Background: The ssDNA cytosine deaminase activity of the HIV restriction factor APOBEC3A was analyzed.
Results: APOBEC3A does not deaminate ssDNA with high processivity.
Conclusion: Deamination motif and not processivity appears more important for mutational inactivation of HIV. APOBEC3A may deaminate genomic DNA during transcription.
Significance: Genomic DNA may undergo enzymatic deoxycytidine deamination as a cost for a deamination-based viral restriction mechanism.
Keywords: DNA Repair, DNA Transcription, Enzyme Mechanisms, HIV, Mutagenesis Mechanisms, Protein-DNA Interaction, Reverse Transcription, Viral Immunology, DNA Deaminases, HIV Restriction Factor
Abstract
APOBEC3A belongs to a family of single-stranded DNA (ssDNA) DNA cytosine deaminases that are known for restriction of HIV through deamination-induced mutational inactivation, e.g. APOBEC3G, or initiation of somatic hypermutation and class switch recombination (activation-induced cytidine deaminase). APOBEC3A, which is localized to both the cytoplasm and nucleus, not only restricts HIV but can also initiate catabolism of cellular DNA. Despite being ascribed these roles, there is a paucity of data available on the biochemical mechanism by which APOBEC3A deaminates ssDNA. Here we assessed APOBEC3A deamination activity on ssDNA and in dynamic systems modeling HIV replication (cytoplasmic event) and DNA transcription (nuclear event). We find that APOBEC3A, unlike the highly processive APOBEC3G, exhibits low or no processivity when deaminating synthetic ssDNA substrates with two cytosines located 5–63 nucleotides apart, likely because of an apparent Kd in the micromolar range (9.1 μm). APOBEC3A was able to deaminate nascently synthesized (−)DNA in an in vitro model HIV replication assay but induced fewer mutations overall in comparison to APOBEC3G. However, the data indicate that the target deamination motif (5′-TC for APOBEC3A and 5′-CC for APOBEC3G) and not the number of mutations best predicted the ability to mutationally inactivate HIV. We further assessed APOBEC3A for the ability to deaminate dsDNA undergoing transcription, which could allow for collateral deaminations to occur in genomic DNA similar to the action of activation-induced cytidine deaminase. That APOBEC3A was able to deaminate dsDNA undergoing transcription suggests a genomic cost of a deamination-based retroviral restriction system.
Introduction
The APOBEC3 (Apo3)2 enzymes are a family of single-stranded DNA (ssDNA) cytosine deaminases found in placental mammals that are a host restriction factor for retroelements and viruses with a ssDNA intermediate, e.g. retroviruses (1–7). In humans there are seven Apo3 family members. Four members (Apo-3B, -3DE, -3F, and -3G) contain two zinc coordinating domains, and three members contain one zinc coordinating domain (Apo-3A, -3C, and -3H) with the consensus sequence His-X-Glu-X23–28-Pro-Cys-X2–4-Cys (8, 9). For the double domain enzymes, only the C-terminal domain is catalytically active (10, 11), except perhaps for Apo3B (12, 13). By deamination of cytosine to uracil in ssDNA, these enzymes can cause mutagenesis of viral transcripts leading to viral inactivation. In humans, Apo3 enzymes are most commonly associated with their ability to restrict HIV. However, this largely occurs only in the absence of the HIV virus infectivity factor (Vif) protein which facilitates the ubiquitination and degradation of many Apo3 members (14–18). Despite being sensitive to Vif-mediated degradation, some members, such as Apo3G, have been documented as having clinical relevance (19, 20), suggesting that Vif-mediated degradation can be avoided at a low level (21). If Apo3 enzymes bypass Vif, they can become incorporated into newly budding virions enabling them to catalyze deaminations of cytosine to uracil on nascent (−)DNA synthesized from the (+)RNA genome in the next host cell (22, 23). Alternatively, Apo3A can restrict incoming HIV particles (6, 7).
During HIV replication, once (−)DNA synthesis begins from reverse transcription of the HIV RNA genome, the RNase H activity of HIV reverse transcriptase begins degrading the RNA template (24). At this moment ssDNA is uncovered at regions between remaining complementary RNA fragments and is vulnerable to cytosine deamination until the (+)DNA is synthesized (22). Apo3G can processively deaminate cytosines through a facilitated diffusion scanning mechanism (25, 26) involving sliding and jumping motions (27, 28) or intersegmental transfer (21). The jumping motions (29) or intersegmental transfers (21) appear to be important for transversing complementary RNA fragments that remain on the (−)DNA during proviral DNA synthesis. It is not known how other Apo3 enzymes scan ssDNA. Synthesis of the (+)DNA strand by reverse transcriptase seals in the uracil lesions as cytosine to thymine transition mutations and the double-stranded DNA (dsDNA) prevents further deaminations from occurring. The primers for (+)DNA synthesis are two RNase-H degradation-resistant polypurine tracts (PPTs) contained in the center and 3′-end of the genomic RNA (30). The amount of time that the (−)DNA is single-stranded is dependent on the proximity of the (−)DNA region to the PPT (22, 31). Accordingly, the PPTs have been found to protect HIV from Apo3G-mediated deamination (31, 32).
For many years after the discovery of the Apo3 family, Apo3A was thought to only have a physiological function of restricting retroelements (33–36). Recently it was shown that Apo3A is the only Apo3 member to be highly expressed in macrophages and monocytes in response to interferon α (37, 38). In these cells Apo3A can induce degradation of naked foreign DNA (39) and can inhibit replication of incoming HIV-1 without the need for prior encapsidation (6, 7). This latter mode of restricting HIV is unique. In the case of Apo3G, it appears to be unable to act as a post-entry barrier to HIV infection (40, 41). Apo3G appears only to be able to deaminate during reverse transcription when it is packaged into the viral particle before infection of the target cell (3, 4).
Apo3A is found both in the cytoplasm and the nucleus (34). This poses a potential problem for genomic DNA that becomes transiently single-stranded during replication and transcription. It has been shown that Apo3A can cause DNA damage and cell cycle arrest in a deamination-dependent manner when expressed in U2OS cells (42). The deamination of genomic DNA may be a mechanism for DNA catabolism where deamination initiates a DNA repair enzyme-dependent degradation pathway (43). Yet the consequences of untargeted deamination may be cell transformation. This is similar to a related family member, activation-induced cytidine deaminase (AID) that initiates somatic hypermutation and class switch recombination in B-cells through deamination of cytosine to uracil in the variable and switch regions of immunoglobulin genes (44). AID deaminates these regions when they are transiently single-stranded during transcription (44). However, AID not only deaminates within the targeted immunoglobulin genes but has been shown to cause deaminations in non-immunoglobulin loci (45, 46) and can induce double-strand breaks through DNA repair pathways that lead to characteristic translocations found in B-cell lymphomas (46). The benefit versus risk ratio for AID is obviously apparent, as individuals suffering from hyper IgM syndrome where the AID gene is inactivated have severe immunodeficiency (47, 48). However, AID access to the nucleus is controlled by phosphorylation (49). Apo3A may pose more of a threat to genomic integrity because it can enter the nucleus through diffusion (34).
Apo3A has recently become a prominent Apo3 family member with identified roles in viral restriction (6, 7) and DNA catabolism (43) and perhaps a collateral role in cell transformation (42). However, it is not known mechanistically how Apo3A interacts with ssDNA to catalyze deaminations of cytosine to uracil. Here we have characterized the deamination mechanisms of Apo3A on ssDNA and two reconstituted systems that represent HIV replication and DNA transcription. We find that Apo3A, in contrast to the processive Apo3G, did not appear to deaminate ssDNA with high processivity and was less effective in causing HIV gene inactivation. However, this deficiency appears to be primarily from the Apo3A-induced mutation sequence context, not a lack of processivity, revealing a previously unrecognized predictor for the efficiency of mutational restriction of HIV. Furthermore, the Apo3A-based viral restriction system may have a genomic cost as Apo3A is able to deaminate dsDNA undergoing transcription.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Recombinant baculovirus for expression of GST-Apo3A, GST-Apo3G, or GST-nucleocapsid was constructed using the pAcG2T vector (BD Biosciences) as previously described (28, 29). Cloning primers for Apo3A are listed in supplemental Table S1. Sf9 cells were infected with recombinant GST-Apo3A, GST-Apo3G, or GST-nucleocapsid virus at a multiplicity of infection of 1 and harvested after 72 h. Cells were lysed, and the GST-tagged proteins were purified as described previously to obtain protein that was cleaved from the GST tag and ∼95% pure (27). Cleaved protein fractions were stored at −80 °C. HIV-1 reverse transcriptase p66/p51 (50) was generously provided by Dr. Stuart F. J. LeGrice (NCI, National Institutes of Health).
Deamination Assays
DNA substrates are listed in supplemental Table S1 and were obtained from Tri-Link Biotechnologies. Apo3A and Apo3G were reacted at 20–50 nm with 100 or 500 nm fluorescein (F)-labeled ssDNA in reverse transcriptase buffer (50 mm Tris, pH 7.5, 40 mm KCl, 10 mm MgCl2, 1 mm DTT). Reactions were incubated for 2.5–30 min at 37 °C. Deaminations were detected through breakage of DNA at abasic sites generated by uracil DNA glycosylase, as described previously (28). Gel band intensities were determined with a Typhoon Trio (GE Healthcare) multipurpose scanner, and integrated gel band intensities were quantified using ImageQuant software (GE Healthcare). The processivity factor was calculated as previously described (28, 51). In brief, the value is determined by comparing the deaminations that occurred at two sites on an ssDNA substrate (5′-C and 3′-C) to the calculated expected value of deaminations that would occur at both motifs if the events were independent (not processive). The expected value was calculated using the probability rule that the frequency of two independent events occurring is their product. The two events considered are the total deaminations occurring at the 5′-proximal C and at the 3′-proximal C. Reactions were performed under single hit conditions, i.e. <15% substrate usage (52), to ensure that a single Apo3 interacts with a given ssDNA substrate. The specific activity was determined under single-hit conditions by calculating the pmol of substrate used/min/μg of enzyme.
