Background: The androgen receptor (AR) is the primary drug target for prostate cancer treatment.
Results: We have identified a novel AR antagonist, the compound 6-(3,4-dihydro-1H-isoquinolin-2-yl)-N-(6-methylpyridin-2-yl)nicotinamide (DIMN) that inhibits the growth of AR-positive prostate cancer cells.
Conclusion: DIMN has been identified as a new lead structure targeting the AR.
Significance: This novel AR antagonist could be a useful therapeutic agent for prostate cancer treatment.
Keywords: Androgen Receptor, Cell Growth, Drug Screening, Gene Expression, Prostate Cancer, Anti-androgen, Nicotinamide Derivative
Abstract
Hormonal therapies, mainly combinations of anti-androgens and androgen deprivation, have been the mainstay treatment for advanced prostate cancer because the androgen-androgen receptor (AR) system plays a pivotal role in the development and progression of prostate cancers. However, the emergence of androgen resistance, largely due to inefficient anti-hormone action, limits the therapeutic usefulness of these therapies. Here, we report that 6-(3,4-dihydro-1H-isoquinolin-2-yl)-N-(6-methylpyridin-2-yl)nicotinamide (DIMN) acts as a novel anti-androgenic compound that may be effective in the treatment of both androgen-dependent and androgen-independent prostate cancers. Through AR structure-based virtual screening using the FlexX docking model, fifty-four compounds were selected and further screened for AR antagonism via cell-based tests. One compound, DIMN, showed an antagonistic effect specific to AR with comparable potency to that of the classical AR antagonists, hydroxyflutamide and bicalutamide. Consistent with their anti-androgenic activity, DIMN inhibited the growth of androgen-dependent LNCaP prostate cancer cells. Interestingly, the compound also suppressed the growth of androgen-independent C4–2 and CWR22rv prostate cancer cells, which express a functional AR, but did not suppress the growth of the AR-negative prostate cancer cells PPC-1, DU145, and R3327-AT3.1. Taken together, the results suggest that the synthetic compound DIMN is a novel anti-androgen and strong candidate for useful therapeutic agent against early stage to advanced prostate cancer.
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer deaths in men in the United States (1, 2). It is well established that androgens, such as testosterone and dihydrotestosterone (DHT),4 play an essential role in the tumorigenesis and progression of androgen-dependent early stage prostate cancer (3). Testosterone, predominantly produced by Leydig cells in the testes, is converted to a more active form, DHT, by the enzyme 5α-reductase in the prostate. Early onset prostate cancer is androgen-dependent; therefore, androgen-ablation therapies that decrease the levels of circulating androgens through chemical or surgical castration have been the mainstay of treatment for androgen-dependent prostate cancer (ADPC). Unfortunately, androgen-ablation therapy is only palliative. After 2–3 years of treatment, the cancer cells progress to a more aggressive form, androgen-independent prostate cancer (AIPC), or to a hormone refractory state known as castration-resistant prostate cancer (CRPC) (4, 5).
The androgen receptor (AR), the mediator of androgen action, is a member of the steroid hormone receptor superfamily and contains a DNA-binding domain and a hormone-binding domain. This receptor is activated by binding with androgens in the cytoplasm and is then translocated into the nucleus, where it regulates the expression of target genes such as prostate-specific antigen (PSA) and NK3 transcription factor locus 1 (NKX3.1) in the human prostate. AR signaling is known to regulate the development and progression of normal, benign, and malignant prostate cells (6–8). The AR is expressed in the vast majority of both ADPC and AIPC, and decreasing levels of AR protein expression reduce both ADPC and AIPC growth, suggesting a critical role of AR signaling in both types of prostate cancers (9–11). Castration resistance is attributed to the high expression of the AR and AR-regulated genes, indicating that AR transcriptional activity is reactivated. AR reactivation in CRPC can be explained by AR gene amplification (12, 13), AR gene mutation (14–17), activation by alternative androgens (18, 19), or ligand-independent AR activation through other factors, including the increased expression of transcriptional co-activators or the activation of kinases and signal transduction pathways that modulate AR function (7, 20–23).
Because of the critical role of the AR in prostate cancer, the AR has been the primary target for the treatment of this disease, and AR antagonists have been used for the treatment of prostate cancer and prostatic hyperplasia. There are two types of AR antagonists, structure-based steroidal and non-steroidal. Cyproterone acetate, one of the steroidal AR antagonists, inhibits androgen action, but it also has weak progestational and glucocorticoid activities (24, 25). Non-steroidal AR antagonists, such as flutamide (hydroxyflutamide) and bicalutamide, have been considered to be less problematic due to their selective blockade of androgen action and fewer side effects (26–28). Bicalutamide is the most widely used of these compounds in the treatment of prostate cancer because it is believed to overcome some problems caused by other anti-androgens (29). However, because the classical AR antagonists are not effective for the treatment of advanced prostate cancers, many efforts have been undertaken to develop newer and better AR antagonists that work effectively on either early stage androgen-dependent or later stage androgen-independent prostate cancer cells.
Searching for new lead scaffolds that induce equal or better biological responses than the current drugs through the same receptor is a challenging goal in drug design. Because new therapeutic targets and their three-dimensional structures have been identified at a dramatic rate, computational screening methods have become quite reliable as a source of chemical starting points in the drug design/discovery process. Compared with the conventional high-throughput screening (HTS) method, virtual screening (VS) extends the screening possibilities to molecules that do not exist physically in the collection but can be purchased. In addition, out of the large number of chemicals screened in silico, only a small subset of chemicals is tested to quantify biological activity based on the computational prediction results. These substantial advantages have made the VS approach increasingly valuable for the identification of novel lead scaffolds that bind to ligand-dependent receptors (30, 31).
Many trials have been conducted to identify new leads for the development of better AR antagonists that could provide new treatments for prostate cancers. Most of the AR antagonists that have been discovered thus far have been developed through ligand-based drug design, which relies on the pharmacophores of known drugs. Because of the characteristics of ligand-based design, most AR antagonists seem to contain the same basic scaffolds as the known drugs, such as bicalutamide and flutamide or other non-steroidal AR agonists (32, 33). As an example, MDV3100, now in phase III clinical trials, was developed from the non-steroidal AR agonist RU59063 by modifying the chemical structures systemically while maintaining the key chemical scaffold (34).
In this study, keeping in mind the possible switch from AR antagonism to agonism induced by similar scaffolds to those of known ligands, we carried out AR structure-based virtual screening to discover a novel chemical scaffold for AR antagonists. We have successfully identified a new lead structure targeting the AR, and verified the biological effects of the compound as AR antagonist that works on early stage prostate cancer cells as well as on late stage cells.
EXPERIMENTAL PROCEDURES
Chemistry
Chemicals were purchased from Aldrich Chemical Co. or Tokyo Chemical Industry Co. Melting points were determined by the capillary method on Electrothermal IA9200 digital melting point apparatus. 1H NMR data were collected on a Varian 300 FT spectrometer and were calibrated with tetramethylsilane. The NMR data are displayed as follows: chemical shifts (δ) are recorded in ppm, coupling constants (J) in hertz (Hz), integrity in the number of protons, and multiplicity in s (singlet), d (doublet), t (triplet), and m (multiplet). Mass spectra were obtained on a Shimadzu LCMS-2010EV utilizing the electron-spray ionization (ESI) method and on a JEOL JNS-DX 303 using the electron-impact (EI) method. IR spectra were recorded on a JASCO-FT IR spectrometer using CHCl3 or KBr pellets. Thin-layer chromatography (TLC) was carried out using plates coated with silica gel 60 F254 purchased from Merck. Column chromatography was performed with Merck silica gel 60 (70–230 mesh).
Chemical Synthesis of DIMN
DIMN was prepared in three steps starting from 6-chloro-nicotinic acid (1) (Fig. 1B). Nicotinic acid chloride, formed by refluxing (1) with thionyl chloride, was treated with pyridylamine (2) to obtain the intermediate (3) with 83% yield. Finally, an SNAr reaction with 1,2,3, 4-tetrahydroisoquinoline (4) in 2-propanol under reflux conditions provided the desired DIMN (5) with 72% yield.
