Background: Wnts activate transcriptional responses via stabilization and nuclear translocation of β-catenin.
Results: FAF1 associates with SCF-β-TrCP E3 ligase complex and promotes cytosolic β-catenin polyubiquitination and degradation.
Conclusion: FAF1 is a scaffold protein that promotes β-TrCP-mediated β-catenin degradation.
Significance: FAF1 may have tumor suppressive activity by antagonizing Wnt/β-catenin signaling.
Keywords: β-Catenin, Cell Signaling, EMT, Ubiquitination, Wnt Signaling, FAF1
Abstract
FAS-associated factor 1 (FAF1) antagonizes Wnt signaling by stimulating β-catenin degradation. However, the molecular mechanism underlying this effect is unknown. Here, we demonstrate that the E3 ubiquitin ligase β-transducin repeat-containing protein (β-TrCP) is required for FAF1 to suppress Wnt signaling and that FAF1 specifically associates with the SCF (Skp1-Cul1-F-box protein)-β-TrCP complex. Depletion of β-TrCP reduced FAF1-mediated β-catenin polyubiquitination and impaired FAF1 in antagonizing Wnt/β-catenin signaling. FAF1 was shown to act as a scaffold for β-catenin and β-TrCP and thereby to potentiate β-TrCP-mediated β-catenin ubiquitination and degradation. Data mining revealed that FAF1 expression is statistically down-regulated in human breast carcinoma compared with normal breast tissue. Consistent with this, FAF1 expression is higher in epithelial-like MCF7 than mesenchymal-like MDA-MB-231 human breast cancer cells. Depletion of FAF1 in MCF7 cells resulted in increased β-catenin accumulation and signaling. Importantly, FAF1 knockdown promoted a decrease in epithelial E-cadherin and an increase in mesenchymal vimentin expression, indicative for an epithelial to mesenchymal transition. Moreover, ectopic FAF1 expression reduces breast cancer cell migration in vitro and invasion/metastasis in vivo. Thus, our studies strengthen a tumor-suppressive function for FAF1.
Introduction
Wnt proteins control many biological processes, including embryonic development and tumorigenesis (1–4). One of the critical components of the canonical Wnt signaling pathway is β-catenin, the levels of which are tightly controlled via protein degradation. In the absence of Wnt ligands, the majority of β-catenin is associated with E-cadherin on the plasma membrane, whereas the levels of intracellular β-catenin are kept low in a phosphorylation-dependent manner by components of the proteasomal degradation pathway. Casein kinase 1 (CK1) and GSK3β sequentially catalyze β-catenin phosphorylation at residues Ser-45, Thr-41, Ser-37, and Ser-33, thereby acting in a destruction complex with adenomatous polyposis coli and Axin. Phosphorylated β-catenin is subsequently recognized by the F-box protein β-transducin repeat-containing protein (β-TrCP),4 a component of the ubiquitin ligase complex that triggers ubiquitin-dependent degradation of β-catenin by the proteasome (5, 6). Wnt ligands induce the disruption of the cytosolic β-catenin degradation complex (7, 8) and thereby enable β-catenin to enhance lymphoid enhancer-binding factor 1 (LEF)/T cell factor-mediated transcription in the nucleus (9, 10).
Wnt/β-catenin signaling among others regulates epithelial to mesenchymal transition (EMT) and hyperactive Wnt signaling can trigger EMT-like programs (2, 11–17). Aberrant activation of the β-catenin-T cell factor cascade was shown to activate EMT in human breast cancer cells by induction of Axin2, which regulates GSK3β, the dominant kinase controlling the stability and activity of Snail, a potent EMT inducer in normal and neoplastic cells (18).
As a member of the Fas death-inducing signaling complex, FAS-associated factor 1 (FAF1) was first identified to potentiate apoptosis in L cells and T cells (19–22). Faf1 gene-targeted mice show embryonic lethality at the two-cell stage (23). In cellular signaling, FAF1 is linked to the regulation of NF-κB activity as well as to valosin containing protein-mediated ubiquitination and proteasomal degradation (24–27). Decreased FAF1 expression was found to be correlated with a high percentage of human gastric carcinomas (28), and genomic loss or deletion of FAF1 has been reported in uterine cervix carcinomas and mantle cell lymphoma (29, 30). Therefore, FAF1 has been postulated to act as a tumor suppressor. We previously identified FAF1 as a potent negative regulator of canonical Wnt signaling and a strong repressor of Wnt/β-catenin-induced osteoblast differentiation and bone formation (31). However, the underlying mechanism by which FAF1 promotes β-catenin degradation is unclear.
In this study, we screened components of the β-catenin destruction complex for their ability to interact with FAF1. FAF1 was shown to form a ternary complex with β-catenin and β-TrCP and thereby to promote β-TrCP-mediated β-catenin ubiquitination and degradation. Moreover, we found FAF1 to inhibit epithelial to mesenchymal transition and invasion/metastasis in breast cancer cells, enforcing the notion of its action as tumor suppressor.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids
FAF1 (#4932), β-TrCP (#4394), CUL1 (#4995), and vimentin (#3932) antibodies were purchased from Cell Signaling Technology. β-Catenin antibody (#610153), E-cadherin (#610181) was purchased from BD Biosciences; Actin (ab8227) was purchased from Abcam. SKIP1 (sc-12073), Myc (sc-40), and HA (sc-805) were from Santa Cruz Biotechnology. Human FAF1 shRNA (1#, TRCN000000424; 2#, TRCN000000424) and human β-TrCP shRNA (TRCN0000006541+TRCN0000315200) MISSION® shRNA Lentiviral Transduction Particles were purchased from Sigma Mission Library, Inc. FAF1, TopFlash-luciferase, FopFlash-luciferase, LEF-luciferase, Wnt, β-catenin, LEF-1, and His6-ubiquitin constructs were described previously (31–38).
Cell Culture
HEK293, HeLa, MCF7, and MDA-MB-231 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Hyclone), nonessential amino acids, l-glutamine, and penicillin/streptomycin in a 5% CO2-containing atmosphere at 37 °C.
Luciferase Reporter Assay
Cells were transfected and lysed as described (36, 39–41), and luciferase activities were measured by a luminometer (Berthold Technologies). Reporter activity was normalized to β-gal activity, resulting from a co-transfected internal control plasmid. Experiments were performed in triplicate.
Transfection, Immunoprecipitation, and Immunoblotting
Cells were transiently transfected using calcium phosphate or Lipofectamine (Invitrogen). 40 h after transfection, cells were lysed with 1 ml of lysis buffer (20 mm Tris-HCl, pH 7.4, 2 mm EDTA, 25 mm NaF, 1% Triton X-100) plus protease inhibitors (Sigma) for 30 min at 4 °C. After centrifugation at 12 × 103 g for 15 min, the lysates were immunoprecipitated with specific antibody and protein A-Sepharose (Zymed Laboratories Inc.) for 3 h at 4 °C. Thereafter, the precipitants were washed three times with washing buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS), and the immune complexes were eluted with sample buffer containing 1% SDS for 5 min at 95 °C and analyzed by SDS-PAGE. Immunoblotting was performed with specific antibody and secondary anti-mouse or anti-rabbit antibodies that were conjugated to horseradish peroxidase (Amersham Biosciences). Proteins were visualized by chemiluminescence.
