have been detected in infiltrating monocytes during the resolution phase of inflammatory reactions (11). The expression of CD163 is regulated by several factors. For example, anti-inflammatory mediators such as glucocorticoids and IL-10 are particularly potent inducers (12–14). However, this is not a common feature of all anti-inflammatory cytokines because IL-4 and IL-13 do not cause an increase in CD163 expression (15, 16). In contrast, pro-inflammatory cytokines (TNF-α and IFN-γ) and the chemokine CXCL4 suppress CD163 expression (12, 16, 17). Furthermore, the pleiotropic cytokines IL-6 and TGF-β, which are both known to exert pro- and anti-inflammatory effects, enhance and suppress the cellular surface expression of CD163, respectively (16, 18). To date, little is known about an endogenous molecule that regulates these cytokines, thus increasing CD163 expression.
α1-Acid glycoprotein (AGP), also referred to a orosomucoid, is an acute phase protein with a molecular mass of 41–43 kDa and is heavily glycosylated (45%) (19). It is biosynthesized by hepatocytes but is also produced by extrahepatic sites such as leukocytes and endothelial cells during an acute phase response, due to the systemic response to a local inflammatory stimulus (20). As a result, the serum concentration of AGP in normal conditions (0.5 mg/ml) is increased by 2–5-fold during an acute phase situation. In the case of hemolysis, it has also been reported that the plasma concentration of AGP is remarkably elevated, as is that of Hp in phenylhydrazine-induced hemolytic mice (21). However, unlike Hp, the physiological function of AGP during hemolysis is not well understood. It is generally thought that AGP possesses anti-inflammatory and immunomodulatory properties (22–25). These experimental findings led us to hypothesize that the local production of AGP at the site of the initial acute phase reaction contributes to the maintenance of homeostasis by reducing the tissue damage associated with an inflammatory process, including hemolysis. However, the molecular mechanism of this process remains poorly understood, largely because the molecular targets that exert these biological functions are unknown.
In this study, to explore the biological function of AGP during hemolysis, the focus was on whether AGP influences the expression of CD163 and exerts a protective role against hemolysis-induced oxidative stress. The effects of AGP on CD163 expression and its mechanism were examined using human monocytic THP-1 cells, human peripheral blood mononuclear cells, and differentiated macrophages like THP-1 (dTHP-1) cells. Furthermore, the suppressive role of AGP on the intravascular oxidative stress via CD163 up-regulation was confirmed using phenylhydrazine-induced hemolytic model mice.
EXPERIMENTAL PROCEDURES
Materials and Animals
Human AGP, human α1-antitrypsin (AAT), human C-reactive protein (CRP), human Hp, human Hb, phorbol 12-myristate 13-acetate, RPMI 1640 medium, lipopolysaccharide, cycloheximide, polymyxin B, β-actin antibody, and fetal bovine serum were purchased from Sigma. Anti-human CD163 antibody (EDHu-1) and anti-human TLR4 antibody (HTA125) were purchased from AbD Serotec (Oxford, UK). The anti-human CD14, anti-human IL-10Rα, anti-human IL-6R antibodies, and isotype control IgG were purchased from R&D Systems, Inc (Minneapolis, MN). The anti-phospho-IkBα, anti-IkBα, anti-phospho-p38, anti-p38, anti-phospho-JNK, anti-JNK, anti-phospho-ERK1/2, and anti-ERK1/2 antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). HRP-goat anti-mouse IgG (H+L) and HRP-goat anti-rabbit IgG (H+L) were purchased from Invitrogen. The animals were maintained under conventional housing conditions. All animal experiments were carried out in accordance with the guideline principles and procedures of Kumamoto University for the care and use of laboratory animals.
Cell Cultures
The human monocytic cell line THP-1 and peripheral blood mononuclear cells (PBMC) were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 100 units of penicillin/ml, and 100 μg of streptomycin/ml. Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Wako) supplemented with 10% FBS and antibiotics. Cells were maintained in a humidified atmosphere of 5% CO2 in air at 37 °C. To obtain macrophage-like differentiated THP-1 cells (dTHP-1), THP-1 cells were exposed to 50 nm phorbol 12-myristate 13-acetate in the culture medium for 48 h. After the incubation, the phorbol 12-myristate 13-acetate-containing medium was aspirated, and adherent (differentiated) cells were resuspended in fresh culture medium and incubated for an additional 24 h. PBMC were obtained from healthy volunteer donors. Informed written consent was obtained from all donors. PBMC were isolated from heparinized venous blood by following the recommended protocols using Ficoll-PaqueTM PLUS (GE Healthcare). Briefly, the whole blood was mixed with 0.9% sodium chloride containing 3% dextran 500 (Sigma) and incubated at room temperature for 30 min to sediment the erythrocytes. After dextran sedimentation, the supernatant was centrifuged at 1,800 rpm for 10 min, and cells were then resuspend in 0.9% sodium chloride, underlaid with Ficoll-PaqueTM PLUS, and centrifuged at 2,800 rpm for 30 min. PBMC recovered from the buffy coat were washed twice in 0.9% sodium chloride and then resuspended in RPMI 1640 medium.
Quantitative Real Time RT-PCR
Total RNA from the cells was isolated using RNAiso PLUS (TaKaRa Bio Inc., Shiga, Japan) according to the recommended protocol. The synthesis of cDNA was performed using PrimeScript® RT master mix (Perfect Real Time) (TaKaRa Bio Inc.). Quantitative real time RT-PCR analysis of CD163, IL-6, IL-10, IFN-γ, TNF-α, heme oxygenase-1, and 18 S ribosomal RNA (18 S rRNA) was performed in an iCycler thermal cycler (Bio-Rad) with an iQ5 qRT-PCR detection system attached (Bio-Rad) using SYBR® Premix Ex TaqTM (Perfect Real Time) (TaKaRa Bio Inc.). PCR amplifications were performed under the following conditions: 95 °C for 3 min, for 40 cycles at 95 °C for 10 s (denaturation step), at 60 °C for 1 min (annealing/extension steps). The primers used are shown in supplemental Table 1. The threshold cycle (Ct) values for each gene amplification were then normalized by subtracting the Ctvalue calculated for 18 S rRNA. The normalized gene expression values were expressed as the relative quantity of gene-specific mRNA compared with 18 S rRNA (fold induction).
Western Blotting
Confluent (90–100%) cells grown on 60-mm dishes were washed twice with ice-cold PBS, lysed at 4 °C in RIPA buffer (150 mm NaCl, 1% Nonidet P-40, 0.15% sodium deoxycholate, 0.1% SDS, 50 mm Tris (pH 8.0), and a 1% protease inhibitor mixture (Nakalai Tesque, Kyoto, Japan)), and centrifuged at 12,000 × g for 10 min at 4 °C. Cell lysates were subjected to SDS-PAGE on 10% polyacrylamide gel. Proteins were electroblotted to a PVDF membrane (Millipore Corp., Bedford, MA). After blocking in 5% skim milk, the membranes were washed three times and treated with the primary antibody for 2 h. After washing three times, the membrane was incubated with a HRP-conjugated secondary antibody for 2 h. Bands were detected using the SuperSignal West Pico Chemiluminescent Substrate (Pierce) or ECL Advance (GE Healthcare), according to the manufacturer's description.
ELISA
After treating the cells with AGP or LPS for 24 h, the culture medium was centrifuged at 1,000 × g for 5 min. Supernatants were collected and analyzed using an ELISA kit for TNF-α, IL-6, and IL-10 (BioLegend, San Diego) following the manufacturer's recommended protocol.
Flow Cytometry
Hb was labeled with fluorescein isothiocyanate (FITC). Hb and FITC were dissolved in 0.1 m NaHCO3 (pH 9.5), followed by mixing for 2 h at room temperature. The solution was desalted by means of a PD-10 desalting column (GE Healthcare). FITC-labeled Hb (FITC-Hb) were pretreated with Hp for 10 min, followed by adding dTHP-1. After incubating at 37 °C for 30 min, dTHP-1 cells were washed twice with ice-cold PBS, and detached by treatment with 0.25% trypsin/EDTA. The resulting cells were analyzed by FACSCalibur (BD Biosciences) using a 488 nm argon laser.