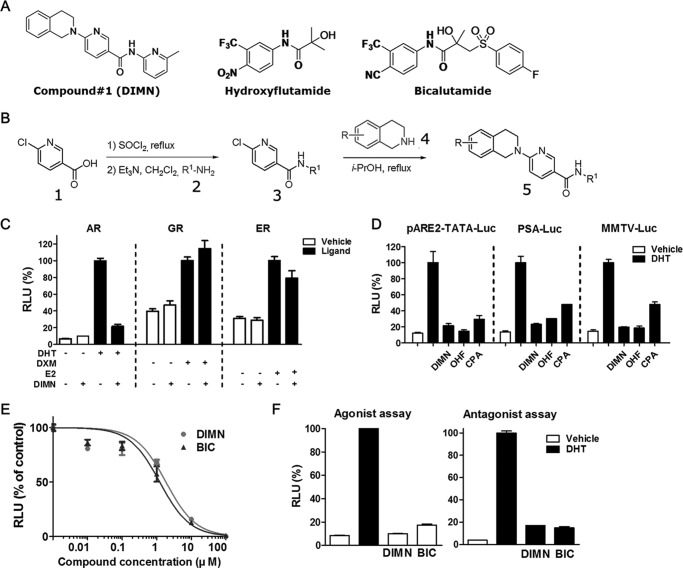
FIGURE 1.
Identification of DIMN as a new AR antagonist. A, chemical structures of DIMN and the classical AR antagonists hydroxyflutamide and bicalutamide. B, chemical synthesis of DIMN. C, selective AR antagonist activity of DIMN. COS-7 cells were co-transfected with, AR/pARE2-TATA-Luc, GR/MMTV-Luc or ER/ERE-Luc. The cells were treated with 1 μm DIMN in the presence of 0.3 nm DHT (dihydrotestosterone), 100 nm DXM (dexamethasone), and 10 nm E2 (estradiol), respectively. D, repression of AR transactivation by DIMN. The transcriptional activity was determined in COS-7 cells transiently co-transfected with pcDNA3.AR and an androgen-responsive luciferase reporter (pARE2-TATA-Luc, PSA-Luc, or MMTV-Luc). After a 24-h transfection, cells were treated with 1 μm OHF, CPA, or DIMN in the presence of 0.3 nm DHT for an additional 24 h. OHF and CPA were used as positive controls for AR antagonism. The luciferase data were normalized for β-galactosidase activity and expressed as a percentage of AR activity in the presence of 0.3 nm DHT only. E, dose-dependent AR antagonistic activities of DIMN. After a 24-h transfection with pcDNA3.AR and pARE2-TATA-Luc, COS-7 cells were treated with various concentrations of BIC or DIMN in the presence of 10 nm DHT and incubated for 24 h. The IC50 values represent the concentrations of compounds that inhibited 50% of the response induced by 10 nm DHT. F, agonistic/antagonistic effect of DIMN on AR transactivation. Cells transfected as in E were treated with 10 μm of DIMN in the absence (white bar) or presence (black bar) of 10 nm DHT. Each value represents the mean ± S.E. of at least three independent experiments. RLU, relative light units.
Reagents
Cyproterone acetate (CPA), bicalutamide (BIC), and 2-hydroxyflutamide (OHF) were purchased from Sigma, Sequoia Research Products Ltd., and LKT Laboratories, Inc., respectively. Radiolabeled dihydrotestosterone ([3H]DHT) ([1,2,4,5,6,7-3H(N)]-dihydrotestosterone (5α-androstan-17β-ol-3-one)) and thymidine ([methyl-3H]thymidine, specific activity: 70–90 Ci (2.59–3.33T Bq/mmol) were obtained from Perkin Elmer Life Science. Antibodies were purchased from Santa Cruz Biotechnology, Inc. (AR (sc-815), PSA (sc-7638) and α-tubulin (sc-5286)) and Epitomics, Inc. (GAPDH (cat. #2251-1)).
Plasmids
The mammalian expression plasmids of the mouse AR (pcDNA3.AR), mouse GR (pcDNA3.GR), pARE2-TATA-Luc, PSA-Luc, MMTV-Luc, GFP-AR, pCR3.1-SRC1, and pSG5-HA-GRIP-1 (SRC-2) have been previously described (35–40). The pcDNA3.ERα (human ERα expression plasmid) and ERE-Luc reporter constructs were kindly provided by Dr. J. W. Lee (Baylor College of Medicine) (41). The mammalian expression plasmids VP-AR1-660, GAL-AR624-919, and 5XGAL4-Luc3 (originally from Dr. Donald McDonnell) were kindly provided as gifts by Dr. Elizabeth M. Wilson (University of North Carolina) (42).
Cell Culture
COS-7, 293T, PPC-1, DU145, HeLa, and MEF (mouse embryonic fibroblast) cells were maintained in Dulbecco's minimum essential medium (Hyclone) supplemented with 10% fetal bovine serum (FBS). LNCaP cells were purchased from the American Type Culture Collection (ATCC CRL-1740). C4–2 and CWR22rv cells were kindly provided by Dr. C. Jung (Chonnam National University Medical School, Republic of Korea). LNCaP, C4–2, and CWR22rv cells were maintained in RPMI 1640 (Hyclone) medium supplemented with 5% FBS. R3327-AT3.1 cells were kindly provided by Mazence, Inc., (Suwon, Republic of Korea) and were maintained in RPMI 1640 (Hyclone) medium supplemented with 10% FBS. All cells were cultured at 37°C in a 95% humidified atmosphere containing 5% CO2.
Transient Transfection Assay
Transfections were carried out using the SuperFect (Qiagen) transfection reagent for COS-7 and 293T cells and the Lipo2000 transfection reagent for PPC-1 cells, according to the instructions of the manufacturer. Cells plated in 24-well plates were transfected with the indicated expression plasmids and a reporter plasmid, along with the β-gal expression plasmid pCMV-β (Clontech). Cells kept in 5% charcoal-stripped FBS (CSS) were treated with chemicals in the presence or absence of the ligand for 24 h and processed as described previously (43). The levels of luciferase activity were normalized to β-gal expression.
Competitive Steroid Binding Assay
The whole-cell binding assay was performed as described previously (44). Briefly, COS-7 cells were transiently transfected with pcDNA3.AR. Twenty-four hours prior to the binding reaction, the cells were placed in phenol red-free DMEM supplemented with 5% CSS and incubated for 2 h at 37 °C with 5 nm 5α-[3H]DHT in the presence and absence of increasing concentrations of unlabeled chemicals. Nonspecific binding of 5α-[3H]DHT was assessed by adding a 100-fold molar excess of unlabeled 5α-DHT. Dose-response data were analyzed using the sigmoidal dose-response function of Prism (GraphPad, San Diego, CA).
Fluorescent Microscopy
HeLa cells plated onto 0.1% gelatin-coated coverslips were transfected with the GFP-AR expression vector. After 16 h, transfected cells were fed with fresh DMEM containing 5% CSS and treated for 1 h with chemicals. Cells were processed for fluorescent microscopy using an Olympus 1 × 70 fluorescent microscope (Tokyo, Japan) as described previously (44).
Northern Blot Analysis
Northern blot analysis was conducted as described previously (45). Random-primed α-32P-labeled PSA, NKX3.1, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA probes were used for hybridization. GAPDH expression was used as an internal control.
Western Blot Analysis
Western blot analysis was conducted as described previously (46). In brief, the LNCaP and C4–2 cells were incubated in RPMI supplemented with 5% CSS for 2 days, and then treated with AR antagonists in the presence of 10 nm or 1 nm DHT for 2 days, respectively (47). The whole cell lysates were separated by SDS-PAGE, transferred to nitrocellulose and subjected to Western blot analysis with anti-AR, anti-PSA, anti-α-tubulin, and anti-GAPDH antibodies. Signals were detected using an ECL kit (Amersham Biosciences Pharmacia).
Thymidine Incorporation Assay
The thymidine incorporation assay was conducted as described previously (37). LNCaP cells were seeded into 96-well plates at a density of 2 × 103 cells per well. The cells were treated with chemicals in the presence of 1 nm DHT for 72 h and then treated with 10 μCi/ml of [3H]thymidine for another 4 h. Cells were harvested onto a glass microfiber filter (Whatman, Inc., Florham Park, NJ) and processed for the measurement of incorporated amount of thymidine into DNA. All values represent the mean ± S.E. of at least three independent experiments.
Cell Viability
The cell growth and cytotoxicity assays were conducted using the CellTiter 96® aqueous non-radioactive cell proliferation assay kit (Promega). Cells were seeded into 96-well plates at a density of 2 × 103 cells per well (LNCaP, C4–2, and MEF) or 5 × 102 cells per well (PPC-1, DU145, and R3327-AT3.1). Cells cultured in media supplemented with 5% CSS (LNCaP), 5% FBS (C4–2 and MEF), or 10% FBS (PPC-1, DU145, and R3327-AT3.1) were treated with indicated chemicals for 4 and 6 days. Combined MTS/PMS (ratio 20:1 by volume, 20 μl/well) solution was added to cells in freshly prepared media. After 2 h, the absorbance at 490 nm was recorded using an ELISA plate reader. Cell viability was also assessed by trypan blue dye exclusion by counting cell numbers as described previously (48). CWR22rv cells (4 × 104 cells per well) were incubated with chemicals for 5 days. All values represent the mean ± S.E. of at least three independent experiments.