Steady State Rotational Anisotropy Assays
Steady state fluorescence depolarization (rotational anisotropy) was measured for Apo3A and Apo3G binding to F-labeled ssDNA-specific for Apo3A (5′-TTC) or Apo3G (5′-CCC) (supplemental Table S1). Reactions were 80 μl and contained F-labeled ssDNA (15 nm) in reverse transcriptase buffer with a titration of Apo3A (0–25 μm) or Apo3G (0–700 nm) to achieve saturation of the substrate. Measurements were made at 21 °C using a QuantaMaster QM-4 spectrofluorometer (Photon Technology International) with a dual emission channel. Samples were excited with vertically polarized light at 494 nm (7-nm band pass), and vertical and horizontal emissions were measured at 520 nm (7-nm band pass).
Model HIV Replication Assay
The model in vitro HIV replication assay and subsequent detection of Apo3-catalyzed deamination was performed as described previously (29). In brief, a synthetic (+) RNA genome was created that contained a PPT, 120 nt of the catalytic domain of the HIV-1 protease (prot), and lacZα. The PPT served as an internal second strand ((+) DNA) primer for the reaction (24). The prot was used to identify specific amino acid changes that could be induced by Apo3 enzyme-mediated deamination, and the lacZα was used as a reporter gene for mutations by blue/white screening. The HIV-1 clone from which the protease gene was amplified was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; p93TH253.3 from Dr. Feng Gao and Dr. Beatrice Hahn (53). To begin the reaction, the RNA genome (50 nm) was annealed to a 24-nt DNA primer (29) and incubated with nucleocapsid (3.0 μm), reverse transcriptase (2.4 μm), and dNTPs (500 μm) in reverse transcriptase buffer in the presence or absence of 200 nm Apo3A or Apo3G. Each of the components of the reverse transcription reactions were at estimated physiological ratios to the HIV genome as found in the HIV-1 literature (24, 54, 55) or Apo3G literature (56). Analyses used the sequences of 25 mutated clones for each condition tested. DNA sequencing was carried out at the National Research Council of Canada (Saskatoon, Saskatchewan, Canada).
Multiangle Light Scattering
Apo3A (300 μg) was subjected to size exclusion chromatography using a Superdex 200HR10/300 column (GE Healthcare) connected to an Agilent 1200 HPLC system. A solution containing 20 mm HEPES, pH 7.3, and 150 mm NaCl was used as the elution buffer. Chromatography and detection were performed as previously reported (29) at the Keck Foundation Biotechnology Resource Laboratory at Yale University (57). Data analysis to determine molecular masses was performed with ASTRA software (58).
Transcription-dependent Deamination Reactions
Transcription-dependent deamination reactions were performed as described previously (59, 60). Specific to our assay, dsDNA (30 nm) containing a T7 RNA polymerase promoter and either a single 5′-TTC, 5′-CCC, or 5′-AGC motif in the non-transcribed strand (supplemental Table S1) were reacted with 300 nm Apo3A, Apo3G, or AID, respectively, in the presence of ribonucleotide triphosphates (500 μm), Promega T7 polymerase (1 unit), and DNase-free RNase A (5 ng/μl, Roche Applied Science) in transcription buffer (50 mm Tris, pH 7.4, 10 mm MgCl2, 1 mm DTT) at 37 °C. Sequencing of the non-transcribed strand for detection of deamination was performed using Thermo Sequenase (Affymetrix) as described previously (60).
RESULTS
Deamination of ssDNA by Apo3A
Each Apo3 enzyme has a di- or trinucleotide sequence in which it prefers to catalyze deaminations. For Apo3A, the preferred dinucleotide sequence has been determined to be 5′-TC (34). Whereas Apo3G has a strong preference for 5′-CCC (22, 23, 61) (underlined C is preferentially deaminated) another family member AID has a promiscuous recognition sequence 5′- WRC (60) (where W is A or T, and R is A or G). To determine whether Apo3A has a conservative or more promiscuous target motif, we determined the specific activity of the enzyme on ssDNA substrates (85 nt) containing 5′-TTC, 5′-ATC, 5′-CTC, 5′-GTC, or 5′-ACC motifs embedded within sequences containing no other cytosines but were otherwise random (Table 1). Apo3A appeared to deaminate all 5′-TC-containing sequences about equally and was not discriminatory of the 5′-base by more than 2-fold (Table 1, specific activities of 3.1–6.1 pmol/μg/min). However, there is a 100-fold decrease in activity when the 5′-ACC sequence was used, suggesting that the dinucleotide 5′-TC is necessary for optimal deamination activity (Table 1).
TABLE 1.
Specific activity of Apo3A for various trinucleotide deamination motifs
| Deamination motif | Specific activity |
|---|---|
| pmol/μg/min | |
| 5′-ATC | 4.6 ± 1.5 |
| 5′-TTC | 6.1 ± 1.9 |
| 5′-CTC | 4.1 ± 2.5 |
| 5′-GTC | 3.1 ± 2.5 |
| 5′-ACC | 0.07 ± 0.01 |
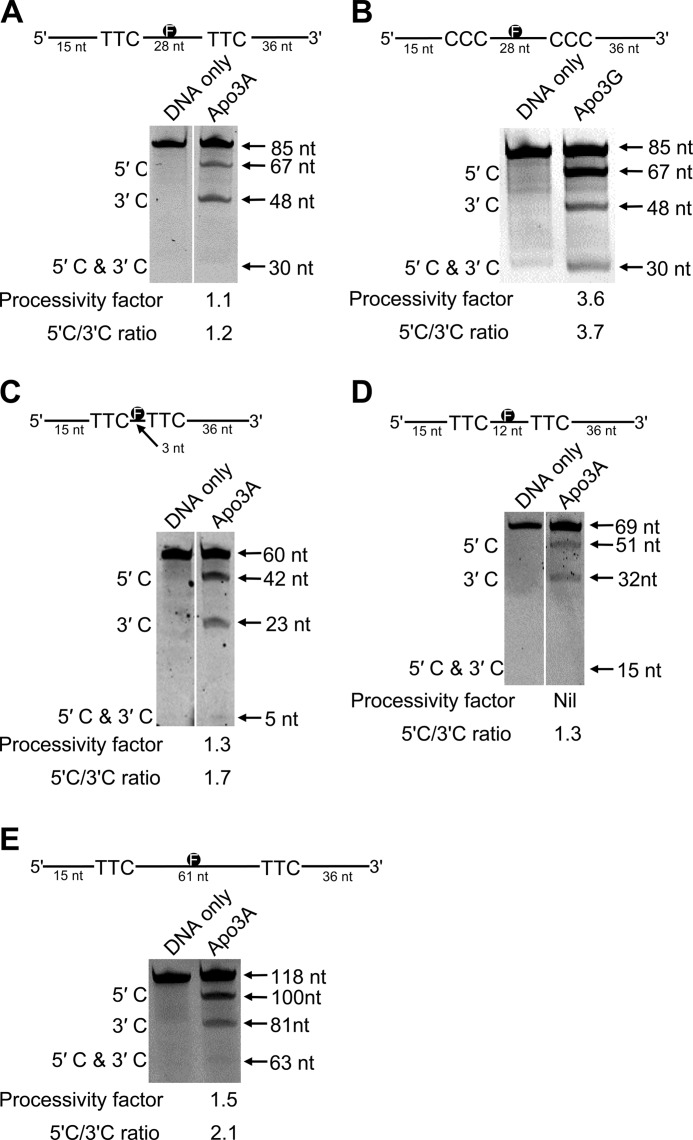
Based on these data (Table 1), we designed a series of substrates containing 5′-TTC motifs to examine whether Apo3A was able to processively deaminate cytosine residues, i.e. if Apo3A can deaminate two cytosine residues in a single enzyme-substrate encounter. The synthetic substrates have two 5′-TTC motifs spaced either 3, 12, 28, or 61 nt apart and contain an F-labeled thymine in between the deamination motifs (Fig. 1, schematic). The internal F-label allows identification of deaminations that occurred at both 5′-TTC motifs on the same ssDNA substrate. All reactions were performed under “single hit” conditions (<15% substrate usage) ensuring that reactions were catalyzed by a single Apo3 enzyme (52). Under these conditions, Apo3G has been shown to deaminate cytosines processively using a three-dimensional search that is characteristic of facilitated diffusion (25, 28, 29). In this type of processive movement, the enzyme can slide and make microscopic dissociations and reassociations with the DNA (termed hopping, jumping, or intersegmental transfer), without diffusing into the bulk solution to efficiently search for target sequences where catalysis takes place (25, 28, 29). Apo3G has also been shown to prefer deamination toward the 5′-end of linear ssDNA due to a catalytic orientation specificity (27, 62, 63). It is not known if these hallmark features of Apo3G are unique or found in other Apo3 family members.
FIGURE 1.