Animal Experiments
5- to 6-week-old ddY, C3H/HeN, and C3H/HeJ Yok mice (Japan SLC, Shizuoka Japan) were intravenously injected with saline or AGP (5 mg/200 μl saline/animal). Hemolysis was induced by the intraperitoneal administration (1 mg/10 g of body weight) of a fleshly prepared phenylhydrazine solution. For administration of anti-CD163 antibody (Santa Cruz Biotechnology) to phenylhydrazine-induced hemolytic mice, 40 μg/mouse anti-CD163 antibody was peritoneally administered into mice 1 h before phenylhydrazine administration. As a negative control, isotype-matched control IgG (Santa Cruz Biotechnology) was administered at the same time point. Phenylhydrazine hydrochloride was dissolved in PBS at a concentration of 10 mg/ml, and the pH was adjusted to pH 7.4 with NaOH. For quantitative real time RT-PCR and Western blotting, liver tissue was dissolved in RNAiso PLUS and RIPA buffer, respectively. Blood was collected in heparinized tubes using an SV Total RNA Isolation System (Promega). For the TBARS assay and derivatives of reactive oxidative metabolites, plasma from blood collected in heparinized capillary tubes was assayed using a TBARS assay kit (Cayman Chemical Co., Ann Arbor, MI) and FREE carpe diem (Diacron International s. r. l., Grosseto, Italy) following the manufacturer's recommended protocols. All animal experiments were carried out in accordance with the guideline principles and procedures of Kumamoto University for the care and use of laboratory animals.
Data Analyses
Data are shown as the means ± S.D. for the indicated number of samples. The overall differences between groups were determined by one-way of analysis of variance.
RESULTS
Up-regulation of CD163 by AGP
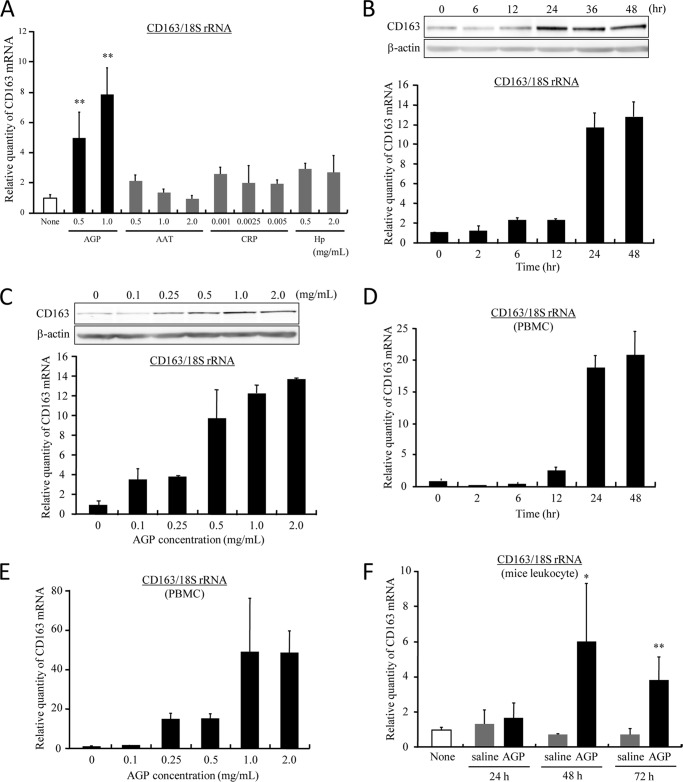
During hemolysis, the concentration of acute phase proteins becomes elevated in response to Hb-induced oxidative inflammation (21). To evaluate the effect of acute phase proteins on the expression of CD163, dTHP-1 cells were treated with four types of representative acute phase proteins (AGP, CRP, AAT, and Hp) at the concentration observed in normal or acute phase conditions, respectively (Fig. 1A). Interestingly, the expression of CD163 mRNA was significantly up-regulated only by AGP, although the other proteins had no effect on induction. The expression of CD163 mRNA and protein was enhanced by AGP in a time- and concentration-dependent manner (Fig. 1, B and C). In particular, it was increased from the normal serum concentration (0.5 mg/ml) and saturated at the acute phase concentration range (1.0–2.0 mg/ml) (Fig. 1C). In the case of human PBMC, CD163 mRNA expression was also up-regulated in a time- and concentration-dependent manner as well as dTHP-1 (Fig. 1, D and E). Similar results were obtained for nondifferentiated THP-1 (supplemental Fig. 1). Moreover, when AGP was intravenously injected into normal mice, the production of CD163 mRNA was also induced in mice leukocytes after 48 h (Fig. 1F).
FIGURE 1.
AGP enhances CD163 expression. A, dTHP-1 cells were treated with AGP, AAT, CRP, and Hp for 24 h at normal or acute phase concentrations. The level of expression of CD163 was evaluated by quantitative RT-PCR. Results are the means ± S.D. of three separate experiments. Statistically significant induction was compared with not treated cells (**, p < 0.01) (None). B, dTHP-1 cells were treated with AGP (1.0 mg/ml) each time. C, dTHP-1 cells were treated for 24 h at each concentration. The expression level of CD163 was evaluated by Western blotting (upper panel) and quantitative RT-PCR (lower panel). D, human PBMC were treated with AGP (1.0 mg/ml) each time. E, human PBMC were treated for 24 h at each concentration. F, ddY mice were injected intravenously with saline or AGP (5 mg/100 μl saline/body). After each of the indicated times, blood was collected from the inferior vena cava. The expression of CD163 in leukocytes was evaluated by quantitative RT-PCR. Results are the means ± S.D. of 4–5 separate experiments. Statistically significant induction was compared with AGP (**, p < 0.01; *, p < 0.05).
AGP Protects against Hemolysis-induced Oxidative Stress via Enhancing CD163 Expression
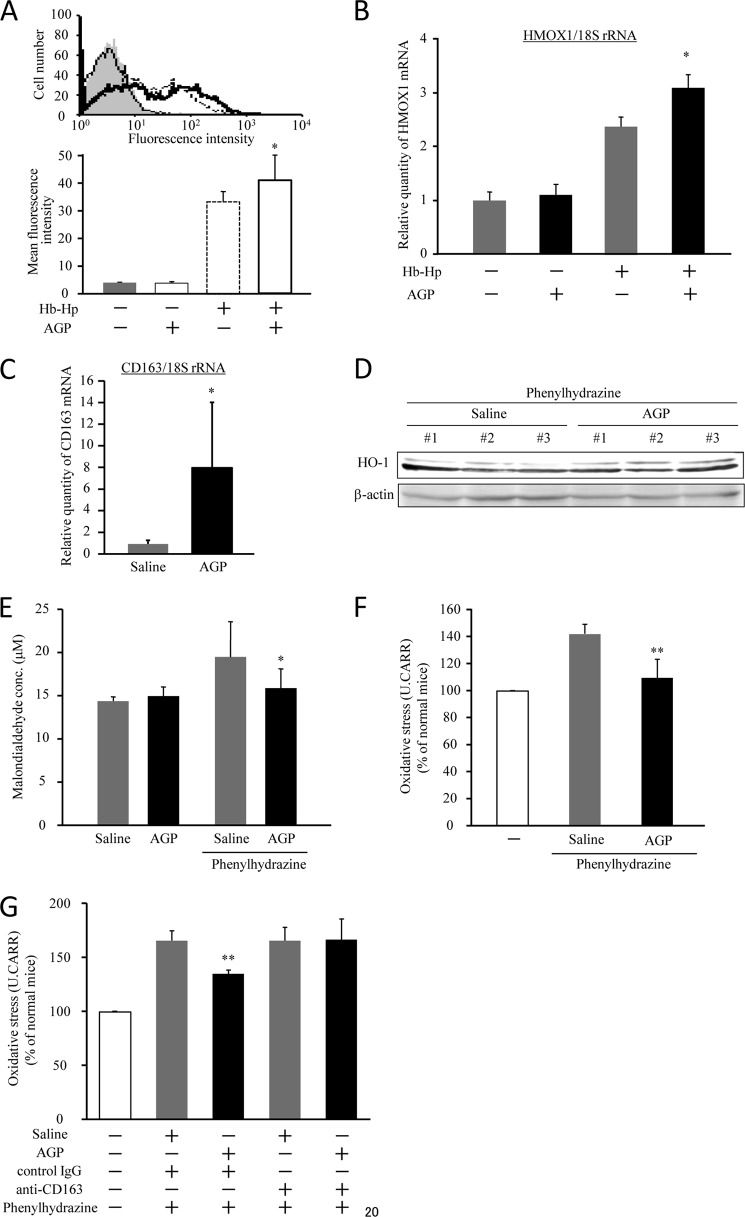
As described above, heme derived from liberated Hb in plasma has a potent oxidizing effect that can induce considerable tissue damage. If AGP accelerates the uptake of Hb via the induction of CD163, it would be possible to reduce hemolysis-induced oxidative stress. Thus, in the next series of experiments, we evaluated the cytoprotective effect conferred by AGP due to augmenting the uptake of Hb. To characterize the uptake of Hb by dTHP-1 cells, we performed a flow cytometric assay using FITC-labeled Hb. An elevated Hb uptake was observed in AGP-treated cells (Fig. 2A). Moreover, we indirectly measured Hb uptake to evaluate the transcriptional induction of heme oxygenase-1 (HO-1), which is induced to metabolize heme following Hb uptake. As shown in Fig. 2B, a significant induction of HO-1 was observed in AGP-treated cells. These data indicate that AGP facilitates the uptake of liberated Hb.