Statistical Analysis
To identify significant differences, the data were analyzed using GraphPad Prism (GraphPad Software, Inc.). Single comparisons between two experimental groups were performed using an unpaired Student's t test. Data are shown as the means ± S.E. of the mean (S.E.). For all statistical analyses, p < 0.05 was used as the criterion to determine statistical significance.
RESULTS
AR Structure-based Virtual Screening for AR Antagonists
A key feature of ligand-dependent receptors for use in rational drug design is the ligand-binding domain (LBD), which we have used in AR structure-based drug discovery. Crystal structures of the AR LBD bound to ligands have been determined, but no structural information about the nature of the antagonist-induced conformational change exists because of the lack of defined crystallization of the wild-type AR-antagonist complex to date. We therefore selected the structure of the AR-metribolone (R1881) complex for screening (code from Protein Data Bank: 1E3G). Since R1881 is one of the compounds known to bind most tightly to the AR, the bound LBD structure would be expected to offer some information on the native AR in its strongest binding state.
A chemical library containing over 200,000 drug-like molecules extracted from commercial and in-house databases was docked into the AR LBD using the docking algorithm FlexX. The binding affinity between the chemicals and the AR LBD was predicted by five different scoring functions and a consensus score. To verify the prediction confidence of our docking system, a root-mean-square deviation (RMSD) calculation was performed by taking into account the binding coordinate of R1881 in the AR LBD. The use of FlexX resulted in the RMSD value of 0.721, which is almost identical to the RMSD value of the native AR-R1881 structure, indicating that our docking program is highly confident. Following the screening, 54 compounds were acquired and numbered in order of their consensus scores (data not shown).
To test whether the selected 54 compounds exhibit agonistic/antagonistic activity toward the AR, we performed transient transfection assays using a reporter system for the AR, pARE2-TATA-Luc, which contains two AREs of the androgen target gene C3. The results revealed that several compounds significantly inhibited the DHT-induced transcriptional activation of AR at a concentration of 1 μm in the presence of 0.3 nm DHT (supplemental Fig. S1, upper panel). Among them, compound #1 (6-(3,4-dihydro-1H-isoquinolin-2-yl)-N-(6-methylpyridin-2-yl)nicotinamide), designated as DIMN, showed the smallest agonistic effect (supplemental Fig. S1, bottom panel) with a significant antagonistic effect. Moreover, this compound survived elimination on the basis of Lipinski's rule, the novelty of chemical structure, and other parameters affecting the successful outcome of lead optimization (supplemental Fig. S1). Therefore, we selected DIMN for further study, which has a completely novel scaffold compared with the previously known agonists and antagonists (Fig. 1A) of the AR, and prepared the compound in three steps starting from 6-chloro-nicotinic acid (1) (Fig. 1B). The chemical 6-chloro-nicotinic acid (1) and 1,2,3,4-tetrahydroisoquinoline (4) showed no agonistic or antagonistic effect on AR transactivation (data not shown).
Identification of DIMN as a New AR Antagonist
We first investigated whether the inhibitory effect of DIMN was AR-specific by testing its effect on the transactivation of the steroid receptor GR and ER. COS-7 cells were co-transfected with plasmids expressing the GR and ER along with MMTV-Luc and ERE-Luc, respectively. DIMN specifically inhibited the transactivation of the AR, but not of the GR and ER (Fig. 1C). DIMN was further analyzed for AR antagonistic activity by transient transfection assays using several reporter systems for the AR. The PSA-Luc and MMTV-Luc plasmids contain the prostate specific antigen (PSA) and mouse mammary tumor virus (MMTV) long terminal repeat promoters, respectively, which are natural AR target promoters (35, 49). DIMN inhibited androgen-induced AR transactivation at 1 μm concentration in all of the tested reporter systems (Fig. 1D); this inhibitory potency was similar to that of hydroxyflutamide (OHF). In addition, DIMN inhibited AR transactivation in a dose-dependent manner with the IC50 value at 3 μm (Fig. 1E), which is comparable to the IC50 value of BIC (1.6 μm). However, even at 10 μm concentration, DIMN showed little agonistic effect in contrast to BIC, which showed some agonistic effect (Fig. 1F) as previously reported (50). These results suggest that DIMN has a strong AR-specific antagonistic effect with little agonistic effect.
Binding of DIMN to AR
To characterize the binding of the compound DIMN to the AR, we performed competitive androgen binding assay using 5α-[3H]DHT (a labeled androgen) and AR expressed in COS-7 cells. We used several known competitors (unlabeled DHT, BIC, and OHF) of DHT as positive controls (38, 51). The IC50 (the concentration of ligand that were able to inhibit AR-DHT binding by 50%) of DIMN was 1–2 μm, while the IC50 values of unlabeled DHT, OHF, and BIC were 1–2 nm, 0.4–0.5 μm, and 0.9 μm, respectively (Fig. 2A). DIMN showed 2–4-fold lower AR binding activity than OHF and 1–2-fold lower binding activity than BIC. The compound DIMN could not bind to the AR more tightly than the conventional AR antagonists BIC and OHF, but could bind nearly as well as those antagonists, suggesting that it competes with androgen for AR binding in a similar fashion.
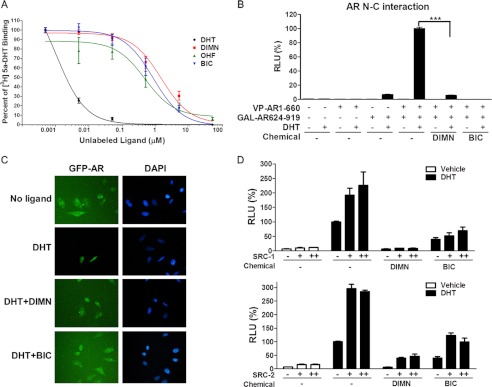
FIGURE 2.
Effect of DIMN on androgen binding to AR and on androgen-induced AR activation steps. A, effect of DIMN on 5α-[3H]DHT binding to the AR. The binding inhibition was determined in COS-7 cells transiently transfected with pcDNA3.AR. The results are presented as percent binding relative to 5α-[3H]DHT alone and are shown for unlabeled DHT, BIC, OHF, and DIMN. B, inhibitory effects of DIMN on the AR N/C interaction. A mammalian two-hybrid assay was performed in PPC-1 cells transfected with 5XGAL4-Luc3, VP-AR1–660, and GAL-AR624–919. The interaction between the AR N and C termini was assessed after the addition of 10 μm of the indicated compound in the presence of 10 nm DHT. Error bars indicate the standard deviation. ***, p < 0.001. C, inhibitory effect of DIMN on the nuclear translocation of GFP-AR. HeLa cells transfected with the GFP-AR expression plasmid were grown on gelatin-coated coverslips. The subcellular localization of the GFP-AR in living cells was observed and recorded by fluorescence microscopy after a 1-h treatment with 10 μm BIC or DIMN in the presence of 10 nm DHT. D, inhibition of SRC-1- and SRC-2-mediated enhancement of AR transactivation by DIMN. 293T cells were co-transfected with pcDNA3.AR, pARE2-TATA-Luc, and increasing amounts of pCR3.1 SRC-1 or pSG5-HA-SRC-2 (+, 300 ng; ++, 600 ng). After a 24-h transfection, cells were treated with 10 μm BIC or DIMN in the presence of 10 nm DHT. Each value represents the mean ± S.E. of at least three independent experiments. BIC and OHF were used as positive controls.
Molecular Basis for the Anti-androgenic Effect of DIMN
Upon ligand binding, AR dissociates from heat shock proteins and translocates into the nucleus, binding to its target gene promoters as a homodimer formed by the intermolecular N/C interaction of two AR molecules. To explore the anti-androgenic effects of DIMN induced through mechanisms other than the inhibition of androgen binding to the AR, we investigated the ability of DIMN to inhibit any of the AR activation steps, such as the N/C interaction, nuclear translocation, and co-activator recruitment.
The effect of DIMN on the AR N/C interaction was tested using a mammalian two-hybrid system. PPC-1 cells were transfected with plasmids encoding the VP-AR1–660 (containing AR residues 1–660) and GAL-AR624–919 (containing AR residues 624–919) fusion proteins in conjunction with a luciferase reporter gene regulated by tandem Gal4-responsive elements (5XGAL4-Luc3) (42). The DHT-induced N/C interaction was inhibited strongly by DIMN. However, its inhibitory effect on the N/C interaction was weaker than that of BIC, which almost completely abolished this interaction (Fig. 2B). The compound induced no N/C interaction of AR in the absence of DHT (Fig. 2B) as OHF and BIC (52, 53). These results suggest that DIMN inhibits the dimerization of the AR.