Analysis of Apo3A processivity on ssDNA. A and B, shown is deamination of an 85-nt F-labeled ssDNA substrate by Apo3A (A) and Apo3G (B). Two 5′-TTC (A) or two 5′-CCC (B) motifs are embedded within the ssDNA sequence spaced 28 nt apart. Single deaminations of the 5′-C and 3′-C were detected as the appearance of labeled 67- and 48-nt fragments, respectively; double deamination of both C residues on the same molecule resulted in a 30-nt labeled fragment (5′-C & 3′-C). C, shown is deamination of a 60-nt F-labeled ssDNA substrate by Apo3A. Two 5′-TTC motifs are embedded within the ssDNA sequence spaced 3 nt apart. Single deaminations of the 5′-C and 3′-C were detected as the appearance of labeled 42- and 23-nt fragments, respectively; double deamination of both C residues on the same molecule resulted in a 5-nt labeled fragment (5′-C & 3′-C). D, deamination of a 69-nt F-labeled ssDNA substrate by Apo3A is shown. Two 5′-TTC motifs are embedded within the ssDNA sequence spaced 12-nt apart. Single deaminations of the 5′-C and 3′-C were detected as the appearance of labeled 51- and 32-nt fragments, respectively; double deamination of both C residues on the same molecule resulted in a 15-nt labeled fragment (5′-C & 3′-C). E, deamination of a 118-nt F-labeled ssDNA substrate by Apo3A is shown. Two 5′-TTC motifs are embedded within the ssDNA sequence spaced 61 nt apart. Single deaminations of the 5′-C and 3′-C were detected as the appearance of labeled 100- and 81-nt fragments, respectively; double deamination of both C residues on the same molecule resulted in a 63-nt labeled fragment (5′-C & 3′-C). The measurements of processivity (processivity factor) and polarity (5′-C/3′-C ratio) are shown below the gel. A zero processivity factor means that no band could be detected during analysis. The expected size of the 5′-C & 3′-C band was determined using an identical substrate that had been deaminated to near completion. Values are an average from at least two independent experiments, and the S.E. for the processivity factors are 0.25 (A), 0.09 (B), 0.46 (C), and 0.57 (E).
On the substrate used to determine the specific activity (85-nt), the 5′-TTC motifs are separated by 28 nt (Fig. 1A, schematic). We used this substrate and an equivalent one for Apo3G having 5′-CCC motifs in place of the 5′-TTC motifs (Fig. 1B). The specific activities for Apo3A and Apo3G on these substrates were 6.1 and 21 pmol/μg/min, respectively. On this substrate, Apo3A catalyzed near to no processive deaminations, as demonstrated by the processivity factor of 1.1 (Fig. 1A, under the gel). The processivity factor is a ratio of the observed double deaminations (Fig. 1A, 5′-C & 3′-C band) to the calculated expected value if the double deaminations were from independent events of two enzymes, i.e. not processive (see “Experimental Procedures”). Therefore, a processivity factor of 1 indicates that the two deaminations on the substrate occurred by independent events. Importantly, if deaminations were allowed to take place outside of single-hit conditions (>15% substrate usage), then double deamination events were clearly seen on the gel indicating that the substrate can be acted upon by Apo3A in two independent events and be deaminated to near completion (data not shown). Apo3A also does not show a preference for deamination of either the 3′- or 5′-proximal motif (Fig. 1A, 5′-C/3′-C ratio is 1.2). The processive enzyme Apo3G demonstrates very different deamination characteristics on its cognate substrate (Fig. 1B). Apo3G has a processivity factor of 3.6 (Fig. 1B, gel). This means that Apo3G is ∼4-fold more likely to catalyze a processive deamination of two 5′-CCC motifs than deaminate each motif in a separate enzyme-substrate encounter. Apo3G also shows a characteristic preference for deamination of cytosine motifs at the 5′-end (Fig. 1B, 5′-C/3′-C ratio is 3.7) (28).
To determine whether Apo3A could deaminate C residues processively if the distance between the 5′-TTC motifs was changed, we tested substrates that had either a 3- or 12-nt distance between deamination motifs (Fig. 1, C and D). For the substrates with the 5′-TTC motifs 3 nt apart, Apo3A had a low processivity (Fig. 1C, processivity factor 1.3), suggesting that minimal local scanning can take place. However, when the substrate had the 5′-TTC motifs 12 nt apart, Apo3A did not catalyze processive deaminations as demonstrated by no double deamination band being detectable under single-hit conditions, which precluded the calculation of a processivity factor (Fig. 1D, 5′-C & 3′-C). Based on data from a mutant Apo3G that prefers to jump more than slide, the jumping movement appears to favor deamination motifs that are spaced farther apart (29). On a substrate with the 5′-TTC motifs separated by 61 nt, Apo3A demonstrates a processivity factor of 1.5 and a polarity preference of 2.1 for the 5′-proximal C (Fig. 1E). That the processivity factor is above 1 for this substrate indicates that Apo3A may be able to catalyze processive deaminations of distantly spaced cytosines a small proportion of the time (Fig. 1E). All together the data (Fig. 1, A, C, and D) indicate that Apo3A has a limited ability for local sliding (Fig. 1C) and distal jumping (Fig. 1E), suggesting that the large majority of deaminations catalyzed by Apo3A are non-processive.
Interaction of Apo3A with ssDNA
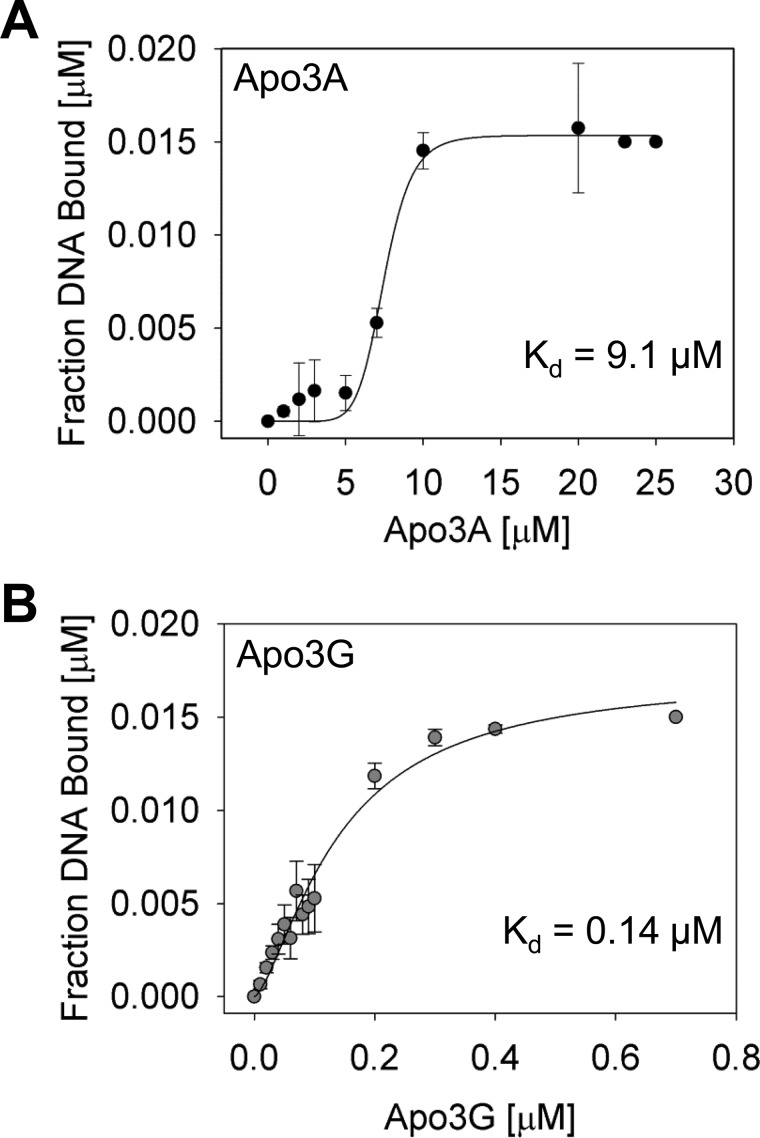
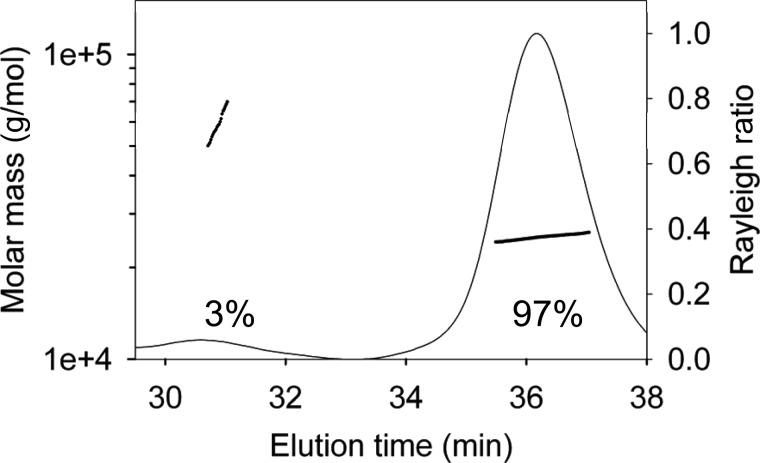
The inability of Apo3A to deaminate ssDNA with a high processivity may be due to a weak interaction with the ssDNA substrate. The apparent equilibrium dissociation constant (Kd) of Apo3A from the DNA substrate used in Fig. 1A (85 nt) was 9.1 μm (Fig. 2A). In contrast, Apo3G binds to its cognate deamination substrate with an apparent Kd of 0.14 μm (Fig. 2B). Apo3A showed an ∼65-fold reduced capacity to bind ssDNA than Apo3G, consistent with the decreased likelihood of Apo3A to catalyze processive deaminations. Furthermore, both Apo3A and Apo3G were best fit to a sigmoid curve during regression analysis. Consistent with these binding data, Apo3G exists as monomers and dimers in solution and oligomerizes to dimers and higher order structures when bound to ssDNA (62, 64). Using multiangle light scattering, Apo3A in solution was found to exist almost entirely (97%) as a monodisperse monomer (Fig. 3, 25 kDa), but the elution profile also had a small (3%) polydisperse dimer population (Fig. 3, 53 kDa). Taken together, the binding (Fig. 2A) and multiangle light scattering (Fig. 3) data suggest that Apo3A may also form dimers or other oligomeric forms on ssDNA.