FIGURE 2.
AGP decreases intravascular oxidative stress by up-regulation of CD163. A, dTHP-1 cells were treated with AGP for 24 h and then incubated with FITC-Hb (1 μg/ml) in the presence of equimolar Hp for 30 min. The uptake of FITC-Hb was assessed by a flow cytometer. The results are the means ± S.D. of five separate experiments (lower panel). Statistically significant induction was compared with Hb-Hp in the absence of AGP (*, p < 0.05). A representative experiment is shown (upper panel). Each histogram indicates untreated (gray filled), AGP (solid line), FITC-Hb-Hp (dotted line), and AGP + FITC-Hb-Hp (bold line), respectively. B, dTHP-1 cells were treated with AGP for 24 h and incubated with Hb (1 μg/ml) in the presence of an equimolar amount of Hp for 4 h. The level of expression of HO-1 mRNA was evaluated by quantitative RT-PCR. The results are the means ± S.D. of six separate experiments. Statistically significant induction was compared with Hb-Hp in the absence of AGP (*, p < 0.05). C–F, ddY mice were injected intravenously with saline or AGP (5 mg/100 μl saline/body) and subsequently administrated phenylhydrazine by intraperitoneal injection after 48 h. 24 h after phenylhydrazine administration, liver and blood were collected. The expression of CD163 (C) and HO-1 (D) in liver was evaluated by quantitative RT-PCR and Western blotting, respectively. Oxidative stress in plasma was measured by means of a TBARS assay (E) and a derivatives of reactive oxidative metabolites test (F). For administration of anti-CD163 antibody (Santa Cruz Biotechnology) to phenylhydrazine-induced hemolytic mice, 40 μg/mouse anti-CD163 antibody was intraperitoneally administered into mice 1 h before phenylhydrazine administration. As a negative control, isotype matched control IgG (G). Results are the means ± S.D. of 4–5 separate experiments. Statistically significant reduction was compared with saline-administered hemolytic model mice (**, p < 0.01; *, p < 0.05).
Furthermore, we investigated the issue of whether AGP has the ability to suppress oxidative stress derived from liberated Hb by causing an increase of CD163 expression using phenylhydrazine-induced acute hemolytic model mice. To construct the hemolysis model, phenylhydrazine was administered intraperitoneally to cause hemolysis at 48 h after AGP or a saline administration. Based on blood parameters, such as hematocrit, hemoglobin, and red blood cell counts, and after another 24 h, the phenylhydrazine administration groups were judged to be in a state of hemolysis (supplemental Table 2). Moreover, the administration of AGP caused a further significant decrease in these blood parameters in comparison with saline group (supplemental Table 2). In these conditions, we estimated the expression levels of CD163 and HO-1 in the liver. Both CD163 and HO-1 were significantly induced in hemolytic model mice that received the AGP treatment. We also evaluated oxidative stress in plasma of the hemolytic model mice using a TBARS assay to estimate the extent of lipid peroxidation and a derivatives of reactive oxidative metabolites test to assay hydroperoxide levels of reactive oxygen metabolites. Each assay showed that AGP significantly decreased oxidative stress in the hemolysis-induced group (Fig. 2, E and F). To demonstrate the essential role of CD163 for AGP-induced attenuation of oxidative stress, we administered an anti-CD163 antibody to phenylhydrazine-induced hemolytic mice. 40 μg/mouse anti-CD163 antibody was peritoneally administered into mice 1 h before phenylhydrazine administration. As a negative control, we used isotype-matched control IgG. As a result, administration of anti-CD163 antibody completely suppressed the attenuation of plasma oxidative stress by AGP administration, whereas administration of control IgG did not (Fig. 2G). Taken together, these data indicate that AGP enhances the scavenging of liberated Hb from the blood circulation through the up-regulation of CD163 and hence exerts a protective role against hemolysis-derived oxidative stress.
Enhancement of CD163 Expression through Induction of IL-6 and IL-10
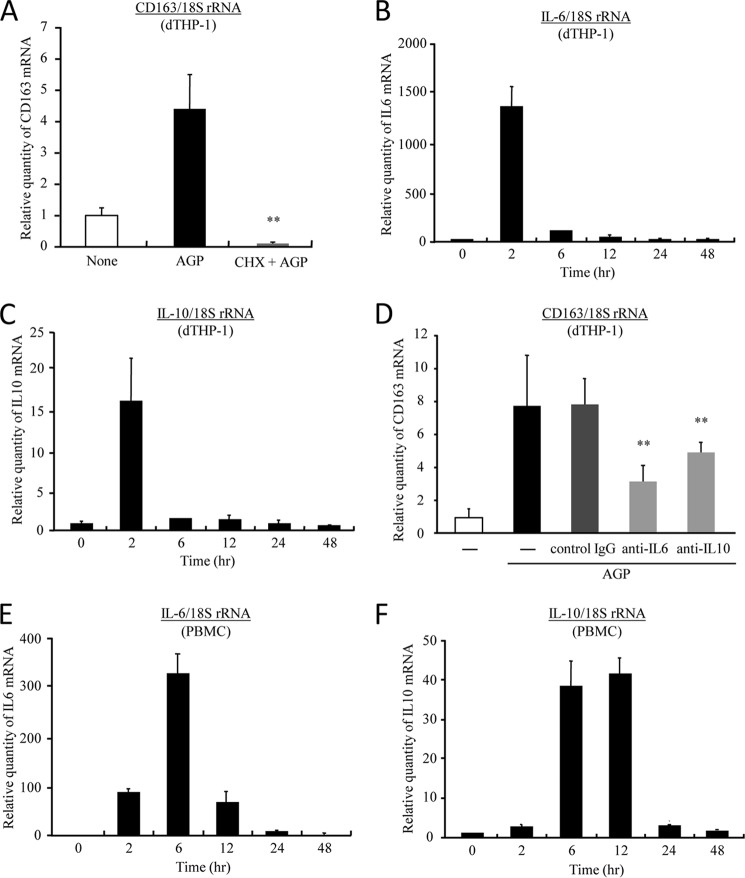
Next, to verify whether the regulation of CD163 by AGP is direct or indirect, a cycloheximide chase assay was carried out in dTHP-1 cells. By pretreatment with cycloheximide, the expression of CD163 mRNA was remarkably inhibited, indicating that CD163 is indirectly regulated by AGP (Fig. 3A). The levels of IL-6 and IL-10, which are well known as CD163 up-regulating factors, were both increased at 2 h after the AGP treatment (Fig. 3, B and C). In addition, both the neutralizing antibody of IL-6 or IL-10 receptors significantly inhibited the AGP-induced up-regulation of CD163 (Fig. 3D). These data indicate that AGP induces CD163 expression through the production of IL-6 and IL-10. Similar results were obtained in human PBMC and nondifferentiated THP-1 (Fig. 3, E and F, and supplemental Fig. 1). IFN-γ, which is known to function as a CD163 down-regulating factor, was not detected at any time in AGP-treated cells (data not shown).