The effect of DIMN on the dynamics of the subcellular distribution of the AR was tested using a GFP-AR fusion protein. When GFP-AR was overexpressed in HeLa cells in the absence of androgen, the fusion protein was mostly distributed in the cytoplasmic compartment, but, in the presence of 10 nm DHT, GFP-AR was predominantly localized in the nucleus, as previously described for the native AR (54) (Fig. 2C). The inhibitory effect of DIMN on the nuclear import of the AR was assessed by adding 10 μm DIMN in addition to 10 nm DHT. The distribution of GFP-AR protein in cells treated with both DHT and DIMN was dispersed between the nuclear and the cytoplasmic compartments, similar to cells challenged with 10 μm BIC (55, 56). These results suggest that DIMN interferes with the nuclear translocation of the AR.
The elevated expression of SRC-1 and SRC-2 has been reported to enhance AR activity in the development of more aggressive prostate cancers (reviewed in Ref. 57). Therefore, we tested the effects of DIMN on the action of the AR co-activators SRC-1 and SRC-2 via transient transfection assays using a reporter system with pARE2-TATA-Luc. As shown in Fig. 2D, overexpression of SRC-1 and SRC-2 enhanced the transcriptional activity of the AR induced by 10 nm DHT. A 10 μm dose of DIMN could inhibit the SRC-1- and SRC-2-mediated enhancement of AR transactivation. BIC exhibited a similar effect on AR transcriptional activity enhanced by the co-activators SRC-1 and SRC-2, consistent with previous reports (53).
Suppression of Androgen-induced AR Target Gene Expression by DIMN in Prostate Cancer Cells
Because DIMN has anti-androgenic activity, we assessed its effect on the expression of the AR target genes PSA and NKX3.1 (58, 59) in androgen-dependent LNCaP and androgen-independent C4–2 prostate cancer cells (Fig. 3), both of which express functional endogenous AR (60). The mRNA levels of PSA, which is a prostate-specific tumor marker (61), were reduced by treatment with DIMN in the absence or presence of DHT in both LNCaP and C4–2 cells, and this reduction of PSA mRNA levels by DIMN was comparable to or greater than that induced by BIC (Fig. 3A). Interestingly, BIC activated the expression of PSA in the absence of DHT in C4–2 cells, as previously reported in LNCaP (62). The mRNA levels of NKX3.1 showed similar patterns to those of PSA upon DIMN treatment in both LNCaP and C4–2 cells (Fig. 3A).
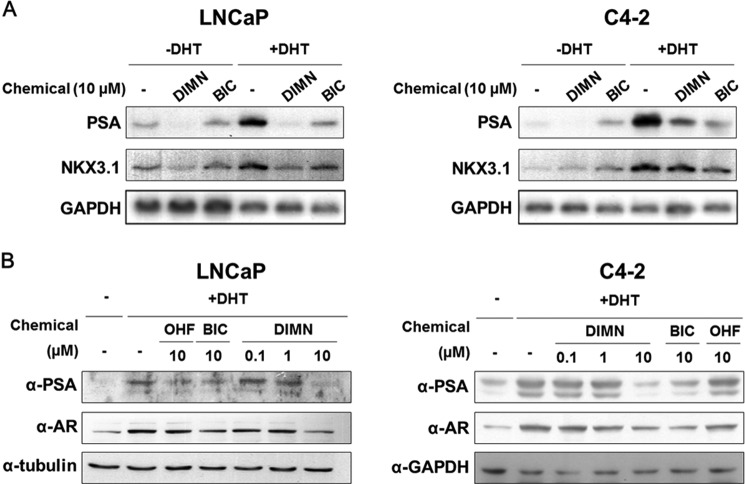
FIGURE 3.
Effects of DIMN on AR and AR target gene expression in prostate cancer cells. A, suppression of AR target gene expression by DIMN in LNCaP and C4–2 cells. Human prostate cancer LNCaP and C4–2 cells maintained in media containing 5% CSS were treated with 10 μm DIMN or BIC in the absence or presence of 10 nm DHT for 48 h prior to harvest. Total RNA was analyzed by Northern blot analysis using cDNA probes for androgen receptor target genes (PSA and NKX3.1). GAPDH expression was used as an internal control. B, suppression of AR and PSA protein expression by DIMN in LNCaP and C4–2 cells. The protein expression levels of AR and PSA were determined by Western blot analysis. The cells maintained as in A were treated with the indicated concentrations of OHF, BIC, or DIMN in the presence of DHT (LNCaP, 10 nm; C4–2, 1 nm) for 48 h prior to harvest. Specific antibodies against the AR and PSA were used for Western blot analysis. Tubulin or GAPDH expression was used as an internal control.
The change in PSA protein levels was also assessed by Western blot analysis. The expression levels of endogenous PSA induced in the presence of DHT were reduced by treatment with DIMN in a dose-dependent manner in both LNCaP and C4–2 cells (Fig. 3B). DIMN reduced PSA protein levels more effectively than the AR antagonists BIC and OHF at the same concentration (10 μm). Interestingly, DIMN also reduced the protein level of the AR, as previously reported with BIC and OHF (53, 63, 64). Taken together, these results suggest that the compound DIMN inhibits AR function in prostate cancer cells and inhibit the expression of endogenous AR target genes in a similar fashion to the conventional AR antagonists, BIC and OHF.
Inhibition of the Growth of Prostate Cancer Cells by DIMN
To assess the effect of DIMN on the androgen-induced proliferation of prostate cancer cells, we measured the proliferation rate of LNCaP cells by the MTS assay. The growth of LNCaP cells induced by 1 nm DHT was highly inhibited by treatment with 10 μm DIMN as well as with BIC and OHF treatment (Fig. 4A, left panel). To confirm this effect of DIMN on the proliferation rate of LNCaP cells, we also performed thymidine incorporation assay (Fig. 4A, right panel). DNA synthesis, which increased in the presence of DHT, was inhibited ∼20 and 60% by 1 μm and 10 μm DIMN, respectively. However, DNA synthesis was inhibited only 10 and 20% by 1 μm and 10 μm BIC, respectively, and up to 15% by OHF. These results indicated that DIMN effectively inhibit the proliferation of LNCaP cells, which represent the early stage androgen-dependent state, and that it is much more potent than BIC or OHF.
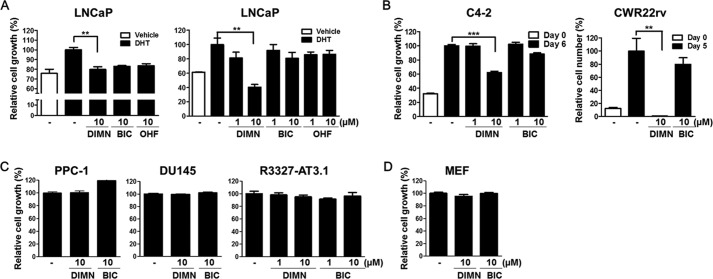
FIGURE 4.
Inhibition of the proliferation of prostate cancer cells by DIMN. A, inhibition of androgen-dependent LNCaP cell growth by DIMN. The inhibitory effect on DHT-induced cell proliferation was evaluated by the MTS colorimetric assay. LNCaP cells seeded into 96-well plates were incubated with 1 nm DHT and 10 μm of the indicated compound for 5 days. The values were compared with those from vehicle-treated cells (left panel). The inhibition of DNA synthesis by DIMN was measured by a [3H]thymidine incorporation assay (right panel). B, inhibition of androgen-independent, but AR-positive, C4-2 and CWR22rv cell growth by DIMN. C4–2 cells were incubated with 1 μm or 10 μm DIMN or BIC for 6 days, and the inhibitory effects on cell proliferation were evaluated by the MTS colorimetric assay (Day 6). CWR22rv cells were incubated with 10 μm DIMN or BIC for 5 days, and cell viability was assessed by trypan blue dye exclusion (Day 5). The white bar represents the starting cell number before chemical treatment (Day 0). Error bars indicate the standard deviation. **, p < 0.01; ***, p < 0.001. C, no inhibitory effect of DIMN was observed on androgen-independent and AR-negative prostate cancer cell growth. For 4 days, PPC-1 and DU145 cells were incubated with 10 μm chemicals, and R3327-AT3.1 cells were incubated with 1 μm or 10 μm chemicals. The inhibitory effects on cell proliferation were evaluated by the MTS colorimetric assay. D, cytotoxic effect of DIMN on MEF cell proliferation. MEF cells were incubated with 10 μm DIMN or BIC in complete medium for 4 days, and the negative effect of DIMN on cell proliferation was evaluated by the MTS colorimetric assay.