FIGURE 2.
Apparent dissociation constant of Apo3A and Apo3G for ssDNA. Binding of Apo3A (A) or Apo3G (B) to ssDNA was studied using fluorescence depolarization (rotational anisotropy). A, binding of Apo3A to an 85-nt ssDNA containing two 5′-TTC deamination motifs is shown. Apo3A binds with an apparent Kd of 9.1 ± 2.5 μm. B, Apo3G binds to a substrate equivalent to that in A, except containing two 5′-CCC deamination motifs, with a ∼65-fold tighter affinity than Apo3A (apparent Kd of 0.14 ± 0.02 μm). Error bars represent the S.E. from three independent experiments.
FIGURE 3.
Determination of Apo3A molecular mass using multiangle light scattering. Purified Apo3A was resolved by size-exclusion chromatography in running buffer with 150 mm NaCl and 50 mm HEPES, pH 7.3. The molecular mass is plotted throughout the eluted peaks (line plot, left y axis). The Rayleigh light-scattering chromatogram shows the protein distribution and is plotted on the right y axis. The median molecular masses and distributions for Apo3A are 97% monomers (25,110 g/mol) and 3% dimers (52,850 g/mol).
Spectra of Apo3A-induced Mutations in a Model HIV Replication Assay
It has not been demonstrated whether an enzyme with zero to low processive behavior, such as Apo3A, is at a disadvantage when considering the extent of mutagenesis that can be induced during HIV replication. To test this, we used a previously established model HIV replication assay (29) in which an RNA is produced by in vitro T7-mediated transcription of a template containing a PPT, 120 nt of the prot of HIV-1, and lacZα. The RNA is reverse-transcribed into a dsDNA “provirus” after a synthetic primer is annealed to the RNA, and HIV-1 reverse transcriptase, nucleocapsid, and dNTPs are added to the reaction in the absence or presence of an Apo3 enzyme. The PPT is used as the second strand primer by reverse transcriptase (30). Afterward, the dsDNA is cloned into a vector for amplification in Escherichia coli in which the lacZα fragment is used to detect mutated clones by blue/white screening (see “Experimental Procedures”). The HIV prot gene is used as a marker of whether the Apo3-induced mutations would inactivate the gene based on results from an extensive protease mutagenesis study conducted by Loeb et al. (65). Because the ssDNA substrate for the Apo3 enzymes must be revealed through the RNase H activity of reverse transcriptase, regions farthest away from the second strand primer (PPT) are single-stranded the longest, i.e. the 5′-end of the (−)DNA strand, and are more likely to incur deaminations (22, 31).
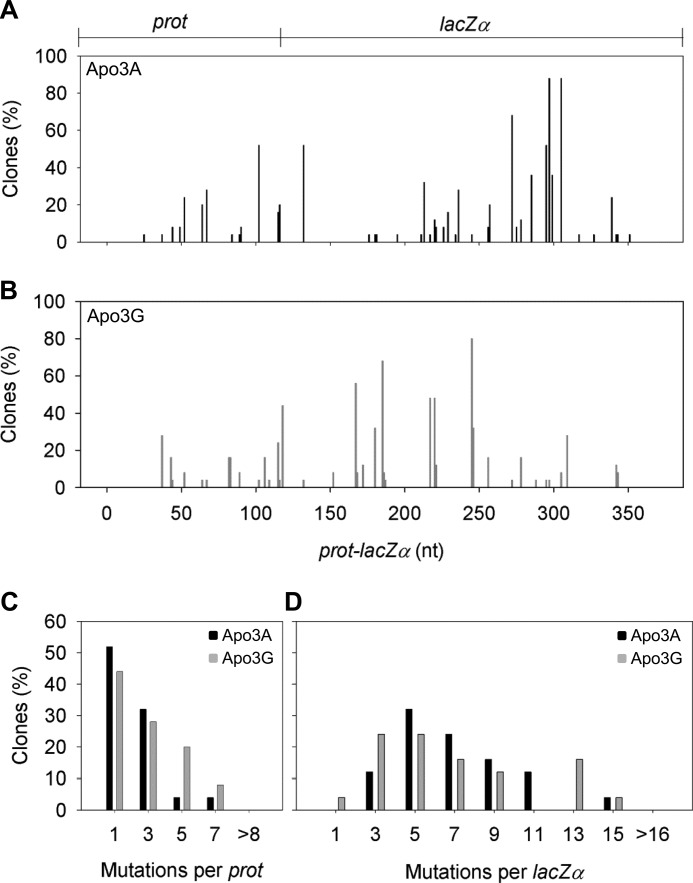
In this system reverse transcriptase causes a population mutation frequency (frequency of white colonies) of 0.10. Apo3A and Apo3G were able to induce an ∼9-fold increase in the mutation frequency to 0.86 and 0.90, respectively. The spectra of mutations across the whole prot-lacZα construct are different for Apo3A and Apo3G due to each enzyme having a distinct deamination motif. The spectra also demonstrate that both Apo3A and Apo3G have more highly mutated sites (>50% of clones) in the lacZα region than the prot region (Fig. 4, A and B), in agreement with the principle that this is due to the lacZα region being single-stranded the longest (31). In this “highly” accessible area, the mutations per lacZα were similar between Apo3G and Apo3A (Fig. 4D). These data suggest that in HIV genomic regions that remain single-stranded for extended periods, processivity is dispensable.
FIGURE 4.
Apo3A- and Apo3G-induced mutational spectra. A and B, shown are spectra of G → A mutations resulting from Apo3A (A)- or Apo3G (B)-catalyzed deaminations occurring during reverse transcription by HIV-1 reverse transcriptase. The DNA substrate formed contains 120 nt of the HIV-1 protease (prot) gene (nt 2282–2401) and 248 nt of the lacZα reporter sequence. Data are represented as the percentage of clones with mutations at a particular site. The x axis denotes the position of the mutation in the construct. C and D, histograms illustrate the per clone numbers of G → A mutations that can be obtained in the prot (C) or lacZα (D) induced by Apo3A (black square) or Apo3G (gray square) deaminations. Values were binned for analysis, and the maximum bin value is shown on the x axis.
When we examined the prot region, which would be less accessible in our system due to its proximity to the PPT, we find a difference in the mutations per clone between Apo3A and Apo3G (Fig. 4C). Where Apo3G has a linear decline in mutations from ∼45%, having 0–1 mutation per prot, 20% having 4–5 mutations per prot, and 9% having 6–7 mutations per prot, Apo3A clones usually had 3 mutations per prot or less (84%). In the prot region, we find that processive scanning of ssDNA appears to have an advantage perhaps because there is less time to search for target deamination motifs. Apo3A would likely have to dissociate and reassociate many times with the cDNA to find a deamination motif. The inefficiency of this process is only apparent near the PPT (compare Fig. 4, C and D).
We analyzed the fidelity of Apo3A for its target site in our “dynamic” deamination system for comparison to results obtained on naked ssDNA substrates (Table 1). Mutated sites across the length of both the prot and lacZα were scored based on the number of clones that contained a given mutation. Mutated sites that were found in more than one clone were described as a multideaminated site, and mutations found in single clones were described as orphan mutations. Apo3A demonstrated that of the total number of multideaminated sites, 70% of them were in a 5′-TC motif, whereas the remaining multideaminated sites were in a 5′-CC (15%), 5′-AC (7.5%), or 5′-GC (7.5%) context. Apo3G demonstrated that of the total number of multideaminated sites, 79% were in a 5′-CC motif, and the other sequence motifs deaminated multiple times were either 5′-TC (17%) or 5′-AC (4%). These data recapitulate the strong preference Apo3A has for its target site (Table 1) and confirm previous findings showing the specificity of the deamination motif for Apo3G (22). The ∼30% of off-target deaminations may be predisposed by the dynamics of HIV replication in which certain regions of (−)DNA are exposed for longer periods of time and incur more deaminations (22, 31, 32).