FIGURE 3.
AGP up-regulates CD163 expression through the induction of IL-6 and IL-10. A, dTHP-1 cells were pretreated with or without cycloheximide (5 μg/ml) for 30 min and subsequently incubated for 24 h with AGP (1.0 mg/ml). B and C, dTHP-1 cells were treated with AGP (1.0 mg/ml) each time. D, dTHP-1 cells were pretreated for 30 min with anti IL-6 receptor (0.5 μg/ml) antibody or anti IL-10 receptor antibody (0.5 μg/ml), and AGP (1.0 mg/ml) was subsequently added, followed by incubation for 24 h. E and F, human PBMC were incubated with AGP (1.0 mg/ml) each time. The level of expression of CD163 (A and D), IL-6 (B and E), and IL-10 (C and F) was evaluated by quantitative RT-PCR. Results are the means ± S.D. of six separate experiments. Statistically significant reduction was compared with AGP-treated cells (A) or isotype control IgG (D) (**, p < 0.01).
Up-regulation of CD163 upon Activation of Cell-surface Toll-like Receptor
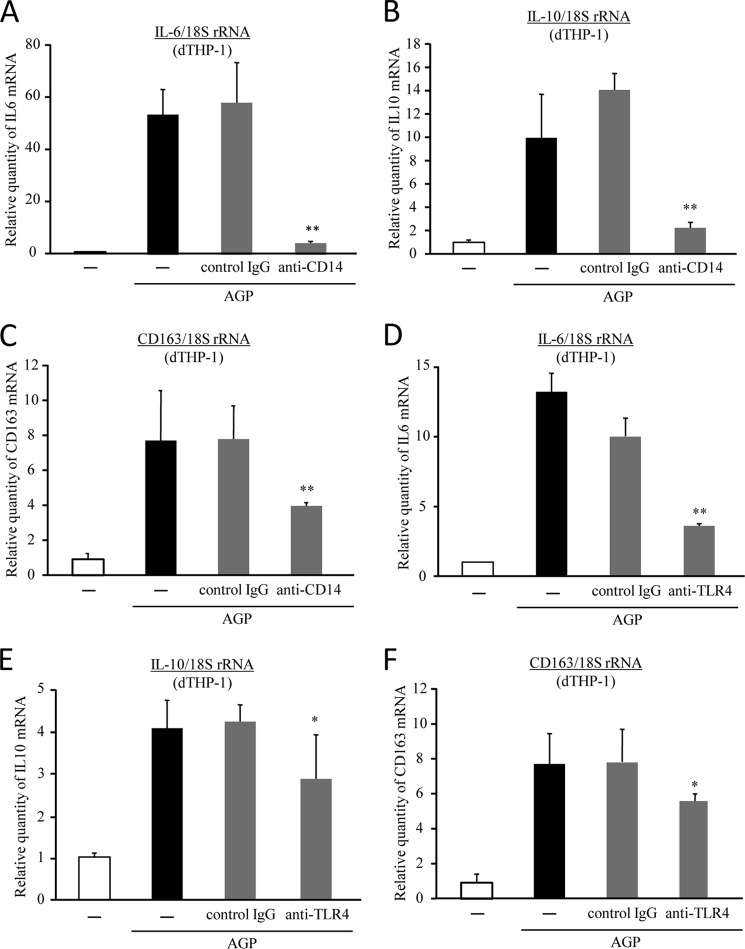
The expression of CD163 is tightly regulated by pro- and anti-inflammatory mediators (16–18). It is noteworthy that the activation of cell surface toll-like receptors, such as TLR4 activation by LPS, has been reported to result in an increase in the production of IL-6 and IL-10 followed by the up-regulation of CD163 expression (26). Furthermore, it has been reported that AGP binds to soluble CD14 (27), which is co-located on cellular membranes with TLR4 and MD2. Based on these findings, we presume that AGP activates TLR4 through binding to CD14. To investigate the involvement of CD14 in the regulation of CD163 by AGP, dTHP-1 cells were pretreated with an anti-CD14 neutralizing antibody. As a result, the induction of IL-6 and IL-10 by AGP was significantly inhibited by the anti-CD14-neutralizing antibody (Fig. 4, A and B). Moreover, similar inhibitions were also observed in CD163 expression (Fig. 4C). In addition, the expressions of IL-6, IL-10, and CD163 were also significantly suppressed by the anti-TLR4-neutralizing antibody (Fig. 4, D–F). We further examined the issue of whether TLR4 signaling is activated by AGP. To accomplish this, we performed reporter assays using HEK293 cells that overexpress TLR4. To evaluate whether AGP stimulates TLR4 downstream signaling, we carried out an IL-8 promoter assay that was already established in HEK293 cells that express TLR4. As reported previously, LPS, a typical TLR4 ligand, strongly activated TLR4 (supplemental Fig. 2). However, AGP weakly but significantly activated TLR4 at an acute phase concentration (1.0 mg/ml). Taken together, these data indicate that AGP enhances CD163 expression through the CD14/TLR4 pathway. Moreover, we confirmed that co-treatment of polymyxin B, which is an LPS-specific inhibitor, did not have an effect on CD163 up-regulation by AGP (data not shown).
FIGURE 4.
Interaction of AGP with CD14 causes TLR4 activation. dTHP-1 cells were pretreated with anti-CD14 (0.5 μg/ml) antibody (A–C) or anti-TLR4 antibody (1.0 μg/ml) (D–F) for 30 min and were subsequently incubated with AGP (1.0 mg/ml) for 24 h. The levels of expression of IL-6 (A and D), IL-10 (B and E), and CD163 (C and F) were evaluated by quantitative RT-PCR. Results are the means ± S.D. of six separate experiments. Statistically significant reduction was compared with isotype control IgG (**, p < 0.01; *, p < 0.05).