To investigate the utility of DIMN as a new generation of AR antagonists for the treatment of CRPC, the more aggressive form of prostate cancer, we next determined the inhibitory effect of DIMN on the proliferation of later stage androgen-independent C4–2 and CWR22rv cells, which grow independently of androgens while expressing AR protein (47, 65). The effect of DIMN on the proliferation rate of C4–2 cells was measured by the MTS assay. The growth of C4–2 cells was effectively inhibited to ∼40% by DIMN, which is more potent than the inhibition by BIC at the same dose, 10 μm (Fig. 4B). We also analyzed cell viability by trypan blue staining in CWR22rv cells, because MTS-based assay resulted in an underestimation of the anti-proliferative effect of DIMN in CWR22rv cells due to the limitation of the method as previously described (66). The viability of CWR22rv cells was completely inhibited by DIMN, whereas there was no significant inhibitory effect on cell viability induced by BIC (Fig. 4B). However, the DIMN showed no inhibitory effect on the cell growth of the AR-negative and androgen-independent prostate cancer cell lines PPC-1, DU145, and R3327-AT3.1 (Fig. 4C). Because DIMN also had an inhibitory effect on the growth of androgen-independent prostate cancer cells that express the AR, we investigated the cytotoxicity of DIMN using mouse embryonic fibroblast (MEF) cells as normal cells. The result showed that DIMN exhibited no cytotoxic effect, similar to BIC (Fig. 4D).
Taken together, these results suggest that the synthetic compound DIMN has an effective inhibitory effect on the growth of AR-positive prostate cancer cells, both androgen-dependent (LNCaP) and androgen-independent (C4–2 and CWR22rv), unlike the conventional AR antagonists BIC and OHF.
Modeling Basis for the Pure Antagonistic Character of DIMN in WT and Mutant ARs
To investigate the basis for the finding that DIMN acts as a pure AR antagonist in the wild type (WT) and mutated ARs, we predicted the binding mode of DIMN through modeling. A series of AR mutations, including T877A and W741C, has been identified from tissue specimens of CRPC patients. In particular, the T877A mutation has been found in patients who were treated with flutamide and eventually became refractory to the treatment (67). The functional significance of the W741C mutation was demonstrated by the bicalutamide-stimulated tumor growth of a prostate xenograft model derived from bicalutamide-treated patients (68).
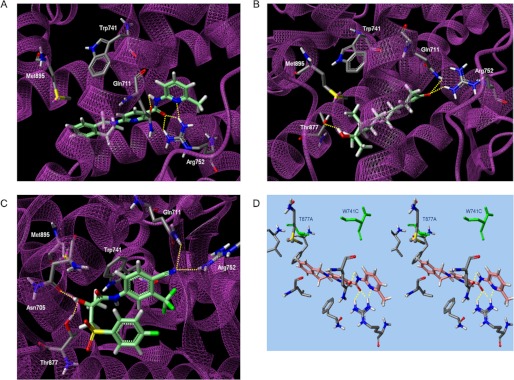
The modeling suggested that DIMN fit well into the narrow cavity and formed four possible hydrogen bonds with the backbones of Gln-711 and Arg-752 (Fig. 5A, supplemental Fig. S2). These interactions are identical to agonist R1881 with high AR binding affinity (Fig. 5B), although R1881 forms another hydrogen bond with Thr-877 (69). Despite of the obvious resemblance of DIMN with agonist, the activity exhibited is solely antagonism which is in turn the ability of a compound to displace H12 from AR LBD. BIC in antagonistic mode, as predicted from in silico simulations and docking model (30, 70), attains an extended conformation with the sulfonyl-linked phenyl ring orienting away from the indole ring of Trp-741 and facing H12. At this structural orientation, the sulfonyl group of BIC overlaps with Met-895 of H12 and possibly displaces the helix due to a steric clash (72, 73). The steric overlap of other antagonists such as OHF with Thr-877 is believed to cause the conformational change for their antagonism in WT AR (74, 75).
FIGURE 5.
Computational analysis of the binding mode of DIMN into the AR. Low-energy binding conformations of DIMN (A), R1881 (B), BIC (C) bound to WT AR, and DIMN (D) bound to point mutated AR by virtual ligand docking. Hydrogen bonds are depicted as dotted lines. The green colored amino acid residues represent W741C and T877A mutation.
However, unexpectedly, BIC has a completely folded conformation with two phenyl rings stacked in the same site that agonist R1881 binds in a docking model (Fig. 5C), which is consistent with the recently illustrated conformation (70). As suggested in the lowest energy state, BIC may act as an agonist even in WT AR, to some extent. The two distinct BIC-AR complexes of either the former extended conformation or the latter folded conformation seem to be accessible due to their comparable binding energy in WT AR (70), presenting a preference of a designed antagonist to have a unfolded scaffold for its full antagonism. Moreover, when W741L/W741C mutation occurs, BIC acts as an agonist since the phenyl moiety of BIC shifts up to occupy the cavity created by the absence of the Trp indole and thus allows H12 to fold, and this fashion is similar to the occurrence of point mutation T877A with the switch to agonist from the antagonists (14, 68, 70).
On the other hand, importantly, DIMN has conserved ligand-receptor hydrogen bonds for high AR affinity and an extended linear structure to extrude H12 because of a steric conflict with the bulky isoquinoline ring. It also locates at a distance unaffected by the change in the size of the active site due to point mutation Trp-741 and Thr-877 (Fig. 5D), supporting the assumption that DIMN works as a pure antagonist regardless of WT or mutated AR.
DISCUSSION
AR antagonists have proven to be useful targets for chemotherapeutic agents in the treatment of prostate cancer. Non-steroidal AR antagonists, such as bicalutamide, have been widely used because of their selective blockade of androgen action and fewer side effects. However, these antagonists cause some side effects due to an increased serum testosterone level by interrupting the negative feedback regulation in the brain (78) and hormone resistance in advanced prostate tumors (67). Therefore, novel potent AR antagonists with fewer negative effects and working on both hormone-dependent and -independent tumors are highly desirable. In an effort to search for such compounds, we performed AR structure-based virtual screening as a tool to discover a new compound, DIMN. DIMN showed strong AR antagonistic effect and little AR agonistic effect. Considering that the agonistic properties of BIC are thought to cause hormone resistance in advanced prostate tumors (79), our data showing that DIMN has little agonistic effect may suggest that this compound could be developed into more effective AR antagonists for the treatment of advanced prostate cancer.
The AR signaling pathway is essential for the growth and progression of both androgen-dependent and androgen-independent prostate cancers. Because of this, AR-mediated signaling and gene expression have been key targets of advanced prostate cancer therapy through the utilization of anti-androgens that prevent AR activation and/or the disruption of endogenous androgen production (80, 81). However, in most cases, these therapies ultimately fail as a result of AR reactivation by various factors, including non-physiological ligands, AR mutants, certain growth factors, and signaling pathways such as PI3K/PTEN/AKT and MAPK (82–89). For example, the PI3K/AKT signaling pathway is well-known to regulate the AR with respect to both expression (90) and transactivation (91–93) and to mediate the proliferation of both androgen-dependent LNCaP and androgen-independent C4–2 and CWR22rv cells (94). CRPCs exhibit a high level of activation of PI3K/AKT signaling, resulting in increased proliferation (95–97). Interestingly, DIMN was able to inhibit the growth of androgen-independent prostate cancer cells as well as androgen-dependent cells (Fig. 4), but only those that expressed the AR. One explanation for this AR-dependent inhibition could be a DIMN-induced decrease in the levels of AR protein (Fig. 3B), although this decrease did not seem to fully explain the strong inhibition of cell growth.
Recent studies have shown that resveratrol, EPI-001, RD162, and MDV3100 also have the ability to inhibit both androgen-independent and androgen-dependent proliferation in prostate cancer cells (71, 76, 87). Resveratrol and EPI-001 have been shown to inhibit the growth of androgen-independent prostate cancer cells by negatively regulating PI3K/AKT pathway-activated AR activity. Similarly, it will be worthwhile to investigate whether and how DIMN disrupts AR signaling, which is known to be activated by various factors in CRPCs, to access the actual pathway of AR inhibition. Further studies are indeed required to characterize the mechanisms of AR antagonist action of DIMN, and such a characterization will help to develop DIMN as favorable treatments for AR-related diseases, including prostate cancer.