Consequences of Apo3A-induced Mutagenesis of the prot
The prot sequence portion of the reverse-transcribed RNA was further analyzed to determine the amino acid sequence changes resulting from Apo3-induced mutagenesis (Table 2). Consistent with different deamination motifs, Apo3A demonstrated a different mutagenesis profile than Apo3G. Mutations of the prot can either be effective in eliminating protease activity (lethal) or induce two types of sublethal mutations. First the mutations may not inactivate the protease and potentially give a selectable advantage to the virus, or second, they may lead to a known selectable advantage, antiretroviral drug resistance. We first analyzed the consequences of mutagenesis of the protease gene at a population level. The two possible amino acids in our sequence that could lead to protease inhibitor resistance, D30N and M46I, were mutated by Apo3A in 24 and 52% of the clones, respectively. These two sites along with E34K (20%) and D35N (28%), which do not inactivate the protease, were the “hot-spot” sites for Apo3A deamination. In general, Apo3A was able to deaminate very few sites that resulted in protease inactivation with the highest percentage mutation of this type being G51K at only 16% (Table 2). Apo3G was characterized by a greater number of inactivating mutations of the protease. The mutation found in the highest percentage of Apo3G deaminated clones was the inactivating mutation G52S at 48% (Table 2). Of the top four hot-spot mutations, three resulted in the inactivating mutations (G52S, 48%; G51R/K, 36%; G40R/E/K, 24%), and only D25N (32%) left the protease with wild-type activity (Table 2).
TABLE 2.
Deamination-induced changes in HIV-1 protease by the action of Apo3A or Apo3G
Protease enzyme activity was inferred from a mutational study carried out by Loeb et al. (65), where plus (+) is active or partially active, and minus (−) is inactive in comparison to wild-type protease. Protease inhibitor resistance information is from the HIV Drug Resistance Database at Stanford University. No recorded value is used to indicate that no clones were found with a mutation at that particular site.
| Protease amino acid position | Nucleotide change (underlined) | Amino acid change | Protease enzyme activity | Protease inhibitor resistance | Mutated clones |
|
|---|---|---|---|---|---|---|
| Apo3G | Apo3A | |||||
| % | ||||||
| 21 | GAA → AAA | Glu → Lys | + | 4 | ||
| 25 | GAT → AAT | Asp → Asn | + | 32 | 4 | |
| 27 | GGA → GAA | Gly → Glu | − | 4 | 8 | |
| GGA → AGA | Gly → Αrg | − | 16 | |||
| 29 | GAT → AAT | Asp → Asn | + | 8 | ||
| 30 | GAT → AAT | Asp → Asn | + | Yes | 8 | 24 |
| 34 | GAA → AAA | Glu → Lys | + | 4 | 20 | |
| 35 | GAT → AAT | Asp → Asn | + | 4 | 28 | |
| 40 | GGG → GGA | Gly → Gly | + | 4 | ||
| GGG → GAG | Gly → Glu | − | 8 | |||
| GGG → AGG | Gly → Αrg | − | 8 | |||
| GGG → AAG | Gly → Lys | − | 8 | |||
| 42 | TGG → TAG | Trp → STOP | − | 16 | 4 | |
| TGG → TGA | Trp → STOP | − | 8 | |||
| 46 | ATG → ATA | Met → Ile | + | Yes | 4 | 52 |
| 48 | GGG → AGG | Gly → Αrg | + | 16 | ||
| GGG → GGA | Gly → Gly | + | 4 | |||
| 49 | GGA → AGA | Gly → Αrg | − | 4 | ||
| 51 | GGA → AAA | Gly → Lys | − | 4 | 16 | |
| GGA → GAA | Gly → Glu | − | 4 | |||
| GGA → AGA | Gly → Αrg | − | 32 | |||
| 52 | GGT → AGT | Gly → Ser | − | 48 | ||
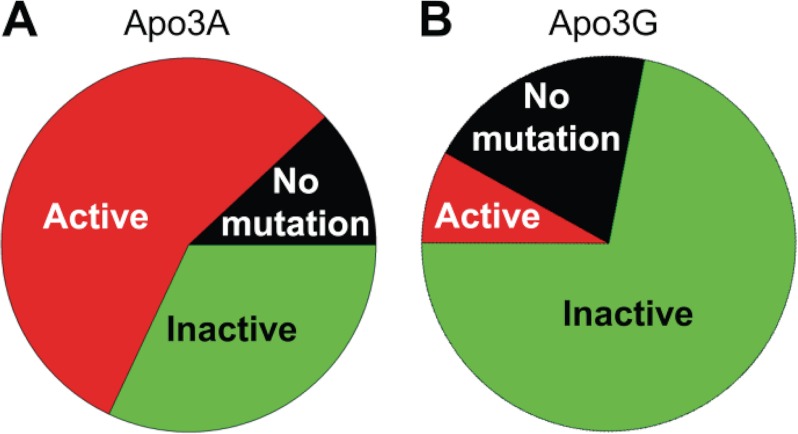
We also examined clones on an individual basis to gauge whether each model provirus synthesized in our assay system would be inactivated after exposure to Apo3A or Apo3G during DNA synthesis. Apo3A-induced mutations inactivated the protease less (Fig. 5A, 32%) than they allowed the protease to retain activity (Fig. 5A, 56%). Apo3A did not induce any mutations in the protease region in 12% of individual clones (Fig. 5A). The main difference between Apo3A and Apo3G is that Apo3G induced inactivating mutations in the majority of individual clones (Fig. 5B, 72%), and only 8% of individual clones coded for an active protease (Fig. 5B). Apo3G was found to catalyze no mutations in the prot gene of 20% of clones (Fig. 5B). Together these data suggest that Apo3A is ineffective in regard to inactivation of the prot gene. Although per clone Apo3G could induce more mutations in the prot region compared with Apo3A (Fig. 4C), both enzymes had prot genes that were unmutated. This suggests that regardless of scanning activity, either highly processive (Fig. 1B, Apo3G) or weakly processive (Fig. 1A, C–E, Apo3A), the temporal dynamics of HIV replication can protect against extensive deamination by Apo3 enzymes (32).
FIGURE 5.
Amino acid changes in the protease resulting from Apo3A- and Apo3G-catalyzed deaminations. Each prot region sequenced was analyzed individually to determine whether deaminations catalyzed by Apo3A (A) or Apo3G (B) were able to induce mutations that led to prot inactivation (Inactive), no inactivation (Active), or no mutations. A, Apo3A was able to inactivate the prot in only 32% of clones and left an active prot (56%) in the majority of clones. Apo3A did not induce any mutations in the prot in 12% of clones. B, Apo3G was effective in inactivating the prot (72% inactive; 8% active) if it catalyzed deaminations in this region. However, there were no mutations in the prot region in 20% of clones.
Deamination Activity of Apo3A on Transcribed dsDNA
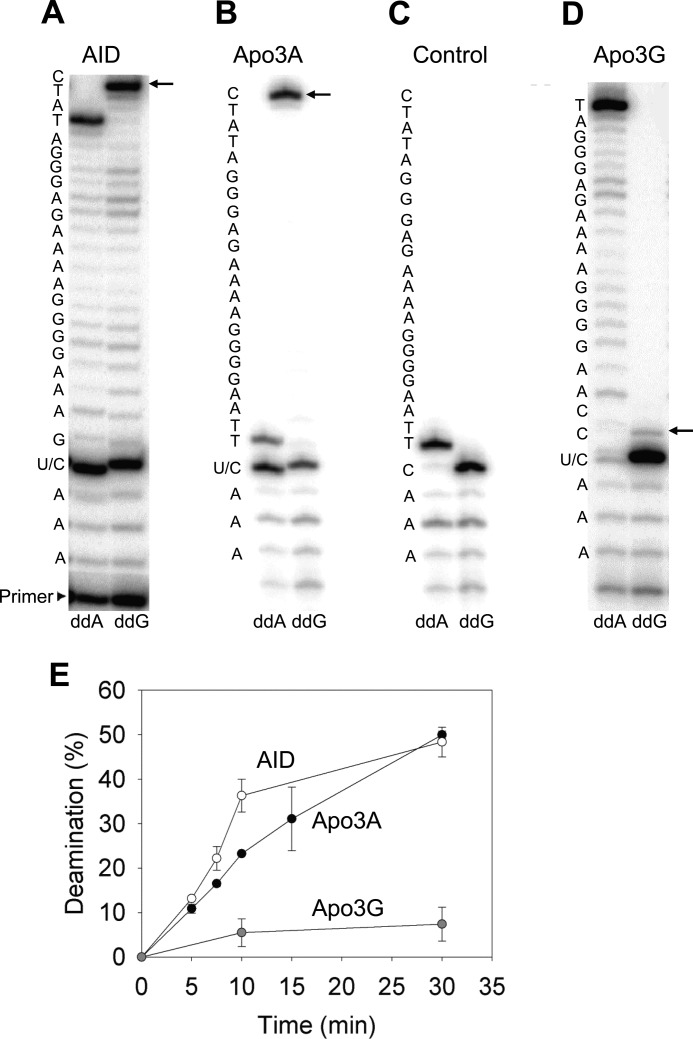
Apo3A localizes to both the cytoplasm and nucleus (34) and could potentially cause deaminations of transiently single-stranded nuclear DNA during transcription, similar to the APOBEC family member AID. To test this we used a 66-nt dsDNA that contained a T7 promoter sequence and a unique deamination motif on the non-transcribed strand as a substrate for T7 RNA polymerase, adapted from an assay used by Pham and colleagues (60). The dsDNA contained a cytosine motif specific for either Apo3A (5′-TTC), Apo3G (5′-CCC), or AID (5′-AGC). We used AID as a positive control (60) to demonstrate that the 5′-AGC motif can be rapidly deaminated (Fig. 6, A and E, 4.6% per min). Deaminations were detected by sequencing the non-transcribed strand in two parallel reactions using either a dG-, dT-, dC-containing dNTP mix with ddA or an dA-, dT-, dC-containing dNTP mix with ddG (see “Experimental Procedures”). If the template strand contained a uracil lesion, then the sequencing polymerase would incorporate a ddA, resulting in a stop band (Fig. 6A, ddA). This would correspond to a decrease in incorporation of ddG in the parallel reaction (Fig. 6A, ddG). Similar to AID, Apo3A had an ability to cause deaminations during transcription but at a reduced rate (Fig. 6, B and E, 2.4% per min). Without the presence of T7 RNA polymerase, no Apo3A-catalyzed deamination was detected (Fig. 6C, note the absence of ddA stop band at U/C position), demonstrating that Apo3A is unable to deaminate dsDNA. As a result, the deaminations must occur when the nontranscribed strand is transiently single-stranded during transcription. We hypothesized that Apo3G should be less able to deaminate dsDNA undergoing transcription due to its size. Apo3G is roughly twice the size of Apo3A and AID (46 kDa compared with 23 and 24 kDa, respectively), which could make access to the transcription bubble (∼9 nt) unlikely (66). As expected, Apo3G was not able to effectively catalyze deaminations during T7-mediated transcription (Fig. 6D). We quantified only 7.4% deamination after 30 min (Fig. 6E). These results demonstrate the ability of Apo3A to catalyze deaminations during transcription and suggests that this mode of deamination could occur in vivo.