Activation of TLR4 Signal Transduction by AGP
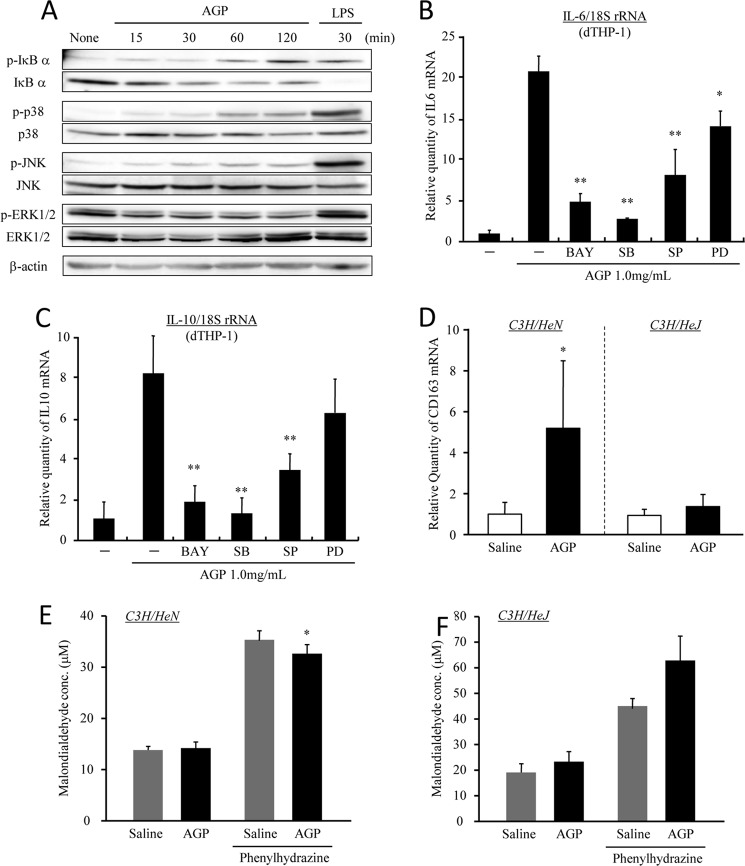
The next series of experiments were designed to elucidate the molecular mechanisms responsible for the signal transduction downstream of TLR4. To clarify the effect of AGP on the activation of endogenous signaling intermediates, such as JNK, p38, and ERK1/2 phosphorylation, as well as IκB-α degradation, which occurs during the TLR4 signaling pathway. After 2 h of incubation with AGP (1.0 mg/ml), IκB-α, JNK and p38 phosphorylation, as well as IκB-α degradation, were elicited in dTHP-1 cells, whereas the phosphorylation of ERK1/2 was not detected (Fig. 5A). To clarify these data, the AGP-induced productions of IL-6 and IL-10 were also examined in the presence of signal inhibitors. As shown in Fig. 5, B and C, the enhancement in IL-6 and IL-10 production by AGP was significantly decreased by the presence of inhibitors for the IκB-α, JNK, and p38 pathways, respectively. Thus, these results show that AGP induces IL-6 and IL-10 via the IκB-α, JNK, and p38 pathways through the activation of TLR4. To confirm the involvement of TLR4 in the enhancement of CD163 expression by AGP, AGP was administered to wild type mice (C3H/HeN) or TLR4 mutant mice (C3H/HeJ). The data showed that the up-regulation of CD163 in liver tissue was observed only in wild type mice (C3H/HeN) but not in C3H/HeJ mice (Fig. 5D). Although the administration of AGP significantly decreased the plasma oxidative stress levels in C3H/HeN mice with hemolysis (Fig. 5E), it was not decreased in the case of C3H/HeJ mice (Fig. 5F). These data indicate that AGP decreased plasma oxidative stress through the up-regulation of CD163 via the activation of TLR4.
FIGURE 5.
AGP induces IL-6 and IL-10 via the activation of TLR4 signaling. A, dTHP-1 cells were treated with AGP or LPS each time. Cells were lysed, and Western blotting was performed using each of the indicated antibodies. B and C, dTHP-1 cells were pretreated with BAY11-7082 (1 nm), SB203580 (1.0 nm), SP600125 (1.0 nm), and PD98059 (10 nm) for 1 h and subsequently incubated with AGP (1 mg/ml) for 2 h. The expression levels of IL-6 (B) and IL-10 (C) were evaluated by quantitative RT-PCR. D–F, C3H/HeN and C3H/HeJ mice were intravenously injected with saline or AGP (5 mg/100 μl saline/animal), and phenylhydrazine was subsequently administered by intraperitoneal injection after 48 h. At 24 h after the phenylhydrazine administration, liver and blood were collected. The expression of CD163 (D) in the liver was evaluated by quantitative RT-PCR. Oxidative stress in plasma from C3H/HeN (E) and C3H/HeJ (F) mice was measured by means of a TBARS assay. Results are the means ± S.D. of six separate experiments. Statistically significant reduction was compared with AGP (**, p < 0.01; *, p < 0.05).
Comparison of the Cytokine Production at the Downstream of TLR4 between AGP and LPS
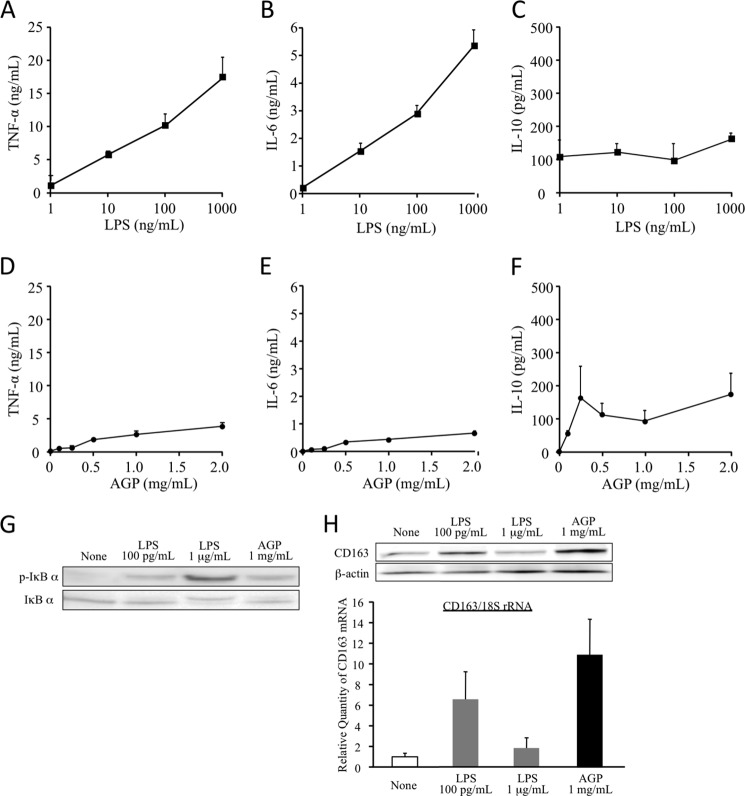
Because LPS-induced monocyte activation via TLR4 is believed to be responsible for inflammatory reactions, the possibility that AGP also exerts pro-inflammatory effects through the activation of TLR4 cannot be excluded. However, it has been reported that AGP exhibits anti-inflammatory effects. Such a discrepancy in TLR4 activation between AGP and LPS might be due to the difference in potential cytokine-inducible ability. Thus, we compared the production of TNF-α, IL-6, and IL-10 in the presence of AGP and LPS. As shown in Fig. 6, LPS strongly induced TNF-α and IL-6 in a concentration-dependent manner (Fig. 6, A and B). Although, unlike these cytokines, the production of IL-10 was different and reached a plateau at a concentration of 1 ng/ml (Fig. 6C). Conversely, AGP induced the production of TNF-α, IL-6, and IL-10 in a concentration-dependent manner from normal serum concentration (0.5 mg/ml) and reached a plateau at an acute phase concentration (1.0–2.0 mg/ml) (Fig. 6, D–F). In addition, the levels of production of TNF-α and IL-6 are quite low as compared with LPS. These data suggest that AGP has the potential to have TLR4 agonistic activity, but its ability is very weak compared with LPS.
FIGURE 6.
Comparison of cytokine production between AGP and LPS. dTHP-1 cells were treated LPS (A–C) or AGP (D--F) at each indicated concentration for 24 h (A–G) or 2 h (H). Cultured medium was collected and centrifuged. The production of TNF-α (A and D), IL-6 (B and E), and IL-10 (C and F) in supernatants was determined by ELISA. Results are the means ± S.D. of six separate experiments. Cells were lysed in RIPA buffer or RNAiso PLUS. The phosphorylation and degradation of IκB-α were evaluated by Western blotting (G). The expressions of CD163 mRNA and protein were analyzed by quantitative RT-PCR (H, lower panel) or Western blotting (H, upper panel).
Actually, when dTHP-1 were treated with either 100 pg/ml or 1.0 μg/ml LPS, at 100 pg/ml LPS, phosphorylation and degradation of IκB-α were slightly induced as was the treatment with 1.0 mg/ml AGP, but 1.0 μg/ml LPS strongly induced phosphorylation and the degradation of IκB-α (Fig. 6G). If this conclusion is valid, CD163 would also be expected to be enhanced by a weak activation of TLR4 induced by a low concentration of LPS and likewise AGP. Thus, we treated dTHP-1 with either 100 pg/ml or 1.0 μg/ml LPS. As expected, 100 pg/ml LPS increased CD163 protein and mRNA but 1.0 μg/ml LPS did not (Fig. 6H). These data imply that AGP up-regulates CD163 through a low level of cytokine production, which does not cause inflammation.