Immunofluorescence
As previously described (32, 42, 43), HeLa cells grown on coverslips were washed twice with PBS, fixed with 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 for 10 min, and blocked with 3% BSA in PBS for 60 min. The cells were then incubated with primary antibodies diluted in TBST (20 mmol/liter Tris-HCl, pH 7.6, 137 mmol/liter NaCl, 0.1% Tween 20) for 3 h and washed twice with PBS and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse or antirabbit antibodies for an additional 40 min. The nuclei were counterstained with 4,6-diamidino-2-phenylindole (Sigma). Samples were examined under an Olympus Fluoview 500 microscope.
Nickel Pulldown
Cells were lysed in 6 m guanidine-HCl, 0.1 m Na2HPO4/NaH2PO4, 10 mm imidazole, followed by nickel bead purification and immunoblot analysis.
Lentiviral Transduction and Generation of Stable Cell Lines
Lentiviruses were produced by transfecting pLKO-1 (for the shRNA-knockdown) or pLV-bc-CMV (for cDNA expression) plasmids together with helper plasmids pCMV-VSVG, pMDLg-RRE (gag/pol), and pRSV-REV into HEK293T cells. Cell supernatants were harvested 48 h after transfection and were either used to infect cells or stored at −80 °C. To obtain stable cell lines, cells were infected at low confluence (20%) for 24 h with lentiviral supernatants diluted 1:1 with normal culture medium in the presence of 5 ng/ml Polybrene (Sigma). 48 h after infection, cells were placed under 5 μg/ml puromycin selection for 1 week and then passaged before use. His-ubiquitin-expressing HEK293T cells were obtained by transfection with His-ubiquitin construct followed by selection in 1 μg/ml neomycin.
Preparation of Cytosolic and Nuclear Fractions
Cytosolic and membrane fractions were prepared using the ProteoExtract kit (Calbiochem) according to the manufacturer's standard procedures.
Embryo Preparation and Tumor Cell Implantation
Dechorionized 2-day postfertilization Casper zebrafish transgenic lines Tg(fli1:GFP) were anesthesized with 0.003% tricaine (Sigma) and positioned on a 10-cm Petri dish coated with 3% agarose. mCherry-labeled MDA-MB-231 cells were trypsinized into single cell suspensions, resuspended in DMEM, kept on ice before injection. The cell suspension was loaded into borosilicate glass capillary needles (1-mm outer diameter × 0.78-mm inner diameter; Harvard Apparatus), and the injections were performed using a Pneumatic Pico pump and a manipulator (WPI) within cell trypsinization. Approximately 400 cells were injected at the duct of Cuvier site in the yolk sac. The zebrafish embryos were subsequently maintained at 33 °C for 6 days after implantation. The number of embryos which displayed tumor cell invasion and micrometastasis in the posterior tail fin area were counted using fluorescent microscopy.
Microscopy and Analysis
Live zebrafish analysis and image acquisition were performed using a Leica MZ16FA stereo microscope. Fixed embryos were imaged in PBST with a Leica SP5 STED confocal microscope. Confocal stacks were processed for maximum intensity projections with Leica software or Adobe Photoshop CS4 software. Overlays were adjusted for brightness and contrast using Adobe Photoshop CS4.
Statistical Analysis
Statistical analyses were performed with a two-tailed unpaired t test. p < 0.05 was considered statistically significant.
RESULTS
Effect of FAF1 on WT and Mutant β-Catenin-induced Transcription
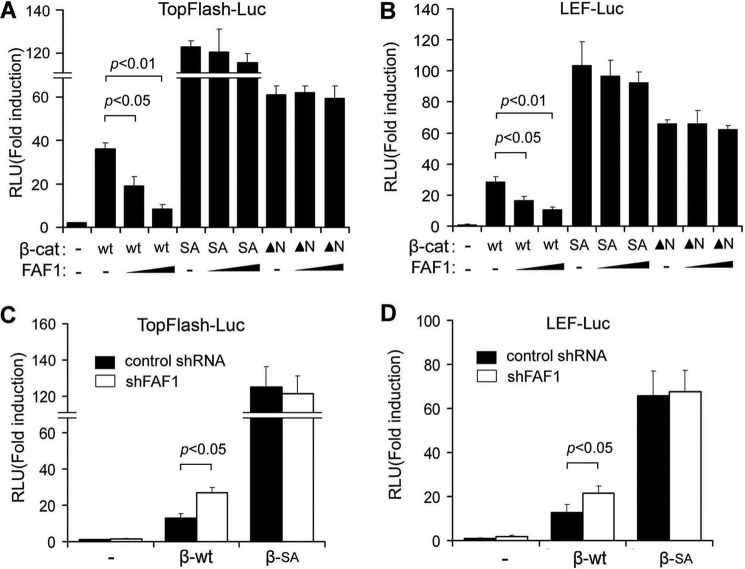
We previously identified FAF1 as a novel inhibitory factor of Wnt signaling by promoting β-catenin polyubiquitination and degradation (31). To investigate the underlying mechanism, we first examined the effects of FAF1 on β-catenin-dependent transcriptional reporters when activated by either wild-type or constitutively active β-catenin mutants (β-catenin SA or β-catenin ▴N). These mutants are resistant to degradation by the adenomatous polyposis coli-Axin-GSK3β destruction complex (5). Consistent with our previous observations (31), ectopic expression of human FAF1 in 293T cells dose dependently inhibited the activation of the Wnt/β-catenin-transcriptional reporter constructs TopFlash-luciferase (44) and LEF-luciferase (45) when activated by wild-type β-catenin. However, FAF1 failed to inhibit the transcriptional activation initiated by constitutively active β-catenin SA or β-catenin ▴N (Fig. 1, A and B). We next tested the effect of FAF1 knockdown on wild type or mutant β-catenin-induced transcriptional responses. As previously reported (31), shRNA-mediated FAF1 depletion potentiated Wnt reporter activity stimulated by wild-type β-catenin. However, FAF1 knockdown showed no effect on β-catenin SA-induced transcriptional responses. Because constitutively active β-catenin is resistant to the (phosphorylation-dependent) degradation and FAF1 knockdown did not interfere with β-catenin phosphorylation (31), these results suggested that FAF1 acts upstream of phosphorylation-dependent polyubiquitination of β-catenin.
FIGURE 1.
A and B, luciferase activity analysis of Wnt reporter in HEK293T cells transfected with TopFlash (A) and LEF-Luc reporters (B) along with plasmids indicated. 36 h after transfection, cells were harvested for luciferase activity analysis. C and D, luciferase activity analysis of Wnt reporter in HEK293T cells transfected with TopFlash reporter (C) and LEF-Luc reporters (D) along with control shRNA or shRNA against FAF1 (shFAF1). 36 h after transfection, cells were harvested for luciferase activity analysis. Each experiment was performed in triplicate, and the data represent mean ± S.D. (error bars) of three independent experiments after normalizing to β-gal activity.