DIMN is a potent anti-androgen that inhibits the proliferation of AR-positive human prostate cancer cells, both androgen-dependent and androgen-independent. Furthermore, DIMN is better than BIC at inhibiting the SRC-1- and SRC-2-mediated enhancement of AR transactivation, although it has a little lower AR binding activity and weaker inhibitory effect on the N/C interaction (Fig. 2). Such AR antagonistic activity of DIMN identifies this class of compounds as potential replacements for the current therapeutic prostate cancer drug, BIC. However, further in vivo study is necessary to confirm that sufficient levels of the compound DIMN is attained in target tissues for a sufficient time to alter AR-regulated processes, which is critical in the final assessment of chemicals as therapeutic drugs. In addition, DIMN also would be expected to increase serum testosterone level due to the loss of negative feedback regulation at the hypothalamus and pituitary in common with conventional AR antagonists (78). Therefore, the in vivo study is also necessary to evaluate whether DIMN acts as selective androgen receptor modulators (SARMs), a class of AR antagonists with peripheral tissue selectivity (77). We are currently conducting in vivo experiments.
In summary, we successfully identified a novel chemical entity, the nicotinamide compound DIMN, as a non-steroidal AR antagonist through receptor-based virtual screening. The potent AR antagonism of this compound has been confirmed by AR-ligand binding competition, the blocking of AR activation steps, the reduced expression of AR target genes, and the considerably inhibited proliferation of AR-expressing prostate cancer cells, either androgen-dependent or androgen-independent. The remarkable potency of the action of the compound DIMN on both prostate cancer cell types as well as their strong anti-androgenic activity suggests that this compound could be potent drug candidate for the treatment of early stage to advanced prostate cancers, potentially replacing currently established anticancer medicines such as BIC.
Supplementary Material
Acknowledgments
We thank Dr. J. W. Lee for the pcDNA3.ERα and ERE-Luc plasmids, Dr. E. M. Wilson for the VP-AR1–660, GAL-AR624–919, and 5XGAL4-Luc3 plasmids, Dr. C. Jung for the C4–2 and CWR22rv cells, and Mazence, Inc., for the R3327-AT3.1 cells.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-2012R1A2A2A01008388, NRF-2011-0015551).

This article contains supplemental Figs. S1 and S2.
- DHT
- dihydrotestosterone
- AR
- androgen receptor
- OHF
- hydroxyflutamide
- BIC
- bicalutamide
- DIMN
- 6-(3,4-dihydro-1H-isoquinolin-2-yl)-N-(6-methylpyridin-2-yl)nicotinamide
- ADPC
- androgen-dependent prostate cancer
- AIPC
- androgen-independent prostate cancer
- CRPC
- castration-resistant prostate cancer
- HTS
- high-throughput screening
- VS
- virtual screening
- RMSD
- root-mean-square deviation
- LBD
- ligand-binding domain.
REFERENCES
- 1. Parkin D. M., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M. J. (2008) Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 [DOI] [PubMed] [Google Scholar]
- 3. Kokontis J. M., Liao S. (1999) Molecular action of androgen in the normal and neoplastic prostate. Vitam. Horm. 55, 219–307 [DOI] [PubMed] [Google Scholar]
- 4. Scher H. I., Steineck G., Kelly W. K. (1995) Hormone-refractory (D3) prostate cancer: refining the concept. Urology 46, 142–148 [DOI] [PubMed] [Google Scholar]
- 5. Eder I. E., Haag P., Bartsch G., Klocker H. (2005) Targeting the androgen receptor in hormone-refractory prostate cancer–new concepts. Future Oncol. 1, 93–101 [DOI] [PubMed] [Google Scholar]
- 6. Heinlein C. A., Chang C. (2004) Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 [DOI] [PubMed] [Google Scholar]
- 7. Rahman M., Miyamoto H., Chang C. (2004) Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clin. Cancer Res. 10, 2208–2219 [DOI] [PubMed] [Google Scholar]
- 8. Setlur S. R., Rubin M. A. (2005) Current thoughts on the role of the androgen receptor and prostate cancer progression. Adv. Anat. Pathol. 12, 265–270 [DOI] [PubMed] [Google Scholar]
- 9. Rowland J. G., Robson J. L., Simon W. J., Leung H. Y., Slabas A. R. (2007) Evaluation of an in vitro model of androgen ablation and identification of the androgen responsive proteome in LNCaP cells. Proteomics 7, 47–63 [DOI] [PubMed] [Google Scholar]
- 10. Wang Q., Li W., Zhang Y., Yuan X., Xu K., Yu J., Chen Z., Beroukhim R., Wang H., Lupien M., Wu T., Regan M. M., Meyer C. A., Carroll J. S., Manrai A. K., Jänne O. A., Balk S. P., Mehra R., Han B., Chinnaiyan A. M., Rubin M. A., True L., Fiorentino M., Fiore C., Loda M., Kantoff P. W., Liu X. S., Brown M. (2009) Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 12. Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., Palotie A., Tammela T., Isola J., Kallioniemi O. P. (1995) In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 [DOI] [PubMed] [Google Scholar]
- 13. Edwards J., Krishna N. S., Grigor K. M., Bartlett J. M. (2003) Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 89, 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taplin M. E., Bubley G. J., Ko Y. J., Small E. J., Upton M., Rajeshkumar B., Balk S. P. (1999) Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 59, 2511–2515 [PubMed] [Google Scholar]
- 15. Veldscholte J., Ris-Stalpers C., Kuiper G. G., Jenster G., Berrevoets C., Claassen E., van Rooij H. C., Trapman J., Brinkmann A. O., Mulder E. (1990) A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 173, 534–540 [DOI] [PubMed] [Google Scholar]
- 16. Wilding G., Chen M., Gelmann E. P. (1989) Aberrant response in vitro of hormone-responsive prostate cancer cells to antiandrogens. Prostate 14, 103–115 [DOI] [PubMed] [Google Scholar]
- 17. Hara T., Miyazaki J., Araki H., Yamaoka M., Kanzaki N., Kusaka M., Miyamoto M. (2003) Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 63, 149–153 [PubMed] [Google Scholar]
- 18. Tan J., Sharief Y., Hamil K. G., Gregory C. W., Zang D. Y., Sar M., Gumerlock P. H., deVere White R. W., Pretlow T. G., Harris S. E., Wilson E. M., Mohler J. L., French F. S. (1997) Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol. Endocrinol. 11, 450–459 [DOI] [PubMed] [Google Scholar]
- 19. Culig Z., Hobisch A., Cronauer M. V., Cato A. C., Hittmair A., Radmayr C., Eberle J., Bartsch G., Klocker H. (1993) Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol. Endocrinol. 7, 1541–1550 [DOI] [PubMed] [Google Scholar]
- 20. Weber M. J., Gioeli D. (2004) Ras signaling in prostate cancer progression. J. Cell. Biochem. 91, 13–25 [DOI] [PubMed] [Google Scholar]
- 21. Gregory C. W., He B., Johnson R. T., Ford O. H., Mohler J. L., French F. S., Wilson E. M. (2001) A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 61, 4315–4319 [PubMed] [Google Scholar]
- 22. Culig Z., Hobisch A., Cronauer M. V., Radmayr C., Trapman J., Hittmair A., Bartsch G., Klocker H. (1994) Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 54, 5474–5478 [PubMed] [Google Scholar]
- 23. Whang Y. E., Wu X., Suzuki H., Reiter R. E., Tran C., Vessella R. L., Said J. W., Isaacs W. B., Sawyers C. L. (1998) Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. U.S.A. 95, 5246–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poyet P., Labrie F. (1985) Comparison of the antiandrogenic/androgenic activities of flutamide, cyproterone acetate, and megestrol acetate. Mol. Cell. Endocrinol. 42, 283–288 [DOI] [PubMed] [Google Scholar]
- 25. Hamann L. G., Farmer L. J., Johnson M. G., Goldman M. E., Mais D. E., Davtian A., Bender S. L., Jones T. K. (1995) Synthesis and biological activity of novel nonsteroidal progesterone receptor antagonists. Ann. N.Y. Acad. Sci. 761, 383–387 [DOI] [PubMed] [Google Scholar]
- 26. Reid P., Kantoff P., Oh W. (1999) Antiandrogens in prostate cancer. Invest. New Drugs 17, 271–284 [DOI] [PubMed] [Google Scholar]
- 27. Anderson J. (2003) The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int. 91, 455–461 [DOI] [PubMed] [Google Scholar]
- 28. Wirth M. P., Hakenberg O. W., Froehner M. (2007) Antiandrogens in the treatment of prostate cancer. Eur. Urol. 51, 306–313 [DOI] [PubMed] [Google Scholar]