DISCUSSION
AGP is an acute phase protein and is frequently used as a marker of inflammation. It is generally thought that AGP plays an important role during inflammatory responses. Despite the accumulation of experimental evidence indicating that AGP exerts the anti-inflammatory and immunomodulatory effects, its molecular mechanism is poorly understood due to the fact that the target molecule that is involved in the function of AGP is not known. Here, we present the first evidence regarding the target molecule in which AGP exhibits a protective function through the enhancement of CD163 that is responsible for Hb scavenging. Based on the present findings, we propose the following mechanism for this sequential response: first, AGP activates TLR4 signaling through an interaction with CD14, which results in an increase in the production of IL-6 and IL-10; second, and these cytokines the up-regulate CD163 expression. This was supported by the in vivo experiments showing that AGP induces CD163 and HO-1 in the liver and consequently inhibits oxidative stress in plasma during hemolysis.
Hemolysis is responsible for the occurrence of a number of diseases (e.g. atherosclerosis, hemoglobinemia, hemoglobinuria, etc.). The liberated Hb is known to generate reactive oxygen species (4), activate endothelial cell pro-inflammatory sequelae (2), and oxidize low density lipoproteins (3). Therefore, the removal of liberated Hb represents a useful strategy for therapeutic intervention in both systemic hemolysis and diseases that are related to local hemolysis. So far, it has been established that Hp provides the first defense line against extracellular Hb by forming an Hb-Hp complex (5) and that CD163 plays a crucial role as a scavenger receptor for Hb-Hp complexes. However, the functional roles of other acute phase proteins in hemolysis are not known with certainty. In addition to the contribution of Hp, we propose a further defense system for hemolysis in which AGP also plays a key role in the exclusion of liberated Hb through enhancing CD163 expression. Thus, it is interesting to note that AGP and Hp, both of which are acute phase proteins, appear to act in a concerted manner to protect against Hb-derived tissue injury during hemolysis.
One of the interesting observations in this study is that AGP is an endogenous ligand for TLR4. No reports have appeared to date regarding the relationship between AGP and TLR4. In addition, few substances, such as palmitate, heparin sulfate, and serum amyloid A3 (28–30), have been identified as endogenous ligands for TLR4. Based on the findings of this study, it appears that AGP is a new member of this group. In this study, AGP caused TLR4 activation via the interaction with CD14. CD14 acts as a multifunctional molecule (31), such as promoting innate immunity to Gram-negative bacteria by transferring LPS to the signaling receptor complex MD-2·TLR4. It is well known that CD14 is either expressed on the surface of myelomonocytic cells (membrane CD14, mCD14) or is present in the circulation in the form of a soluble molecule (sCD14) (32). Because it has been reported that AGP binds to sCD14 (27), we expected that AGP would also bind to mCD14, and subsequently activate TLR4 signaling. In fact, pretreatment with an anti-CD14-neutralizing antibody resulted in the dramatic inhibition of AGP-induced IL-6 and IL-10 production, which resulted in a decrease in CD163 expression (Fig. 3). These data support our conclusion that AGP causes TLR4 signaling via the interaction with CD14.
We also found that NF-κB, p38, and JNK inflammatory pathways contribute to the up-regulation of IL-10 and IL-6 by AGP. However, we did not examine the issue of whether AGP directly up-regulates CD163 via these inflammatory signaling pathways. Therefore, it will be necessary to ascertain the regulation of CD163 by AGP under conditions where these inflammatory pathways are blocked, for instance, in the presence of inhibitors for NF-κB, p38, and JNK. It is known that NF-κB, p38, and JNK are involved in the downstream signaling pathway of oxidative stress. Because AGP attenuated oxidative stress in hemolytic model mice (Fig. 2), it would be expected that AGP treatment would inhibit those inflammatory signaling pathways. However, NF-κB, p38, and JNK were activated by AGP in THP-1 cells (Fig. 5). Such an inconsistency between in vivo and in vitro data may be explained as follows: NF-κB, p38, and JNK were activated by AGP before the induction of CD163. However, due to the decreased oxidative stress after CD163 induction, the activation of those inflammatory signaling pathways would be suppressed. This point would be a future investigation.
Another important finding is that only AGP up-regulates CD163 expression among acute phase proteins, including CRP, AAT, and Hp (Fig. 1). Despite the fact that little is known concerning the precise mechanism of how CD14 recognizes its ligand, such as microbial ligands, CD14 prefer a polyanion as a ligand (33–35). Thus, electrostatic interactions between CD14 and its ligand could play a crucial role in the substrate recognition process. AGP is the most negatively charged plasma protein (pI = 2.7–3.2), due to the presence of a number of sialic acids at the terminal of glycan chains (12% of the carbohydrate moieties) (20, 36). The pI values of CRP, AAT, and Hp are 7.9–9.0, 5.37, and 6.32, respectively, due to the lower sialic acid content (37, 38). Such differences in negative potential between AGP and other acute phase proteins could contribute to the affinity to CD14 and hence the activation of TLR4 and enhancement of CD163 expression. Thus, it is plausible that the unique function of AGP in the up-regulation of CD163 expression among acute phase proteins may depend on its highly sialylated carbohydrate chains.
Inflammation is a highly controlled process that is coordinated by maintaining a balance between pro- and anti-inflammatory activities (39). Insulted tissues generate not only pro-inflammatory cytokines but also anti-inflammatory molecules to permit local and systemic inflammation to be alleviated (40). AGP induces monocytes to express pro- and anti-inflammatory cytokines such as TNF-α, IL-6, IL-12, and IL-1 receptor antagonists (27, 41). Therefore, counteracting mechanisms are simultaneously induced by AGP to maintain the homeostasis between pro- and anti-inflammatory activities (42). The findings of this study indicate that AGP increases IL-6, IL-10, and CD163 from normal serum concentrations in a dose-dependent manner, and their levels reach a plateau at the acute phase concentration of AGP (Figs. 1C and 6, E and F). These observations indicate that AGP controls intravascular homeostasis, due not only via the induction of CD163 against small scale hemolysis at a steady state, but it also further enhances CD163 under severe hemolysis. AGP stimulated NF-κB, JNK, and p38 pathways (Fig. 5A). However, the degree of its stimulation was small as compared with LPS. In addition, the stimulating effect of AGP on IL-10 expression was comparable with LPS, although the effect of AGP on TNF-α and IL-6 expression was noticeably lower than that of LPS (Fig. 6). This divergent regulation between AGP and LPS on NF-κB, JNK, and p38 pathways followed by TNF-α, IL-6, and IL-10 induction was supposed to have a different outcome on CD163 expression. A low level of TNF-α production could protect mice against a subsequent lethal challenge of TNF-α (43), and it is also possible that the cytoprotective effect of AGP is mediated through the low level secretion of TNF-α or some other cytokines. Of course, a high concentration of LPS becomes toxic by overstimulating TLR4 signaling, leading to an excessive inflammatory response that results in adverse reactions such as septic shock. However, the recognition of commensal bacteria by TLR4 and TLR2 under normal steady-state conditions plays an important beneficial role in the control of intestinal epithelial homeostasis by inducing the production of cytoprotective heat shock proteins (e.g. HSP25 and -72) (44). Similarly, AGP may prevent Hb-induced oxidative stress by up-regulation of CD163 without causing an excessive inflammatory response.
It has been reported that the expression of CD163 is regulated by a variety of factors. For example, a genomic analysis of the CD163 promoter region revealed the presence of several binding sites for transcription factors in the 5′-flanking region (45), including three putative glucocorticoid receptor binding sites. In fact, monocytes that had been treated with glucocorticoids or derived from patients on glucocorticoid pulse therapy showed a strongly induced CD163 expression (12, 15, 46, 47). Taking the collective findings into consideration, with the evidence that glucocorticoid also up-regulates AGP synthesis (20), glucocorticoids might not only regulate CD163 expression directly but also indirectly via enhancing AGP production. Because glucocorticoid pulse therapy is widely used in the treatment of hemolysis, glucocorticoid-induced AGP may also contribute its clinical effects via the further enhancement of CD163 expression.