β-TRCP Associates with FAF1 and Is Required for FAF1 in Suppressing Wnt Signaling
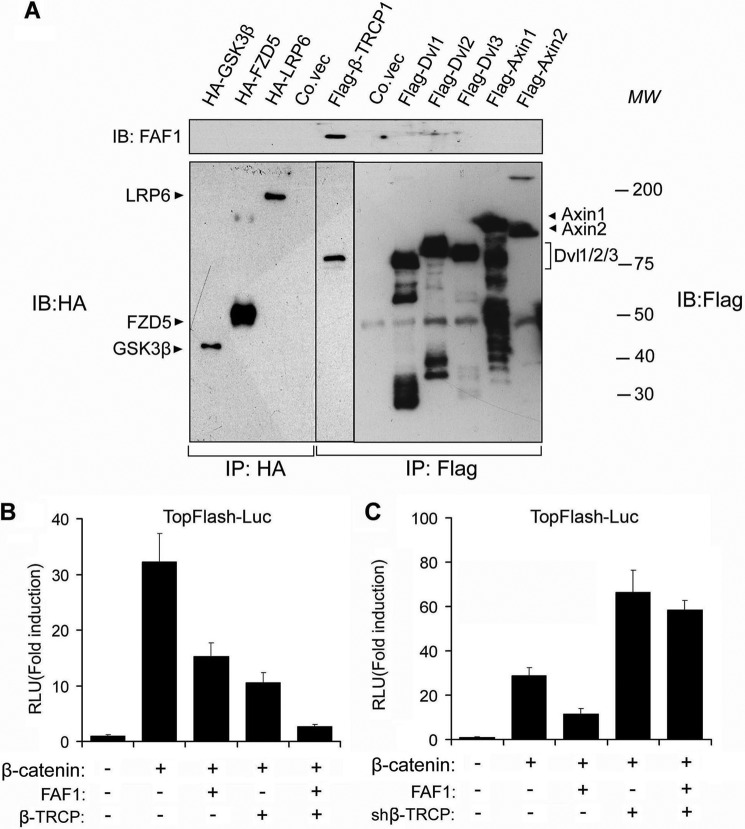
We next analyzed the ability of FAF1 to interact with canonical Wnt pathway components acting upstream of β-catenin phosphorylation. For this, tagged Wnt components were expressed in HEK293T cells, and FAF1 co-immunoprecipitation assays were performed. Immunoblotting showed that the endogenous FAF1 efficiently associated with the F-box containing β-catenin E3 ligase β-TrCP1, but not with Wnt co-receptors (LRP6, Frizzeld5 (FZD5)), the scaffolds Dishevelled1/2/3, or the β-catenin destruction complex (Axin1/2, GSK3β) (Fig. 2A). To functionally examine the role of β-TrCP in FAF1-mediated modulation of Wnt signaling, we tested the effect of β-TrCP on FAF1-mediated repression of the β-catenin-induced transcriptional response. Whereas ectopic expression of β-TrCP was found to facilitate FAF1 in antagonizing β-catenin signaling, shRNA-mediated depletion of endogenous β-TrCP abolished the inhibitory effect of FAF1 (Fig. 2, B and C). In summary, these results indicate that β-TrCP binds FAF1 and is required for FAF1-induced repression of Wnt/β-catenin signaling.
FIGURE 2.
FAF1 inhibiting canonical Wnt signaling requires β-TrCP. A, HEK293T cells were transfected with expression constructs for Wnt signaling components GSK3β, FZD5, LRP6, β-TrCP, Dvl1/2/3, Axin1, and Axin2 as indicated. 48 h after transfection, cells were harvested for immunoprecipitated (IP) with anti-HA or anti-FLAG followed by immunoblot (IB) analysis. B and C, HEK293T cells were transfected with TopFlash-Luc along with β-catenin, FAF1, and β-TrCP (B) or shβ-TrCP (C) plasmids as indicated. 36 h after transfection, cells were harvested for luciferase activity analysis. shβ-TrCP, shRNA-mediated β-TrCP knockdown. Each experiment was performed in triplicate, and the data represent mean ± S.D. (error bars) of three independent experiments after normalizing to β-gal activity.
FAF1 Interacts with Skp1-Cul1-F-box Protein (SCF)-β-TRCP Complex
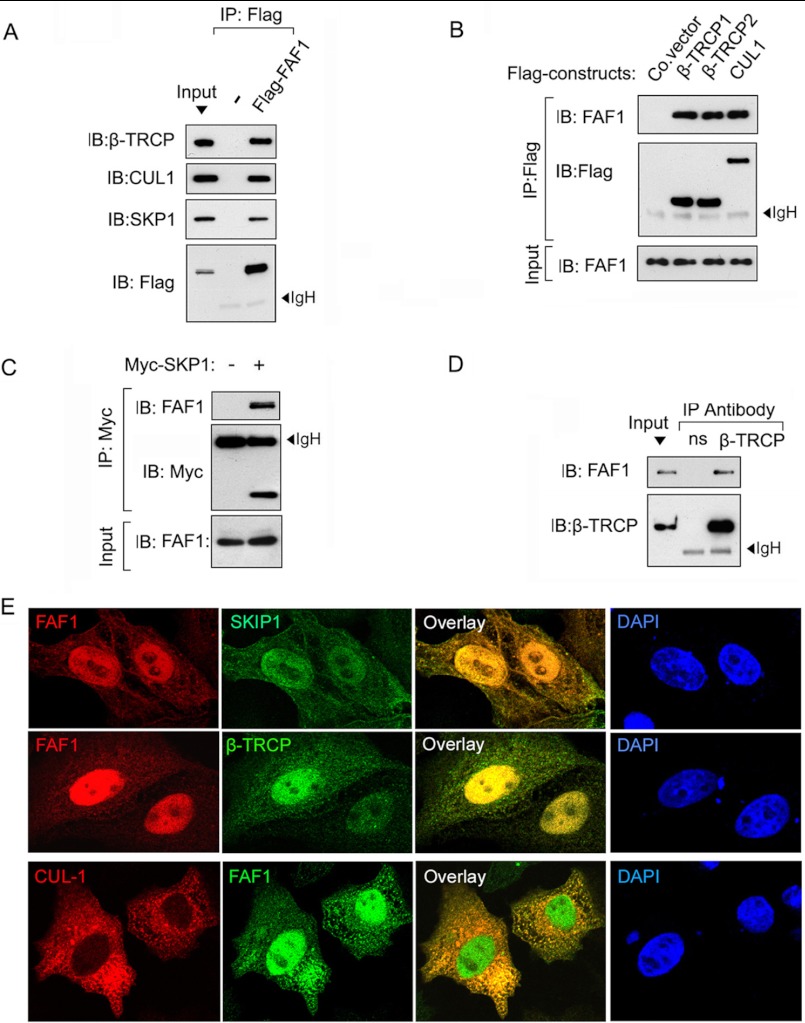
Because β-TrCP is an essential component of the SCF-β-TrCP ubiquitin ligase complex, we tested whether FAF1 interacts with the complex. Indeed, ectopically expressed FLAG-tagged FAF1 could be co-precipitated with endogenous β-TrCP, CUL1, and SKP1 in HEK293T cells (Fig. 3A). Conversely, endogenous FAF1 was found to co-precipitate with ectopically expressed FLAG-β-TrCP1/2, FLAG-CUL1, and Myc-SKP1 in HeLa cells (Fig. 3, B and C). Importantly, in nontransfected HEK293T cells, endogenous FAF1 co-immunoprecipitated with endogenous β-TrCP (Fig. 3D), suggesting that the two proteins interact at physiological conditions. To further consolidate this interaction, we investigated the subcellular localization of FAF1 and SCF complex components in HeLa cells. When co-expressed, FAF1 partially co-localized with SKIP, β-TrCP, and CUL1 (Fig. 3E). These data further support that FAF1 binds to the SCF-β-TrCP complex.