FIGURE 6.
Deamination of dsDNA undergoing transcription by Apo3A and AID. The non-transcribed strand of a dsDNA substrate that underwent T7 RNA polymerase-mediated transcription in the presence of AID (A), Apo3A (B), or Apo3G (D) was sequenced using the primer elongation dideoxynucleotide termination assay. The sequence is denoted to the left of each gel. The U/C label indicates the cytosine embedded within the preferred deamination motif for AID, Apo3A, or Apo3G. When the C is deaminated to U, a stop band will be present at that position in the ddA lane. In the ddG lane, the presence of a U allows synthesis to bypass the U/C site, and the next C in the sequence will have a stop band (arrow) with an intensity that is proportional to the amount of C deamination. Reactions were allowed to proceed for 30 min. C, a dideoxynucleotide termination assay was performed as described for B but in the absence of T7 RNA polymerase. E, shown is the time course of deamination of dsDNA during T7 RNA polymerase-mediated transcription illustrating that Apo3A (black circle) is 2-fold less active than AID (○) and the absence of significant deamination activity of Apo3G (gray circle). Error bars represent the S.E. from at least two independent experiments.
DISCUSSION
Apo3A was recently demonstrated to be a viral restriction factor in macrophages (7) and monocytes (6). Yet there is little biochemical information available for Apo3A to understand the mechanism by which the enzyme catalyzes deaminations and searches for deamination motifs. Here we characterized Apo3A deamination activity in various in vitro systems. We found on naked ssDNA that Apo3A is weakly processive (Fig. 1, A and C–E). Despite this, Apo3A was able to induce mutations in nascent ssDNA produced through reverse transcription and deaminated dsDNA undergoing transcription.
Deamination of ssDNA by Apo3A
Our data indicate that Apo3A can, albeit inefficiently, jump long distances (at least 63 nt, Fig. 1E, processivity factor 1.5) and scan locally (at least 5-nt, Fig. 1C, processivity factor 1.3, but less than 15 nt, Fig. 1D). Although we can only infer the movement of Apo3A on ssDNA based on its deamination kinetics (Fig. 1, C and E), the data support the hypothesis that Apo3A must have exerted its limited scanning capability on the other DNA substrates we tested, but the 5′-TTC motifs were not spaced optimally to detect this in the form of deaminations (Fig. 1, A and D). This polarized form of processivity appears to exemplify facilitated diffusion (26). In this mode of DNA scanning, common for enzymes that must search DNA nonspecifically to find their target catalytic sequence, the enzyme can move by one-dimensional sliding and three-dimensional microscopic dissociations and reassociations (termed hopping (<20-nt) or jumping (>20-nt)) with the DNA (25, 26). However, because Apo3A is only ∼1.5-fold more likely to make a processive deamination than one that is nonprocessive (Fig. 1, C and E) and the apparent Kd is in the micromolar range (Fig. 2A), we assume that most interactions of Apo3A with DNA result in rapid dissociation of the enzyme from the substrate. This is consistent with its specific activity being ∼3-fold less than the processive enzyme Apo3G (6.1 and 21 pmol/μg/min, respectively).
That Apo3A is not highly processive did not prevent ample deaminations from occurring in the lacZα region of the cDNA in our HIV replication assay (Fig. 4, A and D). In this region, which is exposed as ssDNA for the longest time, there was about an equal amount of mutations per clone for Apo3A and the highly processive enzyme Apo3G (Fig. 4D). Based on our deamination data (Fig. 1, A and C–E), it appears that Apo3A would have to make multiple associations with the (−)DNA to induce these multiple mutations. The data (Fig. 4D) suggest that there is little consequence of this inefficient search mechanism if the DNA is single-stranded for an extended time. However, there is an inefficiency that becomes apparent when the DNA does not remain single-stranded for an extended time, such as the prot region nearest the PPT (Fig. 4C). Apo3A demonstrated a decreased ability to catalyze numerous deaminations per prot region compared with the processively scanning Apo3G. Apo3G had >3 mutations in 28% of clones, whereas Apo3A had >3 mutations in only 8% of clones (Fig. 4C). However, at a population level, both Apo3A and Apo3G induced the same mutation frequency (white colonies, 0.86 and 0.90, respectively, data not shown). A high number of white colonies, illustrating association with many different (−)DNAs, would be expected for an enzyme with low processivity such as Apo3A. Apo3G has a bimodal off rate from ssDNA that includes short and long binding events (62, 63) that can reconcile how a processive enzyme can also cause a high number of clones to be mutated.
Consequences of Apo3A-induced Mutations
When comparing the protease inactivation potential of Apo3A and Apo3G, our data indicate that it is the sequence context in which the mutations occurred that was most important for inactivation of the protease rather than the total number of mutations (Fig. 4, Table 2, and Fig. 5). To establish this, we compared whether the protease was inactivated by a single mutation by Apo3G or Apo3A. In the 5 clones of Apo3G with a single mutation in the protease, 60% resulted in an inactive protease. For Apo3A, for the 9 clones with a single mutation in the protease, only 11% resulted in an inactive protease. When we examined the clones having 2 or 3 Apo3A-induced mutations in the prot, we found that still only 40% of the clones would result in an inactivated protease. For Apo3G, 100% of the clones with more than 1 mutation resulted in an inactive protease. All together, the data indicate that deamination of a 5′-CC motif causes more inactivation of protease than a 5′-TC motif. There appears to be two key differences in the 5′-CC and 5′-TC deamination motifs that account for our observations.
The 5′-CC motif is found in codons for Gly (Table 2, 5′-GGN). The deamination would occur on the complementary DNA strand in the context of 5′-NCC, 5′-CCC, and rarely 5′-CCC (underlined C is deaminated) or any combination (Table 2). These changes mostly resulted in mutation of the Gly to a charged amino acid (Table 2; Arg, Glu, or Lys), which is a nonconservative change that has a high chance to cause gene inactivation, especially if the change takes place near the enzyme active site (65, 67). Furthermore, it has been suggested that Apo3G can result in a high frequency of stop codons being introduced into the HIV coding sequence because the only codon for Trp, 5′-TGG, is an Apo3G deamination motif in the (−)DNA strand (5′-CCA) (22). Deamination of the second C would result in the coding sequence being changed to 5′-TAG. In our prot sequence there is one Trp codon, and Apo3G did induce mutagenesis to form a stop codon 16% of the time (Table 2).
The 5′-TC motif does not have these features that are observed for the 5′-CC motif. The 5′-TC motif most often occurs in the codon for Asp, and the deamination-induced mutation changes it to an Asn (Table 2). This change from an acidic amino acid (Asp) to its amide (Asn) is a conservative change and unlikely to cause enzyme inactivation (65, 67). The only other amino acid change Apo3A induced at a high level is the Met to Ile mutation (Table 2), which is also considered to be a conservative change (65, 67). Apo3A could cause a stop codon to be formed at position 42 in 4% of clones (Table 2), but this is an off-target site, i.e. not 5′-TC. However, the Trp codon occurred with a surrounding sequence context of 5′-A TGG A, which would read as 5′-T CCA T in the (−)DNA, and enabled Apo3A to induce mutagenesis of the codon to 5′-TGA in 8% of clones (Table 2). This suggests that the 5′-TC motif is inherently less likely to cause gene inactivation, as demonstrated by our data on Apo3A-induced mutagenesis of HIV-1 protease (Table 2, Fig. 5A). This has implications for other Apo3 family members that prefer to deaminate 5′-TC motifs. Apo3F has been shown to have negligible ability to restrict HIV when stably expressed (68) and supports the notion that the mutations induced by deamination of 5′-TC motifs could be ineffective.
It is particularly striking that in addition to Apo3A inducing a number of non-inactivating mutations in the prot, Apo3A is able to induce such a large number of drug-resistant mutations at M46I (Table 2, 52% of clones) and D30N (Table 2, 24% of clones). The data could imply that even if Apo3A were able to mutationally inactivate an HIV provirus, it would likely harbor these drug-resistant mutations and upon integration could act as a repository for viral recombination, as has been demonstrated for Apo3G (69). A recent analysis of how Apo3A restricts HIV replication suggests that Apo3A, in addition to or as a result of deaminating (−)DNA, decreases viral transcripts (6). This may be an evolutionarily favored use of Apo3A in which the provirus formation is blocked, and integration is avoided to suppress Apo3A contributing to viral evolution or drug resistance. However, a caveat to this reasoning is that the mutation frequencies obtained in our model HIV replication assay may differ in vivo where the effect of these mutations on viral fitness could add a limiting factor.