- 29. Lefort M., Díaz Curiel M., Carrascal M. T., Méndez-Dávila C., de la Piedra C. (2005) Comparative effects of bicalutamide (Casodex) versus orchidectomy on bone mineral density, bone remodeling, and bone biomechanics in healthy rats. Urol Int. 74, 301–307 [DOI] [PubMed] [Google Scholar]
- 30. Söderholm A. A., Viiliäinen J., Lehtovuori P. T., Eskelinen H., Roell D., Baniahmad A., Nyrönen T. H. (2008) Computationally identified novel diphenyl- and phenylpyridine androgen receptor antagonist structures. J. Chem. Inf. Modeling 48, 1882–1890 [DOI] [PubMed] [Google Scholar]
- 31. Axerio-Cilies P., Lack N. A., Nayana M. R., Chan K. H., Yeung A., Leblanc E., Guns E. S., Rennie P. S., Cherkasov A. (2011) Inhibitors of androgen receptor activation function-2 (AF2) site identified through virtual screening. J. Med. Chem. 54, 6197–6205 [DOI] [PubMed] [Google Scholar]
- 32. Kinoyama I., Taniguchi N., Kawaminami E., Nozawa E., Koutoku H., Furutani T., Kudoh M., Okada M. (2005) N-Arylpiperazine-1-carboxamide derivatives: a novel series of orally active nonsteroidal androgen receptor antagonists. Chem. Pharm. Bull. 53, 402–409 [DOI] [PubMed] [Google Scholar]
- 33. Kinoyama I., Taniguchi N., Toyoshima A., Nozawa E., Kamikubo T., Imamura M., Matsuhisa A., Samizu K., Kawanimani E., Niimi T., Hamada N., Koutoku H., Furutani T., Kudoh M., Okada M., Ohta M., Tsukamoto S. (2006) (+)-(2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-dimethyl-N-[6-(trifluoromethyl)pyridin-3- yl]piperazine-1-carboxamide (YM580) as an orally potent and peripherally selective nonsteroidal androgen receptor antagonist. J. Med. Chem. 49, 716–726 [DOI] [PubMed] [Google Scholar]
- 34. Jung M. E., Ouk S., Yoo D., Sawyers C. L., Chen C., Tran C., Wongvipat J. (2010) Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J. Med. Chem. 53, 2779–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee Y. S., Kim H. J., Lee H. J., Lee J. W., Chun S. Y., Ko S. K., Lee K. (2002) Activating signal cointegrator 1 is highly expressed in murine testicular Leydig cells and enhances the ligand-dependent transactivation of androgen receptor. Biol. Reprod 67, 1580–1587 [DOI] [PubMed] [Google Scholar]
- 36. Chattopadhyay S., Gong E. Y., Hwang M., Park E., Lee H. J., Hong C. Y., Choi H. S., Cheong J. H., Kwon H. B., Lee K. (2006) The CCAAT enhancer-binding protein-α negatively regulates the transactivation of androgen receptor in prostate cancer cells. Mol. Endocrinol. 20, 984–995 [DOI] [PubMed] [Google Scholar]
- 37. Suh J. H., Shong M., Choi H. S., Lee K. (2008) CR6-interacting factor 1 represses the transactivation of androgen receptor by direct interaction. Mol. Endocrinol. 22, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee H. J., Chattopadhyay S., Gong E. Y., Ahn R. S., Lee K. (2003) Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 75, 40–46 [DOI] [PubMed] [Google Scholar]
- 39. Oñate S. A., Tsai S. Y., Tsai M. J., O'Malley B. W. (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 40. Hong H., Kohli K., Garabedian M. J., Stallcup M. R. (1997) GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 17, 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S. K., Anzick S. L., Choi J. E., Bubendorf L., Guan X. Y., Jung Y. K., Kallioniemi O. P., Kononen J., Trent J. M., Azorsa D., Jhun B. H., Cheong J. H., Lee Y. C., Meltzer P. S., Lee J. W. (1999) A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J. Biol. Chem. 274, 34283–34293 [DOI] [PubMed] [Google Scholar]
- 42. Langley E., Kemppainen J. A., Wilson E. M. (1998) Intermolecular NH2-/carboxyl-terminal interactions in androgen receptor dimerization revealed by mutations that cause androgen insensitivity. J. Biol. Chem. 273, 92–101 [DOI] [PubMed] [Google Scholar]
- 43. Hong C. Y., Park J. H., Seo K. H., Kim J. M., Im S. Y., Lee J. W., Choi H. S., Lee K. (2003) Expression of MIS in the testis is downregulated by tumor necrosis factor α through the negative regulation of SF-1 transactivation by NF-κB. Mol. Cell. Biol. 23, 6000–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang J., Bohl C. E., Nair V. A., Mustafa S. M., Hong S. S., Miller D. D., Dalton J. T. (2006) Preclinical pharmacology of a nonsteroidal ligand for androgen receptor-mediated imaging of prostate cancer. J. Pharmacol. Exp. Ther. 317, 402–408 [DOI] [PubMed] [Google Scholar]
- 45. Suh J. H., Gong E. Y., Hong C. Y., Park E., Ahn R. S., Park K. S., Lee K. (2008) Reduced testicular steroidogenesis in tumor necrosis factor-α knockout mice. J. Steroid Biochem. Mol. Biol. 112, 117–121 [DOI] [PubMed] [Google Scholar]
- 46. Hong C. Y., Gong E. Y., Kim K., Suh J. H., Ko H. M., Lee H. J., Choi H. S., Lee K. (2005) Modulation of the expression and transactivation of androgen receptor by the basic helix-loop-helix transcription factor Pod-1 through recruitment of histone deacetylase 1. Mol. Endocrinol. 19, 2245–2257 [DOI] [PubMed] [Google Scholar]
- 47. Ai J., Wang Y., Dar J. A., Liu J., Liu L., Nelson J. B., Wang Z. (2009) HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol. Endocrinol. 23, 1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Butler L. M., Agus D. B., Scher H. I., Higgins B., Rose A., Cordon-Cardo C., Thaler H. T., Rifkind R. A., Marks P. A., Richon V. M. (2000) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 60, 5165–5170 [PubMed] [Google Scholar]
- 49. Lee H. J., Hwang M., Chattopadhyay S., Choi H. S., Lee K. (2008) Hepatocyte nuclear factor-3 α (HNF-3α) negatively regulates androgen receptor transactivation in prostate cancer cells. Biochem. Biophys. Res. Commun. 367, 481–486 [DOI] [PubMed] [Google Scholar]
- 50. Kawata H., Arai S., Nakagawa T., Ishikura N., Nishimoto A., Yoshino H., Shiraishi T., Tachibana K., Nakamura R., Sato H. (2011) Biological properties of androgen receptor pure antagonist for treatment of castration-resistant prostate cancer: optimization from lead compound to CH5137291. Prostate 71, 1344–1356 [DOI] [PubMed] [Google Scholar]
- 51. Gao W., Kim J., Dalton J. T. (2006) Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands. Pharm. Res. 23, 1641–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kemppainen J. A., Langley E., Wong C. I., Bobseine K., Kelce W. R., Wilson E. M. (1999) Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol. Endocrinol. 13, 440–454 [DOI] [PubMed] [Google Scholar]
- 53. Masiello D., Cheng S., Bubley G. J., Lu M. L., Balk S. P. (2002) Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 277, 26321–26326 [DOI] [PubMed] [Google Scholar]
- 54. Jenster G., van der Korput H. A., van Vroonhoven C., van der Kwast T. H., Trapman J., Brinkmann A. O. (1991) Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol. Endocrinol. 5, 1396–1404 [DOI] [PubMed] [Google Scholar]
- 55. Georget V., Térouanne B., Nicolas J. C., Sultan C. (2002) Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 41, 11824–11831 [DOI] [PubMed] [Google Scholar]
- 56. Térouanne B., Paris F., Servant N., Georget V., Sultan C. (2002) Evidence that chlormadinone acetate exhibits antiandrogenic activity in androgen-dependent cell line. Mol. Cell. Endocrinol. 198, 143–147 [DOI] [PubMed] [Google Scholar]
- 57. Heinlein C. A., Chang C. (2002) Androgen receptor (AR) coregulators: an overview. Endocr. Rev. 23, 175–200 [DOI] [PubMed] [Google Scholar]
- 58. Yoon H. G., Wong J. (2006) The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol. Endocrinol. 20, 1048–1060 [DOI] [PubMed] [Google Scholar]
- 59. Nelson P. S., Clegg N., Arnold H., Ferguson C., Bonham M., White J., Hood L., Lin B. (2002) The program of androgen-responsive genes in neoplastic prostate epithelium. Proc. Natl. Acad. Sci. U.S.A. 99, 11890–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thalmann G. N., Sikes R. A., Wu T. T., Degeorges A., Chang S. M., Ozen M., Pathak S., Chung L. W. (2000) LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate 44, 91–103 [DOI] [PubMed] [Google Scholar]
- 61. Kim J., Coetzee G. A. (2004) Prostate specific antigen gene regulation by androgen receptor. J. Cell. Biochem. 93, 233–241 [DOI] [PubMed] [Google Scholar]