Here, we found that AGP caused a significant decrease in hemoglobin levels in comparison with the saline group in hemolytic model mice. Under such circumstances, AGP administration enhanced the increase in CD163 and HO-1 in the liver of hemolysis-induced mice (Fig. 2, C and D). These data indicate that AGP enhances the clearance of Hb from the circulating blood through the up-regulation of CD163. This was supported by the fact that administration of anti-CD163 antibody tended to suppress the AGP-induced enhancement of Hb clearance (supplemental Table 3). At the same time, we also found that AGP administration decreased hematocrit as a consequence of the reduction in red blood cell count in hemolytic model mice as compared with the saline group. Although the mechanism of this reduction in red blood cell count is unclear from the present limited data, the possibility that AGP induces further enhancement of hemolysis cannot be excluded. Further investigations will be needed to clarify this issue.
In addition, there is direct evidence that demonstrates the essential role of CD163 for the AGP-induced attenuation of oxidative stress using anti-CD163 antibody (Fig. 2G). In addition, to confirm the involvement of TLR4 in enhancement of CD163 expression and AGP-induced attenuation of oxidative stress using C3H/HeN or C3H/HeJ, we found that AGP decreased plasma oxidative stress through the up-regulation of CD163 via the activation of TLR4. However, the administration of AGP to C3H/HeJ mice with phenylhydrazine-induced hemolysis tended to increase oxidative stress (Fig. 5F). At the same time, plasma hemoglobin levels were decreased by AGP administration in hemolysin-induced C3H/HeN mice compared with the saline group (8.68 ± 0.14 g/dl for the AGP group and 9.10 ± 0.19 g/dl for the saline group), similar to the ddY mice, whereas hemoglobin levels in plasma were increased by AGP administration in hemolysin-induced C3H/HeJ mice compared with the saline group (9.92 ± 0.10 g/dl for the AGP group and 9.64 ± 0.02 g/dl for the saline group; supplemental Table 4). A previous study demonstrated that AGP acts as an important component of the capillary barrier. For instance, Schnitzer and Pinney (48)demonstrated that AGP binds to cultured microvascular endothelium, which increased the negative charge of glycocalyx on the endothelial surface, which regulates vascular permeability. Moreover, Haraldsson and Rippe (49) reported that AGP suppressed the renal filtration of albumin via interaction with the glomerulus. The same group also reported that AGP is one of the major components of the glomerular endothelial cell coat, which is essential for glomerular filtration (50). Taking these observations into consideration, the renal excretion of Hb might also be decreased in AGP-administered C3H/HeJ mice. Therefore, the decrease in Hb clearance by the inhibition of both renal excretion and CD163 scavenging in C3H/HeJ mice with AGP treatment might cause a further accumulation of Hb in the general circulation, which leads to an enhancement in oxidative stress in the plasma.
In conclusion, we demonstrate here, for the first time, that AGP enhances the uptake of Hb through the up-regulation of CD163, and it may exert a protective role against hemolysis-derived oxidative stress. Because dedicated Hb scavenging and detoxification systems could serve as a method for therapeutic intervention in hemolysis, the biological implications of AGP-induced CD163 may not be limited to hemolysis but could contribute to several diseases related to local hemolysis such as atherosclerosis. Therefore, AGP or its inducible agents have potential for use as a therapy against those diseases.
Supplementary Material
This study was supported in part by Grants-in-aid for Scientific Research 20390161 and 23458100 from Japan Society for the Promotion of Science.

This article contains supplemental Figs. 1 and 2 and Tables 1–4.
- Hp
- haptoglobin
- AGP
- α1-acid glycoprotein
- PBMC
- and peripheral blood mononuclear cell
- TBARS
- thiobarbituric acid-reactive substance
- AAT
- α1-antitrypsin
- CRP
- C-reactive protein.
REFERENCES
- 1. Rother R. P., Bell L., Hillmen P., Gladwin M. T. (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin. A novel mechanism of human disease. JAMA 293, 1653–1662 [DOI] [PubMed] [Google Scholar]
- 2. Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G. M., Eaton J. W., Balla G. (2002) Pro-oxidant and cytotoxic effects of circulating heme. Blood 100, 879–887 [DOI] [PubMed] [Google Scholar]
- 3. Grinshtein N., Bamm V. V., Tsemakhovich V. A., Shaklai N. (2003) Mechanism of low density lipoprotein oxidation by hemoglobin-derived iron. Biochemistry 42, 6977–6985 [DOI] [PubMed] [Google Scholar]
- 4. Buehler P. W., D'Agnillo F. (2010) Toxicological consequences of extracellular hemoglobin. Biochemical and physiological perspectives. Antioxid. Redox. Signal. 12, 275–291 [DOI] [PubMed] [Google Scholar]
- 5. Kato G. J. (2009) Haptoglobin halts hemoglobin's havoc. J. Clin. Invest. 119, 2140–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boretti F. S., Buehler P. W., D'Agnillo F., Kluge K., Glaus T., Butt O. I., Jia Y., Goede J., Pereira C. P., Maggiorini M., Schoedon G., Alayash A. I., Schaer D. J. (2009) Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J. Clin. Invest. 119, 2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buehler P. W., Abraham B., Vallelian F., Linnemayr C., Pereira C. P., Cipollo J. F., Jia Y., Mikolajczyk M., Boretti F. S., Schoedon G., Alayash A. I., Schaer D. J. (2009) Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood 113, 2578–2586 [DOI] [PubMed] [Google Scholar]
- 8. Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., Moestrup S. K. (2001) Identification of the hemoglobin scavenger receptor. Nature 409, 198–201 [DOI] [PubMed] [Google Scholar]
- 9. Schaer D. J., Schaer C. A., Buehler P. W., Boykins R. A., Schoedon G., Alayash A. I., Schaffner A. (2006) CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107, 373–380 [DOI] [PubMed] [Google Scholar]
- 10. Law S. K., Micklem K. J., Shaw J. M., Zhang X. P., Dong Y., Willis A. C., Mason D. Y. (1993) A new macrophage differentiation antigen, which is a member of the scavenger receptor superfamily. Eur. J. Immunol. 23, 2320–2325 [DOI] [PubMed] [Google Scholar]
- 11. Zwadlo G., Voegeli R., Schulze Osthoff K., Sorg C. (1987) A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp. Cell Biol. 55, 295–304 [DOI] [PubMed] [Google Scholar]
- 12. Sulahian T. H., Högger P., Wahner A. E., Wardwell K., Goulding N. J., Sorg C., Droste A., Stehling M., Wallace P. K., Morganelli P. M., Guyre P. M. (2000) Human monocytes express CD163, which is up-regulated by IL-10 and identical to p155. Cytokine 12, 1312–1321 [DOI] [PubMed] [Google Scholar]
- 13. Williams L., Jarai G., Smith A., Finan P. (2002) IL-10 expression profiling in human monocytes. J. Leukocyte Biol. 72, 800–809 [PubMed] [Google Scholar]
- 14. Schaer D. J., Boretti F. S., Schoedon G., Schaffner A. (2002) Induction of the CD163-dependent hemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br. J. Haematol. 119, 239–243 [DOI] [PubMed] [Google Scholar]
- 15. Van den Heuvel M. M., Tensen C. P., van As J. H., Van den Berg T. K., Fluitsma D. M., Dijkstra C. D., Döpp E. A., Droste A., Van Gaalen F. A., Sorg C., Högger P., Beelen R. H. (1999) Regulation of CD163 on human macrophages. Cross-linking of CD163 induces signaling and activation. J. Leukocyte Biol. 66, 858–866 [DOI] [PubMed] [Google Scholar]