FIGURE 3.
FAF1 interacts with SCF complex. A, HEK293T cells were transfected with control or FLAG-FAF1 plasmids as indicated. 48 h after transfection, cells were harvested for immunoprecipitation (IP) with FLAG M2 beads followed by β-TrCP, CUL1, SKP1, and anti-FLAG immunoblot (IB) analysis. B, HEK293T cells were transfected with FLAG-tagged β-TrCP1, β-TrCP2, or CUL1 as indicated. 48 h after transfection, cells were harvested for immunoprecipitation with FLAG M2 beads followed with anti-FAF1 and anti-FLAG IB analysis. C, HEK293T transfected with or without Myc-SKP1 were harvested for immunoprecipitation with Myc antibody followed by FAF1 immunoblot analysis. D, HEK293T cells were harvested for immunoprecipitation with anti-β-TrCP antibody followed with anti-FAF1 IB analysis. E, HeLa cells were transfected with Myc or FLAG-tagged FAF1 and SKP1, β-TrCP as indicated. 36 after transfection, cells were harvested for immunofluorescence analysis.
FAF1 Can Form a Trimeric Complex with β-TRCP and β-Catenin
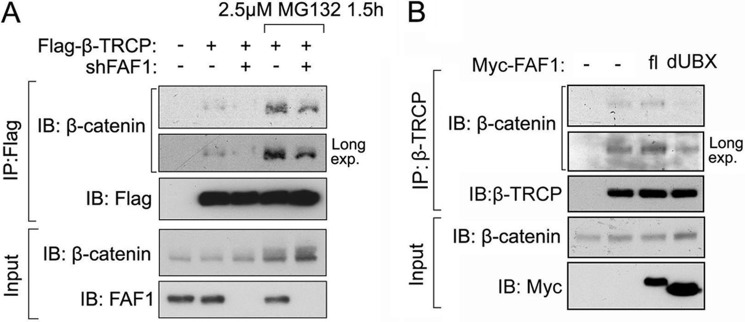
FAF1 was previously demonstrated to bind to β-catenin, the substrate of β-TrCP, upon inhibition of the proteosome (31). Therefore, we asked whether FAF1-β-catenin interaction requires FAF1-β-TrCP association. As both FAF1 and β-TrCP trigger β-catenin degradation, we performed also these interaction studies in the presence of the proteasome inhibitor MG132. As shown in Fig. 4A, in β-TrCP-depleted cells FLAG-FAF1 could bind β-catenin as efficiently as in control cells. Moreover, dUBX, a UBX domain deletion mutant of FAF1, was found not to interact with β-TrCP (Fig. 4B), but did still associate with β-catenin (Fig. 4B). These data indicate that the FAF1-β-catenin interaction is independent of FAF1-β-TrCP association.
FIGURE 4.
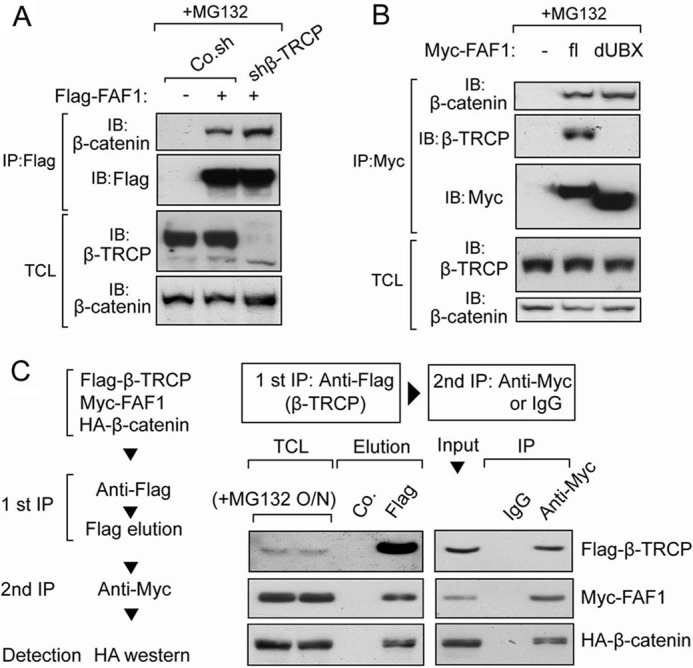
β-TrCP forms a trimeric complex with FAF1 and β-catenin. A, HEK293T cells transfected with or without FLAG-FAF1 were infected with or without lentivirus expressing β-TrCP shRNA as indicated. Cells were treated with MG132 (5 μm) for 4 h and then harvested for immunoprecipitation (IP) with FLAG M2 beads followed by immunoblot (IB) analysis. B, HEK293T cells were transfected with Myc-tagged FAF1 full-length (fl) or truncation that lacks UBX domain (dUBX) as indicated. Cells were treated with MG132 (5 μm) for 4 h and then harvested for immunoprecipitation with anti-Myc antibody followed with immunoblot analysis. C, two-step co-immunoprecipitation of the complex containing β-TrCP, FAF1, and β-catenin is shown. The procedures of the two-step co-immunoprecipitation are outlined in the box on the left, according to Ref. 59. HEK293T cells were transfected with plasmids expressing FLAG-β-TrCP, Myc-FAF1, and HA-β-catenin. Cells were treated with MG132 (5 μm) for 16 h. The first immunoprecipitation was performed using anti-FLAG M2 beads. The complex was eluted with FLAG peptide followed by the second step of co-immunoprecipitation with anti-Myc or control IgG. Protein samples from each step were analyzed by Western blotting separately with anti-FLAG, anti-Myc, and anti-HA.
Because β-TrCP is an E3 ligase that targets β-catenin for degradation (46, 47), we hypothesized that FAF1 might act as a scaffold and facilitate β-TrCP-mediated β-catenin degradation by forming a FAF1-β-TrCP-β-catenin ternary complex. To test this possibility, we performed a two-step co-immunoprecipitation assay in HEK293T cells transfected with FLAG-β-TrCP, Myc-FAF1, and HA-β-catenin. In the first step, anti-FLAG antibody was used to pull down FLAG-β-TrCP, and FLAG peptide was used to elute the complex from the beads. Subsequently, the eluate was immunoprecipitated with anti-Myc or control IgG, followed by Western blotting to detect HA-β-catenin. As shown in Fig. 4C, HA-β-catenin was present in the final Myc-FAF1 immunoprecipitation but not in the IgG control sample. This result indicates that β-TrCP, FAF1, and β-catenin are part of a common ternary complex.