- 62. Lu S., Wang A., Dong Z. (2007) A novel synthetic compound that interrupts androgen receptor signaling in human prostate cancer cells. Molecular Cancer Therapeutics 6, 2057–2064 [DOI] [PubMed] [Google Scholar]
- 63. Veldscholte J., Berrevoets C. A., Brinkmann A. O., Grootegoed J. A., Mulder E. (1992) Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry 31, 2393–2399 [DOI] [PubMed] [Google Scholar]
- 64. Furutani T., Watanabe T., Tanimoto K., Hashimoto T., Koutoku H., Kudoh M., Shimizu Y., Kato S., Shikama H. (2002) Stabilization of androgen receptor protein is induced by agonist, not by antagonists. Biochem. Biophys. Res. Commun. 294, 779–784 [DOI] [PubMed] [Google Scholar]
- 65. Lee S. J., Zhang Y., Lee S. D., Jung C., Li X., Kim H. S., Bae K. H., Jeng M. H., Kao C., Gardner T. (2004) Targeting prostate cancer with conditionally replicative adenovirus using PSMA enhancer. Mol. Ther. 10, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 66. Wang P., Henning S. M., Heber D. (2010) Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PloS one 5, e10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taplin M. E., Rajeshkumar B., Halabi S., Werner C. P., Woda B. A., Picus J., Stadler W., Hayes D. F., Kantoff P. W., Vogelzang N. J., Small E. J. (2003) Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J. Clin. Oncol. 21, 2673–2678 [DOI] [PubMed] [Google Scholar]
- 68. Yoshida T., Kinoshita H., Segawa T., Nakamura E., Inoue T., Shimizu Y., Kamoto T., Ogawa O. (2005) Antiandrogen bicalutamide promotes tumor growth in a novel androgen-dependent prostate cancer xenograft model derived from a bicalutamide-treated patient. Cancer Res. 65, 9611–9616 [DOI] [PubMed] [Google Scholar]
- 69. Matias P. M., Donner P., Coelho R., Thomaz M., Peixoto C., Macedo S., Otto N., Joschko S., Scholz P., Wegg A., Bäsler S., Schäfer M., Egner U., Carrondo M. A. (2000) Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J. Biol. Chem. 275, 26164–26171 [DOI] [PubMed] [Google Scholar]
- 70. Osguthorpe D. J., Hagler A. T. (2011) Mechanism of androgen receptor antagonism by bicalutamide in the treatment of prostate cancer. Biochemistry 50, 4105–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Andersen R. J., Mawji N. R., Wang J., Wang G., Haile S., Myung J. K., Watt K., Tam T., Yang Y. C., Bañuelos C. A., Williams D. E., McEwan I. J., Wang Y., Sadar M. D. (2010) Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 [DOI] [PubMed] [Google Scholar]
- 72. Bohl C. E., Gao W., Miller D. D., Bell C. E., Dalton J. T. (2005) Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 102, 6201–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bohl C. E., Miller D. D., Chen J., Bell C. E., Dalton J. T. (2005) Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J. Biol. Chem. 280, 37747–37754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bohl C. E., Wu Z., Miller D. D., Bell C. E., Dalton J. T. (2007) Crystal structure of the T877A human androgen receptor ligand-binding domain complexed to cyproterone acetate provides insight for ligand-induced conformational changes and structure-based drug design. J. Biol. Chem. 282, 13648–13655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Salvati M. E., Balog A., Shan W., Wei D. D., Pickering D., Attar R. M., Geng J., Rizzo C. A., Gottardis M. M., Weinmann R., Krystek S. R., Sack J., An Y., Kish K. (2005) Structure based approach to the design of bicyclic-1H-isoindole-1,3(2H)-dione based androgen receptor antagonists. Bioorganic Medicinal Chemistry Letters 15, 271–276 [DOI] [PubMed] [Google Scholar]
- 76. Tran C., Ouk S., Clegg N. J., Chen Y., Watson P. A., Arora V., Wongvipat J., Smith-Jones P. M., Yoo D., Kwon A., Wasielewska T., Welsbie D., Chen C. D., Higano C. S., Beer T. M., Hung D. T., Scher H. I., Jung M. E., Sawyers C. L. (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mohler M. L., Bohl C. E., Jones A., Coss C. C., Narayanan R., He Y., Hwang D. J., Dalton J. T., Miller D. D. (2009) Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem. 52, 3597–3617 [DOI] [PubMed] [Google Scholar]
- 78. Miyamoto H., Messing E. M., Chang C. (2004) Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate 61, 332–353 [DOI] [PubMed] [Google Scholar]
- 79. Culig Z., Hoffmann J., Erdel M., Eder I. E., Hobisch A., Hittmair A., Bartsch G., Utermann G., Schneider M. R., Parczyk K., Klocker H. (1999) Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 81, 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gregory C. W., Hamil K. G., Kim D., Hall S. H., Pretlow T. G., Mohler J. L., French F. S. (1998) Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 58, 5718–5724 [PubMed] [Google Scholar]
- 81. Craft N., Sawyers C. L. (1998) Mechanistic concepts in androgen-dependence of prostate cancer. Cancer Metastasis Rev. 17, 421–427 [DOI] [PubMed] [Google Scholar]
- 82. Grigoryev D. N., Long B. J., Njar V. C., Brodie A. H. (2000) Pregnenolone stimulates LNCaP prostate cancer cell growth via the mutated androgen receptor. J. Steroid Biochem. Mol. Biol. 75, 1–10 [DOI] [PubMed] [Google Scholar]
- 83. Yeh S., Lin H. K., Kang H. Y., Thin T. H., Lin M. F., Chang C. (1999) From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 96, 5458–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kang H. Y., Lin H. K., Hu Y. C., Yeh S., Huang K. E., Chang C. (2001) From transforming growth factor-β signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 98, 3018–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miyamoto H., Yeh S., Wilding G., Chang C. (1998) Promotion of agonist activity of antiandrogens by the androgen receptor coactivator, ARA70, in human prostate cancer DU145 cells. Proc. Natl. Acad. Sci. U.S.A. 95, 7379–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yeh S., Miyamoto H., Shima H., Chang C. (1998) From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc. Natl. Acad. Sci. U.S.A. 95, 5527–5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Y., Romigh T., He X., Orloff M. S., Silverman R. H., Heston W. D., Eng C. (2010) Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genetics 19, 4319–4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Feldman B. J., Feldman D. (2001) The development of androgen-independent prostate cancer. Nat. Reviews Cancer 1, 34–45 [DOI] [PubMed] [Google Scholar]
- 89. Rodríguez-Berriguete G., Fraile B., Martínez-Onsurbe P., Olmedilla G., Paniagua R., Royuela M. (2012) MAP Kinases and Prostate Cancer. J. Signal Transduction 2012, 169170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Manin M., Baron S., Goossens K., Beaudoin C., Jean C., Veyssiere G., Verhoeven G., Morel L. (2002) Androgen receptor expression is regulated by the phosphoinositide 3-kinase/Akt pathway in normal and tumoral epithelial cells. Biochem. J. 366, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wen Y., Hu M. C., Makino K., Spohn B., Bartholomeusz G., Yan D. H., Hung M. C. (2000) HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 60, 6841–6845 [PubMed] [Google Scholar]
- 92. Lin H. K., Yeh S., Kang H. Y., Chang C. (2001) Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 7200–7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sharma M., Chuang W. W., Sun Z. (2002) Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J. Biol. Chem. 277, 30935–30941 [DOI] [PubMed] [Google Scholar]
- 94. Eng C. (2003) PTEN: one gene, many syndromes. Human Mutation 22, 183–198 [DOI] [PubMed] [Google Scholar]
- 95. Mikhailova M., Wang Y., Bedolla R., Lu X. H., Kreisberg J. I., Ghosh P. M. (2008) AKT regulates androgen receptor-dependent growth and PSA expression in prostate cancer. Adv. Exp. Med. Biol. 617, 397–405 [DOI] [PubMed] [Google Scholar]
- 96. Wang Y., Mikhailova M., Bose S., Pan C. X., deVere White R. W., Ghosh P. M. (2008) Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene 27, 7106–7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ghosh P. M., Malik S. N., Bedolla R. G., Wang Y., Mikhailova M., Prihoda T. J., Troyer D. A., Kreisberg J. I. (2005) Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr. Relat. Cancer 12, 119–134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.