- 16. Buechler C., Ritter M., Orsó E., Langmann T., Klucken J., Schmitz G. (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and anti-inflammatory stimuli. J. Leukocyte Biol. 67, 97–103 [PubMed] [Google Scholar]
- 17. Gleissner C. A., Shaked I., Erbel C., Böckler D., Katus H. A., Ley K. (2010) CXCL4 down-regulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ. Res. 106, 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pioli P. A., Goonan K. E., Wardwell K., Guyre P. M. (2004) TGF-β regulation of human macrophage scavenger receptor CD163 is Smad3-dependent. J. Leukocyte Biol. 76, 500–508 [DOI] [PubMed] [Google Scholar]
- 19. Schmid K., Kaufmann H., Isemura S., Bauer F., Emura J., Motoyama T., Ishiguro M., Nanno S. (1973) Structure of α1-acid glycoprotein. The complete amino acid sequence, multiple amino acid substitutions, and homology with the immunoglobulins. Biochemistry 12, 2711–2724 [DOI] [PubMed] [Google Scholar]
- 20. Fournier T., Medjoubi-N N., Porquet D. (2000) α1-Acid glycoprotein. Biochim. Biophys. Acta 1482, 157–171 [DOI] [PubMed] [Google Scholar]
- 21. Lim S. K., Kim H., Lim S. K., bin Ali A., Lim Y. K., Wang Y., Chong S. M., Costantini F., Baumman H. (1998) Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92, 1870–1877 [PubMed] [Google Scholar]
- 22. Van Molle W., Libert C., Fiers W., Brouckaert P. (1997) α1-Acid glycoprotein and α1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J. Immunol. 159, 3555–3564 [PubMed] [Google Scholar]
- 23. Van Molle W., Denecker G., Rodriguez I., Brouckaert P., Vandenabeele P., Libert C. (1999) Activation of caspases in lethal experimental hepatitis and prevention by acute phase proteins. J. Immunol. 163, 5235–5241 [PubMed] [Google Scholar]
- 24. Kagaya N., Kamiyoshi A., Tagawa Y., Akamatsu S., Isoda K., Kawase M., Yagi K. (2005) Suppression of cell death in primary rat hepatocytes by α1-acid glycoprotein. J. Biosci. Bioeng. 99, 81–83 [DOI] [PubMed] [Google Scholar]
- 25. Kuzuhara H., Nakano Y., Yamashita N., Imai M., Kawamura Y., Kurosawa T., Nishiyama S. (2006) Protective effects of α1-acid glycoprotein and serum amyloid A on concanavalin A-induced liver failure via interleukin-6 induction by ME3738. Eur. J. Pharmacol. 541, 205–210 [DOI] [PubMed] [Google Scholar]
- 26. Weaver L. K., Pioli P. A., Wardwell K., Vogel S. N., Guyre P. M. (2007) Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J. Leukocyte Biol. 81, 663–671 [DOI] [PubMed] [Google Scholar]
- 27. Su S. J., Yang B. C., Wang Y. S., Yeh T. M. (1999) α1-Acid glycoprotein-induced tumor necrosis factor-α secretion of human monocytes is enhanced by serum binding proteins and depends on protein tyrosine kinase activation. Immunopharmacology 41, 21–29 [DOI] [PubMed] [Google Scholar]
- 28. Suganami T., Yuan X., Shimoda Y., Uchio-Yamada K., Nakagawa N., Shirakawa I., Usami T., Tsukahara T., Nakayama K., Miyamoto Y., Yasuda K., Matsuda J., Kamei Y., Kitajima S., Ogawa Y. (2009) Activating transcription factor 3 constitutes a negative feedback mechanism that attenuates saturated fatty acid/toll-like receptor 4 signaling and macrophage activation in obese adipose tissue. Circ. Res. 105, 25–32 [DOI] [PubMed] [Google Scholar]
- 29. Johnson G. B., Brunn G. J., Kodaira Y., Platt J. L. (2002) Receptor-mediated monitoring of tissue well being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 168, 5233–5239 [DOI] [PubMed] [Google Scholar]
- 30. Maru Y. (2009) Logical structures extracted from metastasis experiments. Cancer Sci. 100, 2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schröder N. W., Eckert J., Stübs G., Schumann R. R. (2008) Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology 213, 329–340 [DOI] [PubMed] [Google Scholar]
- 32. Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein. Science 249, 1431–1433 [DOI] [PubMed] [Google Scholar]
- 33. Cunningham M. D., Shapiro R. A., Seachord C., Ratcliffe K., Cassiano L., Darveau R. P. (2000) CD14 employs hydrophilic regions to “capture” lipopolysaccharides. J. Immunol. 164, 3255–3263 [DOI] [PubMed] [Google Scholar]
- 34. Shapiro R. A., Cunningham M. D., Ratcliffe K., Seachord C., Blake J., Bajorath J., Aruffo A., Darveau R. P. (1997) Identification of CD14 residues involved in specific lipopolysaccharide recognition. Infect. Immun. 65, 293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shnyra A., Lindberg A. A. (1995) Scavenger receptor pathway for lipopolysaccharide binding to Kupffer and endothelial liver cells in vitro. Infect. Immun. 63, 865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hochepied T., Berger F. G., Baumann H., Libert C. (2003) α1-Acid glycoprotein. An acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 14, 25–34 [DOI] [PubMed] [Google Scholar]
- 37. Barrabés S., Sarrats A., Fort E., De Llorens R., Rudd P. M., Peracaula R. (2010) Effect of sialic acid content on glycoprotein pI analyzed by two-dimensional electrophoresis. Electrophoresis 31, 2903–2912 [DOI] [PubMed] [Google Scholar]
- 38. Das T., Sen A. K., Kempf T., Pramanik S. R., Mandal C. (2003) Induction of glycosylation in human C-reactive protein under different pathological conditions. Biochem. J. 373, 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawrence T., Willoughby D. A., Gilroy D. W. (2002) Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2, 787–795 [DOI] [PubMed] [Google Scholar]
- 40. Opal S. M., DePalo V. A. (2000) Anti-inflammatory cytokines. Chest 117, 1162–1172 [DOI] [PubMed] [Google Scholar]
- 41. Tilg H., Vannier E., Vachino G., Dinarello C. A., Mier J. W. (1993) Anti-inflammatory properties of hepatic acute phase proteins. Preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1β synthesis by human peripheral blood mononuclear cells. J. Exp. Med. 178, 1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee Y. S., Choi J. W., Hwang I., Lee J. W., Lee J. H., Kim A. Y., Huh J. Y., Koh Y. J., Koh G. Y., Son H. J., Masuzaki H., Hotta K., Alfadda A. A., Kim J. B. (2010) Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J. Biol. Chem. 285, 22174–22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Su S. J., Yeh T. M. (1996) Effects of α1-acid glycoprotein on tissue factor expression and tumor necrosis factor secretion in human monocytes. Immunopharmacology 34, 139–145 [DOI] [PubMed] [Google Scholar]
- 44. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 45. Ritter M., Buechler C., Langmann T., Schmitz G. (1999) Genomic organization and chromosomal localization of the human CD163 (M130) gene. A member of the scavenger receptor cysteine-rich superfamily. Biochem. Biophys. Res. Commun. 260, 466–474 [DOI] [PubMed] [Google Scholar]
- 46. Vallelian F., Schaer C. A., Kaempfer T., Gehrig P., Duerst E., Schoedon G., Schaer D. J. (2010) Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood 116, 5347–5356 [DOI] [PubMed] [Google Scholar]
- 47. Varga G., Ehrchen J., Tsianakas A., Tenbrock K., Rattenholl A., Seeliger S., Mack M., Roth J., Sunderkoetter C. (2008) Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J. Leukocyte Biol. 84, 644–650 [DOI] [PubMed] [Google Scholar]
- 48. Schnitzer J. E., Pinney E. (1992) Quantitation of specific binding of orosomucoid to cultured microvascular endothelium. Role in capillary permeability. Am. J. Physiol. 263, H48–H55 [DOI] [PubMed] [Google Scholar]
- 49. Haraldsson B. S., Johnsson E. K., Rippe B. (1992) Glomerular permselectivity is dependent on adequate serum concentrations of orosomucoid. Kidney Int. 41, 310–316 [DOI] [PubMed] [Google Scholar]
- 50. Haraldsson B., Rippe B. (1987) Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol. Scand. 129, 127–135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.