β-TrCP Is Required for FAF1-induced β-Catenin Ubiquitination
Next we tested whether FAF1 requires β-TrCP to induce β-catenin polyubiquitination. HEK293T cells were transfected with His-ubiquitin, FAF1 wild type, or FAF1-dUBX (which could not associate with β-TrCP) in the absence or presence of Wnt3a. Nickel pulldown of His-ubiquitin-labeled protein showed that, although ectopic expression of full-length FAF1 highly induced β-catenin polyubiquitination in the absence of Wnt3a treatment, FAF1 dUBX deletion had little effect (Fig. 5A). Consistently, FAF1 failed to induce β-catenin polyubiquitination in β-TrCP-depleted cells (Fig. 5B). Taken together, these results demonstrate that the E3 ligase β-TrCP is required for FAF1-induced β-catenin ubiquitination.
FIGURE 5.
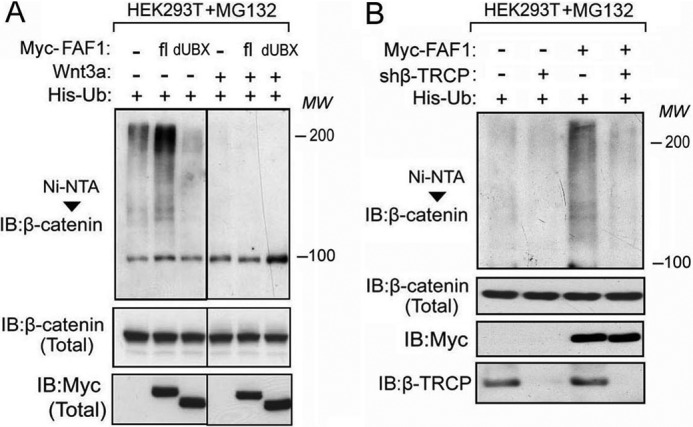
β-TrCP is required for FAF1-induced β-catenin ubiquitination. A, HEK293T cells stably expressing His-Ub were transfected with Myc-tagged FAF1 full-length (fl), truncation lacking UBX domain (dUBX), and Wnt3a as indicated. Cells were treated with MG132 for 4 h and then harvested for nickel-bead pulldown assay followed by immunoblot (IB) analysis. B, His-Ub-expressing HEK293T cells were transfected with or without Myc-FAF1 and infected with shβ-TrCP lentivirus as indicated. Cells were treated with MG132 for 4 h and then harvested for nickel-bead pulldown assay followed by immunoblot analysis.
FAF1 Facilitates Recognition of β-Catenin by β-TRCP
To further verify the scaffolding function of FAF1 for β-catenin and β-TrCP, we expressed ectopic FLAG-tagged β-TrCP and checked its association with endogenous β-catenin in the absence or presence of FAF1 knockdown. In the absence of MG132, we detected a weak association between β-TrCP and β-catenin, and this interaction was increased upon short term MG132 treatment. This is not unexpected as β-TrCP stimulates β-catenin for degradation. Upon depletion of endogenous FAF1, β-TRCP-associated β-catenin was partially decreased (Fig. 6A). To further validate this result, we expressed FAF1 wild type or FAF1-dUBX together with β-TRCP and tested their potential to affect β-TrCP interaction with β-catenin. As shown in Fig. 6B, wild-type FAF1 but not FAF1-dUBX could increase the association between β-TRCP and endogenous β-catenin. Together, these results demonstrate that FAF1 enhances β-catenin degradation by β-TrCP by increasing β-catenin recognition.
FIGURE 6.
FAF1 facilitates the association between β-TrCP and β-catenin. A, HEK293T cells transfected with or without FLAG-β-TrCP were infected with lentivirus expressing FAF1 shRNA as indicated. Cells were treated with MG132 (2.5 μm) for 1.5 h as indicated and harvested for immunoprecipitation (IP) with FLAG M2 beads followed by immunoblot (IB) analysis. B, HEK293T cells were transfected with Myc-tagged FAF1 full-length (fl) or truncation that lacks UBX domain (dUBX) as indicated. 48 h after transfection, cells were harvested for immunoblot analysis.
FAF1 Down-regulation May Correlate with Wnt/β-Catenin Activation during EMT of Breast Cancer Cells
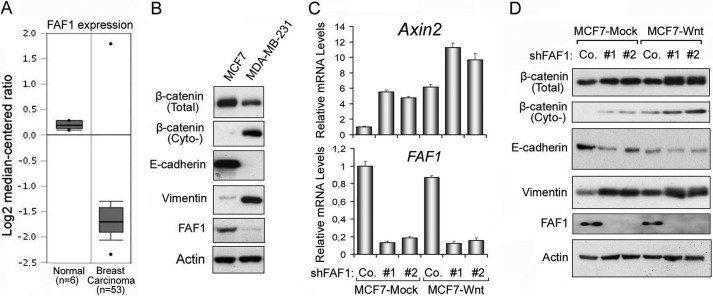
Activation of Wnt/β-catenin signaling triggers many biological events, including epithelial to mesenchymal transition (EMT) (18, 49). Data mining from the oncomine data base (50) indicated that FAF1 expression is significantly down-regulated in human breast carcinoma compared with normal tissue (Fig. 7A). Moreover, we found FAF1 expression to be higher in epithelial-like MCF7 than in mesenchymal-like MDA-MB-231 human breast cancer cells (Fig. 7B). In these cells FAF1 expression inversely correlated with the levels of cytoplasmic β-catenin, and positively and negatively correlated with the epithelial marker E-cadherin and the mesenchymal marker vimentin (51), respectively (Fig. 7B). This suggested that FAF1 might play a negative role in Wnt-induced EMT. To verify this, we depleted endogenous FAF1 in control (MCF7-Mock) and Wnt3a-transfected MCF7 (MCF7-Wnt) cells and examined activation of the β-catenin-Axin2 signaling cascade. As shown in Fig. 7, C and D, FAF1 depletion led to cytosolic β-catenin accumulation, β-catenin-induced Axin2 expression, as well as increased expression of vimentin. These results suggest that loss of FAF1 expression in breast cancer cells may lead to Wnt/β-catenin-induced EMT.
FIGURE 7.
FAF1 down-regulation correlates with Wnt/β-catenin activation during EMT of breast cancer cells. A, comparison between normal breast tissues and breast carcinomas tissues showed that FAF1 expression is down-regulated in breast carcinomas compared with normal tissues. B, immunoblots show FAF1 expression in MCF7 and MDA-MB-231 cells. Note that FAF1 expression is negatively correlated with cytosolic β-catenin levels. C, quantitative-PCR analysis was performed of Axin2 and FAF1 expression in control vector-transfected (MCF7-Mock) or Wnt3a-transfected (MCF7-Wnt3a) cells with a combination of FAF1 depletion mediated by lentivirus infection. D, immunoblottings of cells from C were as indicated.