Potential of Apo3A to Be a Genomic Mutator
For AID, closely spaced deamination events that occur outside the target immunoglobulin genes during transcription can initiate unprogrammed dsDNA strand breakage-induced translocations during DNA repair (70). For example, there are characteristic AID-induced translocations that result in unregulated expression of the proto-oncogene c-myc (46). In combination with the loss of a replication checkpoint control, AID-induced DNA damage can result in cellular transformation (71). Induction of such dsDNA breaks could also be envisioned for Apo3A based on our data showing that Apo3A can catalyze deaminations during transcription of dsDNA (Fig. 6, B and E) and published data showing that Apo3A in a deamination-dependent manner could induce DNA breaks and activate DNA damage responses (42). Although it was reported that these Apo3A-induced DNA breaks occurred during S-phase (42), suggesting that Apo3A accesses ssDNA regions of the genome during DNA replication, it does not exclude the possibility that Apo3A could also deaminate cytosines in various highly transcribed genes outside of S-phase. If Apo3A-catalyzed deaminations were not so numerous as to lead to genomic dsDNA breaks, they may lead to somatic hypermutation if repaired erroneously. Our data (Table 2) demonstrating that Apo3A deaminations induce mostly conservative amino acid changes suggests that most somatic hypermutation events would not lead to gene inactivation. However, this may not be benign as the protein function could be altered and still have negative consequences if it were to occur within a proto-oncogene. In a study of AID mutants it was demonstrated that AID does not function with the highest possible specific activity, suggesting an evolutionary measure has been imposed to control unregulated genomic damage and unprogrammed gene translocations (72). For Apo3A, its low processivity (Fig. 1, A and C–E) and low specific activity (∼3-fold less than Apo3G) could be a means of regulating its ability to cause genomic instability.
Supplementary Material
Acknowledgments
DNA substrates for deamination assays were obtained through the Tri-Link Biotechnologies Research Rewards Program. The SEC-LX/UV/RI instrumentation was supported by National Institutes of Health Award ISI0RR023748-01. We thank Madison Adolph for assistance with preparation of plasmids for DNA sequencing and Yuqing Feng and Anjuman Ara for critical discussion of the manuscript.
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada, a new investigator establishment grant from the Saskatchewan Health Research Foundation, and The Canadian Foundation for Innovation.

This article contains supplemental Table S1.
- Apo3
- apolipoprotein B mRNA-editing, enzyme-catalytic, polypeptide-like 3 (APOBEC3)
- Vif
- viral infectivity factor
- ssDNA
- single-stranded DNA
- PPT
- polypurine tract
- AID
- activation induced deaminase
- F
- fluorescein
- nt
- nucleotide(s).
REFERENCES
- 1. Sheehy A. M., Gaddis N. C., Choi J. D., Malim M. H. (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 [DOI] [PubMed] [Google Scholar]
- 2. Harris R. S., Bishop K. N., Sheehy A. M., Craig H. M., Petersen-Mahrt S. K., Watt I. N., Neuberger M. S., Malim M. H. (2003) DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809 [DOI] [PubMed] [Google Scholar]
- 3. Mangeat B., Turelli P., Caron G., Friedli M., Perrin L., Trono D. (2003) Broad antiretroviral defense by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424, 99–103 [DOI] [PubMed] [Google Scholar]
- 4. Zhang H., Yang B., Pomerantz R. J., Zhang C., Arunachalam S. C., Gao L. (2003) The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu Y. L., Greene W. C. (2008) The APOBEC3 cytidine deaminases. An innate defensive network opposing exogenous retroviruses and endogenous retroelements. Annu. Rev. Immunol. 26, 317–353 [DOI] [PubMed] [Google Scholar]
- 6. Berger G., Durand S., Fargier G., Nguyen X. N., Cordeil S., Bouaziz S., Muriaux D., Darlix J. L., Cimarelli A. (2011) APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7, e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koning F. A., Goujon C., Bauby H., Malim M. H. (2011) Target cell-mediated editing of HIV-1 cDNA by APOBEC3 proteins in human macrophages. J. Virol. 85, 13448–13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarmuz A., Chester A., Bayliss J., Gisbourne J., Dunham I., Scott J., Navaratnam N. (2002) An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 79, 285–296 [DOI] [PubMed] [Google Scholar]
- 9. Harris R. S., Liddament M. T. (2004) Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 4, 868–877 [DOI] [PubMed] [Google Scholar]
- 10. Haché G., Liddament M. T., Harris R. S. (2005) The retroviral hypermutation specificity of APOBEC3F and APOBEC3G is governed by the C-terminal DNA cytosine deaminase domain. J. Biol. Chem. 280, 10920–10924 [DOI] [PubMed] [Google Scholar]
- 11. Navarro F., Bollman B., Chen H., König R., Yu Q., Chiles K., Landau N. R. (2005) Complementary function of the two catalytic domains of APOBEC3G. Virology 333, 374–386 [DOI] [PubMed] [Google Scholar]
- 12. Bogerd H. P., Wiegand H. L., Doehle B. P., Cullen B. R. (2007) The intrinsic antiretroviral factor APOBEC3B contains two enzymatically active cytidine deaminase domains. Virology 364, 486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonvin M., Greeve J. (2007) Effects of point mutations in the cytidine deaminase domains of APOBEC3B on replication and hypermutation of hepatitis B virus in vitro. J. Gen. Virol. 88, 3270–3274 [DOI] [PubMed] [Google Scholar]
- 14. Sheehy A. M., Gaddis N. C., Malim M. H. (2003) The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9, 1404–1407 [DOI] [PubMed] [Google Scholar]
- 15. Stopak K., de Noronha C., Yonemoto W., Greene W. C. (2003) HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12, 591–601 [DOI] [PubMed] [Google Scholar]
- 16. Conticello S. G., Harris R. S., Neuberger M. S. (2003) The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr. Biol. 13, 2009–2013 [DOI] [PubMed] [Google Scholar]
- 17. Marin M., Rose K. M., Kozak S. L., Kabat D. (2003) HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9, 1398–1403 [DOI] [PubMed] [Google Scholar]
- 18. Marin M., Golem S., Rose K. M., Kozak S. L., Kabat D. (2008) Human immunodeficiency virus type 1 Vif functionally interacts with diverse APOBEC3 cytidine deaminases and moves with them between cytoplasmic sites of mRNA metabolism. J. Virol. 82, 987–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vázquez-Pérez J. A., Ormsby C. E., Hernández-Juan R., Torres K. J., Reyes-Terán G. (2009) APOBEC3G mRNA expression in exposed seronegative and early stage HIV-infected individuals decreases with removal of exposure and with disease progression. Retrovirology 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. An P., Bleiber G., Duggal P., Nelson G., May M., Mangeat B., Alobwede I., Trono D., Vlahov D., Donfield S., Goedert J. J., Phair J., Buchbinder S., O'Brien S. J., Telenti A., Winkler C. A. (2004) APOBEC3G genetic variants and their influence on the progression to AIDS. J. Virol. 78, 11070–11076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nowarski R., Britan-Rosich E., Shiloach T., Kotler M. (2008) Hypermutation by intersegmental transfer of APOBEC3G cytidine deaminase. Nat. Struct. Mol. Biol. 15, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 22. Yu Q., König R., Pillai S., Chiles K., Kearney M., Palmer S., Richman D., Coffin J. M., Landau N. R. (2004) Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat. Struct. Mol. Biol. 11, 435–442 [DOI] [PubMed] [Google Scholar]
- 23. Suspène R., Sommer P., Henry M., Ferris S., Guétard D., Pochet S., Chester A., Navaratnam N., Wain-Hobson S., Vartanian J. P. (2004) APOBEC3G is a single-stranded DNA cytidine deaminase and functions independently of HIV reverse transcriptase. Nucleic Acids Res. 32, 2421–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coffin J. M., Hughes S. H., Varmus H. E. (1997) Retroviruses, pp. 121–160, Cold Spring Harbor Laboratory Press, Plainview, NY: [PubMed] [Google Scholar]
- 25. Halford S. E., Marko J. F. (2004) How do site-specific DNA-binding proteins find their targets? Nucleic Acids Res. 32, 3040–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berg O. G., Winter R. B., von Hippel P. H. (1981) Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry 20, 6929–6948 [DOI] [PubMed] [Google Scholar]
- 27. Chelico L., Prochnow C., Erie D. A., Chen X. S., Goodman M. F. (2010) Structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J. Biol. Chem. 285, 16195–16205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chelico L., Pham P., Calabrese P., Goodman M. F. (2006) APOBEC3G DNA deaminase acts processively 3′- → 5′- on single-stranded DNA. Nat. Struct. Mol. Biol. 13, 392–399 [DOI] [PubMed] [Google Scholar]
- 29. Feng Y., Chelico L. (2011) Intensity of deoxycytidine deamination of HIV-1 proviral DNA by the retroviral restriction factor APOBEC3G is mediated by the noncatalytic domain. J. Biol. Chem. 286, 11415–11426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rausch J. W., Le Grice S. F. (2004) Binding, bending, and bonding. Polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. Int. J. Biochem. Cell Biol. 36, 1752–1766 [DOI] [PubMed] [Google Scholar]