Ectopic FAF1 Expression Reduces Breast Cancer Cell Migration in Vitro and Invasion/Metastasis in Vivo
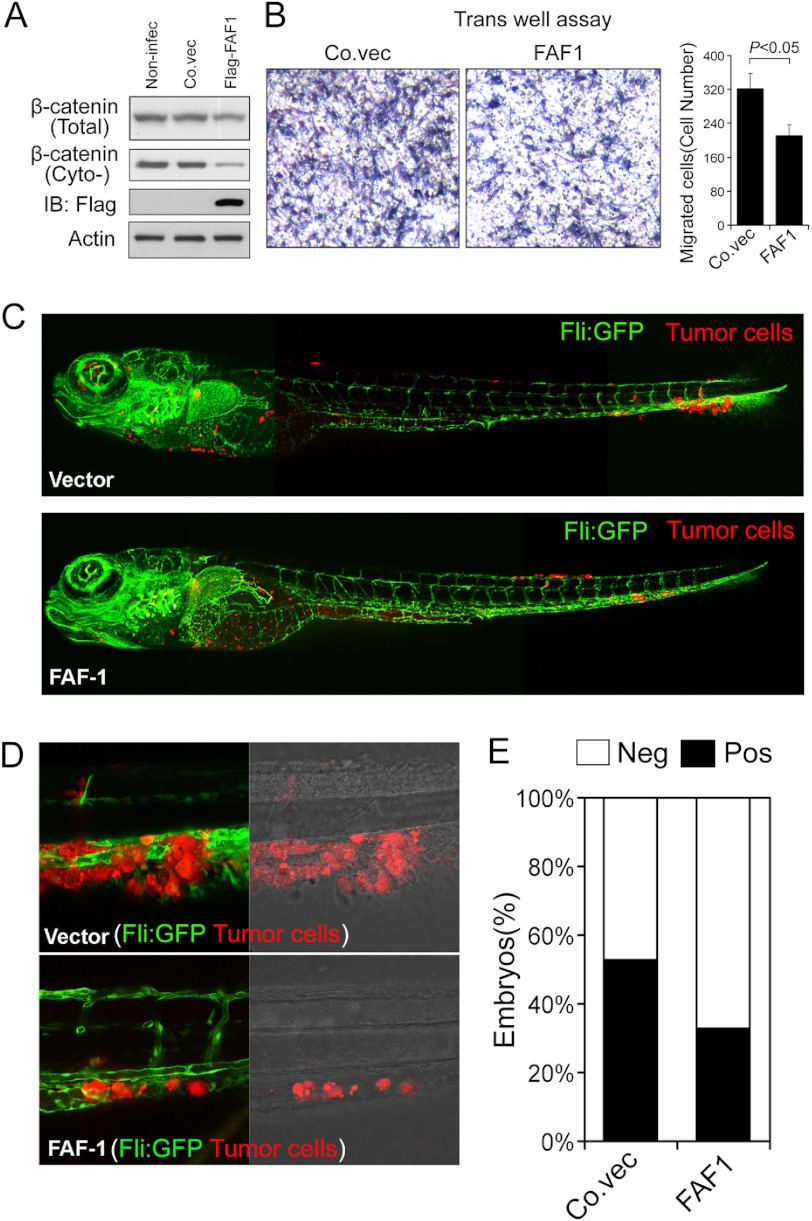
Wnt/β-catenin stimulates EMT, invasion, and metastasis of breast cancer cells (52–54). Active Wnt/β-catenin signaling also correlates with poor prognosis in breast cancer patients (55, 56). Importantly, highly metastatic MDA-MB-231 cells showed reduced cytosolic β-catenin level upon ectopic FAF1 expression, (Fig. 8A) as well as mitigated migratory ability (Fig. 8B). We next examined the effect of FAF1 in vivo using a zebrafish embryo xenograft invasion-metastasis model (57). Control or ectopic FAF1-expressing MDA-MB-231 cells were injected into the ducts of Cuvier 48 h postfertilization and analyzed over 6 days after injection. 53% of embryos showed invasion and metastasis in the case of control cells, whereas FAF1 expression significantly reduced this amount to 37% (Fig. 8, C and D). Taken together, these results indicate that FAF1 reduces soluble β-catenin and inhibits breast cancer invasion and metastasis.
FIGURE 8.
Ectopic FAF1 expression reduces breast cancer cell migration in vitro and invasion/metastasis in vivo. A, noninfected (Non-infec) and control vector (Co.vec) or FLAG-FAF1 infected MDA-MB-231 cells were harvested for immunoblotting (IB). Cytosolic (Cyto-) fraction was isolated for soluble β-catenin detection. B, control vector or FAF1-infected MDA-MB-231 cells were assayed for Transwell migration. Migrated cells were counted from four random fields, and mean values ± S.D. (error bars) were calculated (p = 0.032 < 0.05). Representative results are shown on the left. C–E, MDA-MB-231 cells stably infected with control vector (Vector) or FAF1 expression vector were injected into the blood circulation of 48-h post fertilization zebrafish. Representative images of zebrafish at 6 days after injection are shown (C). Invasion and experimental micrometastasis were detected in the posterior tail fin over 6 days (D). The final percentage of embryos displaying metastasis at 6 days after injection is shown (E).
DISCUSSION
Although FAF1 was initially identified as a member of the FAS death-inducing signaling complex (19), subsequent work has revealed that FAF1 functions in diverse biological processes. FAF1 has been shown to play an important role in normal development and neuronal cell survival, whereas FAF1 down-regulation may contribute to multiple aspects of tumorigenesis. In the field of molecular cellular signaling, FAF1 was first demonstrated to inhibit NF-κB activation induced by TNF-α, interleukin-1β (IL-1β), or lipopolysaccharide (LPS) through multiple mechanisms (24, 25). Recent studies identified FAF1 as a suppressor of canonical Wnt signaling in osteoblast differentiation and bone formation although the detailed mechanism remained unclear (31).
To uncover the mechanism of action underlying the ability of FAF1 to promote β-catenin degradation, we examined multiple essential components of the Wnt signaling cascade for their ability to interact with FAF1 and thereby identified β-TrCP as a strong FAF1-binding factor. FAF1 was also found to interact with SKP1 and CUL1, two other components of the β-catenin ubiquitination apparatus. β-TrCP cooperated with FAF1 in antagonizing Wnt signaling, and loss of endogenous β-TrCP attenuated FAF1-induced β-catenin polyubiquitination. Moreover, we could show that FAF1 forms a ternary trimeric complex with β-TrCP and β-catenin. Thus, FAF1 can function as a scaffold and thereby facilitate β-catenin recognition by β-TrCP. As a consequence, FAF1 depletion results in accumulation of cytosolic β-catenin, and potentiation of Wnt-induced EMT in human breast cancer cells. In mesenchymal-like highly metastatic breast cancer cells, ectopic expression of FAF1 was found to block the accumulation of soluble β-catenin and to antagonize tumor cell invasion and metastasis in zebrafish in vivo. It should be noted that E-cadherin, a strong binding partner of β-catenin in the membrane, can restrict the level of soluble β-catenin. However, we found ectopic FAF1 not to affect E-cadherin expression or to bind E-cadherin.5 We do not exclude the possibility that FAF1 may regulate other signaling pathways involved in breast cancer progression, in addition to the Wnt/β-catenin pathway.
Our results suggest that down-regulation of FAF1 may contribute to the aberrant activation of Wnt/β-catenin signaling during breast cancer progression. In addition to breast cancer, a decreased expression of FAF1 may also occur in many other types of cancer (58), including human gastric carcinomas and uterine cervix carcinomas (28). For instance, recurrent monoallelic and homozygous deletion of FAF1 has been reported in mantle cell lymphoma (30). Furthermore, single nucleotide polymorphism testing revealed that FAF1 may be associated with a genetic locus implicated in susceptibility to Crohn disease, an inflammatory bowel disorder that conveys an increased risk for colorectal cancer (60). Considering that Wnt/β-catenin signaling is a tumor promoting pathway, it would be worthwhile to figure out whether FAF1 loss of expression leads to aberrant Wnt/β-catenin activation in the cases mentioned above.
Finally, Faf1 gene-targeted mice are embryonic lethal and based on the pro-apoptotic function of FAF1, it was postulated that it might act as a tumor suppressor in cancers of varying origins (30, 48, 58, 60). Our studies on the inhibitory role of FAF1 in canonical Wnt signaling in breast cancer cells strengthen this notion.
Acknowledgments
We thank Dr.Wenyi Wei and Dr. Xi He of Harvard Medical School and Dr. C. Liu of Novartis Institutes for Biomedical Research for constructs.
This work was supported by the Netherlands Organization for Scientific Research Grant NWO 918.66.066 and by the Centre for Biomedical Genetics.
L. Zhang, F. Zhou, Y. Li, Y. Drabsch, J. Zhang, H. van Dam, and Peter ten Dijke, unpublished results.
- β-TrCP
- β-transducing repeat-containing protein
- dUBX
- UBX domain deletion mutant of FAF1
- EMT
- epithelial to mesenchymal transition
- FAF1
- FAS-associated factor 1
- SCF
- Skp1-Cul1-F-box protein
- LEF
- lymphoid enhancer-binding factor.
REFERENCES
- 1. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 2. Reya T., Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 3. Moon R. T., Kohn A. D., De Ferrari G. V., Kaykas A. (2004) WNT and β-catenin signalling: diseases and therapies. Nat. Rev. Genet. 5, 691–701 [DOI] [PubMed] [Google Scholar]
- 4. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 6. Nelson W. J., Nusse R. (2004) Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 9. Behrens J., von Kries J. P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382, 638–642 [DOI] [PubMed] [Google Scholar]
- 10. Brunner E., Peter O., Schweizer L., Basler K. (1997) Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385, 829–833 [DOI] [PubMed] [Google Scholar]
- 11. Li Y., Welm B., Podsypanina K., Huang S., Chamorro M., Zhang X., Rowlands T., Egeblad M., Cowin P., Werb Z., Tan L. K., Rosen J. M., Varmus H. E. (2003) Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 100, 15853–15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu B. Y., McDermott S. P., Khwaja S. S., Alexander C. M. (2004) The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 101, 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rowlands T. M., Pechenkina I. V., Hatsell S. J., Pestell R. G., Cowin P. (2003) Dissecting the roles of β-catenin and cyclin D1 during mammary development and neoplasia. Proc. Natl. Acad. Sci. U.S.A. 100, 11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bafico A., Liu G., Goldin L., Harris V., Aaronson S. A. (2004) An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 6, 497–506 [DOI] [PubMed] [Google Scholar]
- 15. Teulière J., Faraldo M. M., Deugnier M. A., Shtutman M., Ben-Ze'ev A., Thiery J. P., Glukhova M. A. (2005) Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development 132, 267–277 [DOI] [PubMed] [Google Scholar]
- 16. Ayyanan A., Civenni G., Ciarloni L., Morel C., Mueller N., Lefort K., Mandinova A., Raffoul W., Fiche M., Dotto G. P., Brisken C. (2006) Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 103, 3799–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kemler R., Hierholzer A., Kanzler B., Kuppig S., Hansen K., Taketo M. M., de Vries W. N., Knowles B. B., Solter D. (2004) Stabilization of β-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development 131, 5817–5824 [DOI] [PubMed] [Google Scholar]
- 18. Yook J. I., Li X. Y., Ota I., Hu C., Kim H. S., Kim N. H., Cha S. Y., Ryu J. K., Choi Y. J., Kim J., Fearon E. R., Weiss S. J. (2006) A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 8, 1398–1406 [DOI] [PubMed] [Google Scholar]
- 19. Chu K., Niu X., Williams L. T. (1995) A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc. Natl. Acad. Sci. U.S.A. 92, 11894–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yanagisawa J., Takahashi M., Kanki H., Yano-Yanagisawa H., Tazunoki T., Sawa E., Nishitoba T., Kamishohara M., Kobayashi E., Kataoka S., Sato T. (1997) The molecular interaction of Fas and FAP-1: a tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J. Biol. Chem. 272, 8539–8545 [DOI] [PubMed] [Google Scholar]
- 21. Fröhlich T., Risau W., Flamme I. (1998) Characterization of novel nuclear targeting and apoptosis-inducing domains in FAS-associated factor 1. J. Cell Sci. 111, 2353–2363 [DOI] [PubMed] [Google Scholar]
- 22. Ryu S. W., Lee S. J., Park M. Y., Jun J. I., Jung Y. K., Kim E. (2003) Fas-associated factor 1, FAF1, is a member of Fas death-inducing signaling complex. J. Biol. Chem. 278, 24003–24010 [DOI] [PubMed] [Google Scholar]
- 23. Adham I. M., Khulan J., Held T., Schmidt B., Meyer B. I., Meinhardt A., Engel W. (2008) Fas-associated factor (FAF1) is required for the early cleavage-stages of mouse embryo. Mol. Hum. Reprod. 14, 207–213 [DOI] [PubMed] [Google Scholar]
- 24. Park M. Y., Jang H. D., Lee S. Y., Lee K. J., Kim E. (2004) Fas-associated factor-1 inhibits nuclear factor-κB (NF-κB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-κB. J. Biol. Chem. 279, 2544–2549 [DOI] [PubMed] [Google Scholar]
- 25. Park M. Y., Moon J. H., Lee K. S., Choi H. I., Chung J., Hong H. J., Kim E. (2007) FAF1 suppresses IκB kinase (IKK) activation by disrupting the IKK complex assembly. J. Biol. Chem. 282, 27572–27577 [DOI] [PubMed] [Google Scholar]
- 26. Song E. J., Yim S. H., Kim E., Kim N. S., Lee K. J. (2005) Human Fas-associated factor 1, interacting with ubiquitinated proteins and valosin-containing protein, is involved in the ubiquitin-proteasome pathway. Mol. Cell. Biol. 25, 2511–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altomare D. A., Menges C. W., Pei J., Zhang L., Skele-Stump K. L., Carbone M., Kane A. B., Testa J. R. (2009) Activated TNF-α/NF-κB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl. Acad. Sci. U.S.A. 106, 3420–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bjørling-Poulsen M., Seitz G., Guerra B., Issinger O. G. (2003) The pro-apoptotic FAS-associated factor 1 is specifically reduced in human gastric carcinomas. Int. J. Oncol. 23, 1015–1023 [PubMed] [Google Scholar]