- 31. Suspène R., Rusniok C., Vartanian J. P., Wain-Hobson S. (2006) Twin gradients in APOBEC3 edited HIV-1 DNA reflect the dynamics of lentiviral replication. Nucleic Acids Res. 34, 4677–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu C., Saenz D. T., Fadel H. J., Walker W., Peretz M., Poeschla E. M. (2010) The HIV-1 central polypurine tract functions as a second line of defense against APOBEC3G/F. J. Virol. 84, 11981–11993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogerd H. P., Wiegand H. L., Doehle B. P., Lueders K. K., Cullen B. R. (2006) APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells. Nucleic Acids Res. 34, 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H., Lilley C. E., Yu Q., Lee D. V., Chou J., Narvaiza I., Landau N. R., Weitzman M. D. (2006) APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr. Biol. 16, 480–485 [DOI] [PubMed] [Google Scholar]
- 35. Bogerd H. P., Wiegand H. L., Hulme A. E., Garcia-Perez J. L., O'Shea K. S., Moran J. V., Cullen B. R. (2006) Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc. Natl. Acad. Sci. U.S.A. 103, 8780–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muckenfuss H., Hamdorf M., Held U., Perkovic M., Löwer J., Cichutek K., Flory E., Schumann G. G., Münk C. (2006) APOBEC3 proteins inhibit human LINE-1 retrotransposition. J. Biol. Chem. 281, 22161–22172 [DOI] [PubMed] [Google Scholar]
- 37. Refsland E. W., Stenglein M. D., Shindo K., Albin J. S., Brown W. L., Harris R. S. (2010) Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues. Implications for HIV-1 restriction. Nucleic Acids Res. 38, 4274–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koning F. A., Newman E. N., Kim E. Y., Kunstman K. J., Wolinsky S. M., Malim M. H. (2009) Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J. Virol. 83, 9474–9485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stenglein M. D., Burns M. B., Li M., Lengyel J., Harris R. S. (2010) APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nat. Struct. Mol. Biol. 17, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santoni de Sio F. R., Trono D. (2009) APOBEC3G-depleted resting CD4+ T cells remain refractory to HIV1 infection. PLoS One 4, e6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamata M., Nagaoka Y., Chen I. S. (2009) Reassessing the role of APOBEC3G in human immunodeficiency virus type 1 infection of quiescent CD4+ T-cells. PLoS Pathog. 5, e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landry S., Narvaiza I., Linfesty D. C., Weitzman M. D. (2011) APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 12, 444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suspène R., Aynaud M. M., Guétard D., Henry M., Eckhoff G., Marchio A., Pineau P., Dejean A., Vartanian J. P., Wain-Hobson S. (2011) Somatic hypermutation of human mitochondrial and nuclear DNA by APOBEC3 cytidine deaminases, a pathway for DNA catabolism. Proc. Natl. Acad. Sci. U.S.A. 108, 4858–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peled J. U., Kuang F. L., Iglesias-Ussel M. D., Roa S., Kalis S. L., Goodman M. F., Scharff M. D. (2008) The biochemistry of somatic hypermutation. Annu. Rev. Immunol. 26, 481–511 [DOI] [PubMed] [Google Scholar]
- 45. Liu M., Duke J. L., Richter D. J., Vinuesa C. G., Goodnow C. C., Kleinstein S. H., Schatz D. G. (2008) Two levels of protection for the B cell genome during somatic hypermutation. Nature 451, 841–845 [DOI] [PubMed] [Google Scholar]
- 46. Robbiani D. F., Bothmer A., Callen E., Reina-San-Martin B., Dorsett Y., Difilippantonio S., Bolland D. J., Chen H. T., Corcoran A. E., Nussenzweig A., Nussenzweig M. C. (2008) AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell 135, 1028–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Durandy A., Peron S., Fischer A. (2006) Hyper-IgM syndromes. Curr. Opin. Rheumatol. 18, 369–376 [DOI] [PubMed] [Google Scholar]
- 48. Minegishi Y., Lavoie A., Cunningham-Rundles C., Bédard P. M., Hébert J., Côté L., Dan K., Sedlak D., Buckley R. H., Fischer A., Durandy A., Conley M. E. (2000) Mutations in activation-induced cytidine deaminase in patients with hyper IgM syndrome. Clin. Immunol. 97, 203–210 [DOI] [PubMed] [Google Scholar]
- 49. McBride K. M., Gazumyan A., Woo E. M., Barreto V. M., Robbiani D. F., Chait B. T., Nussenzweig M. C. (2006) Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 103, 8798–8803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Le Grice S. F., Grüninger-Leitch F. (1990) Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur. J. Biochem. 187, 307–314 [DOI] [PubMed] [Google Scholar]
- 51. Chelico L., Pham P., Goodman M. F. (2009) Stochastic properties of processive cytidine DNA deaminases AID and APOBEC3G. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Creighton S., Bloom L. B., Goodman M. F. (1995) Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 262, 232–256 [DOI] [PubMed] [Google Scholar]
- 53. Gao F., Robertson D. L., Morrison S. G., Hui H., Craig S., Decker J., Fultz P. N., Girard M., Shaw G. M., Hahn B. H., Sharp P. M. (1996) The heterosexual human immunodeficiency virus type 1 epidemic in Thailand is caused by an intersubtype (A/E) recombinant of African origin. J. Virol. 70, 7013–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Briggs J. A., Simon M. N., Gross I., Krausslich H. G., Fuller S. D., Vogt V. M., Johnson M. C. (2004) The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 11, 672–675 [DOI] [PubMed] [Google Scholar]
- 55. Zhu P., Chertova E., Bess J., Jr., Lifson J. D., Arthur L. O., Liu J., Taylor K. A., Roux K. H. (2003) Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. U.S.A. 100, 15812–15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu H., Chertova E., Chen J., Ott D. E., Roser J. D., Hu W. S., Pathak V. K. (2007) Stoichiometry of the antiviral protein APOBEC3G in HIV-1 virions. Virology 360, 247–256 [DOI] [PubMed] [Google Scholar]
- 57. Folta-Stogniew E., Williams K. R. (1999) Determination of molecular masses of proteins in solution. Implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 10, 51–63 [PMC free article] [PubMed] [Google Scholar]
- 58. Wyatt P. J. (1993) Light-Scattering and the Absolute Characterization of Macromolecules. Anal. Chim. Acta 272, 1–40 [Google Scholar]
- 59. Bransteitter R., Pham P., Scharff M. D., Goodman M. F. (2003) Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl. Acad. Sci. U.S.A. 100, 4102–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pham P., Bransteitter R., Petruska J., Goodman M. F. (2003) Processive AID-catalyzed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 424, 103–107 [DOI] [PubMed] [Google Scholar]
- 61. Beale R. C., Petersen-Mahrt S. K., Watt I. N., Harris R. S., Rada C., Neuberger M. S. (2004) Comparison of the differential context dependence of DNA deamination by APOBEC enzymes. Correlation with mutation spectra in vivo. J. Mol. Biol. 337, 585–596 [DOI] [PubMed] [Google Scholar]
- 62. Chelico L., Sacho E. J., Erie D. A., Goodman M. F. (2008) A model for oligomeric regulation of APOBEC3G cytosine deaminase-dependent restriction of HIV. J. Biol. Chem. 283, 13780–13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Senavirathne G., Jaszczur M., Auerbach P. A., Upton T. G., Chelico L., Goodman M. F., Rueda D. (2012) Single-stranded DNA scanning and deamination by APOBEC3G cytidine deaminase at single molecule resolution. J. Biol. Chem. 287, 15826–15835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shlyakhtenko L. S., Lushnikov A. Y., Li M., Lackey L., Harris R. S., Lyubchenko Y. L. (2011) Atomic force microscopy studies provide direct evidence for dimerization of the HIV restriction factor APOBEC3G. J. Biol. Chem. 286, 3387–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd (1989) Complete mutagenesis of the HIV-1 protease. Nature 340, 397–400 [DOI] [PubMed] [Google Scholar]
- 66. Temiakov D., Anikin M., McAllister W. T. (2002) Characterization of T7 RNA polymerase transcription complexes assembled on nucleic acid scaffolds. J. Biol. Chem. 277, 47035–47043 [DOI] [PubMed] [Google Scholar]
- 67. Betts M. J., Russel R. B. (2003) Amino Acid Properties and Consequences of Substitutions, pp. 289–316, John Wiley & Sons, Chichester, UK [Google Scholar]
- 68. Miyagi E., Brown C. R., Opi S., Khan M., Goila-Gaur R., Kao S., Walker R. C., Jr., Hirsch V., Strebel K. (2010) Stably expressed APOBEC3F has negligible antiviral activity. J. Virol. 84, 11067–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mulder L. C., Harari A., Simon V. (2008) Cytidine deamination induced HIV-1 drug resistance. Proc. Natl. Acad. Sci. U.S.A. 105, 5501–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nussenzweig A., Nussenzweig M. C. (2010) Origin of chromosomal translocations in lymphoid cancer. Cell 141, 27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Robbiani D. F., Bunting S., Feldhahn N., Bothmer A., Camps J., Deroubaix S., McBride K. M., Klein I. A., Stone G., Eisenreich T. R., Ried T., Nussenzweig A., Nussenzweig M. C. (2009) AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol. Cell 36, 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang M., Yang Z., Rada C., Neuberger M. S. (2009) AID upmutants isolated using a high-throughput screen highlight the immunity/cancer balance limiting DNA deaminase activity. Nat. Struct. Mol. Biol. 16, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.