- 29. Hidalgo A., Baudis M., Petersen I., Arreola H., Piña P., Vázquez-Ortiz G., Hernández D., González J., Lazos M., López R., Pérez C., García J., Vázquez K., Alatorre B., Salcedo M. (2005) Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer 5, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beà S., Salaverria I., Armengol L., Pinyol M., Fernández V., Hartmann E. M., Jares P., Amador V., Hernández L., Navarro A., Ott G., Rosenwald A., Estivill X., Campo E. (2009) Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood 113, 3059–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L., Zhou F., van Laar T., Zhang J., van Dam H., Ten Dijke P. (2011) Fas-associated factor 1 antagonizes Wnt signaling by promoting β-catenin degradation. Mol. Biol. Cell 22, 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou F., Zhang L., Wang A., Song B., Gong K., Zhang L., Hu M., Zhang X., Zhao N., Gong Y. (2008) The association of GSK3β with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J. Biol. Chem. 283, 14506–14515 [DOI] [PubMed] [Google Scholar]
- 33. Zhang L., Gao X., Wen J., Ning Y., Chen Y. G. (2006) Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J. Biol. Chem. 281, 8607–8612 [DOI] [PubMed] [Google Scholar]
- 34. Zhou F., Zhang L., Gong K., Lu G., Sheng B., Wang A., Zhao N., Zhang X., Gong Y. (2008) LEF-1 activates the transcription of E2F1. Biochem. Biophys. Res. Commun. 365, 149–153 [DOI] [PubMed] [Google Scholar]
- 35. Zhang L., Zhou H., Su Y., Sun Z., Zhang H., Zhang L., Zhang Y., Ning Y., Chen Y. G., Meng A. (2004) Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306, 114–117 [DOI] [PubMed] [Google Scholar]
- 36. Zhou F., Zhang X., van Dam H., ten Dijke P., Huang H., Zhang L. (2012) Ubiquitin-specific protease 4 mitigates Toll-like/interleukin-1 receptor signaling and regulates innate immune activation. J. Biol. Chem. 287, 11002–11010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou F., van Laar T., Huang H., Zhang L. (2011) APP and APLP1 are degraded through autophagy in response to proteasome inhibition in neuronal cells. Protein Cell 2, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou F., Gong K., van Laar T., Gong Y., Zhang L. (2011) Wnt/β-catenin signal pathway stabilizes APP intracellular domain (AICD) and promotes its transcriptional activity. Biochem. Biophys. Res. Commun. 412, 68–73 [DOI] [PubMed] [Google Scholar]
- 39. Zhang L., Shi S., Zhang J., Zhou F., ten Dijke P. (2012) Wnt/β-catenin signaling changes C2C12 myoblast proliferation and differentiation by inducing Id3 expression. Biochem. Biophys. Res. Commun. 419, 83–88 [DOI] [PubMed] [Google Scholar]
- 40. Zhang L., Huang H., Zhou F., Schimmel J., Pardo C. G., Zhang T., Barakat T. S., Sheppard K. A., Mickanin C., Porter J. A., Vertegaal A. C., van Dam H., Gribnau J., Lu C. X., Ten Dijke P. (2012) RNF12 controls embryonic stem cell fate and morphogenesis in zebrafish embryos by targeting Smad7 for degradation. Mol. Cell 46, 650–661 [DOI] [PubMed] [Google Scholar]
- 41. Zhou F., Huang H., Zhang L. (2012) Bisindoylmaleimide I enhances osteogenic differentiation. Protein Cell 3, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu J., Ye Q., Yu B., Wang X., Zhou F. (2012) Contact printing a biomimetic catecholic monolayer on a variety of surfaces and derivation reaction. Chemical Commun. 48, 398–400 [DOI] [PubMed] [Google Scholar]
- 43. Zhou F., Gong K., Song B., Ma T., van Laar T., Gong Y., Zhang L. (2012) The APP intracellular domain (AICD) inhibits Wnt signalling and promotes neurite outgrowth. Biochim. Biophys. Acta 1823, 1233–1241 [DOI] [PubMed] [Google Scholar]
- 44. Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 45. Hsu S. C., Galceran J., Grosschedl R. (1998) Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol. Cell. Biol. 18, 4807–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitagawa M., Hatakeyama S., Shirane M., Matsumoto M., Ishida N., Hattori K., Nakamichi I., Kikuchi A., Nakayama K. (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winston J. T., Strack P., Beer-Romero P., Chu C. Y., Elledge S. J., Harper J. W. (1999) The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marini P., Schmid A., Jendrossek V., Faltin H., Daniel P. T., Budach W., Belka C. (2005) Irradiation specifically sensitises solid tumour cell lines to TRAIL-mediated apoptosis. BMC Cancer 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheel C., Eaton E. N., Li S. H., Chaffer C. L., Reinhardt F., Kah K. J., Bell G., Guo W., Rubin J., Richardson A. L., Weinberg R. A. (2011) Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Finak G., Bertos N., Pepin F., Sadekova S., Souleimanova M., Zhao H., Chen H., Omeroglu G., Meterissian S., Omeroglu A., Hallett M., Park M. (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 [DOI] [PubMed] [Google Scholar]
- 51. Gilles C., Polette M., Mestdagt M., Nawrocki-Raby B., Ruggeri P., Birembaut P., Foidart J. M. (2003) Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res. 63, 2658–2664 [PubMed] [Google Scholar]
- 52. Howe L. R., Brown A. M. (2004) Wnt signaling and breast cancer. Cancer Biol. Ther. 3, 36–41 [DOI] [PubMed] [Google Scholar]
- 53. Micalizzi D. S., Farabaugh S. M., Ford H. L. (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J. Mammary Gland Biol. Neoplasia 15, 117–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gebeshuber C. A., Sladecek S., Grunert S. (2007) β-Catenin/LEF-1 signalling in breast cancer: central players activated by a plethora of inputs. Cells Tissues Organs 185, 51–60 [DOI] [PubMed] [Google Scholar]
- 55. Prasad C. P., Rath G., Mathur S., Bhatnagar D., Parshad R., Ralhan R. (2009) Expression analysis of E-cadherin, Slug and GSK3β in invasive ductal carcinoma of breast. BMC Cancer 9, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Logullo A. F., Nonogaki S., Pasini F. S., Osório C. A., Soares F. A., Brentani M. M. (2010) Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma: association with progression. Oncol. Rep. 23, 313–320 [PubMed] [Google Scholar]
- 57. He S., Lamers G. E., Beenakker J. W., Cui C., Ghotra V. P., Danen E. H., Meijer A. H., Spaink H. P., Snaar-Jagalska B. E. (2012) Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Menges C. W., Altomare D. A., Testa J. R. (2009) FAS-associated factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle 8, 2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harada J., Kokura K., Kanei-Ishii C., Nomura T., Khan M. M., Kim Y., Ishii S. (2003) Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J. Biol. Chem. 278, 38998–39005 [DOI] [PubMed] [Google Scholar]
- 60. Weersma R. K., Stokkers P. C., Cleynen I., Wolfkamp S. C., Henckaerts L., Schreiber S., Dijkstra G., Franke A., Nolte I. M., Rutgeerts P., Wijmenga C., Vermeire S. (2009) Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am. J. Gastroenterol. 104, 630–638 [DOI] [PubMed] [Google Scholar]